Abstract
Staphylococcus aureus are gram-positive bacteria that cause clinically significant infections in humans. Severe S. aureus infections are particularly problematic in hospitalized patients and reoccur despite therapeutic measures. The absence of natural protective immune responses and the lack of high-throughput approaches to identify S. aureus antigens have imposed constraints in the development of effective vaccines. Here, we showed that vaccination with the genetically attenuated S. aureus mutant, inactivated using UV irradiation rather than heat, significantly increased survival and diminished bacterial burden and kidney abscesses when mice were challenged with virulent methicillin-sensitive or methicillin-resistant S. aureus. Protection conferred by immunization could be transferred to the naive host and was not observed in B-cell–deficient mice. Using a novel S. aureus whole-proteome microarray, we show that immunoglobulin G antibody responses to 83 proteins were observed in the immunized mice. These results suggest that protection against S. aureus infections requires antibody responses to the wide repertoire of antigens/virulence factors. Vaccination using UV-irradiated genetically attenuated S. aureus induces humoral immunity and provides a vaccine strategy for pathogens that fail to induce protective immunity. We also describe a novel, high-throughput technology to easily identify S. aureus antigens for vaccine development.
Severe bacterial infections are a significant cause of morbidity and mortality in humans. Staphylococcus aureus are gram-positive bacteria that are a leading cause of invasive infections in humans, particularly in hospitalized patients [1, 2]. The most frequent cause of infection-associated morbidity and mortality is S. aureus sepsis [2, 3]. S. aureus persists in inadequately treated abscesses and infections can reoccur in patients who have received therapy [4–6]. Patients diagnosed with chronic granulomatous disease or hyperimmunoglobulin E (Job) syndrome are also predisposed to recurrent and life-threatening S. aureus infections [7–9].
Although antibiotic therapy is currently used to treat S. aureus infections, the emergence of antibiotic-resistant strains is rapidly exhausting available treatment options [10, 11]. Treatment of methicillin-resistant S. aureus (MRSA) infections often requires longer hospital stays and imposes a tremendous financial burden [12]. MRSA isolates with acquired resistance to vancomycin have also been reported [13–15]. These observations raise concerns that the incidence of S. aureus infections will continue to increase, particularly if there are no improvements in our current therapeutic approaches. The lack of an effective Food and Drug Administration–approved vaccine for S. aureus limits our ability to prevent these infections [16] and emphasizes the importance of identifying preventive therapies.
Because mouse models of infection recapitulate the human disease, studies have attempted to use these models to identify S. aureus antigens that can serve as vaccines [17, 18]. Vaccines consisting of 4 surface-associated proteins [19], antibodies to the surface polysaccharide [20, 21], clumping factor [22], or toxoid derivative of α-hemolysin [23, 24] were suggested to decrease the severity of infections. However, these vaccines have not been approved for use in humans. A few studies have also disputed the role of T and B cells in S. aureus immunity [25–27]. Vaccination with heat-inactivated S. aureus have failed to provide immunity to challenging infections [25–27]. Furthermore, the myriad of diseases caused by S. aureus, the presence of unique virulence determinants among S. aureus strains [28] and the lack of high-throughput approaches to easily identify S. aureus antigens continue to pose challenges in the development of a universal vaccine with broad-spectrum activity. Here, we show that vaccination with the virulence-attenuated strain that was inactivated using UV irradiation conferred significant protection against virulent methicillin-sensitive S. aureus (MSSA) and MRSA. Proteins that stimulated immunoglobulin G (IgG) antibody responses include a wide repertoire of S. aureus antigens or virulence factors.
MATERIALS AND METHODS
Ethics Statement
All animal experiments were approved by the Institutional Animal Care and Use Committee (protocol 13311) and performed using guidelines in the Guide for the Care and Use of Laboratory Animals (8th edition) [29].
Bacterial Strains and Growth Conditions
The wild-type (WT) S. aureus strains used were clinical isolates Newman and LAC. The Newman Δstp1 mutant was constructed previously [30]. The LAC Δstp1 mutant was constructed as described elsewhere [30]. Routine cultures of S. aureus and Escherichia coli were performed in tryptic soy broth or Luria-Bertani broth at 37°C, respectively.
Animal Infection
The mice sepsis/renal abscess model of S. aureus infection was used as described elsewhere [30]. Six-week-old female WT C57BL/6J mice or homozygous muMT (μMT) (NOD.129S2 [B6]-Ighmtm1Cgn/DoiJ) mice were purchased from Jackson Laboratories; interleukin 6 (IL-6) knockout (KO) mice were bred at Seattle Children's Research Institute. S. aureus strains, cultured overnight in tryptic soy broth, were washed twice with phosphate-buffered saline (PBS) and diluted to an optical density at 600 nm (OD600) of 0.56 (1–3 × 107 colony-forming units [CFU]/100 µL). The mice were then injected with 100 µL of the bacterial suspension, intravenously via the tail vein or retro-orbitally, and their survival was monitored for 2 weeks after infection. At the end of the experiment, kidneys were harvested, visually examined for abscesses, and homogenized for enumeration of CFUs.
Animal Immunization
For inactivation of S. aureus, 5 mL of overnight, S. aureus at an OD600 of 0.6 in PBS was either treated with UV irradiation (254 nm; HL-2000 Hybrilinker) for 40 minutes or heat inactivated at 100°C for 2 hours. To confirm the lack of viability after UV irradiation or heat killing, 200 µL of the undiluted bacterial suspension was plated on tryptic soy agar (TSA) and incubated at 37°C, overnight. Under these inactivation conditions, live bacterial CFUs were not recovered. For immunization, a single dose of UV-killed or heat-killed (HK) S. aureus (100 µL) was injected intravenously via the tail vein or retro-orbitally. At 20 days after injection, mice were challenged with a lethal dose of WT S. aureus, and monitoring of survival, examination for abscesses, and enumeration of CFUs were performed as described above.
To collect serum, 100–200 μL of blood obtained from each mouse by retro-orbital bleeding was allowed to clot at room temperature for 1–4 hours and then at 4°C overnight. The samples were centrifuged at 4000 rpm for 10 minutes, and serum was transferred to a fresh tube and stored at −20°C.
Statistical Analysis
The log-rank test was used to evaluate differences in survival of infected mice, and the Student t test or the Mann–Whitney U test was used to evaluate statistical differences between groups. Differences were considered statistically significant at P < .05. These tests were performed using GraphPad Prism software, version 5.00 for Windows.
