Abstract
Background
Exercise confers short-term benefits for individuals with Parkinson disease (PD).
Objective
The purpose of the study was to compare short- and long-term responses among 2 supervised exercise programs and a home-based control exercise program.
Design
The 16-month randomized controlled exercise intervention investigated 3 exercise approaches: flexibility/balance/function exercise (FBF), supervised aerobic exercise (AE), and home-based exercise (control).
Setting
This study was conducted in outpatient clinics.
Patients
The participants were 121 individuals with PD (Hoehn & Yahr stages 1–3).
Interventions
The FBF program (individualized spinal and extremity flexibility exercises followed by group balance/functional training) was supervised by a physical therapist. The AE program (using a treadmill, bike, or elliptical trainer) was supervised by an exercise trainer. Supervision was provided 3 days per week for 4 months, and then monthly (16 months total). The control group participants exercised at home using the National Parkinson Foundation Fitness Counts program, with 1 supervised, clinic-based group session per month.
Measurements
Outcomes, obtained by blinded assessors, were determined at 4, 10, and 16 months. The primary outcome measures were overall physical function (Continuous Scale—Physical Functional Performance [CS-PFP]), balance (Functional Reach Test [FRT]), and walking economy (oxygen uptake [mL/kg/min]). Secondary outcome measures were symptom severity (Unified Parkinson's Disease Rating Scale [UPDRS] activities of daily living [ADL] and motor subscales) and quality of life (39-item Parkinson's Disease Quality of Life Scale [PDQ-39]).
Results
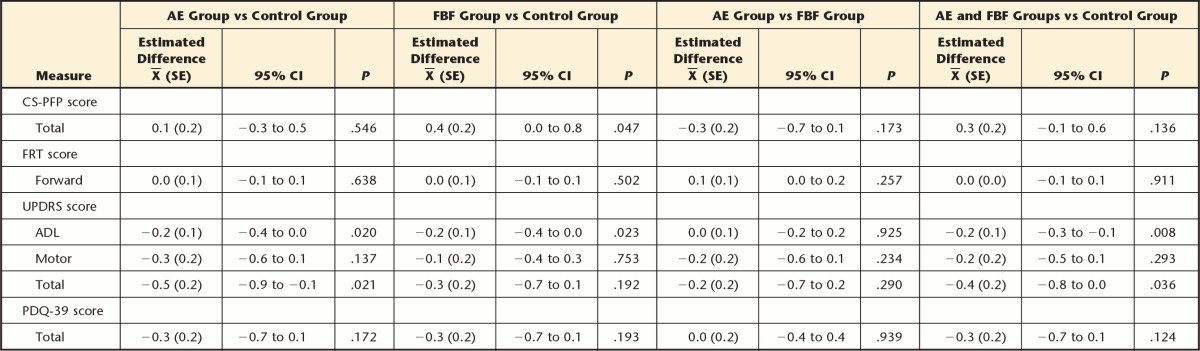
Of the 121 participants, 86.8%, 82.6%, and 79.3% completed 4, 10, and 16 months, respectively, of the intervention. At 4 months, improvement in CS-PFP scores was greater in the FBF group than in the control group (mean difference=4.3, 95% confidence interval [CI]=1.2 to 7.3) and the AE group (mean difference=3.1, 95% CI=0.0 to 6.2). Balance was not different among groups at any time point. Walking economy improved in the AE group compared with the FBF group at 4 months (mean difference=−1.2, 95% CI=−1.9 to −0.5), 10 months (mean difference=−1.2, 95% CI=−1.9 to −0.5), and 16 months (mean difference=−1.7, 95% CI=−2.5 to −1.0). The only secondary outcome that showed significant differences was UPDRS ADL subscale scores: the FBF group performed better than the control group at 4 months (mean difference=−1.47, 95% CI=−2.79 to −0.15) and 16 months (mean difference=−1.95, 95% CI=−3.84 to −0.08).
Limitations
Absence of a non-exercise control group was a limitation of the study.
Conclusions
Findings demonstrated overall functional benefits at 4 months in the FBF group and improved walking economy (up to 16 months) in the AE group.
Parkinson disease (PD) is a chronic, progressive disorder affecting about 1% of people over age 60 years and an estimated 4% of people over the age of 80 years in industrialized countries,1 and is anticipated to affect 8 to 9 million people worldwide by 2030.2 Traditional management of PD has been by pharmacology or surgery, or both. Evidence from various exercise approaches3–11 demonstrates benefits of exercise in preserving aspects of function and quality of life.12–16 However, a Cochrane review of 2001 noted that “there is insufficient evidence to support or refute the efficacy of any given form of physiotherapy over another in Parkinson's disease,” further stating, “Therefore a consensus must be found as to ‘best practice' physiotherapy for Parkinson's disease.”17 Despite considerable efforts, there is not yet sufficient evidence. Furthermore, most interventions studied were relatively short-term, benefits were reported immediately after the intervention, and few authors reported postintervention follow-up. Available postintervention data (typically at 12–26 weeks)15 suggest benefits are lost after supervision is terminated. With no strategies in place to facilitate ongoing exercise, this finding is not surprising because PD is chronic and progressive. Ongoing exercise is likely necessary to combat declines in strength, flexibility, and balance and their functional consequences.
A next important step is to compare interventions, not just in terms of immediate benefits, but also in terms of long-term outcomes. The program with the greatest short-term benefits after supervised exercise may not be the program with greatest benefits over the long term. Thus, this investigation was designed to compare short- and long-term effects of 2 supervised exercise programs with those of a control program.
The 3 exercise approaches investigated were: (1) a flexibility/balance/function program (FBF) specifically designed for people with PD; (2) a standard aerobic endurance program (AE); and (3) as the control, a home-based program of exercises recommended by the National Parkinson Foundation.18 The FBF and AE programs were supervised 3 times a week for 4 months, with tapered supervision for 1 month, and then once monthly to 16 months. The control program was supervised once a month for 16 months.
The FBF intervention was based on exercises used in a previous investigation demonstrating improvement of spinal flexibility and balance in people with PD.8 That program was enriched with exercises designed to enhance postural control and overall function.19 At 4 months, we expected to see the greatest benefits with this program compared with the other 2 programs. However, we were concerned that participants could have difficulty adhering to this program once supervised intervention was completed.
Endurance exercise has known health benefits20 and potentially could benefit people with PD. Improved endurance could lead to improvements in overall function, in particular for those functions that require endurance. We did not anticipate that endurance exercise would be as successful in improving those functional activities that require flexibility and balance as would PD-specific exercises. However, long-term adherence might be easier with this exercise regimen. Hence at 10 and 16 months, it was possible that participants in the AE group would perform better than those in the FBF group.
The exercises outlined in Fitness Counts,18 developed by the National Parkinson Foundation, were chosen as a control because these exercises are commonly given to patients by their neurologists without specific supervision or follow-up. Because of the limited supervision, we anticipated that this program would be less successful than the supervised exercise programs in improving all outcome measures.
Because of the degenerative nature of PD, long-term exercise habits are essential; otherwise, the participant is likely to quickly lose any gains achieved. Yet within 3 months to a year, only 50% or fewer of individuals with a variety of conditions still adhere to an exercise program.21,22 Barriers to exercise have been examined23–26 and include poor exercise self-efficacy, poor sense of control over exercise behaviors, unfavorable self-concept, failure to exercise in the past, insufficient knowledge and skill, and anxiety. Among factors related to exercise adherence,27–31 perhaps most important is readiness or willingness to change.32,33 Based on theoretical constructs, investigators have developed approaches for assisting individuals to develop regular exercise habits.21,23,34–36 These constructs were instrumental in developing the current investigation.
In summary, this investigation compared 2 supervised exercise programs and a control exercise program, both in the short term (4 months) and the long term (10 and 16 months). Strategies were in place to enhance long-term adherence following the supervised period of exercise. Comparisons were made at 4, 10, and 16 months, with the primary endpoints at 4 and 10 months. Our primary hypotheses were:
Overall function: The FBF and AE groups would each have greater improvement on the Continuous Scale—Physical Functional Performance Test (CS-PFP) compared with the control group. This hypothesis was based on the expectation that both improved flexibility and balance and improved endurance would translate to improvements in functional ability.
