Abstract
Cells engage sophisticated programs of DNA damage response (DDR) and repair to guard against genetic mutations. While there is significant knowledge concerning DDR in interphase cells, much less is known about these processes in mitosis. Direct interaction between MDC1, a master DDR organizer, and a marker of DNA damage, histone γH2AX, is required to trigger robust repair. Here we show that the DNA damage-induced interaction between MDC1 and γH2AX is attenuated in mitosis. Furthermore, inhibition in the activity of the core mitotic regulator CDK1, either by pharmacological inhibition or siRNA attenuation, enhances MDC1-γH2AX colocalization in mitosis. Our findings offer key new insights into how DDR is controlled during mitosis.
Keywords: Mitosis, CDK1, MDC1, γH2AX, DDR, DSB
Introduction
Among various types of DNA damage, the DNA double strand break (DSB) is the most detrimental type, as no intact strand is left as a template for repair unless cell processes duplicate genomic material during proliferation. Eukaryotic cells have evolved an intricate DNA damage response (DDR) system to ensure the error-free duplication and separation of genomic information during the cell cycle. A primary sensor for DSB is H2AX, a variant of histone H2A, which is a component of the nucleosome (1). Upon DSB, the MRE11-RAD50-NBS1 (MRN) complex recognizes free ends of DNA and recruits activated ataxia telangiectasia-mutated (ATM) to phosphorylate H2AX on Serine 139. This phosphorylated H2AX, termed γH2AX, directly interacts with an adaptor protein, mediator of DNA damage checkpoint 1 (MDC1), to initiate a cascade of DDR, amplifying and transducing the signal to downstream effectors to induce cell cycle arrest and DNA repair (2). Alternatively, cells can undergo apoptosis or permanent senescence to prevent the propagation of mutated daughter cells (3).
One hallmark of DDR is the accumulation and retention of repair proteins, which form microscopically discernible foci at the lesion sites. Such foci formation is hierarchical and depends on direct interaction between upstream and downstream factors (4). Over the past decade, extensive studies have generated a wealth of information on the mechanism of DDR in interphase cells; however, the precise mechanism regulating DDR during mitosis has yet to be unveiled. Previously, our lab has reported mitotic arrest as a novel source of DSBs (5). We hypothesize that the increase in DSBs during prolonged mitosis is due to lack of adequate DDR and accumulation of spontaneous inherent DNA damage. Supporting our hypothesis, recent research suggests an interesting model of incomplete mitotic DDR, wherein lesions are marked but downstream DDR is delayed until after mitotic exit (6). Accordingly, major mitotic kinases have been shown to actively inhibit DNA damage checkpoints (7). In particular, cyclin-dependent kinase 1 (CDK1) exhibits inhibitory effects on DSB repair shown in yeast and cell-free Xenopus laevis systems (8, 9). However, how the master organizer MDC1 is regulated during mitosis is unknown. On the one hand, MDC1 colocalizes with γH2AX in human osteosarcoma U2OS cell line after γ-irradiation (6). On the other hand, MDC1 physically binds to anaphase-promoting complex/cyclosome (APC/C) complex and promote activation thereof (10). Consistently, siRNA downregulation of MDC1 blocks metaphase-anaphase transition (11). The dual role of MDC1 in mitosis indicates an unknown regulator. Here, we report that CDK1 activity reduces MDC1-γH2AX interaction during mitosis.
Materials and Methods
Cell culture and treatments
All cell lines were obtained from ATCC and authenticated by short tandem repeat profiling. Early passage cells were used for experimentation and maintained in McCoy’s 5A medium, supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum. Nocodazole and RO3306 were used at 200 nM and 2 μM, respectively. γ-irradiation was done in a Cs-137 Gamma cell. CDK1 knockdowns were performed using Stealth Select siRNA by RNAiMax transfection (Life Technologies). Final concentration of siRNA oligonucleotides was 20 nM. All analyses and further treatments were done 48 h post transfection.
Immunofluorescent staining
Cells received 2 Gy of γ-irradiation and recovered for 30 m before fixation with 2% formaldehyde/PBS at RT, washed and blocked with staining buffer (3% BSA, 0.2% Triton™ X-100 (Sigma Aldrich). Samples were incubated with primary and secondary antibodies for 1 h each at RT before Hoechst counterstaining. Confocal microscopy images were taken from Zeiss LSM Meta 510.