Hybridization and Analysis of Serum Samples Using S. aureus Proteome Microarray
The S. aureus whole-proteome microarray chips were prepared by Antigen Discovery, using methods described elsewhere [31–35] (see supplemental information). Briefly, entire open reading frames from S. aureus USA300 genomic DNA were PCR amplified, cloned into T7 expression vectors, transcribed, and translated in vitro, followed by microarray chip printing. Eight serum samples, including 6 from UVΔstp1-immunized mice and 2 from naive mice, were analyzed for reactivity to the S. aureus proteome microarray, in duplicate. Briefly, serum from each mouse was diluted 1:100 in 1 × blocking buffer/10% E. coli lysate. The microarrays were blocked, hybridized with diluted serum samples overnight, incubated with biotinylated goat anti-mouse IgG-specific secondary antibody, and visualized by hybridization with streptavidin-PBXL3. Microarrays were scanned, and fluorescence intensities were quantified using ScanArray software version 4 (Perkin Elmer). The duplicate data sets were averaged, and the mean, standard deviation, standard error, and P value generated from t test for each protein was calculated as described elsewhere [31–35]. A reactive antigen is defined as any protein whose mean signal intensity is greater than the mean of the negative controls plus 2 standard deviations.
RESULTS
S. aureus Deficient in Expression of the Serine/Threonine Phosphatase Stp1: Virulence in IL-6 KO Mice (IL6−/−; Il6tm1Kopf)
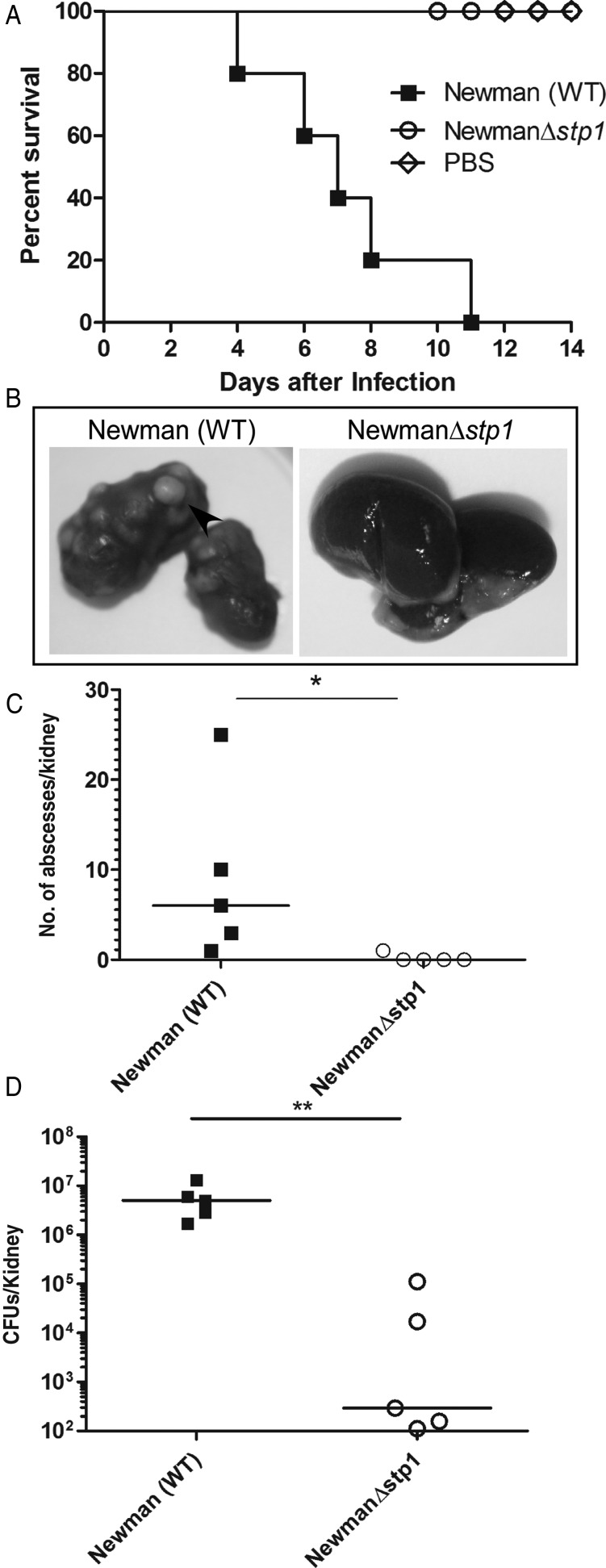
We recently reported that MSSA deficient in expression of a serine/threonine phosphatase (Δstp1) was severely attenuated for systemic infection in C57BL/6J mice [30]. The goal of this study was to determine whether mutants that are attenuated for virulence, such as Δstp1, can to induce protection against lethal S. aureus systemic infection. Because infection with Δstp1 altered IL-6 expression in kidneys of infected mice [30], it was of interest to first confirm that the attenuated virulence observed with this strain is independent of IL-6 expression. To this end, we compared the virulence potential of Δstp1 with that of WT Newman in IL-6 KO mice, using the mouse sepsis/renal abscess model, as described elsewhere [30]. The Kaplan–Meier survival curve shown in Figure 1A indicates that the Δstp1 mutant was significantly attenuated for virulence compared with WT Newman (n = 5; P = .002). Kidneys obtained from Δstp1-infected mice had no visible abscesses, in contrast to kidneys of mice infected with WT Newman (Figure 1B and C) (P = .01). The total number of CFUs isolated from the kidneys of Δstp1-infected mice were also significantly lower than in mice infected with WT Newman (Figure 1D) (P = .007). Together, our results indicate that Stp1-deficient MSSA are attenuated for virulence independent of IL-6 expression.
Figure 1.
The Stp1 mutant is attenuated for virulence in interleukin 6 (IL-6) knockout mice. Six-week-old IL-6 knockout mice were intravenously injected with 1–3 × 107 colony-forming units (CFUs) of Staphylococcus aureus wild-type (WT) Newman, Δstp1 or phosphate-buffered saline (PBS) (control) (n = 5/group). A, Kaplan–Meier survival curve shows that 100% of mice infected with the WT succumbed to infection, in contrastto mice infected with Δstp1 (P = .002), B, Kidneys were harvested from the mice that were moribund or at the end of the experiment. A representative from 5 infected kidney pairs is shown. C, D, A large number of abscesses were seen in the kidneys of mice infected with WT Newman, whereas mice infected with the Δstp1 strain showed no visible abscesses; the numbers of abscesses per kidney (C) and bacterial CFUs obtained from infected kidney pairs (D) were significantly different (P < .05).* P < .05; ** P < .01.
