Abstract
Background and purpose
The risk of revision due to infection after primary total hip arthroplasty (THA) has been reported to be increasing in Norway. We investigated whether this increase is a common feature in the Nordic countries (Denmark, Finland, Norway, and Sweden).
Materials and methods
The study was based on the Nordic Arthroplasty Register Association (NARA) dataset. 432,168 primary THAs from 1995 to 2009 were included (Denmark: 83,853, Finland 78,106, Norway 88,455, and Sweden 181,754). Adjusted survival analyses were performed using Cox regression models with revision due to infection as the endpoint. The effect of risk factors such as the year of surgery, age, sex, diagnosis, type of prosthesis, and fixation were assessed.
Results
2,778 (0.6%) of the primary THAs were revised due to infection. Compared to the period 1995–1999, the relative risk (with 95% CI) of revision due to infection was 1.1 (1.0–1.2) in 2000–2004 and 1.6 (1.4–1.7) in 2005–2009. Adjusted cumulative 5–year revision rates due to infection were 0.46% (0.42–0.50) in 1995–1999, 0.54% (0.50–0.58) in 2000–2004, and 0.71% (0.66–0.76) in 2005–2009. The entire increase in risk of revision due to infection was within 1 year of primary surgery, and most notably in the first 3 months. The risk of revision due to infection increased in all 4 countries. Risk factors for revision due to infection were male sex, hybrid fixation, cement without antibiotics, and THA performed due to inflammatory disease, hip fracture, or femoral head necrosis. None of these risk factors increased in incidence during the study period.
Interpretation
We found increased relative risk of revision and increased cumulative 5–year revision rates due to infection after primary THA during the period 1995–2009. No change in risk factors in the NARA dataset could explain this increase. We believe that there has been an actual increase in the incidence of prosthetic joint infections after THA.
The outcome of hip replacement surgery and the survival of implants have improved during the last decades (Herberts and Malchau 2000, Liu et al. 2009, Fevang et al. 2010). However, an increase in the risk of revision due to infection after THA has also been reported in recent years (Kurtz et al. 2008, Dale et al. 2009, Pedersen et al. 2010b). We wanted to assess whether the increase in risk of revision due to infection is a common feature in the Nordic countries, and we therefore assessed time trends and risk factors for revision due to infection after primary total hip arthroplasty (THA) in the Nordic countries (Denmark, Finland, Norway, and Sweden). The aim was to compare revision rates due to infection in different time periods and different patient and implant groups, and to investigate factors that influence the risk of revision due to infection.
Materials and methods
The Nordic Arthroplasty Register Association dataset
The NARA dataset contains merged individual-based data from the Danish, Finnish, Norwegian, and Swedish arthroplasty registers (Herberts et al. 1989, Havelin et al. 2000, Lucht 2000, Puolakka et al. 2001, Malchau et al. 2005, Havelin et al. 2009). In each register, the data selected were transformed according to a common set of definitions, and revisions were linked to the primary procedures. The data were de-identified nationally before the anonymous data were merged into the NARA dataset. The data were treated in full confidentiality and in compliance with the regulations of each country (Havelin et al. 2009).
The inclusion criteria in the present study were primary THAs and first revisions from the period 1995 through 2009, with complete information on the following parameters: year of primary surgery and first revision, age, sex, diagnosis (osteoarthrosis (OA), inflammatory hip disease, hip fracture, childhood hip disease, femoral head necrosis, or other diagnoses), prosthesis (monoblock or modular), and type of fixation (uncemented, cemented, hybrid, or inverse hybrid, with plain or antibiotic-loaded cement). Primary THA was defined as the first total hip prosthesis regardless of cause of the arthroplasty. The endpoint was revision due to infection, and revision was defined as removal or exchange of the whole or part(s) of the prosthesis. Infection as the cause of revision was determined and reported by the surgeon immediately after surgery, based on the preoperative clinical manifestations and samples in addition to peroperative evaluation. The national datasets were harmonized according to these definitions. Of the 459,540 primary arthroplasties in the NARA dataset, 7,450 resurfacing arthroplasties were not considered as THAs. Of the 452,090 THAs, 3,397 were excluded due to unknown type of fixation, as were 16,525 THAs due to incomplete information on the risk factors. 432,168 THAs met the inclusion criteria. Denmark contributed 83,853 primary THAs, Finland 78,106, Norway 88,455, and Sweden 181,754 (Table 1).
Table 1.
Patient and procedure characteristics for the primary THAs included, and number of primary THAs excluded over the 3 time periods
| 1995-1999 | 2000-2004 | 2005-2009 | 1995-2009 | |
|---|---|---|---|---|
| Number of THAs included | 113,280 | 147,823 | 171,065 | 432,168 |
| Age (%) | ||||
| <40 years | 2 | 1 | 1 | 1 |
| 40–59 years | 17 | 18 | 17 | 17 |
| 60–69 years | 29 | 29 | 32 | 30 |
| 70–79 years | 38 | 37 | 35 | 36 |
| 80–89 years | 14 | 15 | 15 | 15 |
| ≥90 years | 1 | 1 | 1 | 1 |
| Sex (%) Female | 63 | 62 | 61 | 61 |
| Diagnosis (%) | ||||
| Osteoarthritis | 76 | 80 | 83 | 80 |
| Hip fracture | 10 | 7 | 6 | 8 |
| Inflammatory disease | 5 | 4 | 2 | 4 |
| Childhood hip disease | 4 | 4 | 3 | 3 |
| Femoral head necrosis | 2 | 2 | 2 | 2 |
| Other diagnoses | 2 | 3 | 3 | 3 |
| Prosthesis (%) | ||||
| Monoblock | 22 | 10 | 2 | 10 |
| Modular | 78 | 90 | 98 | 90 |
| Fixation (%) | ||||
| Uncemented | 13 | 16 | 30 | 21 |
| Cemented | 76 | 71 | 56 | 67 |
| Hybrid | 10 | 10 | 6 | 9 |
| Inverse hybrid | 1 | 3 | 8 | 4 |
| Cement (%) | ||||
| No???? | 13 | 16 | 30 | 21 |
| With antibiotics | 71 | 79 | 69 | 73 |
| Without antibiotics | 15 | 5 | 1 | 6 |
| Country (%) | ||||
| Denmark | 14 | 20 | 22 | 21 |
| Norway | 23 | 21 | 19 | 20 |
| Sweden | 45 | 42 | 41 | 42 |
| Finland | 19 | 17 | 18 | 18 |
| Number of THAs excluded | 10,540 | 3,303 | 6,169 | 9,922 (4.4%) |
Statistics
Descriptive statistics were used for presentation of the patient and procedure characteristics. Adjusted Cox regression analyses were performed to assess relative risk of revision due to infection and to estimate adjusted cumulative 5-year probability (risk) of revision. Unadjusted cumulative 5-year risks of revision due to infection were estimated by the Kaplan-Meier (KM) method. The study population was divided into 5-year periods (1995–1999, 2000–2004, and 2005–2009). The cases were observed until first revision, death, emigration, or December 31, 2010. We also investigated changes in the revision rates due to deep infection as a function of the year of operation, to give a graphical display of the relationship based on a generalized additive model for survival data (Hastie and Tibshirani 1990). Adjusted hazard rate ratios, as a measure of relative risk, were estimated, with 95% confidence intervals (CIs) for time periods and risk factors. In the Cox analyses we adjusted for age, sex, diagnosis, modularity of the prosthesis, and fixation, and the influence on revision risk of each of these factors was assessed. Separate Cox analyses were performed on a homogenous subgroup of hips with cemented modular THAs with antibiotics in the cement on patients with OA, as this combination was common throughout the 3 time periods in all 4 countries.
