Abstract
Background. An association between infections and vascular events has been observed, but the specific effect of influenza and influenza-like illnesses on triggering acute myocardial infarction (AMI) is unclear.
Methods. Episodes of first AMI from 1 January 2003 through 31 July 2009 were identified using linked anonymized electronic medical records from the Myocardial Ischaemia National Audit Project and the General Practice Research Database. Self-controlled case series analysis was used to investigate AMI risks after consultation for acute respiratory infection. Infections were stratified by influenza virus circulation, diagnostic code, and vaccination status to assess whether influenza was more likely than other infections to trigger AMI.
Results. Of 22 024 patients with acute coronary syndrome, 11 208 met the criterion of having had their first AMI at the age of ≥40 years, and 3927 had also consulted for acute respiratory infection. AMI risks were significantly raised during days 1–3 after acute respiratory infection (incidence ratio, 4.19 [95% confidence interval, 3.18–5.53], with the effect tapering over time. The effect was greatest in those aged ≥80 years (P = .023). Infections occurring when influenza was circulating and those coded as influenza-like illness were associated with consistently higher incidence ratios for AMI (P = .012).
Conclusions. Influenza and other acute respiratory infections can act as a trigger for AMI. This effect may be stronger for influenza than for other infections.
Clinical trials registration. NCT01106196.
(See the Editorial Commentary by Siriwardena, on pages 1636–8.)
Coronary heart disease is the leading cause of death worldwide and a considerable source of morbidity: it is estimated that 124 000 people in the United Kingdom have an acute myocardial infarction (AMI) each year [1]. Accumulating evidence suggests that the short-term risk of acute cardiovascular events, including AMI, may be increased following exposure to acute respiratory infections [2]. There is consistent ecological evidence to support a temporal association between influenza virus circulation and cardiovascular mortality, after adjustment for potential environmental confounding factors [3–5]. However, the specific role of influenza virus infection as a trigger of vascular events at the individual level is less clear. This has important implications for the use of influenza vaccination and antivirals to prevent and treat infections in vascular risk groups.
A previous self-controlled case series study in the General Practice Research Database (GPRD) found an increased risk of AMI in the first 3 days after consultation for a systemic respiratory tract infection [6]. Accurate data on timing of both exposure and outcome are crucial for self-controlled case series. Primary care data sets have accurate information on timing of consultations for acute respiratory infections, but data on timing of illnesses, such as AMI, that present to secondary care are likely to be less precise. In the current study, we use a newly established data linkage between GPRD [7] and the Myocardial Ischaemia National Audit Project (MINAP), a cardiac disease registry database capturing detailed information on all acute coronary syndromes presenting to hospitals in England and Wales [8], to improve the quality of AMI data. As well as reporting definite AMIs that fulfill internationally agreed criteria [9], MINAP allows accurate distinction between ST-elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI), which may be important when considering potential pathophysiological mechanisms. Crucially, AMI events in MINAP are dated with precision, which removes the risk of retrospective recording.
We used self-controlled case series analysis to investigate whether acute respiratory infections likely to be caused by influenza, assessed on the basis of levels of influenza virus circulation, medical codes used to classify infections, and influenza vaccination status, were more liable than other infections to trigger AMI.
METHODS
This study was approved by the Independent Scientific Advisory Group of the GPRD (protocol 09_034), the Cardiovascular Disease Research Using Linked Bespoke Studies and Electronic Records (CALIBER) Scientific Oversight Committee (CALIBER identification 09-08), and the University College London Research Ethics committee (application 2219/001).
Data Sources
We used linked data from the GPRD and MINAP, brought together by the CALIBER program [10]. The GPRD contains anonymized longitudinal primary care records from >5 million currently active patients registered at approximately 640 primary care practices throughout the United Kingdom [11]. These patients compose a representative sample of around 8% of the UK population. Data are available on demographic and lifestyle factors, illness consultations, prescribing patterns, investigations, and referrals. Quality control and regular auditing are central to GPRD, and the data set has been extensively validated [11].
Just over half of GPRD practices are linked to MINAP. In 2011, the MINAP data set contained records on 873 000 patients [12] from all hospitals in England and Wales that admit acute coronary syndrome patients. Records include information on medical history, date and time of symptom onset, diagnosis on admission and discharge, investigations, and treatments. Quality is monitored continuously through assessment of the completeness of 20 key data entry fields (currently 99% complete nationally) and an annual data validation study [12].
Study Participants and Follow-up
Patients registered with a GPRD practice during the study period, from 1 January 2003 through 31 July 2009, who experienced AMI at age ≥40 years were potentially eligible to participate. Patients entered the study at the latest of the following events: their GP registration date, the practice “up-to-standard” date (defined as the date when a practice is considered to have data of sufficiently high quality for research), or 1 January 2003. Patients left the study at the earliest of the following events: their date of death, their date of leaving the practice, or 31 July 2009.