Increased Survival in Δstp1-Infected Mice After Challenge With WT S. aureus
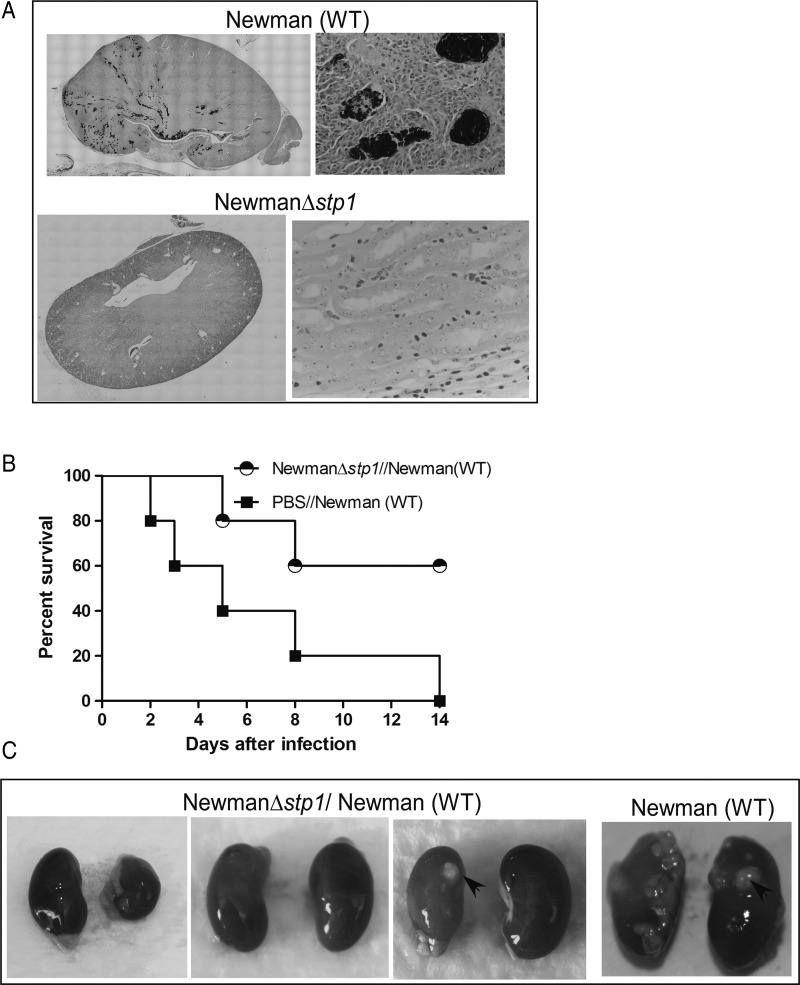
We tested our hypothesis that immunization with Δstp1 may induce protection against lethal S. aureus infection. Support for the use of Δstp1 is provided by observations that the mutant was significantly attenuated for virulence in C57BL/6J [30] and IL-6 KO mice (Figure 1), Gram-stained sections of kidneys did not indicate the presence of bacteria (Figure 2A), and a large number of genes are regulated by Stp1 [30]. To test our hypothesis, C57BL/6J mice were infected with live Δstp1, as described elsewhere [30]. At 20 days after immunization with Δstp1, mice (n = 6) were challenged with a lethal dose of WT Newman. As controls, naive mice (n = 6) treated with PBS were challenged with WT Newman. The survival of the infected mice was monitored for 2 weeks after infection. The results shown in Figure 2B indicate that 60% of mice previously immunized with Δstp1 survived the infection, in contrast to naive mice (Figure 2B) (P = .04). The Δstp1-immunized mice also had fewer kidney abscesses than the naive mouse (Figure 2C). These results suggested that Δstp1 can provide protection against subsequent lethal infections.
Figure 2.
Mice immunized with the Stp1 mutant exhibit increased survival. A, Histological sections of renal tissue from wild-type (WT) C57BL/6J mice infected with Staphylococcus aureus (WT or Δstp1) were examined 5 days after infection for the presence of staphylococci. Kidneys were fixed in formalin, embedded in paraffin, thin sectioned, stained with Brown and Brenn (B&B), and examined by microscopy, as described elsewhere [30]. S. aureus are seen in abscess lesions in kidneys of mice infected with WT S. aureus but not with the Δstp1 mutant (see purple B&B-stained bacteria), B, Six-week-old WT C57BL/6J mice (n = 6/group) were intravenously injected with either 1–3 × 107 CFUs of S. aureus Newman, Δstp1 or phosphate-buffered saline (PBS) (control). At 20 days after infection, both groups of mice were intravenously challenged with a lethal lose of S. aureus WT Newman. Kaplan–Meier survival curve shows that 100% of naive or PBS-treated mice succumbed to infection, in contrast to 60% of mice that survived the infection when immunized with the Δstp1 mutant (P = .04). C, At 2 weeks after infection, large number of abscesses were seen in the kidneys of the mouse infected with WT S. aureus, whereas mice infected with the Δstp1 strain showed fewer abscesses. Three infected kidney pairs from mice that were immunized with Δstp1 and survived the challenge with WT S. aureus are shown.