Balance: The FBF group would have greater improvement on the Functional Reach Test (FRT) compared with the other groups. This hypothesis was based on findings from our prior investigation.8
Movement efficiency: The FBF group would have better economy of walking than the other groups. The causes of reduced economy of walking in people with PD are unknown; however, we theorized that improved thoracic flexibility from the FBF program might result in improved walking economy.
Our secondary hypotheses were:
The FBF and AE groups would each perform better than the control group on the activities of daily living (ADL) and motor subscales of the Unified Parkinson's Disease Rating Scale (UPDRS) because of the impact of these 2 exercise programs on function.
At 4 months, the FBF and AE groups would perform better on the 39-item Parkinson's Disease Questionnaire (PDQ-39) quality of life scale because of the supervised exercise being performed 3 times a week.
Method
Study Design
This was a randomized controlled exercise study for people with early- or mid-stage PD. Participants were enrolled between August 2003 and April 2009; the last participants reached 16 months in July 2010.
Participants were randomly assigned to 1 of 3 groups: (1) supervised flexibility/balance/function exercise (FBF), (2) supervised aerobic exercise (AE), and (3) home exercise (control). Primary outcome measures were: physical function as measured by the CS-PFP,37 balance as measured by the FRT,38 and walking economy (energy cost of walking, or oxygen uptake [V̇o2] in mL/min/kg).39 Secondary outcome measures were: UPDRS ADL and motor subscales and a measure of quality of life (PDQ-39).40 Endpoints were at 4 months (the end of the supervised exercise period for the AE and FBF groups), 10 months, and 16 months. Primary endpoints were at 4 and 10 months.
This investigation was designed with sample sizes sufficient to detect clinically important differences among groups on several relevant outcome measures. Initial sample size estimates (PASS software, NCSS LLC, Kaysville, Utah)41 were based on reported mean changes and standard deviations of measures investigated in the previous exercise intervention study utilizing a similar program,8 as well as other change scores available at the time of the grant submission. Included were FRT and functional axial rotation,8 CS-PFP,37 and UPDRS (total and motor and ADL subscale) scores.42 Approximately halfway through study accrual, updated information on effect sizes for the CS-PFP, UPDRS, and FRT was used to re-estimate the required sample size. Based on a one-way analysis of variance (ANOVA) design with 3 groups and a minimally detectable effect size (f statistic) of 0.4 at 16 months (a ratio of 0.4 of the between-group standard deviation of mean change when comparing 16-month and baseline measures' within-group standard deviation of change), it was estimated that 26 participants completing the study per group were needed to achieve at least 90% power, with α=.05 (2-sided). An effect size of 0.4 translates to between-group standard deviations for mean change between baseline and 16 months of 0.68 cm for the FRT (within-group SD=1.7 cm), 5.92 points for the UPDRS total score (within-group SD=14.8 points), and 3.2 points for the CS-PFP (within-group SD=8 points). Accounting for an estimated 30% attrition rate over 16 months led us to randomize 38 participants per group.
Participants
All participants had primary PD diagnosed by a movement disorders specialist using the UK Brain Bank criteria,43 were in Hoehn and Yahr stages 1 through 3,44 lived in the community, and ambulated independently. Study exclusion criteria were: uncontrolled hypertension, on-state freezing or exercise limitations from other disorders, and Mini-Mental State Examination45 score of less than 24. Most participants were recruited by their treating movement disorder neurologist at the University of Colorado. Other methods included advertisements, presentations at PD support groups, and meetings with other community neurologists. All participants gave informed consent prior to the study.
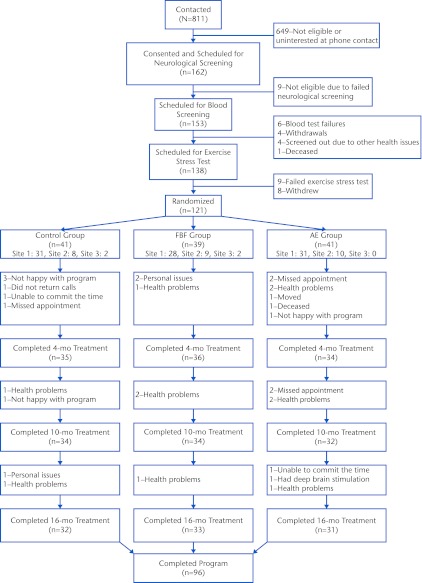
A telephone screen ruled out exclusions related to health. A movement disorders neurologist confirmed the primary PD diagnosis. Participants performed a submaximal graded exercise test (GXT) to determine whether they could exercise safely at intensities up to 85% of age-predicted maximal heart rate (HRmax).39 Eligible volunteers then underwent baseline testing, followed by randomization. Computer-generated randomization assignments were designed by one of the researchers (A.E.B.). Randomization was stratified by sex and blocked to ensure balance across groups over time; the randomization assignments were kept in opaque, sealed envelopes and unsealed by a research assistant after baseline testing. Of the 811 volunteers contacted, 162 provided consent, and 121 were randomized (Fig. 1).
Figure 1.
CONSORT diagram of flow of participants in the study. The control group participated in a home-based program of exercises recommended by the National Parkinson Foundation,18 the AE group participated in a standard aerobic endurance program, and the FBF group participated in a flexibility/balance/function program specifically designed for people with Parkinson disease. Not all participants completed all of the time points, resulting in some fluctuating numbers in the flow diagram; this fluctuation was taken care of by the analysis approach.
Baseline Testing and Outcome Measures
All testing took place at the University of Colorado and was performed by study personnel who were blinded to group allocation. The first test session took place at a time of day when participants had their best response to PD medications; subsequent sessions were conducted as close to that time as possible.
In one session, energy expenditure (V̇o2, mL/min/kg) was measured at 4 walking speeds in 0.5-mph increments (walking economy).6 The maximum speed was based on the participant's fastest tolerable speed during the graded exercise test. A heart rate monitor was worn throughout the test. First, a resting measurement was obtained with the participant sitting in a chair for 5 minutes. Then the participant walked for 5 minutes at each of 4 different speeds, beginning with the slowest speed. Oxygen uptake was measured during the last 2 minutes of each stage using an automated indirect calorimeter system (ParvoMedics TruMax 2400 metabolic cart, Sandy, Utah).
In a second session, the CS-PFP was administered by experienced physical therapists.37 The CS-PFP, a performance-based measure of physical function, quantifies 16 common functional activities. Examples include making a bed, unloading groceries, climbing 3 steps onto a platform while carrying luggage (simulating getting onto a bus), and getting up and down from the floor. For each task, the individual chooses the amount of weight, speed, and distance covered. As such, tasks are performed at the participant's perceived capacity. Tasks are performed consecutively; thus, the CS-PFP measures the cumulative effect of functional performance. Tasks are scored using an algorithm that takes into account weight carried, time to complete the task, and sometimes distance. This test is reliable and valid for people with and without PD.46,47
Performance on the FRT, a test of balance in older adults,38 was measured as described previously.8 The FRT is predictive of falls48 and can be used reliably with individuals who have PD.49 Participants performed 2 practice trials and 3 test trials.
Secondary outcome measures included the UPDRS and PDQ-39. When this study was initiated, the UPDRS was considered the gold standard for quantifying overall severity of PD.50 The UPDRS total score and ADL and motor subscale scores were utilized.42 The 39-item quality-of-life scale (PDQ-39), developed for people with PD, was completed by the participants.40 Changes in levodopa (used in data analysis) were monitored using the levodopa equivalent (mg/day).51
Interventions
Interventions took place at 1 of 3 sites. The majority of participants exercised on the University of Colorado campus. However, some participants (Fig. 1) exercised in a facility an hour south or 45 minutes northwest of Denver. All personnel who supervised the exercise sessions were trained by the primary investigator (M.S.), received written materials outlining the exercise protocols in detail,19,52–55 and co-treated with the primary investigator periodically to ensure consistency when implementing exercise protocols.
Individuals assigned to the FBF and AE groups participated in supervised exercise 3 days a week for 4 months. In month 5, supervision was tapered (described below). Thereafter, participants were asked to participate in a supervised exercise session once a month. The control group participants exercised under supervision during an initial individual session and then once a month for 16 months. All participants were encouraged to perform their prescribed exercise program a total of 5 to 7 days a week throughout the 16 months.