Outcome
The primary outcome was a first AMI (STEMI or NSTEMI) recorded in MINAP during the study period. We defined STEMI and NSTEMI on the basis of troponin and electrocardiography findings, following the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force definition of myocardial infarction [9]. MINAP records relating to subsequent AMIs were excluded. We also excluded records in which a previous history of AMI was noted in the MINAP record and confirmed in the linked GPRD record. When no symptom onset date was recorded (31.5% of records), we substituted the date of call for help (11.6%), arrival of help (0.2%), arrival of services (0.8%), admission (16.1%), reperfusion (0.06%), cardiac arrest (0.05%), referral for investigation (0.6%), local angiogram (0.2%), or first local intervention (0.03%). The median number of days from symptom onset to hospital discharge was used to estimate symptom onset date for a small number of records (1.5%) in which only discharge date was entered. Records with no dates attached (0.4%) were excluded.
Exposure
We extracted data on primary care consultations for acute respiratory infections during the study period that were coded using 4 Read codelists compiled with methods described by Dave and Petersen [13]. The main codelist (codelist 1) contained codes describing acute respiratory infections with a systemic component (eg, “acute bronchitis,” “tracheitis,” and “pneumonia”) to capture more severe acute respiratory infections presenting to general practice. Any organism-specific codes that did not relate to influenza (eg, staphylococcal pneumonia) were then excluded to define a subset of codes in which influenza was a plausible diagnosis (codelist 2). Codelists 3 (specific influenza-like illness [ILI] codes only) and 4 (all other codes for comparison) were subsets of codelist 1. Lists are available on request. Any consultations for acute respiratory infection occurring within 28 days of a previous consultation were excluded because they were considered to be likely related to the same illness.
We considered that the probability of a respiratory illness being caused by influenza would vary according to (1) circulating levels of influenza virus, (2) medical codes used to classify respiratory illness, and (3) influenza vaccination status. Therefore, to identify episodes of acute respiratory infection likely to be caused by influenza, we first identified the peak week of influenza virus circulation for each influenza season, using weekly proportions of nose and throat specimens testing positive for influenza virus by reverse-transcription polymerase chain reaction. These specimens were taken from patients with ILI attending about 85 sentinel general practices in England, under the Health Protection Agency/RCGP swabbing scheme. In separate analyses, we labeled 2, 3, and 4 weeks on either side of this peak week as influenza virus circulation weeks. Effects were compared between weeks of circulations and noncirculation of influenza virus by use of episodes of acute respiratory infection that were extracted using codelist 2. Second, we compared the effect of episodes coded with an ILI code with the effects of episodes coded with another acute respiratory infection code (codelist 3 vs codelist 4).
Third, we examined patients' influenza vaccination status, to categorize episodes of acute respiratory infection as occurring in individuals who were vaccinated against influenza, in individuals who had residual protection against influenza, or in individuals who were unvaccinated against influenza (the group in which ARI was most likely to be caused by influenza). For “vaccinated” episodes, influenza vaccination had been received at least 14 days before the illness in the same year (defined as 1 September through 31 August, to encompass the start of the influenza vaccination season). For episodes of “residual protection,” a patient had received influenza vaccination 1–5 years before. For “unvaccinated” episodes, the patient had not received influenza vaccine in the past 5 years. For this analysis, codelist 2 was used to extract episodes of acute respiratory infection, to maximize sensitivity. Finally, we examined the effect of illness episodes with increasing numbers of the following indicators of influenza: illness during influenza virus circulation weeks, ILI code, and unvaccinated status.
Self-Controlled Case Series Analysis
Patients included in self-controlled case series analysis had records of both AMI and consultation for acute respiratory infection. We examined the incidence of AMI in exposed periods (defined as periods following consultation for acute respiratory infection) as compared to baseline or “unexposed” periods (defined as all other periods during follow-up) for each person. This method was chosen because it uses within-person comparisons, thereby implicitly controlling for fixed confounders [14], a major advantage when using routinely collected data. The exposure period (during which an acute inflammatory stimulus such as influenza virus infection could plausibly provoke a systemic effect) was divided into 1–3, 4–7, 8–14, 15–28, and 29–91 days following an acute respiratory consultation. We excluded from baseline the period from the day of consultation up to 14 days before because an AMI occurring in this time window may affect the subsequent likelihood of attending the GP (and because self-controlled case series analysis requires that the probability of exposure is not affected by the occurrence of an outcome event) [14]. Incidence ratios were calculated for AMIs occurring in each exposure period, compared with baseline periods, using conditional Poisson regression. We adjusted for age in 5-year age bands and for season in 3-month blocks (ie, January–March, April–June, July–September, and October–December) because these are time-varying confounders associated with AMI and risk of respiratory infection.