Immunoprecipitation
For co-immunoprecipitation, lysates were prepared with Nuclear Complex Co-IP Kit (Active Motif) with minor modifications. For denaturing immunoprecipitation, whole cell extracts were denatured by boiling in denaturing buffer before immunoprecipitation.
Results and Discussion
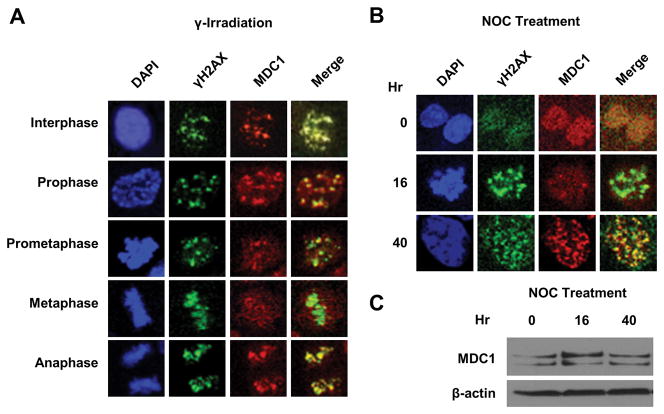
Considering that foci formation is required for efficient repair of DSB, we first examined MDC1-γH2AX colocalization patterns in various phases of mitosis. Asynchronous HCT116 were subjected to 2 Gy of γ-irradiation, which efficiently induced DSBs without preventing mitosis (12). In contrast to the formation of distinct foci in interphase and anaphase cells, there was a progressive loss of colocalization of MDC1 with γH2AX during prometaphase and metaphase, as evidenced by the increased diffuse immunostaining of MDC1 (Fig. 1A). The colocalized MDC1-γH2AX foci returned upon commencement of anaphase. The distinct localization pattern of MDC1 coincided with dynamics of CDK1 activity, which reaches its peak in late prophase and declines in anaphase (13). The relatively short time frame in which the MDC1 foci reappeared in anaphase suggests that their formation is posttranslationally regulated.
Figure 1. MDC1-γH2AX colocalization decreases during mitosis.
(A) Immunofluorescent staining of MDC1 and γH2AX in HCT116 cells following γ irradiation.
(B) Immunofluorescent staining of MDC1 and γH2AX during nocodazole (NOC) treatment. Representative prometaphase (16 h) and post-mitotic (40 h) cells were shown. The mitotic indices at the corresponding times points are shown in Suppl. Fig. S1. (C) Western blot of MDC1 in HCT116 cells at corresponding time points in (B).
To confirm such colocalization indeed decreases in prometaphase, we introduced prometaphase block by nocodazole treatment and investigated MDC1-γH2AX colocalization. Consistently, MDC1 failed to colocalize with γH2AX in mitotically arrested cells (Fig. 1B; 16 h), whereas such colocalization was restored in postmitotic cells (Fig. 1B; 40 h). We also collected cells from the corresponding time points and measured MDC1 protein levels, which were not decreased in cells arrested in mitosis at 16 h (Fig 1C; Suppl. Fig. S1). These observations suggest that the decreased colocalization of MDC1- γH2AX occurs in prometaphase and is likely due to posttranslational modification of MDC1.
