Abstract
Background
The results of primary total hip arthroplasties (THAs) after pediatric hip diseases such as developmental dysplasia of the hip (DDH), slipped capital femoral epiphysis (SCFE), or Perthes’ disease have been reported to be inferior to the results after primary osteoarthritis of the hip (OA).
Materials and methods
We compared the survival of primary THAs performed during the period 1995–2009 due to previous DDH, SCFE, Perthes’ disease, or primary OA, using merged individual-based data from the Danish, Norwegian, and Swedish arthroplasty registers, called the Nordic Arthroplasty Register Association (NARA). Cox multiple regression, with adjustment for age, sex, and type of fixation of the prosthesis was used to calculate the survival of the prostheses and the relative revision risks.
Results
370,630 primary THAs were reported to these national registers for 1995–2009. Of these, 14,403 THAs (3.9%) were operated due to pediatric hip diseases (3.1% for Denmark, 8.8% for Norway, and 1.9% for Sweden) and 288,435 THAs (77.8%) were operated due to OA. Unadjusted 10-year Kaplan-Meier survival of THAs after pediatric hip diseases (94.7% survival) was inferior to that after OA (96.6% survival). Consequently, an increased risk of revision for hips with a previous pediatric hip disease was seen (risk ratio (RR) 1.4, 95% CI: 1.3–1.5). However, after adjustment for differences in sex and age of the patients, and in fixation of the prostheses, no difference in survival was found (93.6% after pediatric hip diseases and 93.8% after OA) (RR 1.0, CI: 1.0–1.1). Nevertheless, during the first 6 postoperative months more revisions were reported for THAs secondary to pediatric hip diseases (RR 1.2, CI: 1.0–1.5), mainly due to there being more revisions for dislocations (RR 1.8, CI: 1.4–2.3). Comparison between the different diagnosis groups showed that the overall risk of revision after DDH was higher than after OA (RR 1.1, CI: 1.0–1.2), whereas the combined group Perthes’ disease/SCFE did not have a significantly different risk of revision to that of OA (RR 0.9, CI: 0.7–1.0), but had a lower risk than after DDH (RR 0.8, CI: 0.7–1.0).
Interpretation
After adjustment for differences in age, sex, and type of fixation of the prosthesis, no difference in risk of revision was found for primary THAs performed due to pediatric hip diseases and those performed due to primary OA.
Total hip arthroplasties (THAs) after pediatric hip disease have often been reported to have inferior results as compared to THA resulting from primary osteoarthritis of the hip (OA) (Dudkiewicz et al. 2002, Sanchez-Sotelo et al. 2002). This may be explained by morphological deformities in the proximal femur or in the acetabulum, due to the disease or previous surgery, causing technical difficulties in performing the joint replacement (Sugano et al. 1998, Chougle et al. 2006). In addition, THAs in hips after pediatric hip diseases have often been combined with predictors of inferior outcome such as young age and inferior uncemented prostheses (Sugano et al. 1998, Furnes et al. 2001). However, the survival of THAs after developmental dysplasia of the hip (DDH) reported to the Norwegian Arthroplasty Register was not found to be inferior to that of THAs after OA, if adjustments for age and for type of implant were performed (Engesaeter et al. 2008). Also, in the Danish Arthroplasty Register the long-term implant survival for patients with childhood hip diseases were encouraging, although an increased risk of revision because of dislocation in the first 6 postoperative months for patients with acetabular dysplasia was found (Thillemann et al. 2008).
We now report the results of primary THA after pediatric hip diseases (DDH, slipped capital femoral epiphysis (SCFE), and Perthes’ disease), and compare them with the results of THAs due to OA, using data from the national hip arthroplasty registers in Denmark, Norway, and Sweden.