Our initial analysis examined the risk of AMI occurring after any acute respiratory infection classified using codelist 1, compared with other periods. We stratified by age, sex, type of AMI (STEMI vs NSTEMI), and history of previous vascular disease (defined by a history of angina, percutaneous coronary intervention, coronary artery bypass graft, peripheral vascular disease, or cerebrovascular disease recorded in MINAP) and tested for heterogeneity, using the Cochran Q statistic. Subsequent analyses examined AMI risks occurring after episodes of respiratory illness most likely to be caused by influenza, to explore whether any triggering effect was specific to influenza. Again, heterogeneity in incidence ratios for respiratory infections judged more and less likely to be influenza was investigated using the Cochran Q statistic. We performed several sensitivity analyses, including adjustment for age in 1-year age bands, excluding records involving patients who died during hospitalization, excluding records for which the symptom onset date was inferred from the discharge date, and including records with missing discharge diagnoses. All analyses were done using Stata, release 11 (StataCorp, College Station, TX).
RESULTS
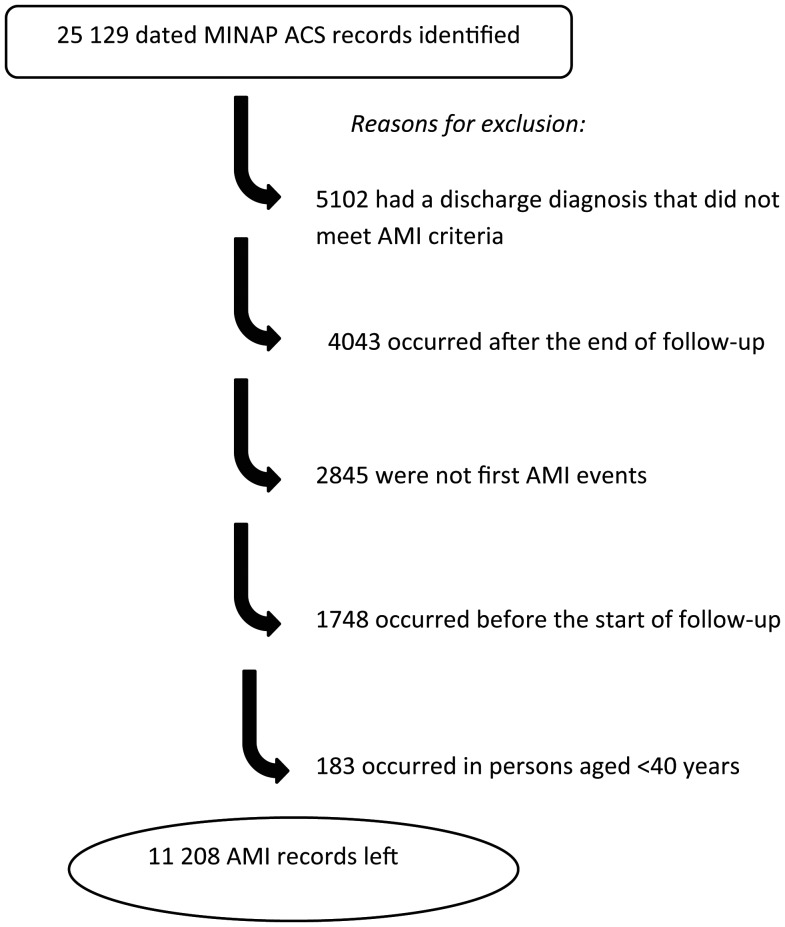
A total of 25 129 potential myocardial infarction records were identified in the MINAP database for 22 024 persons. Of these, 13 921 records were dropped (Figure 1).
Figure 1.
Selection of records on initial acute myocardial infarctions (AMIs) from the Myocardial Ischaemia National Audit Project (MINAP) database on acute coronary syndrome (ACS).
Of the remaining 11 208 AMI records, corresponding to 11 208 persons, 3927 (35.0%) were for individuals who had also consulted for an acute respiratory infection and were included in analyses. These individuals had 8204 episodes of acute respiratory infection (mean, 2.1 episodes per person). Their median age was 73.1 years (interquartile range, 62.5–81.4 years), and 60% were male. Characteristics of study participants are shown in Table 1.
Table 1.
Characteristics of Study Participants
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Men | 2360 (60.1) |
| Women | 1567 (39.9) |
| Age, y | |
| 40–49 | 212 (5.4) |
| 50–59 | 565 (14.4) |
| 60–69 | 873 (22.2) |
| 70–79 | 1122 (28.6) |
| 80–89 | 945 (24.1) |
| ≥90 | 210 (5.3) |
| History of vascular diseasea | |
| Angina | 839 (25.9) |
| PCI | 130 (4.1) |
| CABG | 124 (3.9) |
| Peripheral vascular disease | 144 (4.7) |
| Cerebrovascular disease | 282 (9.1) |
| Type of myocardial infarction | |
| STEMI | 1604 (40.8) |
| NSTEMI | 2323 (59.2) |
| Died in hospitala | |
| Yes | 250 (6.6) |
| No | 3548 (93.4) |
Data are for 3927 participants, unless otherwise indicated.