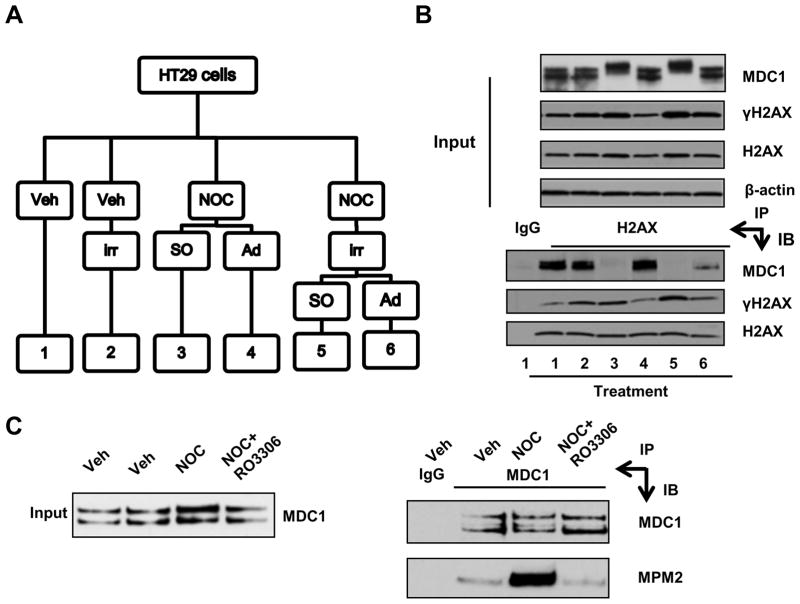
To confirm results of the morphological study, we biochemically determined the affinity between MDC1 and γH2AX in mitotic cells. HT29 cells were synchronized in mitosis by nocodazole treatment before γ-irradiation and shake off, that latter successfully separated mitotic cells from interphase cells that remained attached (Fig. 2A). The purity of the mitotic population was confirmed by FACS analysis (Suppl. Fig. S2). We then immunoprecipitated the H2AX immune complexes with a pan-H2AX antibody, which does not abrogate MDC1-γH2AX binding. Compared to attached cells (Fig. 2B; lanes 4 and 6), significantly less amount of MDC1 was immunoprecipitated with γH2AX in mitotic cells (Fig. 2B; lanes 3 and 5). This result demonstrates that the recruitment of MDC1 to γH2AX is reduced in prometaphase cells, independent of additional γ-irradiation. Furthermore, MDC1 in mitotic cells exhibited retarded mobility upon gel electrophoresis (Fig. 2B; lane 3 and 5, input), suggesting that posttranslational modification weakened MDC1-γH2AX interaction. This observation is in agreement with a previous report showing a reduction of MDC1 pull-down by a γH2AX peptide from mitotic cell lysates compared to non-mitotic cell lysates (6). This decrease of MDC1-γH2AX interaction is also consistent with the finding that APC/C and γH2AX binds to the same MDC1 domain, providing a model of competitive binding to MDC1 between APC/C components and γH2AX (10). Functionally speaking, MDC1 is required for metaphase-anaphase transition through APC/C activation during normal mitosis (11). Taken together, these observations provide an explanation for reduced MDC1-γH2AX interaction in mitosis, indicating that the role of MDC1, as a mitotic regulator, precedes that of a DDR mediator. Specifically, modified MDC1 dissociates from DSB sites in order for timely APC/C activation and weakens mitotic DDR due to insufficient bound MDC1.
Figure 2. MDC1 exhibits decreased interaction with γH2AX in mitosis.
(A) Schematic diagram of treatment ofHT29 cells. Veh, vehicle; irr, γ-irradiation;NOC, nocodazole; SO, shake-off; Ad, adherent cells. (B) Coimmunoprecipitation of MDC1 and γH2AX in HT29 cells. Whole cell lysates were prepared from samples from (A). (C) Denaturing immunoprecipitation of MDC1. HCT116 cells were either treated with nocodazole alone (NOC) or with additionalRO3306 for 1 h before harvest. IP, immunoprecipitation; IB, immunoblot.
Next we sought to identify the regulator(s) of MDC1 localization in mitosis. Previous studies reveal that phosphorylation is a major mechanism of MDC1 regulation (14). Since CDK1 is the definitive mitotic kinase (15), we tested whether MDC1 was phosphorylated by CDK1 in mitosis. We synchronized HCT116 cells in mitosis by nocodazole treatment for 16 h. The CDK1 inhibitor RO3306 was included as a negative control. To exclude contaminating proteins binding to MDC1, we denatured whole cell extracts before immunoprecipitating with an MDC1 antibody. We then used an MPM2 antibody to detect phosphorylation on CDK1 motif(s) in immunoprecipitated MDC1 protein. As shown in Fig. 2C, mitotic cells had a higher level of MPM2 activity than asynchronous cell population. Consistently, CDK1 inhibition reduced both MPM2 abundance and mitotic index to the baseline (Fig. 2C; Suppl. Fig. S3). Our result is consistent with the mitotic phosphoproteomics study, suggesting CDK1 as the candidate kinase of MDC1 in mitosis (16).
