Abstract
Objectives
Depression is the most common psychiatric disorder in patients with chronic kidney disease (CKD). We sought to determine the association of major depression with mortality among diabetic patients with late stage CKD.
Method
The Pathways Study is a longitudinal, prospective cohort study initiated to determine the impact of depression on outcomes among primary care diabetic patients. Subjects were followed from 2001 until 2007 for a mean duration of 4.4 years. Major depression, identified by the Patient Health Questionnaire-9 (PHQ-9), was the primary exposure of interest. Stage 5 CKD was determined by dialysis codes and estimated glomerular filtration rate (<15ml/min). An adjusted Cox proportional hazards multivariable model was used to determine the association of baseline major depression with mortality.
Results
Of the 4128 enrolled subjects, 110 were identified with stage 5 CKD at baseline. Of those, 34 (22.1%) had major depression. Over a period of 5 years, major depression was associated with 2.95-fold greater risk of death (95% CI=1.24–7.02) compared to those with no or few depressive symptoms.
Conclusion
Major depression at baseline was associated with a 3-fold greater risk of mortality among stage 5 CKD diabetic patients. Given the high mortality risk, further testing of targeted depression interventions should be considered in this population.
Keywords: Depression, diabetes, mortality, chronic kidney disease, ESRD
INTRODUCTION
Depression is the most common psychiatric disorder among patients with end-stage renal disease (ESRD) and chronic kidney disease (CKD) and has been associated with increased morbidity and mortality in both cross-sectional and short-term prospective studies.1–8 Depression is largely under-recognized, misdiagnosed, and undertreated in this population.9 Few prospective studies have evaluated depression using validated self-report questionnaires, particularly among patients with diabetes and late stage 5 CKD defined as chronic kidney failure with an estimated glomerular filtration rate (eGFR) less than 15ml/min, or those on dialysis.10–14
It is well known that diabetes is the most common cause of ESRD (44% of cases)15 and is associated with a 15–25% greater mortality risk among ESRD patients compared to those without diabetes.16 In addition patients with diabetes have twice the prevalence of major depression (12%) as the general population.17 We have shown that among patients with diabetes, depression is associated with increased diabetes symptoms,18 poor glycemic control,19 decreased adherence to self-care regimens19, increased cardiovascular disease and cardiovascular disease risk factors,20 increased work disability,21 and mortality.22 Furthermore, depression tends to be chronic in patients with diabetes and the majority of patients (80%) are subject to a relapse of depression over a 5 year period.17 Patients with stage 5 CKD and diabetes represent a population at even greater risk for cardiovascular events, morbidity, and mortality compared to those with diabetes in the general population.23 Depression among the general dialysis population has been associated with increased risk of death, poor compliance with dialysis and medication regimens, and poor quality of life. However, the comorbidity of depression with diabetes has not been well studied among a population-based prospective cohort of those with stage 5 CKD, particularly those treated in a health care system prior to and after initiation of dialysis.24–27
The aim of this study was to evaluate the association of major depression with mortality in with a population-based study of patients with diabetes and late stage CKD treated at a large staff model health maintenance organization. Major depression was evaluated using the Patient Health Questionnaire-9 (PHQ-9),28 and patients were followed prospectively from the time of study enrollment for up to 5 years.
MATERIALS AND METHODS
Subjects and Study Design
The Pathways Epidemiology Study is a longitudinal, prospective population-based cohort study developed by a multidisciplinary team of investigators from the University of Washington and the Group Health Research Institute.19, 20, 29 Patients were recruited from Group Health (GH), a nonprofit health maintenance organization with over 500,000 enrollees who receive medical care provided by 30 primary care clinics located in Western Washington State. Of the 30 clinics available, 9 clinics were selected for the study based on geographic location (40-mile radius of Seattle), large diabetes populations, and increased clinic racial and ethnic diversity. Subjects were prospectively followed from the time of recruitment for the baseline survey (March 2001) until August 31, 2007.