Abbreviations: CABG, coronary artery bypass graft; NSTEMI, non–ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
a Denominator used to calculate the percentage excludes participants with missing data.
The risk of AMI was substantially higher during the initial days following acute respiratory infection (adjusted incidence ratio for days 1–3, 4.19 [95% confidence interval {CI}, 3.18–5.53]), with the effect tapering over time. The effect was most marked in the oldest age groups, with the adjusted incidence ratio rising to 5.94 (95% CI, 3.90–9.04) for days 1–3 after acute respiratory infection among individuals aged ≥80 years (P = .023). There was no evidence of interaction by sex (P = .82), type of AMI (P = .53), or history of vascular disease (P = .73). Table 2 shows adjusted incidence ratios for AMI after acute respiratory infection, stratified by age group, sex, type of infarction, and history of vascular disease.
Table 2.
Age- and Season-Adjusted Incidence Ratios for Acute Myocardial Infarction (AMI) Occurring After Acute Respiratory Infection (ARI) Overall and by Sex, Age, Infarction Type, and History of Vascular Disease
| Model | Adjusted Incidence Ratio (95% Confidence Interval), by Period of Infection Risk in Daysa |
||||||
|---|---|---|---|---|---|---|---|
| 1–3 (n = 52) | Pb | 4–7 (n = 44) | 8–14 (n = 48) | 15–28 (n = 80) | 29–91 (n = 262) | Baseline (n = 3441) | |
| Overall | 4.19 (3.18–5.53) | 2.69 (1.99–3.63) | 1.66 (1.24–2.23) | 1.41 (1.12–1.77) | 1.05 (.92–1.21) | 1.00 | |
| Sex | |||||||
| Men | 4.32 (3.02–6.19) | .82 | 2.01 (1.28–3.16) | 1.28 (.83–1.97) | 1.39 (1.03–1.89) | 0.99 (.82–1.19) | 1.00 |
| Women | 4.05 (2.62–6.27) | 3.66 (2.45–5.46) | 2.22 (1.50–3.29) | 1.44 (1.01–2.06) | 1.16 (.94–1.43) | 1.00 | |
| Age, y | |||||||
| <60 y | 1.46 (.47–4.55) | 1.46 (.54–3.91) | 1.88 (.97–3.65) | 1.50 (.88–2.56) | 0.84 (.59–1.21) | 1.00 | |
| 60–69 y | 3.93 (2.15–7.18) | .13c | 1.89 (.89–4.00) | 1.09 (.51–2.30) | 0.96 (.54–1.71) | 1.03 (.77–1.38) | 1.00 |
| 70–79 y | 4.14 (2.47–6.95) | .1c | 3.55 (2.18–5.78) | 2.31 (1.45–3.66) | 1.81 (1.23–2.65) | 0.96 (.73–1.26) | 1.00 |
| ≥80 y | 5.94 (3.90–9.04) | .023c | 3.18 (1.93–5.25) | 1.40 (.79–2.48) | 1.35 (.88–2.07) | 1.31 (1.04–1.66) | 1.00 |
| Infarction type | |||||||
| STEMI | 4.66 (3.04–7.15) | .53 | 1.76 (.97–3.21) | 1.77 (1.12–2.80) | 1.13 (.74–1.71) | 0.98 (.78–1.23) | 1.00 |
| NSTEMI | 3.89 (2.71–5.60) | 3.25 (2.30–4.60) | 1.60 (1.10–2.33) | 1.58 (1.20–2.09) | 1.10 (.93–1.31) | 1.00 | |
| History of vascular disease | |||||||
| No | 4.32 (3.10–6.02) | .73 | 3.00 (2.12–4.25) | 1.68 (1.18–2.39) | 1.37 (1.03–1.82) | 0.99 (.83–1.17) | 1.00 |
| Yes | 3.89 (2.35–6.42) | 2.03 (1.11–3.69) | 1.62 (.97–2.72) | 1.49 (1.00–2.21) | 1.19 (.95–1.51) | 1.00 | |
Data are for 8204 ARIs.
Abbreviations: NSTEMI, non–ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
a n values denote the no. of AMI events.
b By the Cochran Q test for heterogeneity between the 2 strata, unless otherwise indicated, during days 1–3.
c Compared with <60 y.
There was a higher incidence ratio for AMI after acute respiratory infections occurring during peak weeks of influenza virus circulation as compared to nonpeak weeks (P values varied from .006 to .091, depending on the definition of “peak week”). Although the point estimate for episodes coded as ILI was nearly double that of episodes coded as general acute respiratory infection (incidence ratios, 7.31 [95% CI, 2.72–19.64] vs 4.03 [95% CI, 3.02–5.38] for days 1–3 after infection), this difference was not statistically significant (P = .26). Unvaccinated episodes (thought most likely to represent influenza virus infections) were associated with incidence ratios similar to those for episodes in which there was residual protection (P = .94) and for vaccinated episodes (P = .91). Overall, comparison of acute respiratory infections with at least 1 indicator of influenza to those with no indicators suggested a significantly greater effect on AMI during days 1–3 after infection (incidence ratios, 5.39 [95% CI, 3.89–7.45] vs 2.38 [95% CI, 1.37–4.11]; P = .012). Full details of results stratified by the presence or absence of indicators of influenza are shown in Table 3.