The supervised FBF program consisted of 2 months of flexibility training one-on-one with a physical therapist,52,53 followed by 2 months of small-group exercise (up to 6 participants) that included flexibility, balance, and functional exercises.19 Supervised AE sessions included 5 to 10 minutes of warm-up, 30 minutes of exercise at 65% to 80% of HRmax, and 5 to 10 minutes of cool-down.6 Participants were encouraged to use a treadmill, but were permitted to use a stationary bicycle or elliptical trainer. All except 1 of the participants performed at least some of their exercise on the treadmill. The control program consisted of exercises in the home setting utilizing Fitness Counts, with a single monthly group exercise session supervised by a physical therapist. Details of the exercises are presented in the eTable.
All participants, regardless of group assignment, were assisted in developing long-term exercise habits.55 After randomization and before beginning to exercise, participants met with their trainer to discuss motivation to exercise, potential barriers, and strategies to develop exercise habits. Participants were asked to record supervised and home exercise throughout 16 months. After 4 months, to transition participants to unsupervised exercise, supervision for FBF and AE was tapered (2 sessions a week for 2 weeks, then 1 session a week for 2 weeks). Everyone participated in a monthly exercise session, exercise diaries were reviewed monthly, and strategies were suggested to enhance adherence. Inquiry about adverse events was made at these sessions but could be reported by participants at any time.
Data Analysis
Descriptive analysis included means, standard deviations, and proportions by group at baseline. Comparisons across the treatment groups for categorical variables were made using chi-square tests or their exact counterparts, depending on expected values. Continuous measures or scales were compared using ANOVA. A linear mixed model with main effects for endpoint (baseline and 4, 10, and 16 months), the stratification variable used in randomization (sex), interaction terms between exercise group and endpoint, and levodopa equivalent dose as a time-varying covariate was used to estimate the intervention effect at each time point for each dependent variable. For walking economy, after determining that the V̇o2 × treadmill speed relationship was linear across measured walking speeds of 0.8 to 3.5 mph, we modeled V̇o2 as a function of treadmill speed using a linear mixed model with a random intercept and slope. The other factors in the mixed model for walking economy were the same as above, with the addition of an endpoint × speed interaction and a group × endpoint × speed interaction. For all of the mixed models, it was assumed that the group means at baseline were equal due to randomization, generally a more powerful approach for longitudinal data analysis of a randomized clinical trial that is recommended for routine application by Fitzmaurice et al.56 All analyses were done on an intention-to-treat basis.