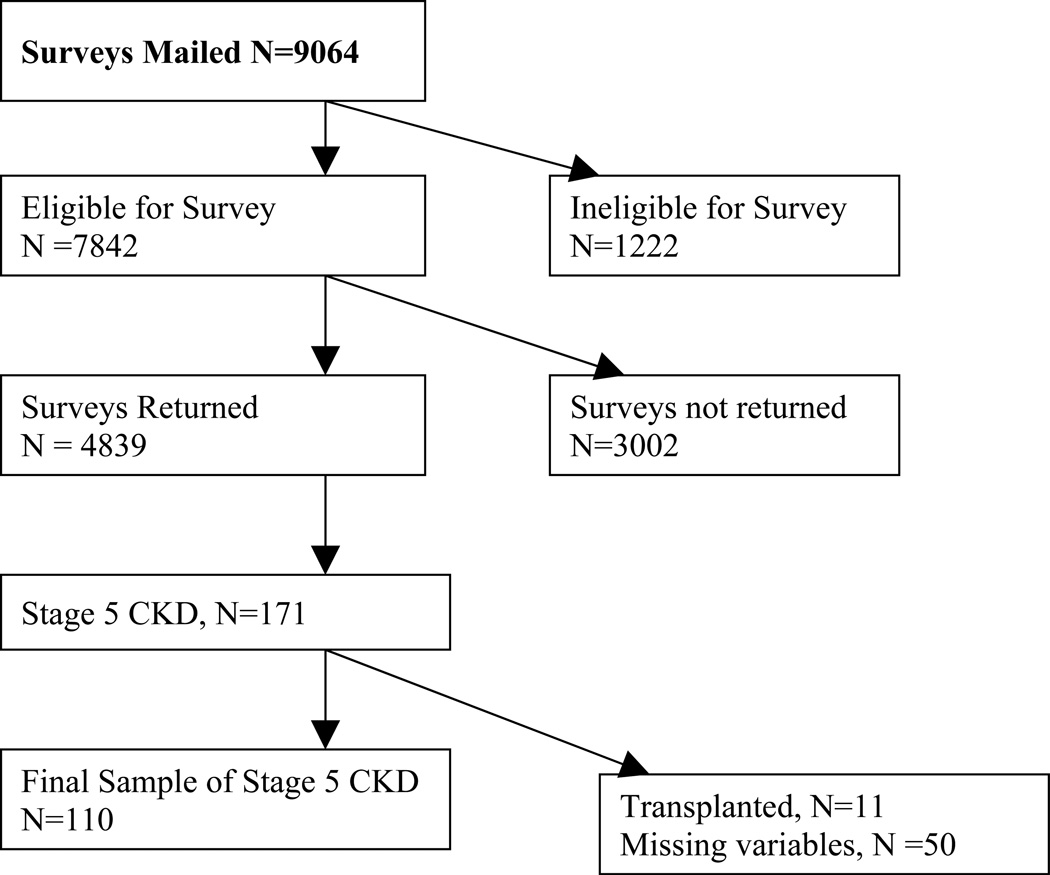
A total of 9063 baseline surveys were mailed to patients identified with diabetes from the GH Diabetes Registry. GH maintains a diabetes registry that includes all patients who have diabetes identified by administrative databases that include pharmacy (insulin or diabetes medications), laboratory (elevated fasting or random glucose or hemoglobin A1c) or hospital discharge data (diagnosis codes for diabetes).30 Of those surveys sent, 7841 met eligibility criteria, and of those, 4839 were returned for a response rate of 62%. Of the respondents, we excluded 711 subjects who did not give permission to review medical records. Of the remaining subjects (n=4128), 128 were found have stage 5 CKD at the time of baseline survey. After excluding 11 patients who received a kidney transplant before baseline and 7 patients with missing covariates, 110 subjects were available for the analysis. 7 patients who received kidney transplants after baseline were censored at transplant date. Patients were considered to have Stage 5 CKD if they met any of the following criteria: eGFR <15 ml/min/ 172 m2 (as estimated by the 4-variable Modification of Diet in Renal Disease [MDRD] equation),31, 32 an International Classification of Diseases, 9th Edition (ICD-9) diagnosis code or procedure code for ESRD (585.5, 585.6, 404.93, v42.0, v45.1, v56.x, 39.27, 39.42–43, 39.49–50, 39.53, 39.93–95, or 54.98), or a CPT code for dialysis (90918, 90919, 90920, 90922, 90923, 90924, 90925, 90935, 90937, 90939, 90941, 90945, 90947, 93990, 90989, 90993, 90999) (Figure 1).