The Cox survival analyses were performed with 1–16 years of follow-up, but the last time period had only 1–6 years of follow-up. To ensure that there was similar follow-up for operations in all 3 time periods, we performed additional analyses with follow-up restricted to 1–6 years for each time period. In addition, we performed separate time trend analyses of revision due to infection for men and women, all age groups, and groups of diagnoses separately. Also, the risk factors were studied in each country separately. Finally, we assessed the risk factors separately within each of the 3 time periods to minimize time-dependent confounding. Additional Cox analyses with the endpoints revision due to aseptic loosening and revision for any cause were performed to relate these to our findings on revision due to infection.
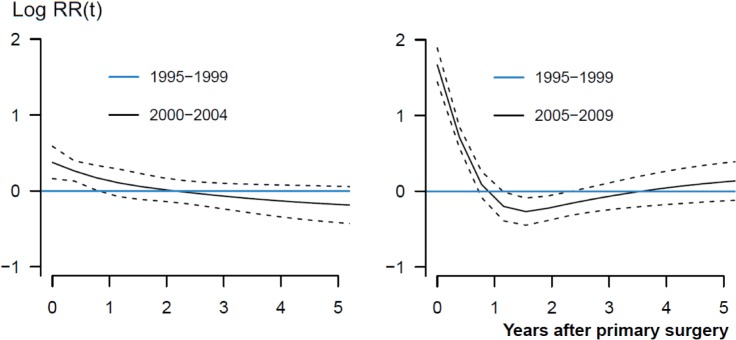
The analyses were performed in accordance with the guidelines for statistical analyses of arthroplasty register data (Ranstam et al. 2011). The proportional-hazard assumptions of the Cox survival analyses were not completely fulfilled. We therefore assessed the proportionality of the main risk factors by smoothed Schoenfeld residuals (Figure 3) (Ranstam et al. 2011). This resulted in assessment of the risk factors before and after 1 year, since adjusted revision rates of the 3 time periods were not fully proportional. Potential overestimation of incidence of revision due to infection through the effect of competing risks (death and revision due to causes other than infection) was assessed by the cumulative incidence function (Gillam et al. 2010). The 3.9% of THAs that were revised for causes other than infection and the 21% of THA patients who died during the follow-up had a negligible effect on the Cox analyses.
Figure 3.
A graphical display of the relationship between relative risk of revision due to infection and time after primary THAs for the period 2000–2004 (left panel) and 2005–2009 (right panel) compared to 1995–1999 (blue lines). Smoothed Schoenfeld residuals adjusted for age, sex, diagnosis, prosthesis and cement (solid lines) with 95% confidence intervals (broken lines).
Bilateral THAs are not independent observations, but were included. The extent of bilaterality was estimated to be 18% and the incidence of revision due to infection was 0.6% in both the first and second hip. Only 0.05% of the bilateral THAs were identified to have had revisions due to infection in both hips. We therefore considered bilaterality to have a negligible influence on the results (Lie et al. 2004, Ranstam and Robertsson 2010, Ranstam et al. 2011).
Values of p < 0.05 were considered to be statistically significant. SPSS software version 18.0 and the R statistical software package were used for the analyses.
Results
2,778 primary THAs (0.6%) were revised due to deep infection. The cumulative 5-year revision rate due to infection, adjusted for year of primary surgery, was 0.62% (0.60–0.65) for the study population and 0.99% (0.83–1.15) for the excluded THAs (4.4% of the total). The implants at use had changed during the study period. In the last 5-year period, there were more uncemented THAs and inverse hybrid THAs and nearly all of the cemented THAs were modular and inserted with cement containing antibiotics (Table 1). There were only minor changes in the distribution of patient-related risk factors over the study period, with the exception that fewer THAs were performed due to inflammatory disease and hip fracture later in the study period (Table 1).