Attenuated Virulence of Stp1 Mutant Derived From MRSA
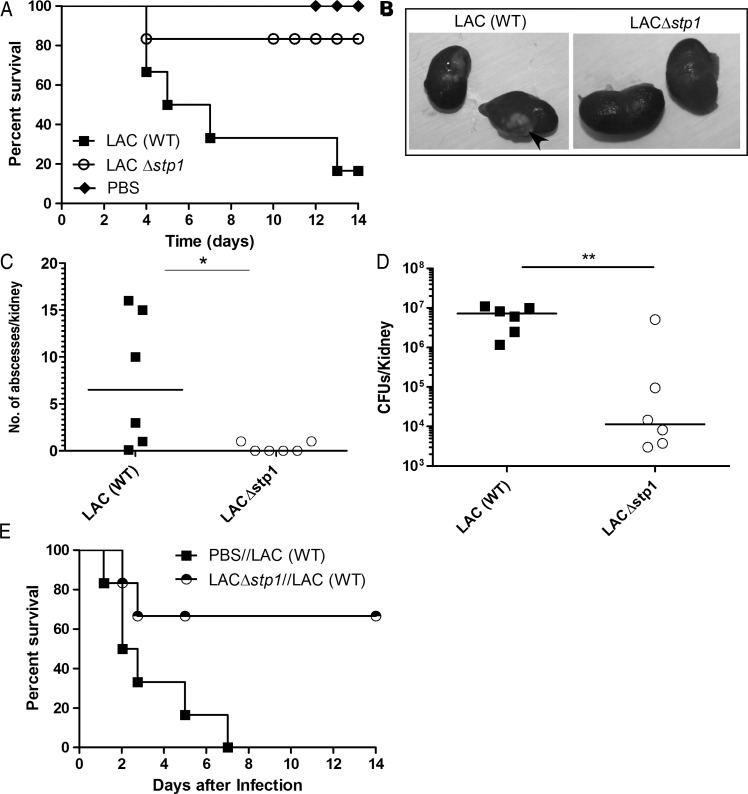
To explore the possibility of using Stp1-deficient S. aureus as a vaccine, we examined the role of Stp1 in the virulence of MRSA. Using methods described elsewhere [30], we derived a Δstp1 mutant from the currently prevalent community-acquired MRSA (USA300 genotype, strain LAC [36, 37]). The virulence potential of the LACΔstp1 was compared with that of the isogenic WT, as described elsewhere [30]. Figure 3A shows that 80% of C57BL/6J mice infected with LACΔstp1 survived the infection, in contrast to the naive mouse infected with WT MRSA LAC (n = 6; P = .04). Mice infected with LACΔstp1 also had significantly fewer abscesses and CFUs in kidneys than mice infected with WT LAC (Figure 3B–D) (P < .05). Similar to our observations with MSSA (Figure 2B), mice immunized with MRSA LACΔstp1 showed increased survival (Figure 3E) (n = 6; P = .02) and decreased kidney abscesses. Taken together, our results suggest that immunization with Δstp1 enabled mice to survive challenging infection with WT MRSA.
Figure 3.
Stp1-deficient methicillin-resistant Staphylococcus aureus (MRSA) exhibit attenuated virulence and immunization provides protection against wild-type (WT) MRSA. Six-week-old WT C57BL/6J mice (n = 6/group) were intravenously injected with MRSA LAC, LACΔstp1, or phosphate-buffered saline (PBS) control. A, Kaplan–Meier survival curve shows that 80% of mice infected with the LACΔstp1 mutant survived the infection, in contrast to 20% of mice infected with WT LAC (P = .04). B, A representative from 6 infected kidney pairs is shown and arrows indicate abscesses on infected kidneys. C, D, Comparing kidney findings in mice infected with WT LAC and those infected with LACΔstp1, there were significant differences in both total number of abscesses per kidney (P < .05) (C) and number of bacterial colony-forming units (CFUs) (P = .008) (D). E, C57BL/6J mice (n = 6/group) immunized with LACΔstp1 survived challenging infections with WT LAC, in contrast to control PBS-treated mice (P = .02). * P < .05; ** P < .01.
Increased Survival in Mice Immunized With UV-Killed Δstp1 Compared With HK Δstp1
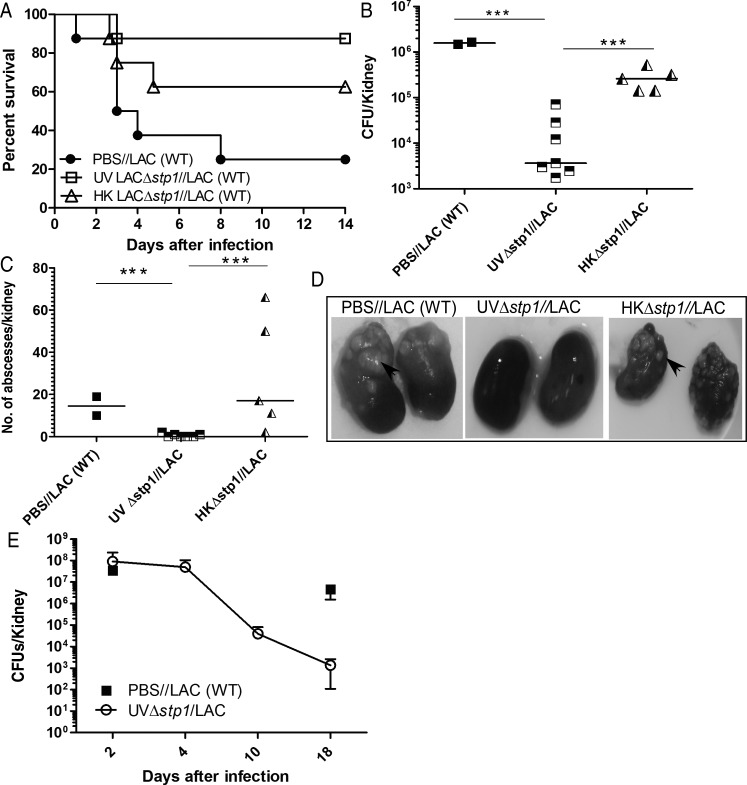
We next tested the hypothesis that heat- or UV-killed Δstp1 might be more suitable as a vaccine. To this end, C57BL/6J mice were immunized with either UV-killed (UV) LACΔstp1, HK LACΔstp1, or PBS (see Material and Methods). Of note, live bacteria were not recovered after UV irradiation or heat killing. Moreover, mice that were immunized with UV LACΔstp1 or HK LACΔstp1 did not exhibit any adverse effects, such as increased numbers of abscesses or increased rates of morbidity or mortality. At 20 days after immunization, vaccinated mice were challenged with WT (LAC), and survival was monitored for 2 weeks after infection. Figure 4A shows that immunization with UV LACΔstp1 induced protection to WT MRSA (n = 8; P = .01). Immunization with UV LACΔstp1 significantly decreased the number of kidney abscesses and bacterial CFUs, compared with immunization with HKΔstp1 or challenge with WT LAC in naive mice (Figure 4B–D). Notably, the survival of mice immunized with HKΔstp1 was not significantly different from that in naive mice infected with WT MRSA (P > .17). To confirm these observations, the experiment was repeated ≥3 independent times (for example, see Supplementary Figure 1A). We observed that immunization with UV LACΔstp1 consistently induced protection against WT S. aureus (Supplementary Figure 1A) (P < .07). In contrast, all mice immunized with HKΔstp1 succumbed to the infection (Supplementary Figure 1A) (P > .7).