Figure 1.
Enrollment in the Pathways Study
Primary Predictor of Interest
The primary predictor of interest was major depression, as determined by the PHQ-9. The PHQ-9 has been validated in dialysis populations against the Structured Clinical Interview for Diagnostic and Statistical Manual IV (SCID IV).14 Using a cutoff value of 10 or greater to define depression illness, the PHQ-9 has a sensitivity of 92%, specificity of 92%, positive predictive value of 71%, negative predictive value of 98% and a kappa value of 0.75 compared to the SCID IV diagnosis of major depression in patients with ESRD.13 In the present study, patient's scores were categorized into those with major depression versus those without major depression. Following the standard definition, major depression was defined as having a score of ≥ 2 on 5 of 9 DSM IV symptoms for 2 weeks or more with at least one of the positive symptoms being a cardinal symptom (i.e. depressed mood or anhedonia). We chose a priori to evaluate major depression verses no major depression. Only 7 subjects were found to have minor depression defined as 2–4 depressive symptoms at least half the days with at least one cardinal symptom. These subjects were included in the reference group of those without major depression.
Other Covariates of Interest
Covariates of interest were obtained from the mailed survey and included socio-demographic characteristics (age, gender, race/ethnicity, educational attainment, and marital status), diabetes type (type 1 or 2), diabetes duration (years), diabetes treatment, height and weight calculated as the body mass index (BMI) in kg/m2, smoking, and sedentary lifestyle as evaluated and defined by the prospectively validated Summary of Diabetes Self-Care Questionnaire.33 The ambulatory medical record was reviewed for the presence and date of comorbid conditions, including cardiovascular disease, peripheral vascular disease, diabetic retinopathy, stroke, and diabetic neuropathy. Glycosylated hemoglobin A1c (HbA1c) was obtained from clinical laboratory databases at baseline enrollment into the study.
Outcome
All-cause mortality was the primary outcome of interest. Mortality was determined using GH automated vital statistics data from 3/1/2001 to 8/31/07. All deaths were validated by comparing GH data to the Washington State Department of Health Death data available from January 1999 to December 2007.22
Statistical Analysis
Kaplan-Meier curves were generated for those with and without major depression. Adjusted Cox proportional hazards regression models were used to analyze the association between major depression and mortality.34 Patients were considered at risk from baseline enrollment and followed longitudinally for a mean of 4.4 years. Patients were censored at the end of the study, at death, at time of transplant, or when lost to follow-up, as determined by disenrollment from GH. The adjusted relative risk (RR) or hazard ratio (HR) was estimated for each covariate. Variables used in multivariable models were chosen a priori or for the observed magnitude of their relationship with major depression and mortality. Proportionality of the Cox models was assessed and confirmed by log (−log) and cumulative Schoenfeld residual plots.
RESULTS
Of the 4128 patients enrolled in the original Pathways study and for whom we had permission to review medical records, 128 had stage 5 CKD at baseline. Of these,11 were transplanted and 7 of the remaining 117were dropped from analyses due to missing covariates.
Table 1 shows the baseline characteristics of the study sample by depression status. The average age was 65.5 years, 45.5% were female, 19.1% were non-white, 39.1% had a high school education or less, and 63.6% were married or living together. The average HbA1c was 7.5± 1.6%. . The mean number of years on dialysis was 2,2 years ± 2.5. The average RxRisk score was $5428± 3455. The mean body mass index (BMI) was 30.6 kg/m2. Only 8.2% of patients smoked at baseline, and 43.6% reported a sedentary lifestyle. Finally, the average duration of diabetes was 15.0 years and 53.3% of patients were on insulin, while 32.5% of patients were on an oral hypoglycemic agent (data not shown).
Table 1.