Model fit was assessed using −2 log likelihood and its associated chi-square test statistic. Intervention effects on the primary outcomes at 4 and 10 months are the principal focus of this report, but differences at the end of the study period (16 months) also were of interest. Effect sizes based on differences between group means and 95% confidence intervals (CIs) are reported along with statistical significance (2-sided P values). All statistical analysis was performed using SAS/BASE and SAS/STAT software, version 9.2 of the SAS System for Windows (SAS Institute Inc, Cary, North Carolina).57
Assessment of Nonignorable Missingness
Overall, 94 (78%) of the participants had complete data on the primary outcome measures. The analyses assume data are missing at random. Analysis of plots of group means over time stratified by the time of the last test completed58 indicated the findings were not biased by missing data (results not shown).
Role of the Funding Source
This work was supported by grants from the National Institutes of Health (R01 HD043770-04, Colorado CTSI TL1 RR025778, P30 DK048520, and NS052487) and the Parkinson's Disease Foundation.
Results
Participants were 121 people in Hoehn and Yahr stages 1 through 3; almost half were in stage 2. The majority were men, married, retired, and with an annual income of more than $50,000. There were no statistically significant differences among the 3 groups for any demographic measures (Tab. 1). Retention was high at 4 months (86.8%), 10 months (82.6%), and 16 months (79.3%) (Fig. 1), at least 10% higher overall than planned for in our sample size calculations.
Table 1.
Baseline Characteristicsa
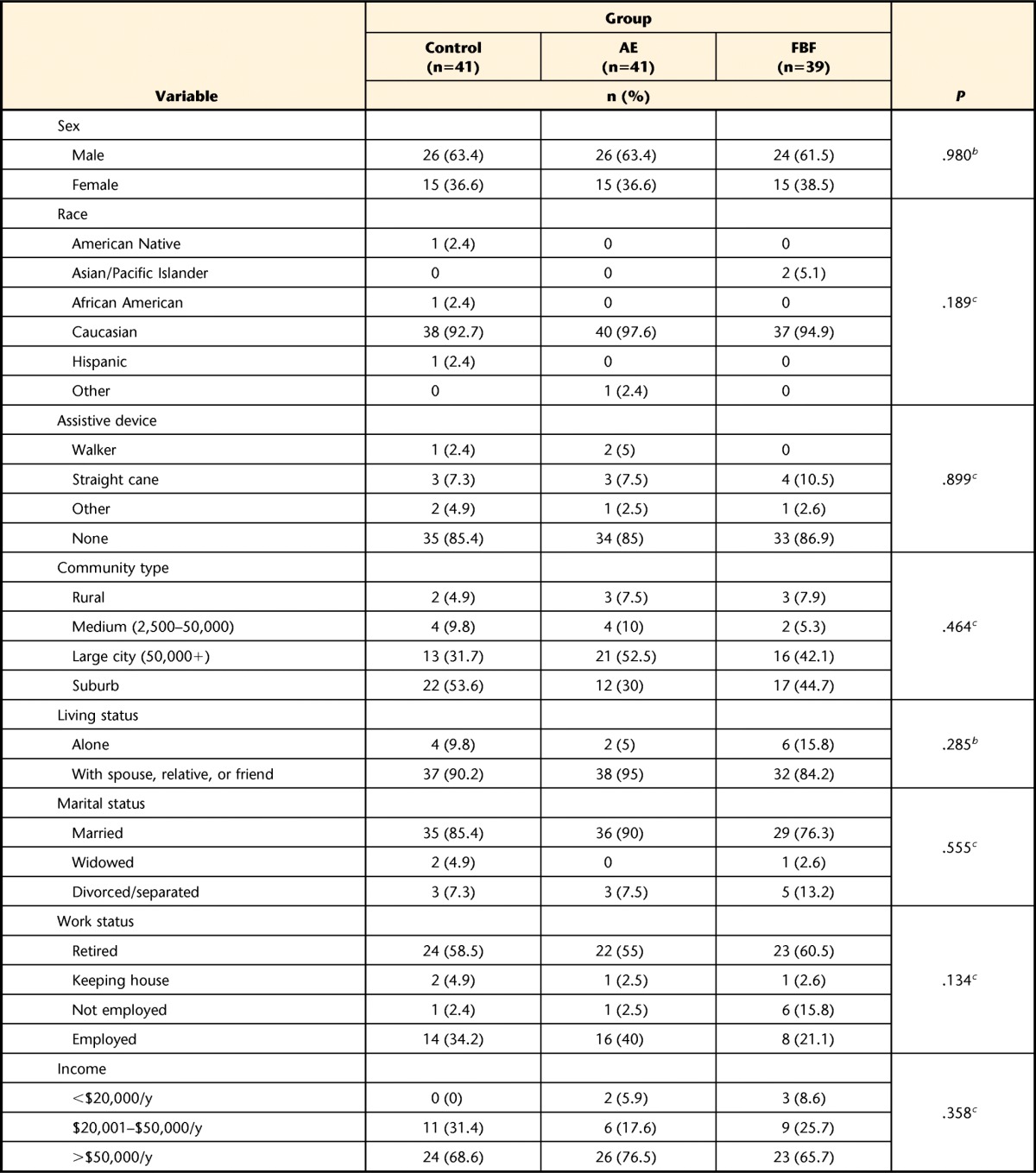
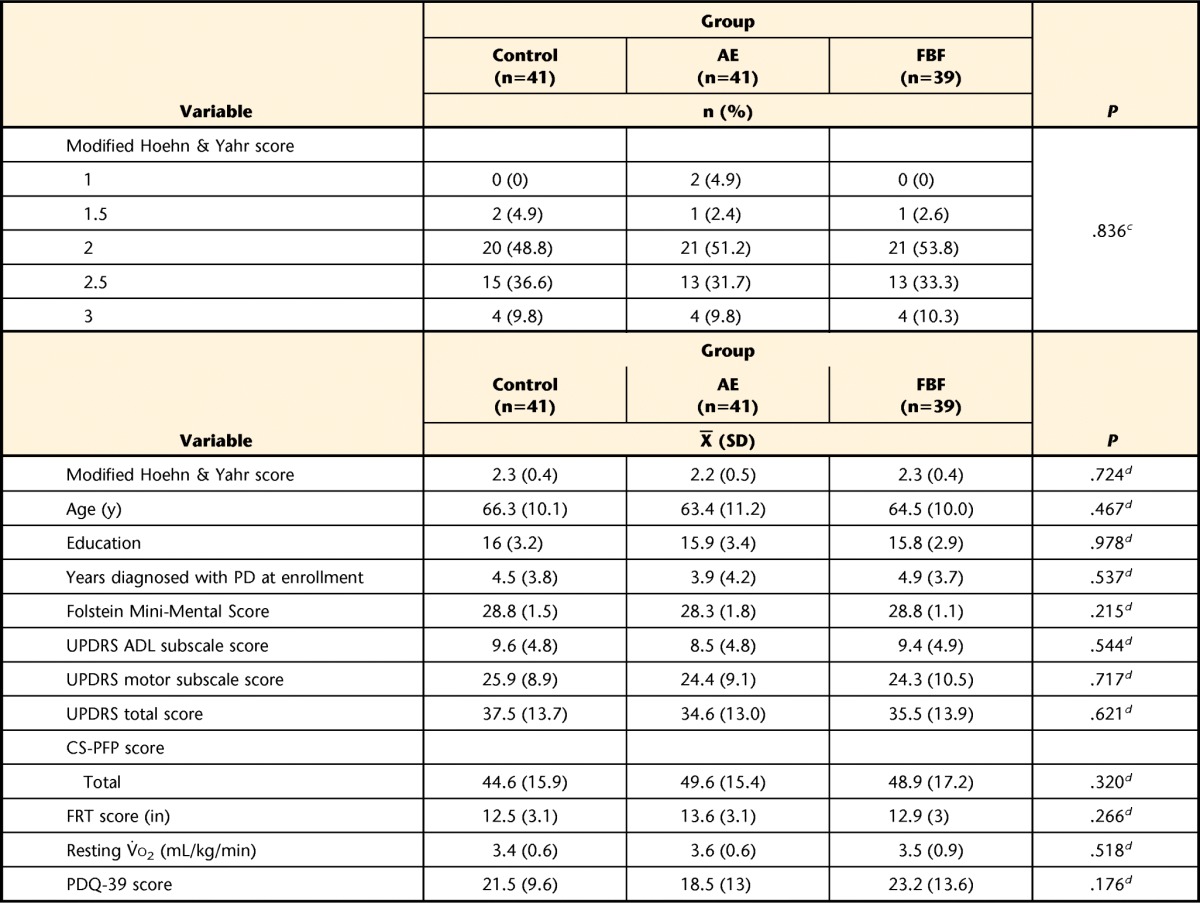
The control group participated in a home-based program of exercises recommended by the National Parkinson Foundation,18 the AE group participated in a standard aerobic endurance program, and the FBF group participated in a flexibility/balance/function program specifically designed for people with Parkinson disease (PD). Subtotals less than the total number of participants indicate some data were not provided by the participants. UPDRS=United Parkinson's Disease Rating Scale, ADL=activities of daily living, CS-PFP=Continuous Scale—Physical Functional Performance Test; FRT=Functional Reach Test, V̇o2=oxygen uptake, PDQ-39=39-item Parkinson's Disease Questionnaire.
b P value from chi-square test.
c Exact chi-square P value.
d P value from analysis of variance.
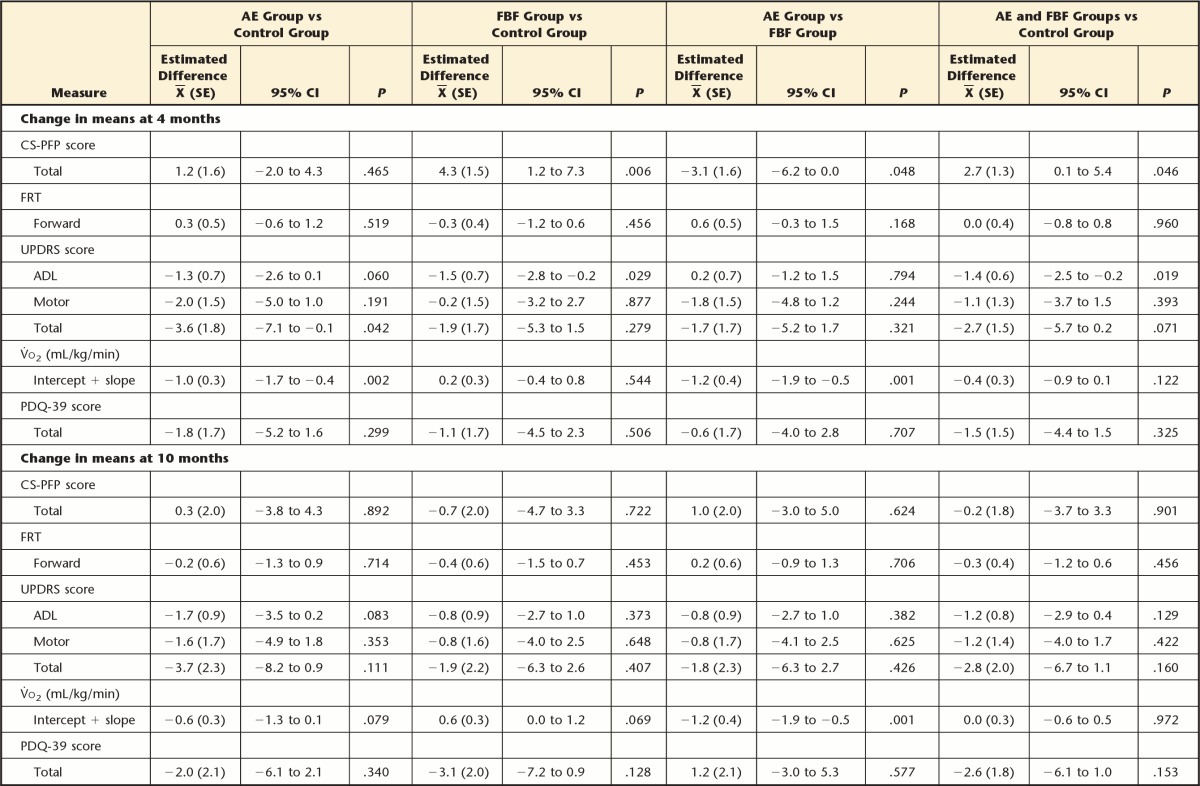
Table 2 includes the adjusted means from the mixed model for each group at each measurement time point. In Table 3, we present the differences between pairs of groups in mean change from baseline. For each group and each outcome, mean change was obtained through the model regression estimates as the adjusted group mean at a given time point minus the adjusted mean at baseline. Differences between groups in change from baseline were obtained using contrasts (subtraction) of the model regression coefficients. The results presented in Table 3 are reported below as the differences, with 95% CI, in mean change from baseline between specific pairs of exercise groups at a given follow-up point.
Table 2.