Table 3.
Age- and Season-Adjusted Incidence Ratios for Acute Myocardial Infarction (AMI) Occurring After Acute Respiratory Infections (ARIs) With and Without Indicators of Influenza
| Adjusted Incidence Ratio (95% Confidence Interval), by Period of Infection Risk in Days |
||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | ARIs, No. | 1–3 | Pb | 4–7 | 8–14 | 15–28 | 29–91 | Baseline |
| ARI onset within 2 weeks of peaka | ||||||||
| Yes | 1278 | 7.79 (4.71–12.90) | .006 | 1.86 (.77–4.51) | 0.87 (.33–2.34) | 1.69 (1.01–2.82) | 0.89 (.62–1.27) | 1.00 |
| No | 6847 | 3.32 (2.37–4.65) | 2.80 (2.04–3.86) | 1.79 (1.32–2.42) | 1.35 (1.05–1.74) | 1.08 (.93–1.25) | 1.00 | |
| ARI onset within 3 weeks of peaka | ||||||||
| Yes | 1733 | 5.95 (3.60–9.81) | .091 | 3.68 (2.12–6.40) | 0.99 (.44–2.23) | 1.54 (.96–2.47) | 1.02 (.76–1.36) | 1.00 |
| No | 6392 | 3.54 (2.53–4.95) | 2.37 (1.66–3.39) | 1.82 (1.33–2.48) | 1.37 (1.06–1.78) | 1.09 (.94–1.26) | 1.00 | |
| ARI onset within 4 weeks of peaka | ||||||||
| Yes | 2226 | 6.34 (4.13–9.72) | .016 | 3.07 (1.80–5.22) | 0.89 (.42–1.88) | 1.32 (.85–2.07) | 1.06 (.82–1.36) | 1.00 |
| No | 5899 | 3.16 (2.19–4.57) | 2.48 (1.73–3.56) | 1.92 (1.40–2.63) | 1.43 (1.10–1.86) | 1.05 (.90–1.23) | 1.00 | |
| Code | ||||||||
| ILI | 410 | 7.31 (2.72–19.64) | .26 | 1.37 (.19–9.74) | 1.56 (.39–6.28) | 0.79 (.20–3.16) | 1.17 (.67–2.05) | 1.00 |
| General ARI | 7796 | 4.03 (3.02–5.38) | 2.61 (1.91–3.57) | 1.66 (1.24–2.24) | 1.43 (1.13–1.81) | 1.06 (.92–1.23) | 1.00 | |
| Vaccination status | ||||||||
| Unvaccinated | 1590 | 4.15 (2.20–7.83) | 1.90 (.85–4.29) | 1.87 (.99–3.51) | 1.91 (1.21–3.03) | 1.08 (.79–1.46) | 1.00 | |
| Residual protection | 1466 | 4.30 (2.29–8.07) | .94c | 2.61 (1.29–5.26) | 2.26 (1.27–4.03) | 0.58 (.26–1.31) | 1.15 (.86–1.55) | 1.00 |
| Vaccinated | 5069 | 3.99 (2.79–5.70) | .91c | 2.92 (2.03–4.20) | 1.41 (.95–2.09) | 1.50 (1.13–1.98) | 1.05 (.88–1.25) | 1.00 |
| No. of indicatorsd | ||||||||
| 0 | 3433 | 2.38 (1.37–4.11) | 2.49 (1.56–3.97) | 1.67 (1.08–2.58) | 1.61 (1.17–2.23) | 1.07 (.88–1.31) | 1.00 | |
| 1 | 3771 | 5.53 (3.88–7.88) | .011e | 2.76 (1.79–4.26) | 1.61 (1.04–2.48) | 1.12 (.77–1.63) | 1.03 (.85–1.26) | 1.00 |
| 2 or 3f | 921 | 4.31 (1.92–9.68) | .23e | 2.73 (1.13–6.60) | 1.58 (.65–3.82) | 1.64 (.87–3.07) | 1.08 (.73–1.59) | 1.00 |
| ≥1 | 4692 | 5.39 (3.89–7.45) | .012e | 2.80 (1.89–4.13) | 1.63 (1.10–2.40) | 1.21 (.87–1.68) | 1.05 (.88–1.26) | 1.00 |
Abbreviation: ILI, influenza-like illness.
a Intervals denote ARI onset during the specified no. of weeks before or after the peak week of influenza virus circulation.
b By the Cochran Q test for heterogeneity between the 2 strata, unless otherwise indicated, during days 1–3.
c Compared with unvaccinated status.
d Indicators are defined as ARI within 4 weeks of the peak week of influenza virus circulation, presence of an ILI code, and unvaccinated status.
e Compared with 0 indicators.
f Data are pooled because of small numbers.