Time trend of revision due to infection
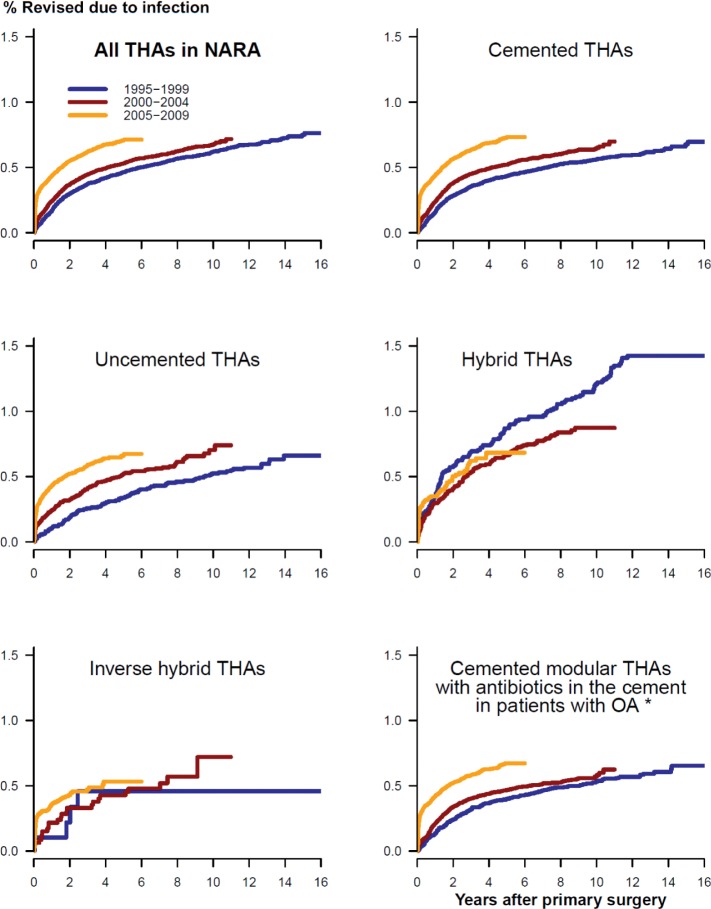
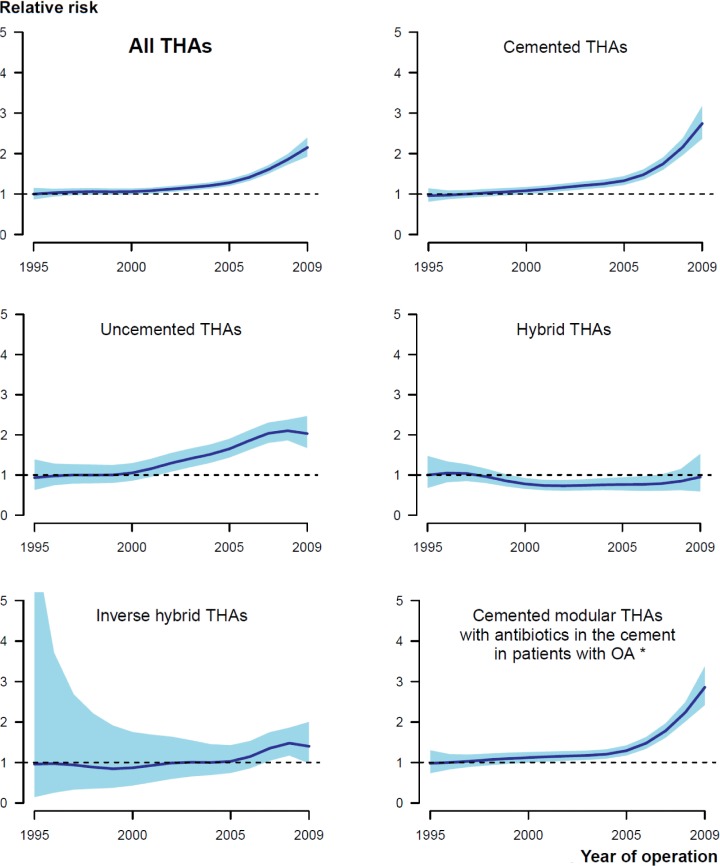
The risk of revision due to infection increased in the period 2005–2009 relative to the period 1995–1999 in the total study population (Table 2; Figures 1 and 2), and in each of the 4 countries separately (Denmark: RR = 1.3 (CI 1.0–1.6); Norway: RR = 1.7 (1.2–2.3); Sweden: RR = 1.5 (1.2–1.9); and Finland: RR = 1.2 (1.0–1.5)). For the period 2000–2004, the risk of revision due to infection only increased in Norway (RR = 1.3 (1.1–1.6)). The overall cumulative 5-year revision rate due to infection also increased, despite the fact that the revision rate for the period 2005–2009 might be an underestimate due to incomplete 5-year follow-up (Table 3 and Figure 1). The subgroup of cemented modular THAs with antibiotic-loaded bone cement in OA patients showed similar results (Tables 2 and 3; Figures 1 and 2).
Table 2.
Relative risk of revision due to infection of primary THAs in the NARA with 1-16 years of follow-up. Adjusted for age, sex, diagnosis, prosthesis, and cement
| Period | Number of THAs included |
Number of THAs revised due to infection |
Adjusted risk ratio for revision due to infection |
95% confidence interval |
p-value | |
|---|---|---|---|---|---|---|
| All THAs | 1995–1999 | 113,280 | 778 | 1 | ||
| 2000–2004 | 147,823 | 937 | 1.1 | 1.0–1.2 | 0.03 | |
| 2005–2009 | 171,065 | 1,063 | 1.6 | 1.4–1.7 | <0.001 | |
| Uncemenxted THAs | 1995–1999 | 15,177 | 87 | 1 | ||
| 2000–2004 | 23,553 | 147 | 1.4 | 1.0–1.8 | 0.03 | |
| 2005–2009 | 51,445 | 308 | 1.9 | 1.5–2.5 | <0.001 | |
| Cemented THAs | 1995–1999 | 86,177 | 538 | 1 | ||
| 2000–2004 | 105,421 | 641 | 1.2 | 1.1–1.3 | 0.006 | |
| 2005–2009 | 96,455 | 619 | 1.7 | 1.5–2.0 | <0.001 | |
| Hybrid THAs | 1995–1999 | 11,369 | 149 | 1 | ||
| 2000–2004 | 15,163 | 125 | 0.8 | 0.6–1.0 | 0.02 | |
| 2005–2009 | 10,390 | 63 | 0.8 | 0.6–1.1 | 0.2 | |
| Inverse hybrid THAs | 1995–1999 | 556 | 4 | 1 | ||
| 2000–2004 | 3,685 | 24 | 1.3 | 0.4–4.0 | 0.6 | |
| 2005–2009 | 12,775 | 73 | 1.6 | 0.5–4.6 | 0.4 | |
| Cemented modular THAs with antibiotics in cement inserted due to OA a |
1995–1999 | 37,848 | 208 | 1 | ||
| 2000–2004 | 69,052 | 374 | 1.1 | 0.9–1.3 | 0.2 | |
| 2005–2009 | 75,929 | 467 | 1.7 | 1.4–2.0 | <0.001 |
a Adjusted for age and sex.
Figure 1.
Adjusted cumulative revision rates for THAs revised due to infection in 3 time periods of primary surgery, for all THAs (upper left panel) and 5 subgroups of THAs. Adjusted for age, sex, diagnosis, prosthesis, and cement. *Adjusted for age and sex only.
Figure 2.