Figure 4.
Mice immunized with UVΔstp1 exhibit increased survival, in contrast to those immunized with heat-killed (HK) Δstp1. Six-week-old wild-type (WT) C57BL/6J mice (n = 8/group) were intravenously injected with either UV-killed or HK LACΔstp1. Controls included phosphate-buffered saline (PBS)–treated mice. At 20 days after infection, all groups of mice were intravenously challenged with a lethal dose of methicillin-resistant Staphylococcus aureus WT LAC. A, Kaplan–Meier survival curve shows that immunization with UV LACΔstp1 enhanced the survival of mice challenged with WT LAC, in contrast to control immunization with PBS (P = .01). Note that protection conferred by UV LACΔstp1 is greater than that for immunization with HK LACΔstp1. Protection conferred by immunization with UV LACΔstp1 was confirmed by repeating the experiment 3 independent times. B–D, Large numbers of abscesses were seen in the kidneys of mice immunized with HK LACΔstp1 or control PBS compared with mice immunized with UV LACΔstp1. The numbers of colony-forming units (CFUs) obtained from kidneys of infected mice were also significantly different (P < .001). E, Mean CFU counts and standard deviations in kidneys of mice immunized with UV LACΔstp1 and challenged with WT LAC (n = 5); CFU counts 18 days after infection from naive mice infected with WT LAC represent data from 3 mice, because naive mice usually succumb to the infection.*** P <.001.
Because immunization with HKΔstp1 did not confer a significant and consistent increase in survival of the infected mice or significant decrease in bacterial burden or kidney abscesses, we did not pursue testing the HK strain as a potential vaccine. Mice immunized with UV LACΔstp1 exhibited a significant decline in CFUs in the infected kidneys during the course of infection (Figure 4E). Furthermore, protection conferred by UVΔstp1 was not strain specific, because immunization with UV MSSA Δstp1 (n = 6/group) provided protection against challenge with both isogenic WT MSSA (Figure 5) (P < .02) and WT MRSA (Supplementary Figure 1B) (P < .05). In addition, immunization with UV-inactivated virulent S. aureus, such as WT LAC or MRSA deficient for the serine/threonine kinase (Δstk1) [30, 38]) (Supplementary Figure 2A), failed to confer significant protection against lethal challenge (Supplementary Figure 2B). These data suggest that immunization with virulence-attenuated strains, such as Δstp1, facilitates the generation of protective immunity.
Figure 5.
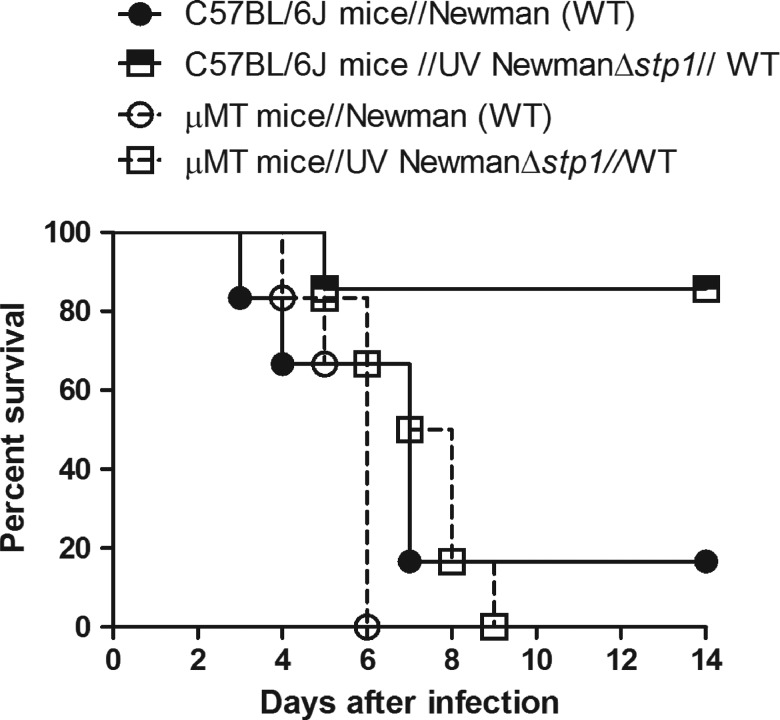
B cells are necessary for the protection induced by UVΔstp1. Six-week-old wild-type (WT) C57BL/6J or B-cell–deficient muMT (µMT) mice (n = 6/group) were intravenously injected with either UVΔstp1 or phosphate-buffered saline (control). At 20 days after infection, all groups of mice were intravenously challenged with a lethal dose of Staphylococcus aureus WT Newman. Kaplan–Meier survival curve shows that both immunized and nonimmunized µMT mice succumbed to S. aureus infection (P = 1), in contrast to UVΔstp1-immunized WT mice.
Critical Role of B Cells in UVΔstp1-Mediated Protection Against S. aureus Infection
We hypothesized that if B cells are important for protection mediated by UVΔstp1, then μMT mice that are deficient in mature B lymphocytes [39] will be susceptible to S. aureus infection despite immunization. To test this hypothesis, C57BL/6J mice and B-cell–deficient μMT mice (n = 6/group) were immunized with either UVΔstp1 or PBS. At 20 days after immunization, mice were challenged with a lethal dose of WT Newman. Figure 5 shows that μMT mice that were immunized with UVΔstp1 succumbed to S. aureus similar to PBS-treated μMT mice. In contrast, C57BL/6J mice immunized with UV NewmanΔstp1 survived challenge against WT Newman (Figure 5) (P < .02). The numbers of CFUs and kidney abscesses were also similar in UVΔstp1- and PBS-treated μMT mice (approximately 107 CFU). Similarly, μMT mice immunized with UV LACΔstp1 succumbed to WT LAC infection within 6 days (data not shown). These data indicate that B cells are essential for the protection induced by UVΔstp1.