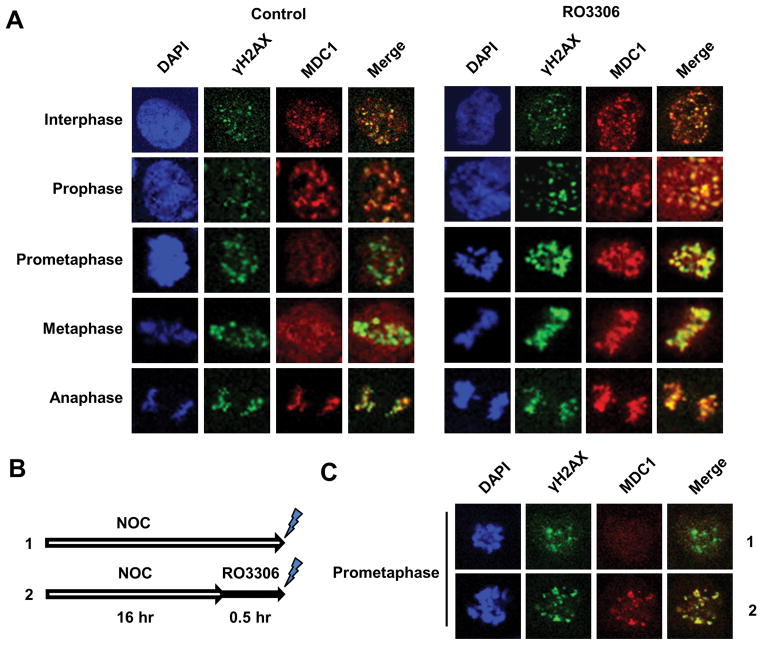
Having established that CDK1 as the putative kinase regulating MDC1, we examined the effect of CDK1 inhibition on MDC1/γH2AX colocalization in mitotic cells. Unlike other CDK1/CDK2 dual inhibitors, RO3306 exhibits a 10-fold selectivity for CDK1 over CDK2 (17). As shown in Fig. 3A, RO3306-treated HCT116 cells demonstrated a significant increase in MDC1-γH2AX colocalization in prometaphase and metaphase, judging by the intensified MDC1 foci compared to control cells. A similar effect was observed in HCT116 cells at a higher irradiation dosage (10 Gy) (Suppl. Fig. S4). We also performed the experiment in HT29 cells, which are proficient in mismatch repair in contrast to HCT116 (18), and showed that CDK1 inhibition too strengthened MDC1-γH2AX colocalization in mitotic cells (Suppl. Fig. S5). Furthermore, we tested whether CDK1 inhibition could enhance MDC1-γH2AX colocalization in prometaphase-blocked HCT116 (Fig. 3B). Indeed, additional treatment with RO3306 restored the colocalization compared to nocodazole treatment alone (Fig. 3C). These findings indicate that CDK1 activity induces the loss of MDC1-γH2AX interaction during mitosis.
Figure 3. Inhibition of CDK1 activity increases MDC1-γH2AX colocalizationin mitosis.
(A) Immunofluorescent staining of MDC1 and γH2AX in HCT116 cells. HCT116 cells were pre-incubated with RO3306 or vehicle for 30 m, before subjected to 2 Gy γ-irradiation. (B) Schematic experimental procedure of (C). HCT116 cells were treated with nocodazole alone (NOC) or with additionalRO3306 30 m before 2 Gy γ-irradiation. (C) Immunofluorescent staining of MDC1 and γH2AX in representative prometaphase cells.