Graphical display of the relationship between year of primary surgery and relative risk of revision due to infection (with 95% CI), for all THAs (upper left panel) and 5 subgroups of THAs. The broken lines represent no difference in relative risk from the beginning of the period (RR = 1). Adjusted for age, sex, diagnosis, prosthesis, and cement. *Adjusted for age and sex.
Table 3.
Adjusted cumulative 5-year revision rates of primary THAs in the NARA. Adjusted for age, sex, diagnosis, prosthesis, and cement
| Period | Number of THAs included |
Cumulative 5-years revision rate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kaplan-Meier infection |
Adjusted infection |
Adjusted aseptic loosening |
Adjusted all revisions |
|||||||
| All THAs | 1995–1999 | 113,280 | 0.54 | (0.49–0.58) | 0.46 | (0.42–0.50) | 1.41 | (1.34–1.49) | 3,34 | (3.22–3.45) |
| 2000–2004 | 147,823 | 0.57 | (0.53–0.61) | 0.54 | (0.50–0.58) | 0.81 | (0.77–0.86) | 3.01 | (2.92–3.10) | |
| 2005–2009 b | 171,065 | 0.73 | (0.68–0.77) | 0.71 | (0.66–0.76) | 1.00 | (0.93–1.07) | 3.30 | (3.19–3.41) | |
| Uncemented THAs | 1995–1999 | 15,177 | 0.36 | (0.26–0.45) | 0.34 | (0.25–0.44) | 1.32 | (1.13–1.50) | 4.39 | (4.05–4.72) |
| 2000–2004 | 23,553 | 0.55 | (0.45–0.65) | 0.52 | (0.43–0.61) | 0.85 | (0.73–0.97) | 4.28 | (4.02–4.54) | |
| 2005–2009 b | 51,445 | 0.70 | (0.61–0.78) | 0.65 | (0.57–0.74) | 1.21 | (1.08–1.34) | 4.24 | (4.02–4.45) | |
| Cemented THAs | 1995–1999 | 86,177 | 0.51 | (0.47–0.56) | 0.43 | (0.38–0.48) | 1.34 | (1.25–1.43) | 2.82 | (2.70–2.94) |
| 2000–2004 | 105,421 | 0.56 | (0.51–0.60) | 0.52 | (0.48–0.57) | 0.74 | (0.68–0.79) | 2.53 | (2.43–2.63) | |
| 2005–2009 b | 96,455 | 0.74 | (0.68–0.81) | 0.74 | (0.67–0.80) | 0.85 | (0.77–0.94) | 2.93 | (2.80–3.07) | |
| Hybrid THAs | 1995–1999 | 11,369 | 0.94 | (0.76–1.12) | 0.88 | (0.70–1.06) | 1.82 | (1.55–2.09) | 4.92 | (4.50–5.34) |
| 2000–2004 | 15,163 | 0.72 | (0.58–0.85) | 0.67 | (0.53–0.80 | 0.98 | (0.81–1.14) | 3.79 | (3.48–4.10) | |
| 2005–2009 b | 10,390 | 0.72 | (0.54–0.90) | 0.67 | (0.50–0.85) | 1.00 | (0.75–1.25) | 3.86 | (3.41–4.31) | |
| Inverse hybrid THAs | 1995–1999 | 556 | 0.77 | (0.02–1.51) | 0.36 | (0–1.38) | 2.36 | (0.97–3.75 | 5.59 | (3.65–7.54) |
| 2000–2004 | 3,685 | 0.53 | (0.29–0.77) | 0.34 | (0–1.27) | 1.64 | (1.19–2.09) | 3.98 | (3.31–4.64) | |
| 2005–2009 b | 12,775 | 0.66 | (0.50–0.83) | 0.43 | (0–1.58) | 1.37 | (1.02–1.72) | 3.67 | (3.20–4.14) | |
| Modular THAs with antibiotics in cement in patients with OAa |
1995–1999 | 37,848 | 0.43 | (0.36–0.49) | 0.40 | (0.33–0.46) | 1.18 | (0.67–1.69) | 2.60 | (2.44–2.77) |
| 2000–2004 | 69,052 | 0.49 | (0.44–0.55) | 0.47 | (0.41–0.52) | 0.69 | (0.39–0.99) | 2.21 | (2.10–2.32) | |
| 2005–2009 b | 75,929 | 0.71 | (0.64–0.77) | 0.67 | (0.60–0.73) | 0.78 | (0.44–1.12) | 2.60 | (2.46–2.75) | |
a Adjusted for age and sex.
b Cumulative 5-year revision rates probably were underestimates due to incomplete 5-year follow-up.
The entire increase in risk of revision due to infection occurred within 1 year of primary surgery, and most notably within the first 3 months after surgery (Table 4; Figures 1 and 3). The increased risk of revision due to infection was found for cemented and uncemented THAs, but not for hybrid THAs and inverse hybrid THAs (Table 2; Figures 1 and 2). The increase in risk of revision due to infection was more gradual through the time periods for uncemented THAs than for cemented THAs, where the main increase in relative risk of revision and cumulative 5-year revision rate was in the last time period (Tables 2 and 3; Figures 1 and 2).
Table 4.
Adjusted relative risks of revision due to infection for 4 different time intervals after primary surgery, for the 3 time periods. Adjusted for age, sex, diagnosis, prosthesis, and cement
| Time after primary surgery |
Number of THAs included |
Number of THAs revised due to infection |
Adjusted risk ratio for revision due to infection |
95% CI | p-value |
|---|---|---|---|---|---|
| 0–3 months | |||||
| 1995–1999 | 113,280 | 74 | 1 | ||
| 2000–2004 | 147,823 | 175 | 1.9 | 1.4–2.4 | <0.001 |
| 2005–2009 | 171,065 | 535 | 4.8 | 3.7–6.2 | <0.001 |
| 3–12 months | |||||
| 1995–1999 | 111,607 | 142 | 1 | ||
| 2000–2004 | 145,625 | 206 | 1.3 | 1.0–1.6 | 0.05 |
| 2005–2009 | 168,019 | 216 | 1.2 | 1.0–1.5 | 0.09 |
| 1–2 years | |||||
| 1995–1999 | 109,178 | 164 | 1 | ||
| 2000–2004 | 142,589 | 195 | 1.1 | 0.9–1.3 | 0.6 |
| 2005–2009 | 164,758 | 175 | 1.0 | 0.8–1.3 | 0.9 |
| > 2 years | |||||
| 1995–1999 | 105,338 | 398 | 1 | ||
| 2000–2004 | 138,270 | 361 | 0.9 | 0.8–1.1 | 0.5 |
| 2005–2009 | 126,131 | 137 | 0.9 | 0.7–1.1 | 0.2 |
The risk of revision due to infection increased similarly for men and women, in all age groups and for the different diagnoses, as well as for the excluded cases.