Baseline Characteristics of Stage 5 CKD Patients by Depression Status
| Characteristics | Total sample | No Major depression |
Major depression |
P-value |
|---|---|---|---|---|
| n (%) | 110 | 88 (80.0) | 22 (20.0) | |
| Age Category | ||||
| Under 55 | 24 (21.8) | 19 (21.6) | 5 (22.7) | 0.99 |
| 55 to 64 | 18 (16.4) | 15 (17.1) | 3(13.6) | |
| 65 to 74 | 34 (30.9) | 27 (30.7) | 7 (31.8) | |
| 75 + | 34 (30.9) | 27 (30.7) | 7 (31.8) | |
| Female | 50 (45.5) | 40 (45.5) | 10 (45.5) | 1.0 |
| Race | ||||
| White | 89 (80.9) | 71 (80.7) | 18 (81.8) | 0.99 |
| Black | 11 (10.0) | 9 (10.2) | 2 (9.1) | |
| All Other | 10 (9.1) | 8 (9.1) | 2 (9.1) | |
| High school education or less | 43 (39.1) | 33 (37.5) | 10 (45.5) | 0.49 |
| Married/living together | 70 (63.6) | 58 (65.9) | 12 (54.6) | 0.32 |
| Years on Dialysis | ||||
| Not on Dialysis | 43 (39.1) | 32 (36.4) | 11 (50.0) | 0.35 |
| 0 to <4 years | 34 (30.9) | 27 (30.7) | 7 (31.8) | |
| 4+ years | 33 (30.0) | 29 (33.0) | 4 (18.2) | |
| Years on Dialysis | 2.2 (2.5) | 2.4 (2.5) | 1.5 (2.3) | 0.66 |
| RxRisk score, $a | 5428 ± 3455 | 5220± 3279 | 6257± 4066 | 0.17 |
| HbA1c >= 8.0 | 38 (34.6) | 28 (31.8) | 10 (45.5) | 0.23 |
| HbA1c, %, mean ± sd | 7.5 ± 1.6 | 7.5 ± 1.6 | 7.8 ± 1.8 | 0.53 |
| Currently smoking | 9 (8.2) | 7 (8.0) | 2 (9.1) | 0.86 |
| Sedentary Lifestyle (<=1 day/ wk exercise) |
48 (43.6) | 33 (37.5) | 15 (68.2) | 0.01 |
| Cardiovascular disease | 67 (60.9) | 55 (62.5) | 12 (54.6) | 0.49 |
| Stroke or Brain Procedure | 16 (14.6) | 10 (11.4) | 6 (27.3) | 0.06 |
| Peripheral Vascular Disease | 24 (21.8) | 20 (22.7) | 4 (18.2) | 0.64 |
| Low Vision/Blindness | 17 (15.5) | 13 (14.8) | 4 (18.2) | 0.70 |
| Neuropathy | 43 (39.1) | 31 (35.2) | 12 (54.6) | 0.10 |
Data are N (%) unless otherwise stated. Column percentages may not add up to 100 due to rounding.
Definitions: CKD= chronic kidney disease, HbA1c = hemoglobin A1c,
RxRisk is a pharmacy-based measure of medical comorbidity. The score is an estimate of expected previous year healthcare costs as a function of age, sex, and the chronic condition classes in which prescription drug fills are observed. Depression and diabetes medications excluded from this modified RxRisk score.
Table 1 also shows the baseline characteristics of the study sample by depression status. Patients with major depression were more likely to have a sedentary lifestyle (p=0.01) and slightly more likely to be female, to have less than a high school education, to be single, to have poorer glucose control, and to have longer duration of diabetes than those with no or few depressive symptoms, although the latter differences were not statistically significant.