Results of sensitivity analyses are shown in Supplementary Table 1. Effect sizes did not change markedly between different analyses, including those restricted to AMI events in which the patient did not die during hospitalization (incidence ratio, 3.56 [95% CI, 2.59–4.90] for days 1–3 after infection).
DISCUSSION
This study, one of the first to use the new GPRD-MINAP data linkage [15], showed a short-term increased risk of AMI in the days following acute respiratory infection, confirming previous findings that were based on GPRD data alone [6]. The effect was greatest for people aged ≥80 years and for acute respiratory episodes judged most likely to be due to influenza virus infection.
A major strength of this study was the use of novel data linkage to improve the accuracy and timing of data on AMI. Previous validation studies of AMI in the GPRD have found that, although AMI diagnoses have a generally high positive predictive value [16], less is known about the accuracy of recording of timing [17]. In one study, 15% of confirmed cases had a date of AMI recorded in GPRD that differed from that provided by the GP [18]. For self-controlled case series analysis using short risk periods, even a small proportion of AMI records with inaccurate dates or false-positive records could introduce considerable bias. Therefore, we used MINAP data [8], which has the advantage of multiple data entry fields for information on timing of AMI, to allow internal validation of the AMI date, as well as improved diagnostic precision. While an AMI can potentially affect the length of the observation period, which goes against assumptions on which the case series method is based [19], exclusion of AMI events in which the patient died during hospitalization did not significantly alter results.
Use of a self-controlled case series eliminated any fixed between-person confounding [20, 21] that may have affected similar analyses that involved case-control or cohort designs. Use of within-person comparisons also reduced the risk of residual bias due to difficulties choosing appropriate controls; this was particularly important in our study, as there may be systematic differences between patients who present to the GP with acute respiratory infection and those who do not. Our results thus apply to infections that were severe enough to cause someone to consult. A final advantage of the self-controlled case series design was the ability to use multiple risk periods [14], which allowed us to identify a gradient of risk for AMI after acute respiratory infection. We did not know the onset date of infections but instead used the date of diagnosis. Because the date of attending the GP is likely to be some time after respiratory symptom onset [22, 23], the true effect on AMI may be even higher than that observed. Conversely, though, it is not possible to establish whether symptoms in any patients for whom acute respiratory infection was diagnosed were actually due to myocardial loss evolving over 1–2 days. Incidence ratios would be artificially inflated by any such misclassification.
Identification of influenza virus infections in primary care data is challenging because cases rarely have microbiological confirmation. While some studies have based influenza diagnosis on codes used by GPs to classify respiratory illnesses [24, 25], this method alone would fail to identify influenza virus infections classified using nonspecific respiratory illness codes. In our study, relatively few illnesses were classified with specific ILI codes, and there was no significant difference between illnesses classed as ILI and those classified as non-ILI. A more powerful method to identify illnesses likely to be caused by influenza was to use linked influenza surveillance data. This showed a greater effect in peak weeks of influenza virus circulation than in nonpeak weeks, strongly suggesting that influenza virus infections are more likely than other respiratory infections to trigger AMI. Varying numbers of weeks included in the “peak” and “nonpeak” periods resulted in similar magnitudes of effect. We also used influenza vaccination data to compare illness among vaccinated individuals with that among unvaccinated individuals but did not see a difference in effect on AMI. This does not mean that influenza vaccination is ineffective at preventing influenza-associated AMI; rather, acute respiratory infections occurring after influenza vaccination are likely to have a different etiology. It is also possible that vaccination status was misclassified for some illnesses: we did not have data on vaccinations occurring in other settings, such as the workplace; influenza vaccine effectiveness is around 70% in healthy adults and lower in frail elderly populations [26]. Our results suggest that acute respiratory infections other than influenza can also be associated with increased AMI risk. However, combining these methods, we found significantly higher incidence ratios for illnesses with at least 1 influenza indicator, compared with those with no indicators of influenza.
Previous large primary care database studies that used either case-control or case-only designs have shown an association between GP attendance with acute respiratory infection and subsequent AMI [6, 27, 28]. Our incidence ratio for AMI in days 1–3 after acute respiratory infection (4.19 [95% CI, 3.18–5.53]) was compatible with although somewhat lower than that of an earlier comparable self-controlled case series study in GPRD (4.95 [95% CI, 4.43–5.53]) [6]. We adjusted for season as a proxy for environmental factors, which would tend to lead to a more conservative effect size. Other studies have focused on serological measures of influenza. While one Chinese study in an unvaccinated population showed significantly higher levels of influenza A and B virus immunoglobulin G in a single serum sample in AMI patients as compared to controls [29], such studies tend to be underpowered to detect any association [30–32]. Another approach is to explore, ideally in trial settings, whether influenza vaccination protects against cardiovascular events. From our previous review [2], pooled analysis of 2 relatively small randomized controlled trials in populations with existing cardiovascular disease [33, 34] suggested a protective although nonsignificant effect of influenza vaccination against cardiovascular-related death (relative risk, 0.51 [95% CI, .15–1.76]). A more recent trial of influenza vaccination as secondary prevention showed a significant reduction in major adverse cardiovascular events (unadjusted hazard ratio, 0.70 [95% CI, .57–.86]) [35]. Several recent observational studies have suggested reductions in AMI following influenza vaccination [36–38], although it may be difficult to disentangle the effect of healthy-user bias in observational vaccine studies.