Time trend of revision due to aseptic loosening and revision for any cause
The adjusted cumulative 5-year revision rate due to aseptic loosening was lower in 2000–2004 and 2005–2009 than in 1995–1999, but the last time period did not have complete 5-year follow-up and would have been an underestimate (Table 3). For uncemented THAs, the cumulative 5-year revision rate due to aseptic loosening did not improve during the study period (Table 3). For revisions due to any cause, there was no improvement in cumulative 5-year revision rate during the study period, except for hybrid THA, despite the incomplete 5-year follow-up in 2005–2009 (Table 3). Compared to other methods of fixation, cemented THA had the lowest cumulative 5-year revision rate for any cause in 2005–2009 (Table 3).
Risk factors for revision due to infection
Male sex and THA performed due to inflammatory disease, hip fracture, or femoral head necrosis were the patient-related risk factors associated with increased risk of revision due to infection (Table 5). Implant-related risk factors that increased the relative risk of revision due to infection were hybrid fixation and plain bone cement (Table 5). The findings were similar when we assessed the risk factors within each time period separately and before and after 1 year after primary surgery. The exception was patients of advanced age at primary THA, who had a higher risk of revision due to infection within the first year after surgery, whereas they had a lower risk of revision due to infection more than 1 year postoperatively.
Table 5.
Adjusted relative risks and adjusted cumulative 5-year revision rates for risk factors for revision due to infection. All risk factors were adjusted mutually for the other risk factors in addition to the year of primary surgery. Follow-up in the risk analyses was 1-16 years
| Number of THAs included |
Number of THAs revised due to infection |
Adjusted risk ratio for revision due to infection |
95% confidence interval |
p-value | Adjusted cumulative 5-years revision rate, infection |
|
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| <40 | 5,590 | 39 | 1 | 0.47 | ||
| 40–51 | 74,107 | 515 | 1.1 | 0.8–1.5 | 0.6 | 0.59 |
| 60–69 | 129,134 | 854 | 1.1 | 0.8–1.5 | 0.7 | 0.58 |
| 70–79 | 157,292 | 1,021 | 1.1 | 0.8–1.5 | 0.6 | 0.62 |
| 80–89 | 63,034 | 337 | 0.9 | 0.7–1.3 | 0.8 | 0.52 |
| ≥90 | 3,011 | 12 | 0.7 | 0.4–1.4 | 0.3 | 0.32 |
| Sex | ||||||
| Female | 266,42 | 1,312 | 1 | 0.46 | ||
| Male | 165,748 | 1,466 | 1.9 | 1.8–2.1 | <0.001 | 0.87 |
| Diagnosis | ||||||
| Osteoarthritis | 345,925 | 2,090 | 1 | 0.54 | ||
| Hip fracture | 33,572 | 327 | 2.1 | 1.9–2.4 | <0.001 | 1.12 |
| Inflammatory disease | 15,771 | 118 | 1.4 | 1.1–1.7 | 0.001 | 0.72 |
| Childhood hip disease | 14,983 | 80 | 0.9 | 0.7–1.2 | 0.6 | 0.51 |
| Femoral head necrosis | 9,671 | 92 | 1.7 | 1.4–2.1 | <0.001 | 0.87 |
| Other diagnoses | 12,246 | 71 | 1.3 | 1.0–1.6 | 0.06 | 0.65 |
| Prosthesis | ||||||
| Modular | 388,371 | 2,475 | 1 | 0.58 | ||
| Monoblock | 43,797 | 303 | 1.1 | 1.0–1.3 | 0.09 | 0.69 |
| Fixation | ||||||
| Uncemented | 90,177 | 542 | 1 | 0.54 | ||
| Cemented | 288,053 | 1,798 | 1.1 | 1.0–1.2 | 0.09 | 0.58 |
| Hybrid | 36,922 | 337 | 1.6 | 1.4–1.8 | <0.001 | 0.79 |
| Inverse hybrid | 17,016 | 101 | 1.0 | 0.8–1.3 | 0.7 | 0.53 |
| Cement | ||||||
| With antibiotics | 316,072 | 1,997 | 1 | 0.58 | ||
| Without antibiotics | 25,921 | 239 | 1.5 | 1.3–1.8 | <0.001 | 0.96 |
Discussion
Our main finding was the higher risk of revision due to infection after primary uncemented and cemented THAs in the 4 Nordic countries for the period 2005–2009 than for the period 1995–1999. This confirms earlier reports from Norway and Denmark (Dale et al. 2009, Pedersen et al. 2010b). The cumulative 5-year revision rate due to infection was also higher in 2005–2009 than in the previous 2 time periods. This was the case even though the revision rates for 2005–2009 probably were underestimates due to the incomplete 5-year follow-up, and they might therefore have been expected to be even higher.
None of the risk factors that we assessed could explain the increased risk of revision due to infection. The incidence of unfavorable risk factors (male sex, hybrid fixation, cement without antibiotics, and THA performed due to inflammatory disease, hip fracture, or femoral head necrosis) did not increase during the study period. In addition, these confounders were adjusted for in the analyses. An increased incidence of prosthetic joint infection would therefore have to be caused by factors that are not registered in the NARA dataset. These may include changes in patient-related factors (i.e. more comorbidity), changes in microbiology (i.e. increased bacterial virulence or more resistant strains), or changes in surgery-related factors (i.e. duration of surgery or changed surgical technique).
The common NARA dataset contains only limited information on comorbidity, which is a well-documented risk factor for infection after THA (Ridgeway et al. 2005, Pulido et al. 2008, Pedersen et al. 2010b, Dale et al. 2011). If THA was performed on more patients with poor health in the later parts of the study period, an increased incidence of prosthetic joint infections could result. In Norway, the comorbidity at THA increased during 2005–2009 (The Norwegian Arthroplasty Register 2010). The incidence of specific comorbidities associated with increased risk of infection after THA, like obesity and diabetes, is increasing in several countries (Pedersen et al. 2010a, Danaei et al. 2011, Haverkamp et al. 2011, Mraovic et al. 2011, Doak et al. 2012, Iorio et al. 2012). Given that the THA patients reported to the NARA are representative of the general population, an increased incidence of prosthetic joint infections requiring revision could result.