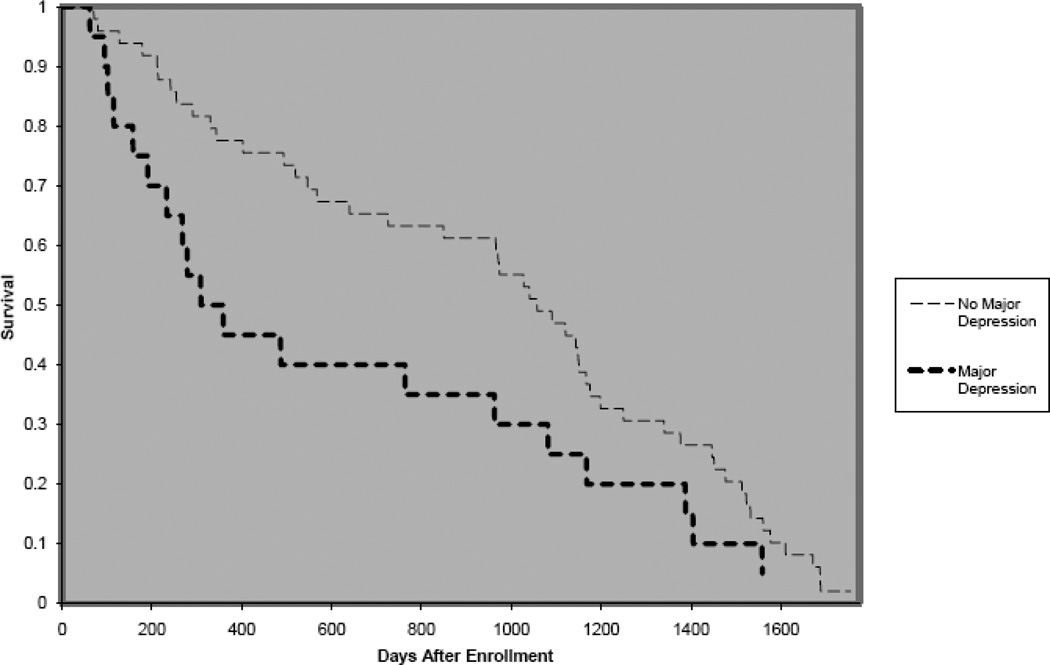
Of the 110 patients, 46 (41.8%) died during the approximately 5 years of follow up. Kaplan-Meier curves (Figure 2) show those with major depression had decreased survival (median survival 3.8 years) compared to those without major depression (median survival 4.7 years).
Figure 2.
Kaplan-Meier Curves of Survival of Stage 5 CKD Patients Stratified by Depression Status
Multivariable analysis was conducted with major depression as the predictor of interest and mortality as the primary outcome. Using Cox proportional hazard modeling, we found major depression was associated with a 2.95-fold greater risk of death (95% CI =1.23–7.02) compared to those without major depression after adjustment for age, sex, education, race, marital status, baseline comorbid conditions, smoking, hemoglobin A1c, BMI, sedentary lifestyle, and years on dialysis. After adjustment, increasing age and decreasing BMI were the only other variables significantly associated with increased mortality in this cohort (Table 2).
Table 2.
Multivariable Analysis of Depression Associated With All-Cause Mortality in Stage 5 CKD Patients
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Characteristics | HR | 95 % CI | HR | 95% CI |
| Major Depression | 1.80 | 1.07–3.02 | 2.95 | 1.234–7.02 |
| Male | 1.68 | 1.05–2.68 | 1.60 | 0.70–3.70 |
| Some College | 1.06 | 0.66–1.70 | 1.63 | 0.68–3.84 |
| Age, < 55 years | 1.0 | Reference | 1.00 | Reference |
| Age 55 to 64 years | 1.15 | 0.37–3.58 | 1.27 | 0.18–8.63 |
| Age 65 to 74 years | 1.38 | 1.63–9.60 | 5.95 | 1.55–22.95 |
| Age 75+ years | 1.60 | 2.06–11.89 | 5.38 | 1.45–19.94 |
| White | 1.0 | Reference | 1.00 | Reference |
| African American | 0.71 | 0.31–1.65 | 0.84 | 0.28–2.58 |
| Other Race/Ethnicity | 1.01 | 0.46–2.22 | 1.13 | 0.26–4.99 |
| Single | 0.56 | 0.33–0.93 | 0.71 | 0.28–1.81 |
| Body Mass Index | 0.95 | 0.90–1.00 | 0.93 | 0.86–0.99 |
Models adjusted for age, sex, education, race, baseline cardiovascular disease, stroke, peripheral vascular disease, retinopathy, neuropathy, , smoking, hemoglobin A1c, body mass index, years on dialysis, sedentary lifestyle, and education.
Definitions: CKD= chronic kidney disease, HR= hazard ratio.