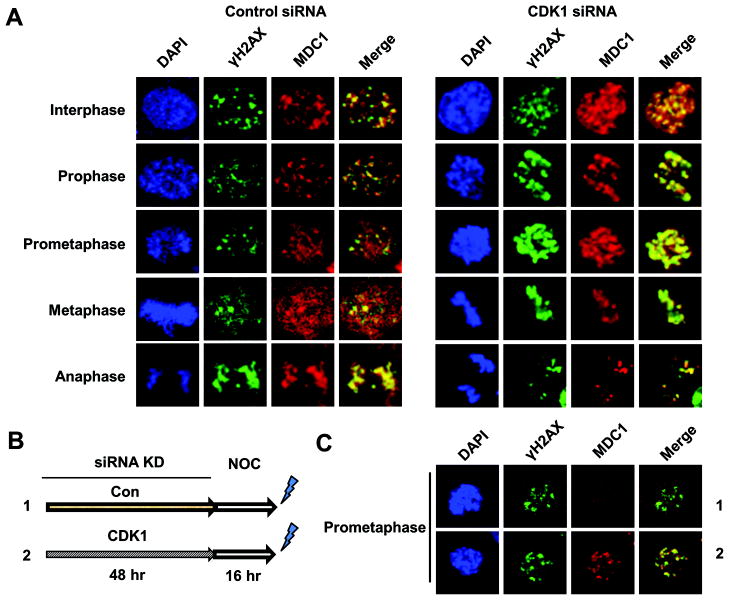
Finally, to confirm the result of pharmacological studies, we used siRNA to downregulate CDK1 in HCT116 cells. CDK1 was effectively knocked down (Suppl. Fig. S6), although the remaining CDK1 protein was sufficient for cell division according to nuclear morphology. Consistently, CDK1 knockdown significantly enhanced MDC1-γH2AX colocalization in prometaphase and metaphase cells compared to control siRNA treatment (Fig. 4A). To test whether low CDK1 activity protects MDC1-γH2AX colocalization in prolonged mitosis, we knocked down CDK1 before nocodazole treatment (Fig. 4B). Interestingly, sustained low CDK1 activity protected MDC1-γH2AX colocalization even when prometaphase was prolonged (Fig. 4C). These data suggest that MDC1 foci disassembly requires a higher CDK1 activity threshold than that necessary for the maintenance of mitosis.
Figure 4. CDK1 downregulation increases MDC1-γH2AX colocalization during mitosis.
(A) Immunofluorescent staining of MDC1 and γH2AX in HCT116 cells. Cells were transfected with non-specific control siRNA or siRNA targeting CDK1. Cells were irradiated with 2Gy γ-irradiation48 h post transfection. (B) Schematic experimental procedure of (C). Following CDK1 knockdown, HCT116 cells were treated with nocodazole (NOC) for 16 h before 2 Gy γ-irradiation. (C) Immunofluorescent staining of MDC1 and γH2AX in representative prometaphase cells as treated in (B).
In this study, we demonstrated that MDC-γH2AX interaction decreases as a cell traverses through two critical phases of mitosis, prometaphase and metaphase. This observation suggests that MDC1-γH2AX interaction is inversely correlated with CDK1 activity. Simona Giunta et al. reported that MDC1 colocalized with γH2AX in U2OS cells during normal mitosis (6). Although our results do not completely agree with theirs, we did observe residual MDC1 foci in prometaphase in the colon cancer cells we used. This discrepancy could be due to that duration of normal prometaphase is not sufficient for a complete MDC foci disassembly, shown as smaller foci accompanied with increased diffuse immunostaining. Supporting this idea, we observed that prometaphase block by nocodazole could further diminish MDC1-γH2AX colocalization. This suggests MDC1 foci disassembly starts at prometaphase, presumably due to the maximized CDK1 activity. Moreover, CDK1 activity reduction, by either pharmacological or genetic manipulation, restored MDC1-γH2AX colocalization in both normal and prolonged mitosis. These data suggest that high CDK1 activity constitutively promotes MDC1 foci disassembly. However, whether phosphorylation of MDC1 by CDK1 induces foci disassembly requires further elucidation. Recently, Zhang et al. reported that high CDK1 activity suppressed mitotic DDR (19). Although we agree with their over-arching hypothesis, the utilization of alsterpaullone, a nonspecific CDK1 inhibitor, as a sole approach to downregulate CDK1 activity weakened their conclusion. In contrast, we used multi-angled approaches to demonstrate the inhibitory role of CDK1 in MDC1 foci formation. Since the sustained MDC1-γH2AX interaction is required to consistent downstream DDR activation, decreased MDC1-γH2AX interaction in mitosis partially explains the incomplete DDR.
In summary, our findings provide new insights into the regulation of DDR in mitosis. In the context of unperturbed mitosis, inherent DSBs are unlikely to sever chromosomes, due to their super-condensed structure. We and others have shown that DSBs induced during mitosis, either by mitotic poisons or γ-irradiation, are actively repaired in postmitotic cells, as shown by late-stage DDR protein foci staining (5, 6). Thus, completion of mitosis is prioritized over DSB repair, which supports our previous findings that prolonged mitosis induces genomic instability. Human cells benefit from timely progression through mitosis rather than repair. To ensure this, a subgroup of MDC1 dissociate from γH2AX foci in a CDK1-dependent manner to promote normal metaphase-anaphase transition.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institutes of Health: DK052230 and CA084197.