Patients and methods
This study is based on the cooperation in the Nordic Arthroplasty Register Association (NARA). Today, this is a collaboration between the national arthroplasty registers in Denmark, Finland, Norway, and Sweden. When this study was started, data from Finland were not available and only THAs from the Denmark, Norway, and Sweden were included. De-identification of the patients, including deletion of the national civil registration numbers, was performed in each national registry. The anonymous data were then merged into a common database (Havelin et al. 2009). This database contains the following data: country, age, sex, operated side, diagnosis, date of primary THA, hospital code, type of fixation of the prosthesis, type of bone cement, date of death (if applicable), and cause and date of eventual revision. For this study, primary THAs performed due to DDH, SCFE, Perthes’ disease, or OA for the period 1995–2009 were selected. In Denmark and Sweden, Perthes’ disease and SCFE were reported separately, while in Norway these 2 diagnoses were reported together.
Statistics
Survival analyses were performed using the Kaplan-Meier method and Cox regression. A Cox multiple regression model was used to study relative revision risks (failure-rate ratios) for the 4 groups of diagnoses, with adjustments for the possible influences of age (< 30, 30–39, 40–49, 50–60, 61–70, 71–80, > 80 years), sex, and the type of fixation of the implant (uncemented, cemented (both stem and cup), hybrid, or reverse hybrid (cemented cup)). Failure (revision) of the implant was defined as surgical exchange or extraction of the entire prosthesis, or any of its parts. Estimates from Cox analyses with the 4 groups of diagnoses were used to construct adjusted survival curves. 95% confidence intervals (CIs) were calculated. For revisions, the surgeon had recorded one or more reasons for failure, but in combination with infection, infection was considered to be the primary cause of revision. Aseptic loosening was otherwise counted as the principal cause of revision when given in combination with other causes. Potential overestimation of incidence of revision due to the effect of competing risks (i.e. death) was assessed by the cumulative incidence function (Gillam et al. 2010). The proportion of deaths was 7.3% for those with pediatric hip disease and 18.3% for those with OA during the follow-up, but imposed a negligible influence on the estimated risks for revisions. The extent of bilateral THAs was about 20% for patients with OA and those with pediatric hip disease, and had minimal effect on the survival estimates. Since the survival curves indicated non-proportionality, we also performed separate analyses for the first 6 months postoperatively (Figure 4) and later. The statistics package SPSS version 19.0 was used for the analyses.
Figure 4.

Survival in the first six months postoperatively with adjustment for age, sex, and type of fixation in the Cox model with any reason for revision as endpoint in the analysis, for THAs after pediatric hip disease and for THAs due primary osteoarthritis.
Results
Epidemiology
In the period 1995–2009, 370,630 primary THAs were reported to the arthroplasty registers of Denmark, Norway, and Sweden. 14,403 THAs (3.9%) were performed due to pediatric hip disease and 288,435 THAs (77.8%) were performed due to primary OA (Table 1). Sweden contributed with 50%, Denmark with 25%, and Norway with 25% of the reported primary arthroplasties. Females constituted 66% of those with pediatric hip disease and 60% of those with OA (p < 0.001). Mean age of those operated due to pediatric hip disorders was 55 years (SD 13), and it was 69 years (SD 10) for patients with OA (p < 0.001) (Table 1).
Table 1.