DISCUSSION
We found that major depression was associated with a 2.95-fold greater risk of mortality compared to those without major depression in a prospective cohort of diabetic patients with stage 5 CKD who received medical care in a large health maintenance organization. This mortality risk associated with major depression in the stage 5 CKD population is greater than that found for the general diabetes population but similar to that for the general dialysis population.35 To our knowledge this is the first study to examine the associations of major depression and mortality among patients with stage 5 CKD in a longitudinal cohort of primary care patients with concomitant diabetes mellitus. A major strength of current study was that it had a validated measure of depression, was population based, prospective, and had significant longitudinal follow up among similarly treated patients in a large HMO setting prior to and after initiation of dialysis, compared to most studies in the literature that only evaluate depression once dialysis has been initiated.
In some, but not all, previous studies, which focused exclusively on the dialysis population, depression and depressive symptoms were associated with an increased risk of mortality in both prevalent and incident general dialysis patients.24, 25, 27, 36. For example, among dialysis patients, Hedayati found a relative risk of 2.07 (95% CI 1.10–3.90) for mortality associated with depression,24 while, Boulware found that depressive symptoms were strongly associated (HR=2.22, 95% CI=1.36–3.60) with all-cause mortality in the CHOICE study.25 Unfortunately, the relative risk associated with death was not reported separately for those with diabetes in either of the preceding studies. Hedayati did find that diabetic CKD patients had a 2-fold greater prevalence of depression, compared to non-diabetic patients; however, this study was a convenience sample of CKD clinic patients and did not assess longitudinal outcomes prospectively.2
Initiation of dialysis is often associated with significant psychological and biologic stress, lifestyle changes, and decreased quality of life. Underscoring the importance of diagnosing depression in this population, the United States Renal Data System (USRDS) initiated a large scale study to evaluate rehabilitation/quality of life and nutrition, which will prospectively screen for depression in ESRD patients using the Patient Health Questionnaire-2 (PHQ-2).15 Depression and psychological stress are theorized to contribute to increased mortality by several mechanisms, some of which include alteration in nutritional status, decreased adherence with prescriptions and medication regimens, and adverse effects on the immunologic system37–39 and hypothalamic-pituitary-adrenal (HPA) axis.40 In addition, depression is thought to increase the risk of suicide and withdrawal from dialysis.40 Dialysis patients reportedly have an 84% greater risk of suicide than the general population.41 However when the diabetic population was assessed, the rate of suicide was lower than that of the general ESRD population (HR= 0.76, 95% CI 0.59–0.99).40 Patients with depression are more likely to withdraw from dialysis compared to patients without depression;27 however, in our cohort only 4 patients withdrew from dialysis or chose not to initiate dialysis and of those, 2 had major depression.
The current study has a number of strengths. The Pathways Study is a prospective, longitudinal cohort study in a large primary care diabetes population for whom a valid and reliable measure of major depression was available, compared to most studies in the literature that have used depression scales measuring severity only. Stage 5 CKD and ESRD were validated by an internal dialysis registry and laboratory data, while death was adjudicated by chart review. The cohort was well characterized and represents a general, population-based, primary care diabetes cohort for which we had medical record reviewed and confirmed comorbid conditions. The current study also enrolled both prevalent and incident dialysis patients and adjusted for dialysis time on dialysis.