Surgery-related risk factors such as duration of surgery, and timing and type of systemic antibiotic prophylaxis are also not included in the NARA dataset. However, both short and long duration of surgery have been shown to be risk factors for infection (Ridgeway et al. 2005, Pulido et al. 2008, Dale et al. 2009, Pedersen et al. 2010b, Dale et al. 2011). Less compliance to guidelines for optimal systemic prophylaxis could also have contributed to an increased incidence of prosthetic joint infections, as could an increase in bacterial resistance to antibiotic prophylaxis (Kerttula et al. 2007, Stefansdottir et al. 2009a, b, Lutro et al. 2010). Finally, changes in operation room ventilation or changed adherence to guidelines of prophylactic routines may also have influenced the trend of revision due to infection (National Institute of Health and Clinical Excellence (NICE) 2008, Dale et al. 2009).
Other confounders not reported to the NARA may have contributed to an increase in reporting of revision due to infection to the registers without reflecting a corresponding increase in true incidence of prosthetic joint infection. Such confounders could be improved reporting of revisions due to infection, changes in revision policy and in the threshold of revision (i.e. new surgical methods), or changes in diagnostics (i.e. improved microbiological detection methods and changed definitions) (Dale et al. 2009, Pedersen et al. 2010b).
Since 2000, in Norway there has been an increase in the reporting of minor revision procedures, such as soft tissue debridement procedures with exchange of removable parts of modular implants and retention of the femoral stem and acetabular cup (Engesæter et al. 2011). Such procedures were reported to the registers as revision procedures because prosthesis parts were exchanged. These minor revisions may have different indications or a lower threshold to be performed than full exchange revisions. Such minor revisions may also be performed and reported earlier postoperatively than full exchange revisions. This may be the reason for the increased risk of revision due to infection in the first year after primary surgery, as found for the latter 2 time periods. In addition, similar operations performed on monoblock prostheses would not be reported because heads and liners were not exchanged. We adjusted for this potential under-reporting of infected monoblock prostheses in the analyses. In addition, the minor partial revisions were most likely used as alternatives to complete exchange procedures rather than alternatives to no revision at all. This is supported by the finding of a higher risk of revision due to infection in 2005–2009 than in 1995–1999 both for the uncemented THAs, which were all modular, and for the more homogenous subgroup of modular THAs inserted with cement containing antibiotics in patients with OA. In addition, in Norway the incidence of major revision due to infection increased during 1995–2009 as well (Engesæter et al. 2011). Thus, we do not think that increased use of modular implants and the changes in revision policy could explain the increased risk of revision due to infection.
There have been improvements in the diagnostics of prosthetic joint infections. Some bacteria such as coagulase-negative staphylococci have been increasingly acknowledged for their pathogenicity (von Eiff et al. 2006). In addition, improvements in bacterial sampling and identification may also have increased the number of infections being identified preoperatively (Trampuz and Widmer 2006, Moojen et al. 2007). The clinical presentation of an aseptic loosening and a low-grade periprosthetic infection may also be similar (Tunney et al. 1998, Ince et al. 2004, Moojen et al. 2010). If knowledge and awareness changed during the study period, there may have been a corresponding change in reporting of infection as the cause of the revision. Unexpectedly positive peroperative bacterial samples would be identified postoperatively and would not be reported to the registers. Some prosthetic joint infections may therefore have been erroneously registered as aseptic loosening in the NARA, but possibly to a lesser extent in the later stages of the study period due to improvements in diagnostics.
Our finding of increased risk of revision due to infection, which is the definition of infection used by the NARA, most probably reflects a true increase in incidence of prosthetic joint infections. To our knowledge, there have been no publications on time trends of the incidence of prosthetic joint infections after primary THA. Kurtz et al. (2008) reported a 2-fold increase in overall incidence of deep infection after THA from 0.66% in 1990 to 1.23% in 2004. This study on “total infection burden” was based on aggregated data, without any linkage between primary THA and revision after discharge and with both primary and revision arthroplasty included in the analyses. For primary THAs only, the authors found a reduced incidence of infection, most probably due to shorter length of hospital stay.
Another manifestation of infection after THA is surgical site infection, which a subject of interest in large infection surveillance programs. The definition of surgical site infection is wider than those of prosthetic joint infection and revision due to infection: the risk pattern is different and the follow-up is more limited than in arthroplasty registers (HELICS 2004, Dale et al. 2011). It may be that the treatment strategy for early postoperative soft tissue infections has become more aggressive in recent years, resulting in an increased revision rate. However, only one fifth of the surgical site infections reported to the Norwegian Surveillance System for Healthcare Associated Infections after primary THAs were reported to the Norwegian Arthroplasty Register for revisions due to infection in the period 2005–2009 (Dale et al. 2011). Both revision due to infection and surgical site infection will be surrogate endpoints of true prosthetic joint infections (Parvizi et al. 2011).
The Dutch National Nosocomial Surveillance Network (PREZIES) reported a decrease in surgical site infections after primary THA between 1996 and 2006 (Mannien et al. 2008), as did the British mandatory surveillance of SSI between 2004 and 2010 (Health Protection Agency 2011). Capture of surgical site infections is highly dependent on length of stay after primary THA or type and length of post-discharge surveillance (Huotari and Lyytikainen 2006). For instance, low-grade prosthetic joint infections, presenting as pain and loosening of the implant at a later stage, will generally be missed in surveillance programs for surgical site infection. The reported decrease in the incidence of surgical site infections may therefore be due to shorter length of stay and limited post-discharge surveillance, and not to a reduction in the incidence of prosthetic joint infections in need of revision (Mannien et al. 2008, Health Protection Agency 2011).
A previous study from Norway found that uncemented THAs had a higher risk of revision due to infection than cemented THAs (Dale et al. 2009). A study from Denmark, in contrast, found that cemented THAs had higher risk of revision due to infection than uncemented THAs (Pedersen et al. 2010b). In the present study, the overall risk of revision due to infection was similar for cemented, inverse hybrid, and uncemented THAs.