Numbers of primary THAs and diagnoses (percentage, mean age, and percentage of females) in Denmark, Norway, Sweden, and altogether, for the period 1995–2009
| Denmark n = 94,252 |
Norway n = 90,686 |
Sweden n = 185,692 |
Total n = 370,630 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | % | Age (years) |
Female (%) |
% | Age (years) |
Female (%) |
% | Age (years) |
Female (%) |
% | Age (years) |
Female (%) |
| Primary OA | 78.1 | 68.8 | 56.3 | 73.6 | 70.9 | 69.0 | 79.8 | 68.8 | 57.0 | 77.8 | 69.3 | 59.6 |
| Pediatric hip dis. | 3.1 | 51.9 | 58.6 | 8.8 | 57.0 | 68.0 | 1.9 | 53.4 | 65.5 | 3.9 | 55.1 | 65.5 |
| DDH | 2.1 | 52.9 | 71.2 | 7.5 | 58.0 | 75.3 | 1.8 | 53.8 | 67.4 | 3.3 | 56.0 | 72.5 |
| Perthes’ | 0.6 | 49.5 | 29.3 | – | – | – | 0.1 | 47.2 | 38.2 | 0.2 | 48.9 | 31.6 |
| SCFE | 0.4 | 49.3 | 32.4 | – | – | – | 0.02 | 53.0 | 38.2 | 0.1 | 49.7 | 32.9 |
| SCFE/Perthes’ | – | – | – | 1.3 | 51.6 | 27.1 | – | – | 0.3 | 51.6 | 27.1 | |
| Other | 18.8 | 68.0 | 65.4 | 17.6 | 69.3 | 70.2 | 18.4 | 71.5 | 71.8 | 18.3 | 70.0 | 69.7 |
| All | 100 | 68.2 | 58.1 | 100 | 69.4 | 69.1 | 100 | 69.0 | 59.9 | 100 | 68.9 | 61.7 |
3.3% of all primary THAs were performed due to previous DDH (12,068 THAs), but there were large differences in the 3 Nordic countries: 7.5% in Norway, 2.1% in Denmark, and 1.8% in Sweden (Table 1). SCFE and Perthes’ disease as a group were reported to be responsible for 0.6% of primary THAs (2,335 THAs), varying from 1.3% in Norway and 1.0% in Denmark to 0.1% in Sweden. In Denmark, Perthes’ disease was responsible for 0.6% of primary THAs and SCFE 0.4%, while in Sweden the numbers were 0.1% and 0.02%, respectively (Table 1).
The mean follow-up was calculated using the reverse Kaplan-Meier method. For the patients with OA, the median follow-up was 5.9 years and for those with pediatric hip disease it was 6.4 years (6.4 years for those with DDH, 7.4 years for those with SCFE, and 6.8 years for those with Perthes’ disease). The mean follow-up was therefore relatively short in all groups.
Uncemented THAs and reversed hybrids were used more commonly in patients with a pediatric hip disorder than in patients who were operated due to primary OA, while cemented THAs were less common (p < 0.001) (Figure 1).
Figure 1.

Type of fixation of the prostheses in patients operated due to osteoarthritis of the hip (OA) (n = 288,435 THAs) and due to pediatric hip disease (n = 14,403 THAs). The patients with pediatric hip disease were younger than the OA patients
Survival
Without any adjustment, and with any reason for revision as endpoint, the 10-year survival of THAs was 94.7% after pediatric hip disease as one group and 96.6% after OA. The overall risk of revision for THAs after previous childhood hip diseases was 1.4 times higher (CI: 1.3–1.5) than after OA (Figure 2). The corresponding risk of aseptic loosening was 1.3 times higher (CI: 1.2–1.4), for dislocation it was 1.2 times higher (CI 1.1–1.5), and for infection the risk ratio for revision was 0.8 times (CI: 0.6–1.0). However, after adjustments in the Cox model for age, sex, and type of fixation of the prosthesis and with any reason for revision as endpoint in the analyses, the 10-year survival of THAs was 93.6% after pediatric hip disease and 93.8% after OA. No statistically significant difference was found between the overall risk of revision for THAs after pediatric hip disease and that after OA (RR 1.04, CI: 1.0–1.1) (Figure 3 and Table 2). Similar results were found with aseptic loosening as endpoint (RR 1.0, CI: 0.9–1.1), with dislocation as endpoint (RR 1.1, CI: 0.9–1.3), and with infection as endpoint in the analyses (RR 0.9, CI: 0.7–1.1) (Table 2).
Figure 2.