Adoptive Transfer of Serum From UVΔstp1-Immunized Mice to Naive Mice
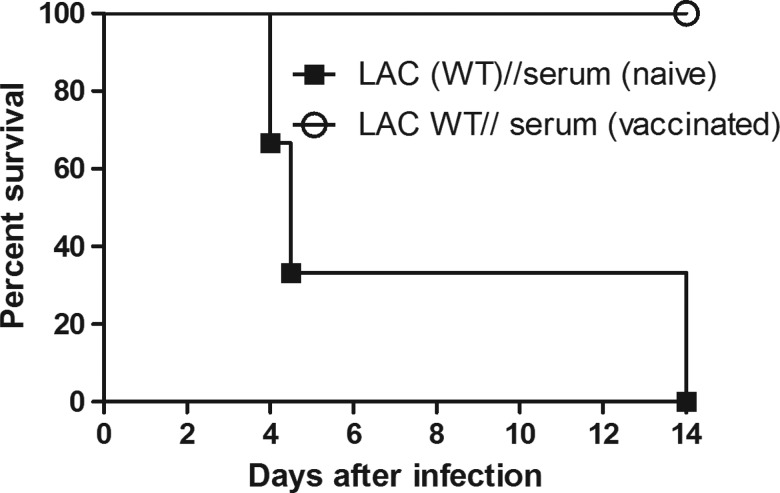
We next investigated the importance of antibody mediated immunity induced by UVΔstp1. To this end, we adoptively transferred serum into naive mice from UVΔstp1-immunized mice that cleared challenging infection with WT S. aureus. C57BL/6J mice (n = 3/group) received serum from UVΔstp1-immunized or nonimmunized (PBS-treated) mice 2 hours before and at days 1, 2, and 3 after infection with WT LAC. The results shown in Figure 6 indicate that mice that received serum from UVΔstp1-immunized mice survived the infection with WT LAC, whereas mice that received serum from naive mice succumbed to the infection (P = .01). This result shows the essential role of the humoral response in protection against systemic S. aureus infection.
Figure 6.
Serum from UVΔstp1-infected mice provides protection to naive mice. Wild-type (WT) C57BL/6J mice (n = 3/group) received serum from naive or UVΔstp1-immunized mice at 2 hours before and 1, 2, and 3 days after a lethal dose of WT LAC (1–3 × 107 colony-forming units). Kaplan–Meier survival curve shows that serum from UVΔstp1-immunized mice provided protection against lethal WT methicillin-resistant Staphylococcus aureus infections, in contrast to serum from phosphate-buffered saline–infected mice (P = .01).
Identification of S. aureus Protective Antigens
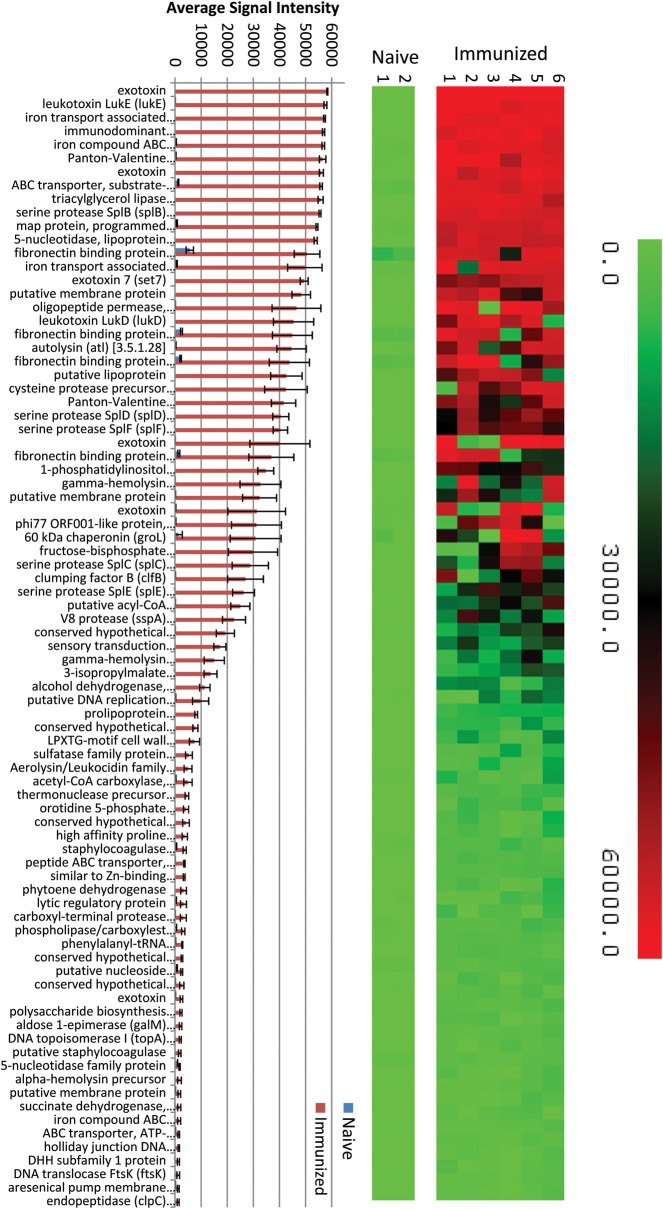
Our results suggest that immunization with UVΔstp1 elicits a B-cell–mediated antibody response that mediates protective immunity against WT S. aureus. For a comprehensive understanding of the nature of the immunogenic proteins, we used a novel S. aureus USA300 whole-proteome microarray with 2509 unique open reading frames (95.5%) of the genome. Serum samples from 6 mice that were immunized with UV LACΔstp1 and challenged with WT LAC and 2 mice that were only treated with PBS were individually hybridized in duplicate to the proteome array. To determine whether immunized mice generated protective IgG antibodies to specific S. aureus antigens, the arrays were probed with an IgG specific secondary antibody. A reactive antigen was defined as any protein whose mean signal intensity is greater than the mean of the negative controls plus 2 standard deviations. Both PBS- and UVΔstp1-immunized mice showed reactivity to the mouse IgG control spots and the 2 S. aureus proteins, Sbi (SAUSA300_2364) and Spa/Protein A (SAUSA300_0113), which bind immunoglobulin [40, 41]. A robust and statistically significant response against 83 S. aureus proteins was observed in serum samples from UVΔstp1-immunized mice, when responses were compared with those in control serum samples (Figure 7; Supplementary Table 1). This included relatively high levels of antibodies against exotoxins, leukotoxins, hemolysins, autolysin, proteases, peptidases, lipoproteins, extracellular matrix and iron-binding proteins, and chaperones (Figure 7; Supplementary Table 1). Of the 83 identified proteins, 49 are novel antigens that had not been identified previously as immunogenic or required for protection against S. aureus infection and include exotoxins, lipoproteins, and cell wall anchor proteins (Supplementary Table 1). Collectively, our studies indicate that immunization with the inactivated Δstp1 mutant generates IgG responses to specific antigens and provides protection against lethal S. aureus systemic infection.
Figure 7.