Limitations
The current study also has several limitations. Because this was a population-based study at a health maintenance organization and not at a dialysis unit, dialysis-specific variables were not available; therefore, analyses were not adjusted for dialysis adequacy (Kt/V), albumin, calcium metabolism or other dialysis parameters. The current study reports on only a small number of stage 5 CKD patients and had limited power to study the association of minor depression with mortality.. Since some studies have found that both minor as well as major depression are associated with adverse outcomes in patients with chronic medical illnesses,42 our analysis could be considered a conservative estimate of the risk of mortality associated with depressive symptoms, since patients with subclinical depressive symptoms or minor depression were included in the reference group. Although results are suggestive of a strong association of depression with mortality in stage 5 CKD patients, a causal relationship cannot be confirmed because this study is observational. Finally, this study used the PHQ-9 questionnaire, not a structured psychiatric interview. However, this questionnaire has been validated against a structured interview in patients with prevalent and incident kidney failure and was found to have high sensitivity and specificity.14
Conclusion
Major depression was associated with an almost 3-fold greater risk of mortality in stage 5 CKD patients with type 2 diabetes followed longitudinally for 5 years in this prospective cohort study. Major depression is highly prevalent in stage 5 CKD patients with diabetes, with approximately one in five patients in this study meeting criteria for this important comorbidity. Additional prospective investigation is needed to determine if depression treatment will improve outcomes in this high risk population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Craven JL, Rodin GM, Littlefield C. The Beck Depression Inventory as a screening device for major depression in renal dialysis patients. Int J Psychiatry Med. 1988;18(4):365–374. doi: 10.2190/m1tx-v1ej-e43l-rklf. [DOI] [PubMed] [Google Scholar]
- 2.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ. Prevalence of Major Depressive Episode in CKD. Am J Kidney Dis. 2009 Jun 2; doi: 10.1053/j.ajkd.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cukor D, Coplan J, Brown C, et al. Depression and anxiety in urban hemodialysis patients. Clin J Am Soc Nephrol. 2007 May;2(3):484–490. doi: 10.2215/CJN.00040107. [DOI] [PubMed] [Google Scholar]
- 4.Hinrichsen GA, Lieberman JA, Pollack S, Steinberg H. Depression in hemodialysis patients. Psychosomatics. 1989 Summer;30(3):284–289. doi: 10.1016/S0033-3182(89)72273-8. [DOI] [PubMed] [Google Scholar]
- 5.Israel M. Depression in dialysis patients: a review of psychological factors. Can J Psychiatry. 1986 Jun;31(5):445–451. doi: 10.1177/070674378603100513. [DOI] [PubMed] [Google Scholar]
- 6.Kimmel PL. Psychosocial factors in chronic kidney disease patients. Semin Dial. 2005 Mar-Apr;18(2):71–72. doi: 10.1111/j.1525-139X.2005.18202.x. [DOI] [PubMed] [Google Scholar]
- 7.Levenson JL, Glocheski S. Psychological factors affecting end-stage renal disease. A review. Psychosomatics. 1991 Fall;32(4):382–389. doi: 10.1016/S0033-3182(91)72038-0. [DOI] [PubMed] [Google Scholar]
- 8.Smith MD, Hong BA, Robson AM. Diagnosis of depression in patients with end-stage renal disease. Comparative analysis. Am J Med. 1985 Aug;79(2):160–166. doi: 10.1016/0002-9343(85)90004-x. [DOI] [PubMed] [Google Scholar]
- 9.Kimmel PL. Depression in patients with chronic renal disease: what we know and what we need to know. J Psychosom Res. 2002 Oct;53(4):951–956. doi: 10.1016/s0022-3999(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 10.Chilcot J, Wellsted D, Da Silva-Gane M, Farrington K. Depression on dialysis. Nephron Clin Pract. 2008;108(4):c256–c264. doi: 10.1159/000124749. [DOI] [PubMed] [Google Scholar]
- 11.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ. Validation of Depression Screening Scales in Patients With CKD. Am J Kidney Dis. 2009 Jun 2; doi: 10.1053/j.ajkd.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chilcot J, Wellsted D, Farrington K. Screening for depression while patients dialyse: an evaluation. Nephrol Dial Transplant. 2008 Aug;23(8):2653–2659. doi: 10.1093/ndt/gfn105. [DOI] [PubMed] [Google Scholar]
- 13.Watnick S, Kirwin P, Mahnensmith R, Concato J. The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis. 2003 Jan;41(1):105–110. doi: 10.1053/ajkd.2003.50029. [DOI] [PubMed] [Google Scholar]