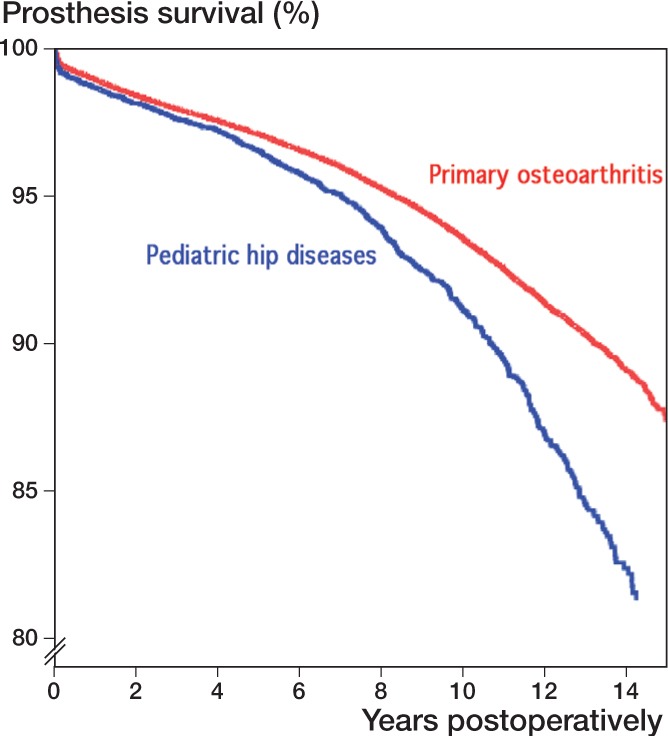
Unadjusted survival curves in the Cox model (Kaplan-Meier curves) with any reason for revision as endpoint in the analysis, for THAs after pediatric hip disease and for THAs due to primary osteoarthritis. The two groups were not comparable, however, due to differences in age, sex, and type of fixation. (The numbers of hips at risk at 14 years were 5,885 for OA and 429 for pediatric hip disease).
Figure 3.

Survival curves with adjustment for age, sex, and type of fixation in the Cox model with any reason for revision as endpoint in the analysis, for THAs after pediatric hip disease and for THAs due to primary osteoarthritis. The numbers of hips at risk at 14 years were 5,885 for OA and 429 for pediatric hip disease.
Table 2.
Results for primary THAs after pediatric hip disease and after primary osteoarthritis. Adjusted for age, sex, and type of fixation in the Cox model, with endpoints as indicated
| Endpoint in the analysis | No. of THAs |
No. of revisions |
10–year survival a |
RR b | 95% CI c | p–value |
|---|---|---|---|---|---|---|
| Any reason for revision | 302,838 | 12,067 | ||||
| Pediatric hip disease | 14,403 | 853 | 93.6% | 1.04 | 0.96–1.12 | 0.4 |
| Primary osteoarthritis | 288 435 | 11,214 | 93.8% | 1 | – | – |
| Aseptic loosening | 302 838 | 5,803 | ||||
| Pediatric hip disease | 14,403 | 396 | 97.2% | 1.00 | 0.89–1.11 | 1.0 |
| Primary osteoarthritis | 288 435 | 5,407 | 97.0% | 1 | – | – |
| Dislocation | 302 838 | 2,641 | ||||
| Pediatric hip disease | 14,403 | 156 | 98.7% | 1.10 | 0.92–1.31 | 0.3 |
| Primary osteoarthritis | 288 435 | 2,485 | 98.8% | 1 | – | – |
| – | ||||||
| Infection | 302 838 | 1,806 | ||||
| Pediatric hip disease | 14,403 | 72 | 99.3% | 0.86 | 0.67–1.10 | 0.2 |
| Primary osteoarthritis | 288 435 | 1,734 | 99.3% | 1 | – | – |
a Cox–adjusted 10–year survival.
b Cox relative revision risk.
c 95% confidence interval for RR.
The first 6 months postoperatively
Restricting the analyses to revisions within 6 months postoperatively using any reason for revision as endpoint and adjusting for differences in age, sex, and type of fixation, a higher risk of revision was found for those operated for pediatric hip disease than for those operated for OA (RR 1.2, CI 1.0–1.5) (Table 3 and Figure 4). More than 6 months postoperatively, however, no differences in revision risk were detected with any reason for revision as endpoint (RR 1.0, CI: 0.9–1.1). This increased risk of early revision was mainly due to increased risk of revision due to dislocation (RR 1.8, CI: 1.4–2.3) (Table 3).
Table 3.