Serum samples from UVΔstp1-vaccinated mice showed conserved antibody responses to 83 Staphylococcus aureus proteins. The S. aureus USA300 proteome array containing 2509 unique open reading frames (ORFs) (95.5%) was used to identify immunoglobulin G responses to S. aureus antigens (see Materials and Methods for details). The heat map and bar graph represent statistically significant reactive antigens (P < .05) observed in serum samples obtained from 6 UVΔstp1-immunized mice and 2 control mice treated with phosphate-buffered saline. Abbreviation: ATP, adenosine triphosphate.
DISCUSSION
S. aureus infections are estimated at 1.8 million cases per year in the United States alone [17]. Because infections due to S. aureus are on the rise in community and healthcare settings [17], efforts to identify a vaccine that can decrease the severity and/or incidence of infections have gained significant interest. Despite numerous vaccine trials that used selected subunit vaccines or high-titer anti–S. aureus antibodies, a vaccine that confers significant protection in human clinical trials has yet to be identified [42]. Such failures have led to substantial controversy over whether S. aureus infections can be prevented by a vaccination approach. The arm of the immune response that is essential for protective immunity against S. aureus infection has also been controversial. Recently, Schmaler et al [25] reported that administration of heat-inactivated S. aureus failed to confer protection, and other studies dispute observations of protective immunity [26]. Consistent with previous observations, we observed that vaccination with heat-inactivated S. aureus did not decrease the bacterial load or the number of kidney abscesses (Figure 4). Moreover, immunization with heat-inactivated S. aureus did not confer a significant and consistent increase in survival against lethal challenge (Figure 4 and Supplementary Figure 1A). Although the reason is not completely understood, it is likely that heat inactivation of pathogen-associated molecular patterns (eg, toxins, peptidoglycan) are likely to affect mechanisms used by the host to mount an effective immune response. Further mechanistic studies are necessary to comprehensively understand why the HK vaccine failed to elicit significant protection and will provide novel information on S. aureus infections and immunity.
Our studies show that vaccination with a virulence-attenuated mutant of S. aureus that was inactivated using UV irradiation conferred significant protection from lethal challenge. The immunity conferred by vaccination was not observed in B-cell–deficient µMT mice (Figure 5) and could be transferred to a naive host (Figure 6). Although immunization with UVΔstp1 conferred a significant increase in survival against lethal challenge, S. aureus were still recovered from the immunized mice, albeit at significantly lower amounts. Because only a single-dose vaccine was administered, boosters with the same vaccine may increase the titer and avidity of the IgG response, further enhancing survival and decreasing bacterial burden. It remains to be determined whether immunization with UV-inactivated attenuated S. aureus provides protection in other immunocompetent hosts or strains of mice as well as in other models of S. aureus infection (eg, skin abscesses, pneumonia).
For the first time, we describe a novel, whole-proteome antigen microarray approach for identification of antibody responses to individual S. aureus proteins. Using these methods, we identified that all vaccinated mice had a widespread IgG antibody response to critical determinants of S. aureus pathogenesis (Figure 7; Supplementary Table 1). Although some of these proteins have been suggested to be immunogenic [43], 49 novel S. aureus immunogenic proteins were identified from the whole-proteome screen and included exotoxins, lipoproteins, and cell wall anchor proteins (Figure 7; Supplementary Table 1). The advantages of whole-proteome microarray serological profiling include its high-throughput nature, the ability to detect antibodies against most (>95%) of the proteome in one assay using a small volume, the ability to perform multiple assays in parallel, the reproducibility of the process, the established methods for statistical analysis, and the ability to identify individual antigenic responses without being limited by discrepancies in methods or preselected antigens. Using the whole-proteome array, we were able to identify antigenic properties of many S. aureus proteins and cross-protective immunity highlights their importance in prevention of lethal sepsis. Identifying a vaccine for S. aureus that can confer significant protection from lethal infections in humans is problematic because 80% of infants become colonized in the gut within the first year of life, and 20%–30% of adult humans are persistently colonized in the nose [43]. Although it is suggested that antibodies elicited during colonization or natural infection are not able to prevent subsequent infections with new or autologous strains of S. aureus [3], the S. aureus whole-proteome microarray described in this study provides the opportunity to compare entire antibody profiles in humans that are susceptible or immune to S. aureus infections and can accelerate vaccine development.
Active immunization of healthy individuals is necessary to prevent future S. aureus infections, whereas passive immunization is critical for prevention of disease in immunocompromised individuals, who are highly susceptible to recurrent S. aureus infections. Attempts to generate a successful vaccine using selected surface-associated S. aureus antigens have failed partly because they did not elicit a broad immune response to confer protection [42]. Although immunization of mice with clumping factor ClfA conferred strain-specific protection from arthritis and lethal sepsis [42, 44], INH-A21 (Veronate), consisting of pooled human immunoglobulin from donors with high antibody titers against ClfA and SdrG (a fibrin-binding protein) [42, 45, 46], failed in phase III clinical trials. These results suggest that antibodies directed to a few virulence factors may be insufficient for protection against S. aureus. Strategies using live virulence-attenuated S. aureus [47] face the risk of potential reversion to the virulent strain. Although strategies that incorporate whole, inactivated attenuated S. aureus provide the host with an opportunity to mount a widespread antibody response, it is unlikely that incorporation of whole bacteria is an acceptable vaccine strategy for administration in humans. However, immunization with inactivated attenuated strains in other animal models of S. aureus infection (eg, skin abscess, pneumonia), combined with high-throughput screening, will facilitate identification of the repertoire of antigens necessary to elicit a widespread antibody response against invasive staphylococci.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr Barry Kreiswirth for the MRSA strain LAC. We thank Mason Craig Bailey for animal support and Byron Z. Schmidt, NyugenThao Binh-Tran, and Melissa de los Reyes for technical assistance. Histopathology was performed at the UW Department of Pathology. We thank Dr Rebecca Hodge for help with microscopy, Dr Akilesh Singh for expert advice, and Christopher Whidbey for critical reading of the manuscript.