We found an incidence of revision due to infection of 0.6%; it is therefore a relatively rare complication after THA. Large populations are required for the study of time trends and risk factors for such rare events. The large NARA dataset offers an opportunity for in-depth studies of revision due to infection even in subgroups with sufficient power. The data are prospective and have a high degree of completeness (Soderman et al. 2000, Pedersen et al. 2004, Espehaug et al. 2006). The completeness of the NARA dataset and the small proportion of cases excluded in the present study (4.4%) also indicate that there was minimal selection bias, even if the relative risk of revision due to infection was higher in the excluded group. The time trend of revision due to infection was similar for the included cases and the excluded cases. The number of variables in the NARA dataset is limited, however, and even though we adjusted for several well-known confounders in our analyses, unmeasured confounding would still be a problem.
Considering the size and quality of the NARA dataset, and the adjustment for several clinically important risk factors, we believe that there has been a true increase in the risk of prosthetic joint infections. The largest increase in relative risk of revision due to infection was for uncemented THAs, but the overall risk of revision due to infection was similar for cemented, uncemented, and inverse hybrid THAs. Male sex, hybrid fixation, cement without antibiotics, and THA performed due to inflammatory disease, hip fracture, or femoral head necrosis were risk factors for revision due to infection.
Acknowledgments
HD and AMF performed the analyses. HD wrote the manuscript. All the authors contributed to interpretation of the analyses and to critical revision of the manuscript.
We thank the surgeons in Denmark, Finland, Norway, and Sweden for conscientious reporting of THAs and the staff of the 4 national registers for their thorough quality assurance of registrations.
No competing interests declared.
References
- Dale H, Hallan G, Espehaug B, Havelin LI, Engeæter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop. 2009;80(6):639–45. doi: 10.3109/17453670903506658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H, Skramm I, Lower HL, Eriksen HM, Espehaug B, Furnes O, Skjeldestad FE, Havelin LI, Engesaeter LB. Infection after primary hip arthroplasty. Acta Orthop. 2011;82(6):646–54. doi: 10.3109/17453674.2011.636671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- Doak CM, Wijnhoven TM, Schokker DF, Visscher TL, Seidell JC. Age standardization in mapping adult overweight and obesity trends in the WHO European Region. Obes Rev. 2012;13(2):174–91. doi: 10.1111/j.1467-789X.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- Engesæter LB, Dale H, Schrama JC, Hallan G, Lie SA. Surgical procedures in the treatment of 784 infected THAs reported to the Norwegian Arthroplasty Register. Acta Orthop. 2011;82(5):530–7. doi: 10.3109/17453674.2011.623572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espehaug B, Furnes O, Havelin LI, Engesæter LB, Vollset SE, Kindseth O. Registration completeness in the Norwegian Arthroplasty Register. Acta Orthop. 2006;77(1):49–56. doi: 10.1080/17453670610045696. [DOI] [PubMed] [Google Scholar]
- Fevang BT, Lie SA, Havelin LI, Engesaeter LB, Furnes O. Improved results of primary total hip replacement. Acta Orthop. 2010;81(6):649–59. doi: 10.3109/17453674.2010.537807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam MH, Ryan P, Graves SE, Miller LN, de Steiger RN, Salter A. Competing risks survival analysis applied to data from the Australian Orthopaedic Association National Joint Replacement Registry. Acta Orthop. 2010;81(5):548–55. doi: 10.3109/17453674.2010.524594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. Generalized additive models. Chapman & Hall, London. 1990.
- Havelin LI, Engesæter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties. Acta Orthop Scand. 2000;71(4):337–53. doi: 10.1080/000164700317393321. [DOI] [PubMed] [Google Scholar]
- Havelin LI, Fenstad AM, Salomonsson R, Mehnert F, Furnes O, Overgaard S, Pedersen AB, Herberts P, Karrholm J, Garellick G. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop. 2009;80(4):393–401. doi: 10.3109/17453670903039544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp D, Klinkenbijl MN, Somford MP, Albers GH, van der Vis HM. Obesity in total hip arthroplasty--does it really matter? A meta-analysis. Acta Orthop. 2011;82(4):417–22. doi: 10.3109/17453674.2011.588859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Protection Agency Sixth report of the mandatory surveillance of surgical site infection in orthopaedic surgery: April 2004 to March 2010. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1287147699571 2011 Available from.
- HELICS. Hospital in Europe Link for Infection Control through Surveillance Surgical Site Infection Surveillance of Surgical Site Infections, Protocol. http://helics.univ-lyon1.fr/ European Centre for Disease Prevention and Control. 2004 Available from.
- Herberts P, Malchau H. Long-term registration has improved the quality of hip replacement: a review of the Swedish THR Register comparing 160,000 cases. Acta Orthop Scand. 2000;71(2):111–21. doi: 10.1080/000164700317413067. [DOI] [PubMed] [Google Scholar]
- Herberts P, Ahnfelt L, Malchau H, Stromberg C, Andersson GB. Multicenter clinical trials and their value in assessing total joint arthroplasty. Clin Orthop. 1989;(249):48–55. [PubMed] [Google Scholar]
- Huotari K, Lyytikainen O. Impact of postdischarge surveillance on the rate of surgical site infection after orthopedic surgery. Infect Control Hosp Epidemiol. 2006;27(12):1324–9. doi: 10.1086/509840. [DOI] [PubMed] [Google Scholar]
- Ince A, Rupp J, Frommelt L, Katzer A, Gille J, Lohr JF. Is “aseptic” loosening of the prosthetic cup after total hip replacement due to nonculturable bacterial pathogens in patients with low-grade infection? Clin Infect Dis. 2004;39(11):1599–603. doi: 10.1086/425303. [DOI] [PubMed] [Google Scholar]
- Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus. hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726–9. doi: 10.1016/j.arth.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Kerttula AM, Lyytikainen O, Karden-Lilja M, Ibrahem S, Salmenlinna S, Virolainen A, Vuopio-Varkila J. Nationwide trends in molecular epidemiology of methicillin-resistant Staphylococcus aureus. Finland, 1997–2004. 2007;7:94. doi: 10.1186/1471-2334-7-94. BMC Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection Burden for Hip and Knee Arthroplasty in the United States. J Arthroplasty. 2008;23(7):984–91. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Lie SA, Engesæter LB, Havelin LI, Gjessing HK, Vollset SE. Dependency issues in survival analyses of 55,782 primary hip replacements from 47,355 patients. Stat Med. 2004;23(20):3227–40. doi: 10.1002/sim.1905. [DOI] [PubMed] [Google Scholar]
- Liu SS, Della Valle AG, Besculides MC, Gaber LK, Memtsoudis SG. Trends in mortality, complications, and demographics for primary hip arthroplasty in the United States. Int Orthop. 2009;33(3):643–51. doi: 10.1007/s00264-008-0549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht U. The Danish Hip Arthroplasty Register. Acta Orthop Scand. 2000;71(5):433–9. doi: 10.1080/000164700317381081. [DOI] [PubMed] [Google Scholar]
- Lutro O, Langvatn H, Schrama J, Hallan G, Dale H, Espehaug B, Sjursen H, Engesæter L. Resistance of staphylococci isolated from infected hip arthroplasties in Norway. http://www.centraloffice-europe.com/nof2010/detail.asp?id=109 NOF Congress 2010 . 2010 Available from.