Results for the first 6 postoperative months for primary THAs after pediatric hip disease and after primary osteoarthritis a
| Endpoint in the analysis | No. of THAs |
No. of revisions |
RR | 95% CI | p–value |
|---|---|---|---|---|---|
| Any reason for revision | 302,838 | 2,289 | |||
| Pediatric hip diseases | 14,403 | 141 | 1.24 | 1.03–1.50 | 0.02 |
| Primary osteoarthritis | 288 435 | 2,148 | 1 | – | – |
| Aseptic loosening | 302 838 | 154 | |||
| Pediatric hip diseases | 14,403 | 9 | 0.95 | 0.46–1.95 | 0.9 |
| Primary osteoarthritis | 288 435 | 145 | 1 | – | – |
| Dislocation | 302 838 | 1,020 | |||
| Pediatric hip diseases | 14,403 | 78 | 1.78 | 1.38–2.30 | < 0.001 |
| Primary osteoarthritis | 288 435 | 942 | 1 | – | – |
| Infection | 302 838 | 637 | |||
| Pediatric hip diseases | 14,403 | 17 | 0.73 | 0.44–1.20 | 0.2 |
| Primary osteoarthritis | 288 435 | 620 | 1 | – | – |
a For full explanation, see Table 2.
DDH compared to OA
A slightly higher overall risk of revision was found for DDH than for OA after adjustment (RR 1.1, CI: 1.0–1.2). The main reason for this difference was increased risk of dislocation (RR 1.2, CI: 1.0–1.4), while we found that there was no statistically significant change in the risk of revision due to aseptic loosening or due to infection (Table 4).
Table 4.
Results for primary THAs after previous DDH and after primary osteoarthritis
| Endpoint in the analysis | No. of THAs |
No. of revisions |
10–year survival a |
RR b | 95% CI c | p–value |
|---|---|---|---|---|---|---|
| Any reason for revision | 300,503 | 11,922 | ||||
| DDH | 12,068 | 706 | 93.3% | 1.09 | 1.00–1.18 | 0.04 |
| Primary osteoarthritis | 288 435 | 11,214 | 93.8% | 1 | – | – |
| Aseptic loosening | 300 503 | 5,803 | ||||
| DDH | 12,068 | 325 | 97.1% | 1.05 | 0.93–1.18 | 0.5 |
| Primary osteoarthritis | 288 435 | 5,407 | 97.0% | 1 | – | – |
| Dislocation | 300 503 | 2,641 | ||||
| DDH | 12,068 | 138 | 98.7% | 1.18 | 0.98–1.42 | 0.08 |
| Primary osteoarthritis | 288 435 | 2,485 | 98.8% | 1 | – | – |
| Infection | 300 503 | 1,806 | ||||
| DDH | 12,068 | 57 | 99.5% | 0.85 | 0.65–1.12 | 0.3 |
| Primary osteoarthritis | 288 435 | 1,734 | 99.3% | 1 | – | – |
For full explanation, see Table 2.
The first 6 months postoperatively, the overall risk of revision was higher for DDH than for OA (RR 1.3, CI: 1.1–1.6), while no difference was found after the first 6 months postoperatively (RR 1.1, CI: 0.98-1.2). A higher risk of early revision due to dislocation was found for DDH than for OA (RR 1.9, CI: 1.5–2.5), but there was no difference in the risk of revision due to aseptic loosening (RR 1.0, CI: 0.48–2.2) or due to infection (RR 0.68, CI: 0.38–1.2).
Perthes’ disease/SCFE compared to OA
The risk of revision for the combined group of patients with Perthes’ disease or SCFE (2,335 THAs) was compared with the risk for OA patients (288,435 THAs). No differences in the results were found (RR 0.85, CI: 0.72–1.0) with any reason for revision as endpoint after adjustment for sex, age, and type of fixation. Similar results were obtained with aseptic loosening as endpoint (RR 0.81, CI: 0.63–1.0), with dislocation as endpoint (RR 0.72, CI: 0.44–1.2), and with infection as endpoint in the analyses (RR 1.0, CI: 0.56–1.6).