Linear Model–Based Meansa
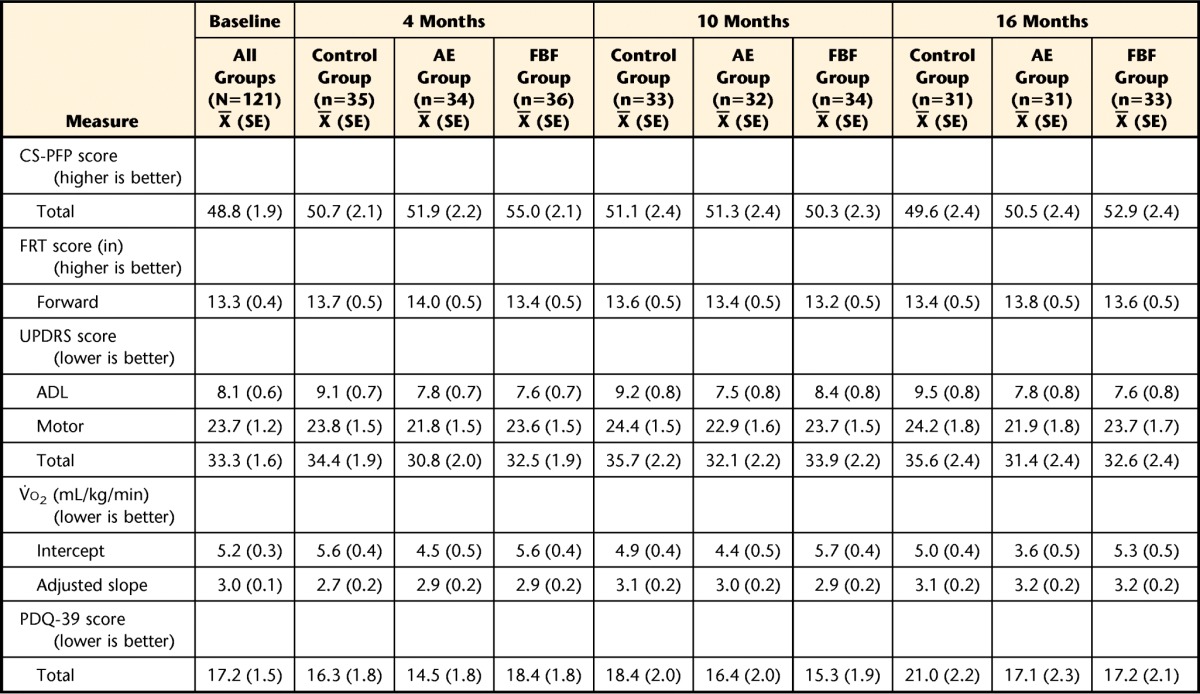
The model likelihood ratio chi square <.001 for all models. Models include the following design variables and covariate adjustments: sex (categorical, 2 levels), time (categorical, 4 levels), group × time interactions (6 dummy variables), levodopa equivalents (continuous). The control group participated in a home-based program of exercises recommended by the National Parkinson Foundation,18 the AE group participated in a standard aerobic endurance program, and the FBF group participated in a flexibility/balance/function program specifically designed for people with Parkinson disease (PD). UPDRS=United Parkinson's Disease Rating Scale, ADL=activities of daily living, CS-PFP=Continuous Scale—Physical Functional Performance Test, FRT=Functional Reach Test, V̇o2=oxygen uptake, PDQ-39=39-item Parkinson's Disease Questionnaire, SE=standard error.
Table 3.
Estimated Mean Differences Between Groups (Treatment Effect)a
The control group participated in a home-based program of exercises recommended by the National Parkinson Foundation,18 the AE group participated in a standard aerobic endurance program, and the FBF group participated in a flexibility/balance/function program specifically designed for people with Parkinson disease (PD). UPDRS=United Parkinson's Disease Rating Scale, ADL=activities of daily living, CS-PFP=Continuous Scale—Physical Functional Performance Test, FRT=Functional Reach Test, V̇o2=oxygen uptake, PDQ-39=39-item Parkinson's Disease Questionnaire, SE=standard error, 95% CI=95% confidence interval.
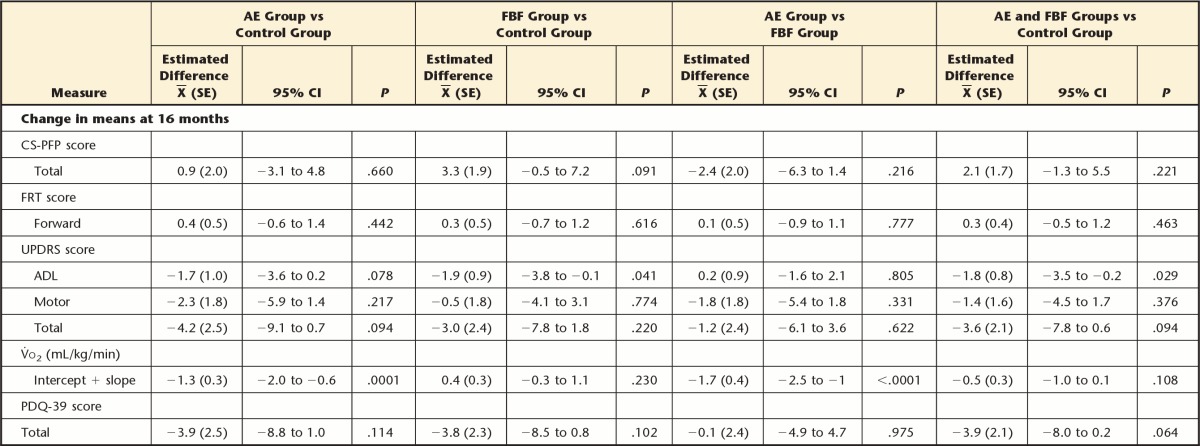
As hypothesized with respect to overall function, at 4 months the FBF group improved on the CS-PFP more than the control group (mean difference=4.3, 95% CI=1.2 to 7.3) and more than the AE group (mean difference=3.1, 95% CI=0.0 to 6.2). Contrary to our hypothesis, the AE group did not improve more than the control group (mean difference=1.2, 95% CI=−2.0 to 4.3) on the CS-PFP. However, at 10 and 16 months, there were no differences between any groups for CS-PFP. There were no differences in FRT scores at any time point (Tab. 3), in contrast to our hypotheses. Also in contrast to the hypotheses, at 4 months, walking economy (ie, reduced energy cost of walking) improved more in the AE group than in the FBF group (mean difference=−1.2 mL/kg/min, 95% CI=−1.9 to −0.5) (Fig. 2). At 10 months, the difference between the AE and FBF groups persisted: the AE group had greater improvements in walking economy than the FBF group (mean difference=−1.21 mL/kg/min, 95% CI=−1.92 to −0.49). At 16 months, the AE group improved more on walking economy than the control group (mean difference=−1.3 mL/kg/min, 95% CI=−2.0 to −0.6) and the FBF group (mean difference=−1.7 mL/kg/min, 95% CI=−2.5 to −1.0).
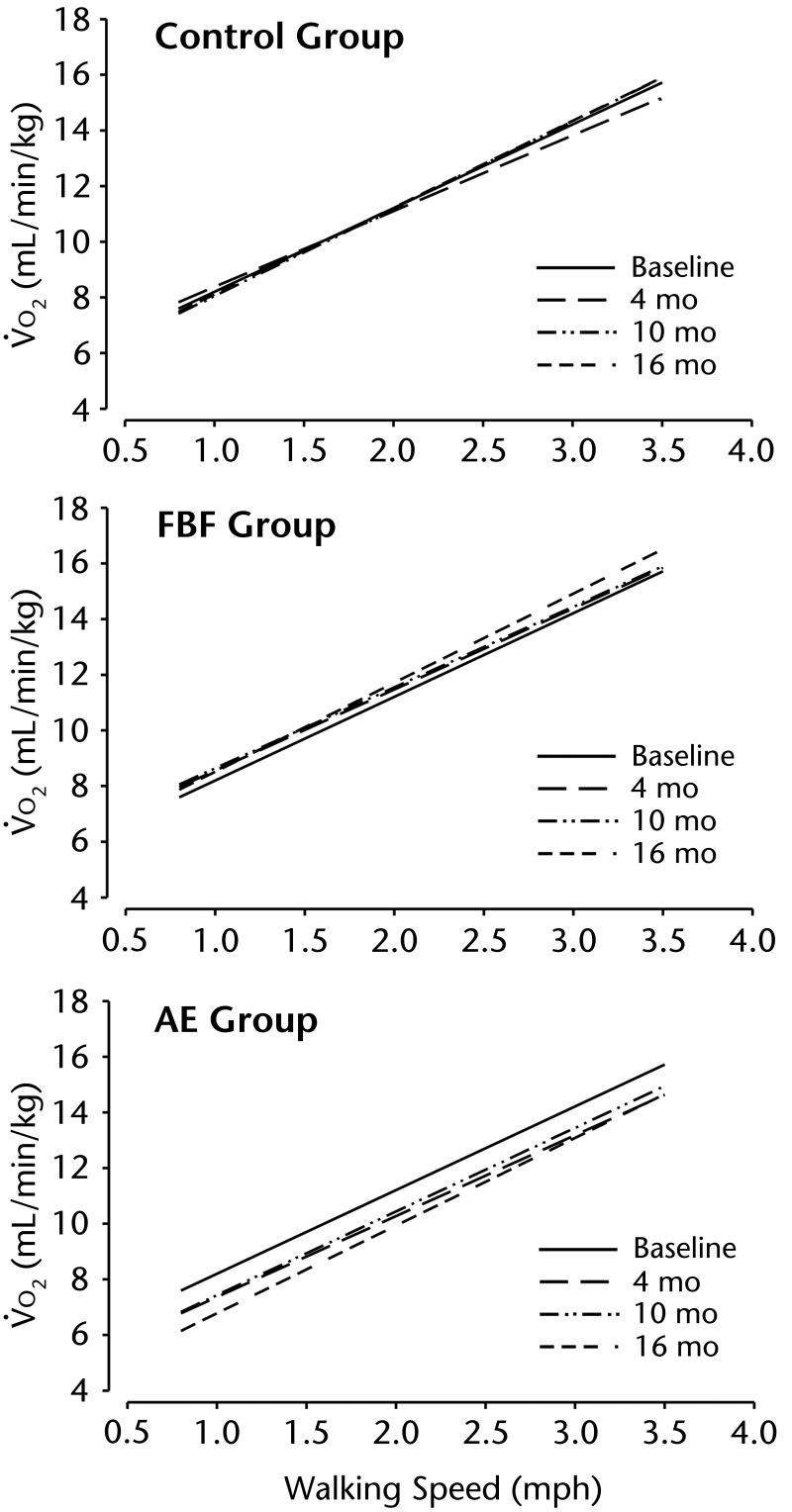
Figure 2.