Influenza vaccine uptake is often poor, especially in high-risk groups, such as those with existing cardiac disease [39]. This study adds to the evidence to support increased efforts to maximize uptake of influenza vaccination in these groups to protect against cardiovascular complications of influenza. Our analysis was restricted to first AMI events. People who had these would not necessarily fall under the vaccination guidelines as people with established cardiac disease. This is a potential argument for lowering the age limit for routine influenza vaccination (currently 65 years in the United Kingdom) to cover those at risk of cardiovascular disease. However, because the triggering effect was greatest in older adults, such a strategy may not be cost-effective. As previously suggested [36], further trial evidence is needed on the use of influenza vaccine for primary prevention of cardiovascular events.
In conclusion, in this self-controlled case series analysis that used a novel data linkage system, we found a consistent association between acute respiratory infections, especially those occurring at times of peak influenza virus circulation, and AMI, after adjustment for age and season.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr Richard Pebody (Centre for Infections, Health Protection Agency) for supplying influenza surveillance data. Swabs came from the RCGP sentinel GP swabbing programme, headed by Dr Douglas Fleming, and swab testing for influenza virus was undertaken by the Respiratory Virus Unit at the Health Protection Agency Centre for Infections, coordinated by Dr Joanna Ellis. We also acknowledge the CALIBER Scientific Oversight Committee for providing helpful comments on study design.
Financial support. This work was supported by a Medical Research Council Clinical Research Training Fellowship (to C. W. G.). The CALIBER research record linkages leading to these results was funded by the Wellcome Trust (086091/Z/08/Z; http://www.wellcome.ac.uk/) and the UK National Institute of Health Research (RP-PG-0407–10314; http://www.nihr.ac.uk/; principle investigator, H. H.). L. S. and S. L. T. are supported by Wellcome Trust grants.
Potential conflicts of interest. L. S. has undertaken paid consultancy work for GSK unrelated to this work. All authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scarborough P, Bhatnagar P, Wickramsinghe K, Smolina K, Mitchell C, Rayner M. Coronary heart disease statistics. British Heart Foundation report. 2010 http://www.bhf.org.uk/publications/view-publication.aspx?ps=1001546 . Accessed 13 March 2012. [Google Scholar]
- 2.Warren-Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9:601–10. doi: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 3.Tillett HE, Smith JW, Gooch CD. Excess deaths attributable to influenza in England and Wales: age at death and certified cause. Int J Epidemiol. 1983;12:344–52. doi: 10.1093/ije/12.3.344. [DOI] [PubMed] [Google Scholar]
- 4.Warren-Gash C, Bhaskaran K, Hayward A, et al. Circulating influenza virus, climatic factors, and acute myocardial infarction: a time series study in England and Wales and Hong Kong. J Infect Dis. 2011;203:1710–8. doi: 10.1093/infdis/jir171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong CM, Chan KP, Hedley AJ, Malik Peiris JS. Influenza-associated mortality in Hong Kong. Clin Infect Dis. 2004;39:1611–7. doi: 10.1086/425315. [DOI] [PubMed] [Google Scholar]
- 6.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–8. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 7.Walley T, Mantgani A. The UK general practice research database. Lancet. 1997;350:1097–9. doi: 10.1016/S0140-6736(97)04248-7. [DOI] [PubMed] [Google Scholar]
- 8.Herrett E, Smeeth L, Walker L, Weston C. The myocardial ischaemia national audit project (MINAP) Heart. 2010;96:1264–7. doi: 10.1136/hrt.2009.192328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.CALIBER Web site. http://caliberresearch.org.uk. Accessed 13 March 2012. [Google Scholar]
- 11.General Practice Research Database Web site. http://www.gprd.com/products/database . Accessed 13 March 2012. [Google Scholar]
- 12.MINAP Steering Group. Myocardial Ischaemia National Audit Project (MINAP): How the NHS cares for patients with heart attack. Tenth Public Report. 2011 London: Padcreative. [Google Scholar]
- 13.Dave S, Petersen I. Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf. 2009;18:704–7. doi: 10.1002/pds.1770. [DOI] [PubMed] [Google Scholar]