In Denmark and Sweden, Perthes’ disease and SCFE were reported separately, and the results of THAs for Perthes’ disease (n = 748) and SCFE (n = 374) were compared separately to the results for OA reported for Denmark and Sweden (n = 222,841). With any reason as endpoint, with adjustment for sex, age, and type of fixation, and with OA patients as reference, no significant differences could be detected either for Perthes’ disease (RR 1.1, CI: 0.86–1.5) or for SCFE (RR 1.1, CI: 0.76–1.5). Similarly, no significant differences compared to OA were found for Perthes’ disease with aseptic loosening as endpoint (RR 1.2, CI: 0.88–1.8), for SCFE with aseptic loosening as endpoint (RR 1.0, CI: 0.64–1.7), for Perthes’ disease with dislocation as endpoint (RR 0.69, CI: 0.30–1.6), for SCFE with dislocation as endpoint (RR 0.81, CI: 0.30–2.2), for Perthes’ disease with infection as endpoint (RR 1.1, CI: 0.43–2.6), or for SCFE with infection as endpoint (RR 1.1, CI: 0.37–3.6).
Perthes’ disease/SCFE compared to DDH
Compared to DDH (n = 12,068), THAs in the combined group of Perthes’ disease and SCFE (n = 2,335) showed a reduced risk of revision with any reason for revision as endpoint (RR 0.82, CI: 0.67–0.99). We also found this reduced risk of revision using dislocation as endpoint (RR 0.56, CI: 0.33–0.95), but not with aseptic loosening (RR 0.84, CI: 0.63–0.1.1) or infection as endpoint (RR 1.1, CI: 0.57–2.0). All the risk ratios given were adjusted for age, sex, and type of fixation.
Perthes’ disease compared to SCFE
Finally, we made comparisons between THAs after Perthes’ disease (n = 748) and THAs after SCFE (n = 374). With any reason as endpoint in the analyses, with adjustment for sex, age, and type of fixation, and with Perthes’ disease as reference, no significant differences in the results could be detected (RR 0.98, CI: 0.64–1.5). No differences could be detected either with aseptic loosening as endpoint (RR 0.85, CI: 0.47–1.5), with dislocation as endpoint (RR 1.3, CI: 0.36–4.7), or with infection as endpoint (RR 1.2, CI: 0.28–5.2).
Discussion
We found no difference in the overall risk of revision of primary THA after pediatric hip diseases and after primary OA, when adjustment was done for differences in age, sex, and type of fixation of the implants. The survival of THAs after previous DDH was, however, inferior to that of THAs due to OA, mainly because of the increased risk of dislocation during the first 6 postoperative months. We found that the risk of revision was lower after Perthes’ disease/SCFE than after DDH.
These good overall results of THAs after pediatric hip disease are in accordance with other registry studies (Engesaeter et al. 2008, Thillemann et al. 2008, Boyle et al. 2012). An increased risk of revision in the first 6 months postoperatively was also reported from the Danish Arthroplasty Register (Thillemann et al. 2008). This increased risk of early revision due to dislocation of THAs after DDH is probably to be expected, since the insertion of a THA in DDH patients is often technically challenging, due to dysplastic acetabulum, narrow femur, shortening, and rotational deformity. The changed anatomy is sometimes caused by previous surgery. Frequently, special components are needed and procedures such as shortening of the femur and acetabular augmentation with superior bone grafting may be considered (Kobayashi et al. 2004, Chougle et al. 2005, Boyle et al. 2012).
The diagnosis DDH in our study included all severities of the disease. Since our dataset depended on the diagnoses reported to the different registers in Denmark, Norway, and Sweden, we had to use the type of data from the register with the least well-differentiated DDH diagnosis (“yes” or “no”). We could therefore only dichotomize regarding the DDH diagnosis and we were thus unable to stratify our results for THAs on the basis of the severity of DDH—for example, using the Crowe or Hartofilakidis classification systems (Yiannakopoulos et al. 2008).
There was a lower risk of revision for THAs after Perthes’ disease/SCFE than for THAs after DDH, either with any reason or with dislocation as endpoint. To the best of our knowledge, such differences have not been shown earlier. This might be explained by there being less morphological deformities in the acetabulum after Perthes’ disease/SCFE than after DDH, and therefore less technically demanding surgery—possibly with less risk of dislocation.