- 14.Watnick S, Wang PL, Demadura T, Ganzini L. Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis. 2005 Nov;46(5):919–924. doi: 10.1053/j.ajkd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Collins AJ, Foley RN, et al. Excerpts from the United States Renal Data System 2008 Annual Data Report. Am J Kidney Dis. 2009 Jan;53(suppl 1)(1):S1–S374. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Young BA, Rudser K, Kestenbaum B, Seliger SL, Andress D, Boyko EJ. Racial and ethnic differences in incident myocardial infarction in end-stage renal disease patients: The USRDS. Kidney Int. 2006 May;69(9):1691–1698. doi: 10.1038/sj.ki.5000346. [DOI] [PubMed] [Google Scholar]
- 17.Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med. 2008 Nov;121(11 Suppl 2):S8–S15. doi: 10.1016/j.amjmed.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludman EJ, Katon W, Russo J, et al. Depression and diabetes symptom burden. Gen Hosp Psychiatry. 2004 Nov-Dec;26(6):430–436. doi: 10.1016/j.genhosppsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004 Sep;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 20.Katon WJ, Lin EH, Russo J, et al. Cardiac risk factors in patients with diabetes mellitus and major depression. J Gen Intern Med. 2004 Dec;19(12):1192–1199. doi: 10.1111/j.1525-1497.2004.30405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Korff M, Katon W, Lin EH, et al. Work disability among individuals with diabetes. Diabetes Care. 2005 Jun;28(6):1326–1332. doi: 10.2337/diacare.28.6.1326. [DOI] [PubMed] [Google Scholar]
- 22.Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005 Nov;28(11):2668–2672. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 23.Young BA, Pugh JA, Maynard C, Reiber G. Diabetes and renal disease in veterans. Diabetes Care. 2004 May;27(Suppl 2):B45–B49. doi: 10.2337/diacare.27.suppl_2.b45. [DOI] [PubMed] [Google Scholar]
- 24.Hedayati SS, Bosworth HB, Briley LP, et al. Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int. 2008 Oct;74(7):930–936. doi: 10.1038/ki.2008.311. [DOI] [PubMed] [Google Scholar]
- 25.Boulware LE, Liu Y, Fink NE, et al. Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: contribution of reverse causality. Clin J Am Soc Nephrol. 2006 May;1(3):496–504. doi: 10.2215/CJN.00030505. [DOI] [PubMed] [Google Scholar]
- 26.Kimmel PL, Weihs K, Peterson RA. Survival in hemodialysis patients: the role of depression. J Am Soc Nephrol. 1993 Jul;4(1):12–27. doi: 10.1681/ASN.V4112. [DOI] [PubMed] [Google Scholar]
- 27.Lopes AA, Bragg J, Young E, et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int. 2002 Jul;62(1):199–207. doi: 10.1046/j.1523-1755.2002.00411.x. [DOI] [PubMed] [Google Scholar]
- 28.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 29.Katon W, Von Korff M, Lin E, et al. Improving primary care treatment of depression among patients with diabetes mellitus: the design of the pathways study. Gen Hosp Psychiatry. 2003 May-Jun;25(3):158–168. doi: 10.1016/s0163-8343(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 30.McCulloch DK, Price MJ, Hindmarsh M, Wagner EH. A population-based approach to diabetes management in a primary care setting: early results and lessons learned. Eff Clin Pract. 1998 Aug-Sep;1(1):12–22. [PubMed] [Google Scholar]
- 31.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 33.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 34.Cox D. Regression models and life tables (with discussion) Journal of Royal Statistical Society: Series B. 1972;34:187–220. [Google Scholar]
- 35.Katon W, Fan MY, Unutzer J, Taylor J, Pincus H, Schoenbaum M. Depression and diabetes: a potentially lethal combination. J Gen Intern Med. 2008 Oct;23(10):1571–1575. doi: 10.1007/s11606-008-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimmel PL. Depression as a mortality risk factor in hemodialysis patients. Int J Artif Organs. 1992 Dec;15(12):697–700. [PubMed] [Google Scholar]
- 37.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006 Jun;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 38.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002 Jun;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 39.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12(5):255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 40.Cukor D, Cohen SD, Peterson RA, Kimmel PL. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007 Dec;18(12):3042–3055. doi: 10.1681/ASN.2007030345. [DOI] [PubMed] [Google Scholar]
- 41.Kurella M, Kimmel PL, Young BS, Chertow GM. Suicide in the United States end-stage renal disease program. J Am Soc Nephrol. 2005 Mar;16(3):774–781. doi: 10.1681/ASN.2004070550. [DOI] [PubMed] [Google Scholar]
- 42.Drayer RA, Piraino B, Reynolds CF, 3rd, et al. Characteristics of depression in hemodialysis patients: symptoms, quality of life and mortality risk. Gen Hosp Psychiatry. 2006 Jul-Aug;28(4):306–312. doi: 10.1016/j.genhosppsych.2006.03.008. [DOI] [PubMed] [Google Scholar]