Walking economy at speeds from 0.8 mph to 4 mph. The control group participated in a home-based program of exercises recommended by the National Parkinson Foundation,18 the AE group participated in a standard aerobic endurance program, and the FBF group participated in a flexibility/balance/function program specifically designed for people with Parkinson disease (PD). Oxygen uptake (V̇o2, in mL/min/kg) is presented for each group at 4 time points (baseline, 4, 10, and 16 months), illustrating the improvement (less oxygen required) for the AE group, but not for the other 2 groups. Walking speeds (increased by 0.5 mph for 4 speeds) are determined for each participant by the maximum walking speed achieved during the graded exercise test.
With regard to secondary outcomes, there were no group differences in the change in PDQ-39 or UPDRS motor subscale scores at any time point. We had hypothesized that both the FBF and AE groups would perform better than the control group on the UPDRS ADL subscale, but only the FBF group performed better than the control group at 4 months (mean difference=−1.47, 95% CI=−2.79 to −0.15) and at 16 months (mean difference=−1.95, 95% CI=−3.84 to −0.08).
Overall group × time interactions are shown in Table 4. Slope of change across the 4 time endpoints was estimated using the regression estimates for each outcome. The AE and FBF groups demonstrated greater improvements on the UPDRS ADL subscale compared with the control group (for each comparison: slope of −0.2, 95% CI=−0.4 to 0.0). The FBF group demonstrated more favorable effects on the CS-PFP than the control group (slope of 0.4, 95% CI=0.0 to 0.8).
Table 4.
Estimated Slope Differences Between Groupsa
The control group participated in a home-based program of exercises recommended by the National Parkinson Foundation,18 the AE group participated in a standard aerobic endurance program, and the FBF group participated in a flexibility/balance/function program specifically designed for people with Parkinson disease (PD). UPDRS=United Parkinson's Disease Rating Scale, ADL=activities of daily living, CS-PFP=Continuous Scale—Physical Functional Performance Test, FRT=Functional Reach Test, PDQ-39=39-item Parkinson's Disease Questionnaire, SE=standard error, 95% CI=95% confidence interval.
Five study-related non-serious adverse events were reported: 3 noninjurious falls (1 in each group) and 2 reports of soreness or pain (both in the AE group). Additionally, 24 nonserious adverse events (not during exercise) were possibly related to the study (2 sprain/strain: 1 in the FBF group and 1 in the AE group; 22 soreness/pain: 9 in the FBF group, 9 in the AE group, and 4 in the control group). One participant died unexpectedly after enrollment but before randomization.
Discussion
This study examined both short-term (4-month) and long-term (10- and 16-month) benefits of exercise for people with early- or mid-stage PD. We embarked on this investigation to determine whether FBF, an exercise program targeted to people with PD, would confer greater benefits than would AE, a general conditioning program, or a control program, and importantly to determine whether the hypothesized differences would persist over the long term after completion of supervised exercise 3 times a week. Study procedures were designed to help participants maintain benefits of exercise once the supervised portion of the study was completed. Immediately following the supervised exercise period (4 months), the FBF program was superior to both the AE and control programs for improving overall function. However, the AE program was superior at 4, 10, and 16 months for improving economy of walking.
Overall Function
The hypothesis that the FBF program would generate better improvements in physical function than the control program was based on findings from a previous 10-week study of flexibility exercises compared to wait-listed controls8 in which exercise improved both flexibility and FRT scores. We believed the program, augmented with balance and functional training, would improve overall functional ability, and, indeed, it did. The mean change from baseline of more than 6 points suggests that the FBF program conferred substantial functional benefits, possibly because of the global nature of the functional training. This change is of particular clinical significance, given that participants in this study were nearing the threshold for disability, as evidenced by their low mean CS-PFP scores.59 However, the difference was not maintained at 10 and 16 months, possibly because participants were not able to adhere sufficiently to this program.
We hypothesized that the AE group participants also would perform better than controls on the CS-PFP because this continuous functional task requires endurance. However, this was not the case, suggesting that endurance training alone is insufficient for improving overall daily function. This finding has important ramifications when designing exercise programs for people with early- and mid-stage PD.
Balance
We anticipated that the FBF group participants would perform better on the FRT, based on the prior investigation utilizing the axial mobility exercise program,8 but they did not. Possibly lack of significant improvement in the present investigation reflected the relatively high functional reach distances at baseline. Of importance, even at 16 months the mean FRT score was not less than at baseline for any of the 3 groups, suggesting that all groups might have benefited to some extent with respect to balance.
Movement Efficiency
We postulated that people with PD might require more V̇o2 (hence reduced walking economy) because of the energy expenditure to overcome the overall stiffness associated with PD. The FBF group did not improve, but the AE group improved significantly and substantially at all 3 time points.
The finding that the AE program improved walking economy was unexpected. Energy cost of walking, when normalized to body weight, is relatively unaffected by factors such as sex and level of fitness (eg, obesity)60 and was not expected to change in response to endurance exercise training. Baseline data from the current study demonstrated abnormally low walking economy (higher energy cost) in patients with PD compared with a control group of people who were healthy.39 Thus, to understand why walking economy improved in response to the AE program, it will be necessary to investigate the mechanisms for impaired walking economy associated with PD. Several possibilities include39,61,62: (1) increased resting energy expenditure, possibly associated with tremors, although this explanation did not explain our observed differences between patients and controls; (2) impaired efficiency of mitochondrial energy production via oxidative phosphorylation; (3) energy cost of ventilation, which has been reported to be increased in patients with PD; and (4) impaired mechanical muscle contraction efficiency, which may be influenced by such factors as muscle fiber type and multi-segment movement coordination. Centrally mediated mechanisms of reduced muscle force production associated with PD63 also might contribute to the increased energy demand.
The reduced economy of movement at baseline in our participants with PD, compared with individuals without PD,39 raises the possibility that reduced economy of movement contributes to the fatigue experienced by many individuals with PD.64 We did not specifically measure fatigue, but this possibility should be considered in future investigations of aerobic conditioning.
Secondary Outcomes
The FBF group had significantly better performance on the UDPRS ADL subscale at both 4 and 16 months. However, the UPDRS ADL change was small and of questionable clinical significance. No other group differences were found. Possibly all participants continued to be active and to benefit from their respective interventions. This interpretation is supported by data from a subset of our participants who participated in a qualitative study 1 year after completing the 16-month parent study.65 Individuals from all 3 of the exercise groups (FBF, AE, and control) indicated that they continued to exercise after completion of the study, although typically at a lower intensity than during the study.