From an epidemiological point of view, it is remarkable that in Norway 7.5% of the primary THAs have been reported to be the result of DDH, while the corresponding figures are only 1.8% in Sweden and 2.1% in Denmark. Increased numbers of THAs due to DDH in Norway have also been reported from NARA earlier (Havelin et al. 2009). Increased annual age- and sex-standardized incidence of THA for primary hip osteoarthritis in females in Norway compared to Denmark, Finland, Iceland, and Sweden has been reported earlier (Lohmander et al. 2006). It could be speculated that undiagnosed DDH in childhood may be the reason behind some of the primary OA reported from Norway. The finding in the present study that DDH appears to be more common in Norwegian females could be a possible contributing factor to the observed increased incidence of THAs in Norwegian females (Lohmander et al. 2006).
Differences in the criteria of diagnoses reported to these 3 national registers could also be considered a possible explanation for variation in the reported incidence of the different diseases. The diagnoses in all 3 registers are based on the decision of the operating surgeon who fills in the form to the register immediately after surgery. In the Danish register, the diagnoses made by the surgeons in patients undergoing primary THA were validated with radiographs by independent observers and the diagnoses were confirmed in 84% of the patients (Pedersen et al. 2004). The DDH diagnosis in the Norwegian Arthroplasty Register was confirmed in 88% of the patients (Engesaeter et al. 2011) and in 91% of the patients for Perthes’ disease/SCFE (Lehmann et al. 2012).
Uncemented implants were used in 43% of patients with pediatric hip disease and in only 15% of those with primary OA. Similar proportions have been reported from the New Zealand Arthroplasty Register (Boyle et al. 2012). The use of uncemented fixation in younger patients is peculiar, since no scientific paper from any of the national joint registers has reported better results with uncemented implants (Clement et al. 2012). Thus, in our analyses statistical adjustments for fixation of the prosthesis were performed to permit comparisons of the results of the THAs linked to the different diagnoses.
In summary, the present study shows that after adjustment for differences in age, sex, and type of fixation of the implants, the results of THAs after pediatric hip disease as a group were not inferior to the results of THAs after primary osteoarthritis. However, the risk of revision of THAs after DDH is higher than the risk of revision of THAs after OA. For the combined group of Perthes’ disease and SCFE, the risk of revision of THAs is the same as that for THAs after OA.
Acknowledgments
This paper is the result of close team work. All the authors participated in the planning and design of the study and in interpretation of the results. The statistician AMF prepared data from each national register, included de-identification of the patients, and merged the selected information into the common dataset. The statistical analyses were performed by LBE and AMF. LBE and IØE wrote the manuscript, but all authors participated in revision of the manuscript.
References
- Boyle MJ, Frampton CM, Crawford HA. Early results of total hip arthroplasty in patients with developmental dysplasia of the hip compared with patients with osteoarthritis. J Arthroplasty. 2012;27(3):386–90. doi: 10.1016/j.arth.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Chougle A, Hemmady MV, Hodgkinson JP. Severity of hip dysplasia and loosening of the socket in cemented total hip replacement. A long-term follow-up. J Bone Joint Surg (Br) 2005;87(1):16–20. [PubMed] [Google Scholar]
- Chougle A, Hemmady MV, Hodgkinson JP. Long-term survival of the acetabular component after total hip arthroplasty with cement in patients with developmental dysplasia of the hip. J Bone Joint Surg (Am) 2006;88(1):71–9. doi: 10.2106/JBJS.D.02689. [DOI] [PubMed] [Google Scholar]
- Clement ND, Biant LC, Breusch SJ. Total hip arthroplasty: to cement or not to cement the acetabular socket? A critical review of the literature. Arch Orthop Trauma Surg. 2012;132(3):411–27. doi: 10.1007/s00402-011-1422-2. [DOI] [PubMed] [Google Scholar]