Financial support. This work was supported by funding from the Seattle Children's Research Institute (L. R. and T. R. T.), the National Institutes of Health (grants R01AI070749 and R01AI100989 to L. R.), and M. J. Murdock Charitable Trust (grant 2009245: JVZ:2/25/2010 to A. M. S.). Support for K. B. is provided by the National Institutes of Health (training grant 5 T32 HD007233-29; principal investigator, Lisa Frenkel).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Styers D, Sheehan DJ, Hogan P, Sahm DF. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2. doi: 10.1186/1476-0711-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 3.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol. 2012;15:92–9. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 5.Chambers HF. Community-associated MRSA—resistance and virulence converge. N Engl J Med. 2005;352:1485–7. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 6.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 7.Freeman AF, Holland SM. Clinical manifestations, etiology, and pathogenesis of the hyper-IgE syndromes. Pediatr Res. 2009;65:32R–7R. doi: 10.1203/PDR.0b013e31819dc8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison CJ. Innate immunity as a key element in host defense against methicillin resistant Staphylococcus aureus. Minerva Pediatr. 2009;61:503–14. [PubMed] [Google Scholar]
- 9.Goebel WS, Dinauer MC. Gene therapy for chronic granulomatous disease. Acta Haematol. 2003;110:86–92. doi: 10.1159/000072457. [DOI] [PubMed] [Google Scholar]
- 10.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–17. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 11.Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–56. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 12.Noskin GA, Rubin RJ, Schentag JJ, et al. National trends in Staphylococcus aureus infection rates: impact on economic burden and mortality over a 6-year period (1998–2003) Clin Infect Dis. 2007;45:1132–40. doi: 10.1086/522186. [DOI] [PubMed] [Google Scholar]
- 13.Weigel LM, Clewell DB, Gill SR, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003;302:1569–71. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 14.Chang S, Sievert DM, Hageman JC, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–7. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 15.Perichon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:4580–7. doi: 10.1128/AAC.00346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011;19:225–32. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spellberg B, Daum R. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol. 2011;34:335–48. doi: 10.1007/s00281-011-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dedent A, Kim HK, Missiakas D, Schneewind O. Exploring Staphylococcus aureus pathways to disease for vaccine development. Semin Immunopathol. 2012;34:317–33. doi: 10.1007/s00281-011-0299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103:16942–7. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenney D, Pouliot KL, Wang Y, et al. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–7. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 21.Maira-Litran T, Kropec A, Goldmann DA, Pier GB. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-beta-(1–6)-glucosamine. Infect Immun. 2005;73:6752–62. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAdow M, Kim HK, Dedent AC, Hendrickx AP, Schneewind O, Missiakas DM. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011;7:e1002307. doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menzies BE, Kernodle DS. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect Immun. 1996;64:1839–41. doi: 10.1128/iai.64.5.1839-1841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–94. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmaler M, Jann NJ, Ferracin F, Landmann R. T and B cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2-MyD88-dependent T cell activation. J Immunol. 2011;186:443–52. doi: 10.4049/jimmunol.1001407. [DOI] [PubMed] [Google Scholar]
- 26.Spellberg B, Ibrahim AS, Yeaman MR, et al. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun. 2008;76:4574–80. doi: 10.1128/IAI.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Kockritz-Blickwede M, Rohde M, Oehmcke S, et al. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am J Pathol. 2008;173:1657–68. doi: 10.2353/ajpath.2008.080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(Suppl 5):S350–9. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, National Research Council. Washington, DC: National Academies Press: 2011. Guide for the care and use of laboratory animals. [Google Scholar]
- 30.Burnside K, Lembo A, de Los Reyes M, et al. Regulation of hemolysin expression and virulence of Staphylococcus aureus by a serine/threonine kinase and phosphatase. PLoS One. 2010;5:e11071. doi: 10.1371/journal.pone.0011071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbour AG, Jasinskas A, Kayala MA, et al. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun. 2008;76:3374–89. doi: 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies DH, Liang X, Hernandez JE, et al. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci U S A. 2005;102:547–52. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyles JE, Unal B, Hartley MG, et al. Immunodominant Francisella tularensis antigens identified using proteome microarray. Proteomics. 2007;7:2172–83. doi: 10.1002/pmic.200600985. [DOI] [PubMed] [Google Scholar]
- 34.Felgner PL, Kayala MA, Vigil A, et al. A Burkholderia pseudomallei protein microarray reveals serodiagnostic and cross-reactive antigens. Proc Natl Acad Sci U S A. 2009;106:13499–504. doi: 10.1073/pnas.0812080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molina DM, Pal S, Kayala MA, et al. Identification of immunodominant antigens of Chlamydia trachomatis using proteome microarrays. Vaccine. 2010;28:3014–24. doi: 10.1016/j.vaccine.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLeo FR, Chambers HF. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest. 2009;119:2464–74. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 38.Tamber S, Schwartzman J, Cheung AL. Role of PknB kinase in antibiotic resistance and virulence in community-acquired methicillin-resistant Staphylococcus aureus strain USA300. Infect Immun. 2010;78:3637–46. doi: 10.1128/IAI.00296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–6. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Jacobsson K, Vasi J, Lindberg M, Frykberg L. A second IgG-binding protein in Staphylococcus aureus. Microbiology. 1998;144(Pt 4):985–91. doi: 10.1099/00221287-144-4-985. [DOI] [PubMed] [Google Scholar]
- 41.Graille M, Stura EA, Corper AL, et al. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proc Natl Acad Sci U S A. 2000;97:5399–404. doi: 10.1073/pnas.97.10.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaffer AC, Lee JC. Vaccination and passive immunisation against Staphylococcus aureus. Int J Antimicrob Agents. 2008;32(Suppl 1):S71–8. doi: 10.1016/j.ijantimicag.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Holtfreter S, Kolata J, Broker BM. Towards the immune proteome of Staphylococcus aureus: the anti-S. aureus antibody response. Int J Med Microbiol. 2010;300:176–92. doi: 10.1016/j.ijmm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Josefsson E, Hartford O, O'Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis. 2001;184:1572–80. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- 45.Vernachio J, Bayer AS, Le T, et al. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob Agents Chemother. 2003;47:3400–6. doi: 10.1128/AAC.47.11.3400-3406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bloom B, Schelonka R, Kueser T, et al. Multicenter study to assess safety and efficacy of INH-A21, a donor-selected human staphylococcal immunoglobulin, for prevention of nosocomial infections in very low birth weight infants. Pediatr Infect Dis J. 2005;24:858–66. doi: 10.1097/01.inf.0000180504.66437.1f. [DOI] [PubMed] [Google Scholar]
- 47.Kim HK, Kim HY, Schneewind O, Missiakas D. Identifying protective antigens of Staphylococcus aureus, a pathogen that suppresses host immune responses. FASEB J. 2011;25:3605–12. doi: 10.1096/fj.11-187963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.