All 3 treatment groups demonstrated remarkably little change in UPDRS motor subscale scores over 16 months. Yet, based on the natural history of PD, the expected rate of increase in the UPDRS motor subscale score in levodopa-treated patients (“on-medication” state) would have been at least 2 to 3 points per year.66 Lack of comparable decline in these data, as well as on other outcome measures, supports the impression that all participants benefited to some degree from their exercise. We also cannot rule out the possibility of a placebo effect, known to be powerful among people with PD.67
Retention of 79.3% at 16 months suggests that long-term intervention studies can be carried out successfully in this population. Furthermore, the low rate of adverse events suggests that patients with PD can engage in relatively vigorous treadmill exercise. These findings contrast with data from a small pilot study, indicating a high rate of falls with treadmill training.68
Several limitations should be acknowledged. It would have been unethical to have a no-exercise control group for a 16-month study, given the growing evidence that exercise benefits people with PD. Cross-sectional data are available across stages of PD for the functional measures used in this study59; however, longitudinal data are lacking for these measures. Such data will allow further interpretation of data in this investigation.
Our study was conducted in Colorado, one of the fittest states in the country. These individuals may be more likely to exercise than people in other areas of the country, even if they are assigned to the control group, possibly affecting applicability of our results to other populations.
With regard to outcomes, when this study was initiated, there was no expectation that exercise might ameliorate the UPDRS motor subscale scores. Hence, we did not collect data in the “off-medication” state. However, we did control for levodopa equivalents, which should have adjusted for any bias due to medication effects. Other measures might provide better estimates of balance in people with PD than does the FRT; evidence in this regard likewise became available after this study was initiated.69
With regard to the interventions, the 3 groups received different degrees of individualized attention and group experience, which could have confounded the findings. On the other hand, the interventions studied are clinically relevant, which was the motivation for implementing them as described.
Finally, we do not have meaningful data on participants' adherence. Although we used exercise diaries, accuracy was insufficient for meaningful interpretation. In future studies, we recommend regular use of activity monitors to quantitatively characterize overall activity (eg, 1 week a month).70,71
From a clinical perspective, the findings suggest that both FBF and AE programs may be important for people with early- and mid-stage PD. Findings support using the FBF program with individuals early in PD to improve overall function and the AE program to improve long-term aerobic endurance. A refresher FBF program could be implemented, should flexibility and function begin to decline. The necessary dose and timing of such a combined intervention are yet to be established. Based on the lack of meaningful decline of any measures over the 16-month study, it appears that the Fitness Counts program (control) also confers some benefits, although to a lesser extent than the supervised programs. Possibly participating in a study with monthly sessions was sufficient for these individuals. Qualitative reports from graduates of the 16-month study65 emphasize that people need ongoing support to maintain regular exercise. We strongly recommend that clinicians find ways to assist individuals with PD to develop and maintain long-term exercise habits, including appropriate exercise programs as well as continued re-evaluation and support.
The Bottom Line
What do we already know about this topic?
Exercise benefits people who are in the early and middle stages of Parkinson disease (PD); however, there is insufficient evidence to determine the best approach. This investigation compared short-term and long-term effects of 2 supervised exercise programs (a PD-specific program and an endurance exercise program) to a control program, recognizing that the program with the greatest short-term benefits after supervised exercise may not be the program with the greatest long-term benefits.
What new information does this study offer?
The findings from this study indicate that the PD-specific and endurance programs confer different benefits, with the endurance program having the greatest long-term benefits. Clinicians can extrapolate from these data to determine appropriate exercise programs for individual patients.
If you're a patient, what might these findings mean for you?
For people with early- or mid-stage PD, different types of exercises provide different short-term and long-term benefits. The greatest long-term benefits may result from endurance training with monthly follow-up visits.
Supplementary Material
Footnotes
Dr Schenkman, Dr Schwartz, and Dr Kohrt provided concept/idea/research design. Dr Schenkman, Dr Barón, Dr Schwartz, and Dr Kohrt provided writing. Dr Hall, Dr Schwartz, Ms Mettler, and Dr Kohrt provided data collection. Dr Schenkman, Dr Barón, Dr Schwartz, Ms Mettler, and Dr Kohrt provided data analysis. Dr Schenkman and Ms Mettler provided project management. Dr Schenkman provided fund procurement. Dr Schwartz and Dr Kohrt provided facilities/equipment. Ms Mettler provided clerical support. Dr Hall provided consultation (including review of manuscript before submission). The authors gratefully acknowledge members of the research team who made this study possible and the participants with Parkinson disease, without whom there would have been no study.
This study was approved by the Institutional Review Board of the University of Colorado.
This research was presented at the Combined Sections Meeting of the American Physical Therapy Association; February 9−12, 2011; New Orleans, Louisiana.
This work was supported by grants from the National Institutes of Health (R01 HD043770-04, Colorado CTSI TL1 RR025778, P30 DK048520, and NS052487) and the Parkinson's Disease Foundation.
This trial is registered at ClinTrials.gov: NCT01257945.
References
- 1. De Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535 [DOI] [PubMed] [Google Scholar]
- 2. Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386 [DOI] [PubMed] [Google Scholar]
- 3. Dibble LE, Hale T, Marcus RL, et al. The safety and feasibility of high-force eccentric resistance exercise in persons with Parkinson's disease. Arch Phys Med Rehabil. 2006;87:1280–1282 [DOI] [PubMed] [Google Scholar]
- 4. Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson's disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. 2009;41:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson's disease: a systematic review across the disability spectrum. J Neurol Phys Ther. 2009;33:14–26 [DOI] [PubMed] [Google Scholar]
- 6. Schenkman M, Hall D, Kumar R, Kohrt WM. Endurance exercise training to improve economy of movement of people with Parkinson disease: three case reports. Phys Ther. 2008;88:63–76 [DOI] [PubMed] [Google Scholar]
- 7. Bergen JL, Toole T, Elliott RG, III, et al. Aerobic exercise intervention improves aerobic capacity and movement initiation in Parkinson's disease patients. NeuroRehabilitation. 2002;17:161–168 [PubMed] [Google Scholar]
- 8. Schenkman M, Cutson TM, Kuchibhatla M, et al. Exercise to improve spinal flexibility and function for people with Parkinson's disease: a randomized, controlled trial. J Am Geriatric Soc. 1998;46:1207–1216 [DOI] [PubMed] [Google Scholar]
- 9. Comella CL, Stebbins GT, Brown-Toms N, Goetz CG. Physical therapy and Parkinson's disease: a controlled clinical trial. Neurology. 1994;44(3 pt 1):376–378 [DOI] [PubMed] [Google Scholar]
- 10. Ellis T, de Goede CJ, Feldman RG, et al. Efficacy of a physical therapy program in patients with Parkinson's disease: a randomized controlled trial. Arch Phys Med Rehabil. 2005;86:626–632 [DOI] [PubMed] [Google Scholar]
- 11. Tickle-Degnen L, Ellis T, Saint-Hilaire MH, et al. Self-management rehabilitation and health-related quality of life in Parkinson's disease: a randomized controlled trial. Mov Disord. 2010;25:194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Goede CJ, Keus SH, Kwakkel G, Wagenaar RC. The effects of physical therapy in Parkinson's disease: a research synthesis. Arch Phys Med Rehabil. 2001;82:509–515 [DOI] [PubMed] [Google Scholar]