- Dudkiewicz I, Salai M, Ganel A, Blankstein A, Chechik A. Total hip arthroplasty in patients younger than 30 years of age following developmental dysplasia of hip (DDH) in infancy. Arch Orthop Trauma Surg. 2002;122(3):139–42. doi: 10.1007/s004020100307. [DOI] [PubMed] [Google Scholar]
- Engesaeter LB, Furnes O, Havelin LI. Developmental dysplasia of the hip-good results of later total hip arthroplasty: 7135 primary total hip arthroplasties after developmental dysplasia of the hip compared with 59774 total hip arthroplasties in idiopathic coxarthrosis followed for 0 to 15 years in the Norwegian Arthroplasty Register. J Arthroplasty. 2008;23(2):235–40. doi: 10.1016/j.arth.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Engesaeter IO, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesaeter LB. Total hip replacement in young adults with hip dysplasia. Acta Orthop. 2011;82(2):149–54. doi: 10.3109/17453674.2011.566146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. J Bone Joint Surg (Br) 2001;83(4):579–86. doi: 10.1302/0301-620x.83b4.11223. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. [DOI] [PubMed] [Google Scholar]
- Gillam MH, Ryan P, Graves SE, Miller LN, de Steiger RN, Salter A. Competing risks survival analysis applied to data from the Australian Orthopaedic Association National Joint Replacement Registry. Acta Orthop. 2010;81(5):548–55. doi: 10.3109/17453674.2010.524594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelin LI, Fenstad AM, Salomonsson R, Mehnert F, Furnes O, Overgaard S, Pedersen AB, Herberts P, Karrholm J, Garellick G. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop. 2009;80(4):393–401. doi: 10.3109/17453670903039544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Saito N, Nawata M, Horiuchi H, Iorio R, Takaoka K. Total hip arthroplasty with bulk femoral head autograft for acetabular reconstruction in DDH. Surgical technique. J Bone Joint Surg (Am) (Suppl 1) 2004;86:11–7. doi: 10.2106/00004623-200403001-00003. [DOI] [PubMed] [Google Scholar]
- Lehmann TG, Engesaeter IO, Laborie LB, Lie SA, Rosendahl K, Engesaeter LB. Total hip arthroplasty in young adults, with focus on Perthes’ disease and slipped capital femoral epiphysis. Follow-up of 540 subjects reported to the Norwegian Arthroplasty Register during 1987–2007. Acta Orthop. 2012;83(2):159–64. doi: 10.3109/17453674.2011.641105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander LS, Engesaeter LB, Herberts P, Ingvarsson T, Lucht U, Puolakka TJ. Standardized incidence rates of total hip replacement for primary hip osteoarthritis in the 5 Nordic countries: similarities and differences. Acta Orthop. 2006;77(5):733–40. doi: 10.1080/17453670610012917. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Johnsen S, Overgaard S, Soballe K, Sorensen HT, Lucht U. Registration in the danish hip arthroplasty registry: completeness of total hip arthroplasties and positive predictive value of registered diagnosis and postoperative complications. Acta Orthop Scand. 2004;75(4):434–41. doi: 10.1080/00016470410001213-1. [DOI] [PubMed] [Google Scholar]
- Sanchez-Sotelo J, Berry DJ, Trousdale RT, Cabanela ME. Surgical treatment of developmental dysplasia of the hip in adults: II. Arthroplasty options. J Am Acad Orthop Surg. 2002;10(5):334–44. doi: 10.5435/00124635-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Sugano N, Noble PC, Kamaric E, Salama JK, Ochi T, Tullos HS. The morphology of the femur in developmental dysplasia of the hip. J Bone Joint Surg (Br) 1998;80(4):711–9. doi: 10.1302/0301-620x.80b4.8319. [DOI] [PubMed] [Google Scholar]
- Thillemann TM, Pedersen AB, Johnsen SP, Soballe K. Implant survival after primary total hip arthroplasty due to childhood hip disorders: results from the Danish Hip Arthroplasty Registry. Acta Orthop. 2008;79(6):769–76. doi: 10.1080/17453670810016830. [DOI] [PubMed] [Google Scholar]
- Yiannakopoulos CK, Chougle A, Eskelinen A, Hodgkinson JP, Hartofilakidis G. Inter- and intra-observer variability of the Crowe and Hartofilakidis classification systems for congenital hip disease in adults. J Bone Joint Surg (Br) 2008;90(5):579–83. doi: 10.1302/0301-620X.90B5.19724. [DOI] [PubMed] [Google Scholar]