- 14.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25:1768–97. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 15.Boggon R, van Staa TP, Timmis A, et al. Clopidogrel discontinuation after acute coronary syndromes: frequency, predictors and associations with death and myocardial infarction—a hospital registry-primary care linked cohort (MINAP-GPRD) Eur Heart J. 2011;32:2376–86. doi: 10.1093/eurheartj/ehr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol. 2010;69:4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the general practice research database: a systematic review. Br J Gen Pract. 2010;60:e128–36. doi: 10.3399/bjgp10X483562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammad TA, McAdams MA, Feight A, Iyasu S, Dal Pan GJ. Determining the predictive value of Read/OXMIS codes to identify incident acute myocardial infarction in the general practice research database. Pharmacoepidemiol Drug Saf. 2008;17:1197–201. doi: 10.1002/pds.1672. [DOI] [PubMed] [Google Scholar]
- 19.Farrington CP, Anaya-Izquierdo K, Whitaker HJ, Hocine MN, Douglas I, Smeeth L. Self controlled case series analysis with event-dependent observation periods. JASA. 2011;106:417–26. [Google Scholar]
- 20.Smeeth L, Donnan PT, Cook DG. The use of primary care databases: case-control and case-only designs. Fam Pract. 2006;23:597–604. doi: 10.1093/fampra/cml025. [DOI] [PubMed] [Google Scholar]
- 21.Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res. 2009;18:7–26. doi: 10.1177/0962280208092342. [DOI] [PubMed] [Google Scholar]
- 22.Da Silva PR, Nguyen VT, Hayward AC. Logistic issues and potential prescribing costs associated with use of neuraminidase inhibitors for the treatment of influenza in primary care. J R Soc Med. 2003;96:66–9. doi: 10.1258/jrsm.96.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ. 1997;314:722–7. doi: 10.1136/bmj.314.7082.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meier CR, Napalkov PN, Wegmuller Y, Jefferson T, Jick H. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis. 2000;19:834–42. doi: 10.1007/s100960000376. [DOI] [PubMed] [Google Scholar]
- 25.Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Am J Epidemiol. 2009;169:382–8. doi: 10.1093/aje/kwn310. [DOI] [PubMed] [Google Scholar]
- 26.Fleming DM, Andrews NJ, Ellis JS, et al. Estimating influenza vaccine effectiveness using routinely collected laboratory data. J Epidemiol Community Health. 2010;64:1062–7. doi: 10.1136/jech.2009.093450. [DOI] [PubMed] [Google Scholar]
- 27.Clayton TC, Thompson M, Meade TW. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2008;29:96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 28.Meier CR, Jick SS, Derby LE, Vasilakis C, Jick H. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet. 1998;351:1467–71. doi: 10.1016/s0140-6736(97)11084-4. [DOI] [PubMed] [Google Scholar]
- 29.Guan XR, Li X, Xin XM, et al. Influenza virus infection and risk of acute myocardial infarction. Inflammation. 2008;31:266–72. doi: 10.1007/s10753-008-9074-2. [DOI] [PubMed] [Google Scholar]
- 30.Mattila KJ. Viral and bacterial infections in patients with acute myocardial infarction. J Intern Med. 1989;225:293–6. doi: 10.1111/j.1365-2796.1989.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 31.Nicholls AC, Thomas M. Coxsackie virus infection in acute myocardial infarction. Lancet. 1977;1:883–4. doi: 10.1016/s0140-6736(77)91203-x. [DOI] [PubMed] [Google Scholar]
- 32.Ponka A, Jalanko H, Ponka T, Stenvik M. Viral and mycoplasmal antibodies in patients with myocardial infarction. Ann Clin Res. 1981;13:429–32. [PubMed] [Google Scholar]
- 33.Ciszewski A, Bilinska ZT, Brydak LB, et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J. 2008;29:1350–8. doi: 10.1093/eurheartj/ehm581. [DOI] [PubMed] [Google Scholar]
- 34.Gurfinkel EP, Leon de la FR, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) study. Eur Heart J. 2004;25:25–31. doi: 10.1016/j.ehj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J. 2011;32:1730–5. doi: 10.1093/eurheartj/ehr004. [DOI] [PubMed] [Google Scholar]
- 36.Gwini SM, Coupland CA, Siriwardena AN. The effect of influenza vaccination on risk of acute myocardial infarction: self-controlled case-series study. Vaccine. 2011;29:1145–9. doi: 10.1016/j.vaccine.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Hung IF, Leung AY, Chu DW, et al. Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis. 2010;51:1007–16. doi: 10.1086/656587. [DOI] [PubMed] [Google Scholar]
- 38.Siriwardena AN, Gwini SM, Coupland CA. Influenza vaccination, pneumococcal vaccination and risk of acute myocardial infarction: matched case-control study. CMAJ. 2010;182:1617–23. doi: 10.1503/cmaj.091891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Health Protection Agency Web site. Influenza vaccination uptake monitoring on behalf of the Department of Health. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/SeasonalInfluenza/EpidemiologicalData/15influsInfluenzavaccinationuptakemonitoring/ . Accessed 12 October 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.