- Malchau H, Garellick G, Eisler T, Karrholm J, Herberts P. Presidential guest address: the Swedish Hip Registry: increasing the sensitivity by patient outcome data. Clin Orthop. 2005;(441):19–29. doi: 10.1097/01.blo.0000193517.19556.e4. [DOI] [PubMed] [Google Scholar]
- Mannien J, van den HS, Muilwijk J, van den Broek PJ, van Benthem B, Wille JC. Trends in the incidence of surgical site infection in the Netherlands. Infect Control Hosp Epidemiol. 2008;29(12):1132–8. doi: 10.1086/592094. [DOI] [PubMed] [Google Scholar]
- Moojen DJ, Spijkers SN, Schot CS, Nijhof MW, Vogely HC, Fleer A, Verbout AJ, Castelein RM, Dhert WJ, Schouls LM. Identification of orthopaedic infections using broad-range polymerase chain reaction and reverse line blot hybridization. J Bone Joint Surg (Am) 2007;89(6):1298–305. doi: 10.2106/JBJS.F.00822. [DOI] [PubMed] [Google Scholar]
- Moojen DJ, van HG, Vogely HC, Burger BJ, Walenkamp GH, Tulp NJ, Schreurs BW, de Meulemeester FR, Schot CS, van d P, I, Fujishiro T, Schouls LM, Bauer TW, Dhert WJ. Incidence of low-grade infection in aseptic loosening of total hip arthroplasty. Acta Orthop. 2010;81(6):667–73. doi: 10.3109/17453674.2010.525201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraovic B, Suh D, Jacovides C, Parvizi J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412–8. doi: 10.1177/193229681100500231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Health and Clinical Excellence (NICE) Surgical site infection, prevention and treatment of surgical site infection, clinical guideline. www.nice.org.uk/nicemedia/pdf/CG74FullGuideline.pdf CG74. 2008 Available from.
- Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992–4. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A, Johnsen S, Overgaard S, Soballe K, Sorensen HT, Lucht U. Registration in the Danish Hip Arthroplasty Registry: completeness of total hip arthroplasties and positive predictive value of registered diagnosis and postoperative complications. Acta Orthop Scand. 2004;75(4):434–41. doi: 10.1080/00016470410001213-1. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Mehnert F, Johnsen SP, Sorensen HT. Risk of revision of a total hip replacement in patients with diabetes mellitus: a population-based follow up study. J Bone Joint Surg (Br) 2010a;92(7)(34):929. doi: 10.1302/0301-620X.92B7.24461. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Svendsson JE, Johnsen SP, Riis A, Overgaard S. Risk factors for revision due to infection after primary total hip arthroplasty. A population-based study of 80,756 primary procedures in the Danish Hip Arthroplasty Registry. Acta Orthop. 2010b;81(5):542–7. doi: 10.3109/17453674.2010.519908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop. 2008;7(466):1710–5. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puolakka TJ, Pajamaki KJ, Halonen PJ, Pulkkinen PO, Paavolainen P, Nevalainen JK. The Finnish Arthroplasty Register: report of the hip register. Acta Orthop Scand. 2001;72(5):433–41. doi: 10.1080/000164701753532745. [DOI] [PubMed] [Google Scholar]
- Ranstam J, Robertsson O. Statistical analysis of arthroplasty register data. Acta Orthop. 2010;81(1):10–4. doi: 10.3109/17453671003587168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranstam J, Karrholm J, Pulkkinen P, Makela K, Espehaug B, Pedersen AB, Mehnert F, Furnes O. Statistical analysis of arthroplasty data. II. Guidelines. Acta Orthop. 2011;82(3):258–67. doi: 10.3109/17453674.2011.588863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg (Br) 2005;87(6):844–50. doi: 10.1302/0301-620X.87B6.15121. [DOI] [PubMed] [Google Scholar]
- Soderman P, Malchau H, Herberts P, Johnell O. Are the findings in the Swedish National Total Hip Arthroplasty Register valid? A comparison between the Swedish National Total Hip Arthroplasty Register, the National Discharge Register, and the National Death Register. J Arthroplasty. 2000;15(7):884–9. doi: 10.1054/arth.2000.8591. [DOI] [PubMed] [Google Scholar]
- Stefansdottir A, Johansson D, Knutson K, Lidgren L, Robertsson O. Microbiology of the infected knee arthroplasty: report from the Swedish Knee Arthroplasty Register on 426 surgically revised cases. Scand J Infect Dis. 2009a;41(11–12):831–40. doi: 10.3109/00365540903186207. [DOI] [PubMed] [Google Scholar]
- Stefansdottir A, Robertsson O, Dahl A, Kiernan S, Gustafson P, Lidgren L. Inadequate timing of prophylactic antibiotics in orthopedic surgery. We can do better. Acta Orthop. 2009b;80(6):633–8. doi: 10.3109/17453670903316868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Norwegian Arthroplasty Register Annual Report 2010. http://nrlweb.ihelse.net/eng/Report_2010.pdf The Norwegian Arthroplasty Register. 2010 Available from.
- Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19(4):349–56. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg (Br) 1998;80(4):568. doi: 10.1302/0301-620x.80b4.8473. [DOI] [PubMed] [Google Scholar]
- von Eiff C, Arciola CR, Montanaro L, Becker K, Campoccia D. Emerging Staphylococcus species as new pathogens in implant infections. Int J Artif Organs. 2006;29(4):360–7. doi: 10.1177/039139880602900405. [DOI] [PubMed] [Google Scholar]