Footnotes
None of the authors have any conflicts of interest.
Supplemental information is available at Cancer Research online.
Author Contributions
B.Y.: contributed to the design of the experiments, performed the experiments and wrote the manuscript.
W.B.D.: contributed to the design of the experiments and performed some of the experiments.
V.W.Y.: contributedto the design of the experiments and wrote the manuscript.
References
- 1.Bassing CH, Chua KF, Sekiguchi J, Suh H, Whitlow SR, Fleming JC, et al. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8173–8. doi: 10.1073/pnas.122228699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123:1213–26. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Ciccia A, Elledge SJ. The DNA Damage Response: Making It Safe to Play with Knives. Molecular Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekker-Jensen S, Mailand N. Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair (Amst) 2010;9:1219–28. doi: 10.1016/j.dnarep.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Dalton WB, Nandan MO, Moore RT, Yang VW. Human cancer cells commonly acquire DNA damage during mitotic arrest. Cancer research. 2007;67:11487–92. doi: 10.1158/0008-5472.CAN-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giunta S, Belotserkovskaya R, Jackson SP. DNA damage signaling in response to double-strand breaks during mitosis. The Journal of cell biology. 2010;190:197–207. doi: 10.1083/jcb.200911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Vugt MaTM, Gardino AK, Linding R, Ostheimer GJ, Reinhardt HC, Ong SE, et al. A mitotic phosphorylation feedback network connects Cdk1, Plk1, 53BP1, and Chk2 to inactivate the G(2)/M DNA damage checkpoint. PLoS biology. 2010;8:e1000287-e. doi: 10.1371/journal.pbio.1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Shim EY, Davis M, Lee SE. Regulation of repair choice: Cdk1 suppresses recruitment of end joining factors at DNA breaks. DNA Repair (Amst) 2009;8:1235–41. doi: 10.1016/j.dnarep.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson SE, Li Y, Chait BT, Gottesman ME, Baer R, Gautier J. Cdk1 uncouples CtIP-dependent resection and Rad51 filament formation during M-phase double-strand break repair. J Cell Biol. 2011;194:705–20. doi: 10.1083/jcb.201103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coster G, Hayouka Z, Argaman L, Strauss C, Friedler A, Brandeis M, et al. The DNA damage response mediator MDC1 directly interacts with the anaphase-promoting complex/cyclosome. J Biol Chem. 2007;282:32053–64. doi: 10.1074/jbc.M705890200. [DOI] [PubMed] [Google Scholar]
- 11.Townsend K, Mason H, Blackford AN, Miller ES, Chapman JR, Sedgwick GG, et al. Mediator of DNA damage checkpoint 1 (MDC1) regulates mitotic progression. J Biol Chem. 2009;284:33939–48. doi: 10.1074/jbc.M109.009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Tran T, Zhang L, Hatcher R, Zhang P. DNA damage-induced mitotic catastrophe is mediated by the Chk1-dependent mitotic exit DNA damage checkpoint. Proc Natl Acad Sci U S A. 2005;102:1065–70. doi: 10.1073/pnas.0409130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavet O, Pines J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J Cell Biol. 2010;189:247–59. doi: 10.1083/jcb.200909144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coster G, Goldberg M. The cellular response to DNA damage: A focus on MDC1 and its interacting proteins. Nucleus (Austin, Tex) 2010;1:166–78. doi: 10.4161/nucl.1.2.11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santamaría D, Barrière C, Cerqueira A, Hunt S, Tardy C, Newton K, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–5. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 16.Dephoure N, Zhou C, Villèn J, Beausoleil Sa, Bakalarski CE, Elledge SJ, et al. A quantitative atlas of mitotic phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10762–7. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10660–5. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–5. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Peng G, Lin SY, Zhang P. DNA damage response is suppressed by high CDK1 activity in mitotic mammalian cells. J Biol Chem. 2011 doi: 10.1074/jbc.M111.267690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.