- 13. Keus SH, Bloem BR, Hendriks EJ, et al. Evidence-based analysis of physical therapy in Parkinson's disease with recommendations for practice and research. Mov Disord. 2007;22:451–460 [DOI] [PubMed] [Google Scholar]
- 14. Lim I, van Wegen E, de Goede C, et al. Effects of external rhythmical cueing on gait in patients with Parkinson's disease: a systematic review. Clin Rehabil. 2005;19:695–713 [DOI] [PubMed] [Google Scholar]
- 15. Goodwin VA, Richards SH, Taylor RS, et al. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23:631–640 [DOI] [PubMed] [Google Scholar]
- 16. Morris ME, Martin CL, Schenkman ML. Striding out with Parkinson disease: evidence-based physical therapy for gait disorders. Phys Ther. 2010;90:280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deane KH, Jones D, Ellis-Hill C, et al. A comparison of physiotherapy techniques for patients with Parkinson's Disease. Cochrane Database Syst Rev. 2001;(1):CD002815. [DOI] [PubMed] [Google Scholar]
- 18. Cianci H; National Parkinson Foundation. Parkinson's disease: fitness counts. Available at: http://www3.parkinson.org/site/DocServer/Fitness_Counts.pdf?docID=188&JServSessionIdr004=bodq224hr2.app338a. Accessed August 3, 2012
- 19. Rodriguez JW, Stelzner DA, Krapfl B, et al. Balance & Function Protocol Instructor's Manual. Denver, CO: Physical Therapy Program, University of Colorado Health Sciences Center; 2006 [Google Scholar]
- 20. US Department of Health and Human Services. Physical activity guidelines advisory committee report, 2008. Available at: http://www.health.gov/paguidelines/Report/pdf/CommitteeReport.pdf. Published June 2008. Accessed August 3, 2012
- 21. Woodard CM, Berry MJ. Enhancing adherence to prescribed exercise: structured behavioral interventions in clinical exercise programs. J Cardiopulm Rehabil. 2001;21:201–209 [DOI] [PubMed] [Google Scholar]
- 22. Dunn AL, Blair SN. Translating evidenced-based physical activity interventions into practice: the 2010 Challenge. Am J Prev Med. 2002;22(suppl 4):8–9 [DOI] [PubMed] [Google Scholar]
- 23. Jensen GM, Lorish CD. Promoting patient cooperation with exercise programs: linking research, theory, and practice. Arthritis Care Res. 1994;7:181–189 [DOI] [PubMed] [Google Scholar]
- 24. Trost SG, Owen N, Bauman AE, et al. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001 [DOI] [PubMed] [Google Scholar]
- 25. Oman RF, King AC. Predicting the adoption and maintenance of exercise participation using self-efficacy and previous exercise participation rates. Am J Health Promot. 1998;12:154–161 [DOI] [PubMed] [Google Scholar]
- 26. Jette AM, Rooks D, Lachman M, et al. Home-based resistance training: predictors of participation and adherence. Gerontologist. 1998;38:412–421 [DOI] [PubMed] [Google Scholar]
- 27. Dalton CC. The concept of readiness to change. J Adv Nursing. 2003;42:108–117 [DOI] [PubMed] [Google Scholar]
- 28. Jette AM, Lachman M, Giorgetti MM, et al. Exercise—it's never too late: the strong-for-life program. Am J Public Health. 1999;89:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dubbert PM, Doyle ME, MacAller H, et al. From supervised to unsupervised exercise: factors associated with exercise adherence. J Aging and Physical Activity. 2003;11:351–368 [Google Scholar]
- 30. Perri MG, Anton SD, Durning PE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. 2002;21:452–458 [PubMed] [Google Scholar]
- 31. Marcus BH, Banspach SW, Lefebre RC, et al. Using the stages of change model to increase the adoption of physical activity among community participants. Am J Health Promot. 1992;6:424–429 [DOI] [PubMed] [Google Scholar]
- 32. Bandura A. Self-efficacy: The Exercise of Control. New York, NY: Worth Publishers; 1997 [Google Scholar]
- 33. Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63:60–66 [DOI] [PubMed] [Google Scholar]
- 34. Fraser SN, Spink KS. Examining the role of social support and group cohesion in exercise compliance. J Behav Med. 2002;25:233–249 [DOI] [PubMed] [Google Scholar]
- 35. Eden KB, Orleans CT, Mulrow CD, et al. Does counseling by clinicians improve physical activity: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137:208–215 [DOI] [PubMed] [Google Scholar]
- 36. Kahn EB, Ramsey LT, Brownson RC, et al. The effectiveness of interventions to increase physical activity: a systematic review. Am J Prev Med. 2002:22(suppl 4):73–107 [DOI] [PubMed] [Google Scholar]
- 37. Cress ME, Buchner DM, Questad KA, et al. Continuous-scale physical functional performance in healthy older adults: a validation study. Arch Phys Med Rehabil. 1996;77:1243–1250 [DOI] [PubMed] [Google Scholar]
- 38. Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45:M192–M197 [DOI] [PubMed] [Google Scholar]
- 39. Christiansen CL, Schenkman ML, McFann K, et al. Walking economy in people with Parkinson's disease. Mov Disord. 2009;24:1481–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res. 1995;4:241–248 [DOI] [PubMed] [Google Scholar]
- 41. PASS 2008 [computer program] Kaysville, UT: NCSS LLC; 2008 [Google Scholar]
- 42. Marchese R, Diverio M, Zucchi F, et al. The role of sensory cues in the rehabilitation of parkinsonian patients: a comparison of two physical therapy protocols. Mov Disord. 2000:15:879–883 [DOI] [PubMed] [Google Scholar]
- 43. Daniel SE, Lee AJ. Parkinson's disease society brain bank, London: overview and research. J Neural Transm Suppl. 1993;39:165–172 [PubMed] [Google Scholar]
- 44. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442 [DOI] [PubMed] [Google Scholar]
- 45. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 46. Cress ME, Buchner DM, Questad KA, et al. Exercise: effects on physical functional performance in independent older adults. J Gerontol A Biol Sci Med Sci. 1999;54:M242–M248 [DOI] [PubMed] [Google Scholar]
- 47. Schenkman M, Cutson TM, Kuchibhatla M, et al. Application of the continuous-scale physical functional performance test to people with Parkinson disease. Neurology Report. 2002;26:130–138 [Google Scholar]
- 48. Duncan PW, Studenski S, Chandler J, Prescott B. Functional reach: predictive validity in a sample of elderly male veterans. J Gerontol. 1992;47:M93–M98 [DOI] [PubMed] [Google Scholar]
- 49. Schenkman M, Cutson TM, Kuchibhatta M, et al. Reliability of impairment and physical performance measures for persons with Parkinson's disease. Phys Ther. 1997;77:19–27 [DOI] [PubMed] [Google Scholar]
- 50. Fahn S, Elton RL, UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Goldstein M.Recent Developments in Parkinson's Disease. Vol 2. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163 [Google Scholar]
- 51. Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653 [DOI] [PubMed] [Google Scholar]
- 52. Schenkman M, Keysor J, Chandler J, et al. Axial Mobility Exercise Program: An Exercise Program to Improve Functional Ability. Durham, NC: Claude D Pepper Older Americans Independence Center, Duke University; 1994 [Google Scholar]
- 53. Chandler J, Laub KC, Keysor J, et al. Axial Mobility Exercise Program: An Exercise Program to Improve Functional Ability. Participant's Manual. Durham, NC: Claude D Pepper Older Americans Independence Center, Duke University; 1993 [Google Scholar]
- 54. Laub KC, Schenkman M. The Axial Mobility Exercise Program [videotape] Durham, NC: Claude D Pepper Older Americans Independence Center, Duke University; 1995 [Google Scholar]
- 55. Stelzner DA, Rodriguez JW, Krapfl B, et al. Instructor's Adherence Protocol, Instructor's Guidelines for Assisting Participants. Denver, CO: Physical Therapy Program, University of Colorado Health Sciences Center; 2004 [Google Scholar]
- 56. Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis New York, NY: John Wiley & Sons Inc; 2004 [Google Scholar]
- 57. SAS/BASE, SAS/STAT (for Windows) [computer program]. Version 9.2. Cary, NC: SAS Institute Inc; 2002–2008 [Google Scholar]
- 58. Fairclough D. Design and Analysis of Quality of Life Studies in Clinical Trials. 2nd ed. Boca Raton, FL: Chapman and Hall/CRC; 2010 [Google Scholar]
- 59. Schenkman M, Ellis T, Christiansen C, et al. Profile of functional limitations and task performance among people with early- and middle-stage Parkinson disease. Phys Ther. 2011;91:1339–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Browning RC, Baker EA, Herron JA, Kram R. Effects of obesity and sex on the energetic cost and preferred speed of walking. J Appl Physiol. 2006;100:390–398 [DOI] [PubMed] [Google Scholar]
- 61. Perrault H. Efficiency of movement in health and chronic disease. Clin Invest Med. 2006;29:117–121 [PubMed] [Google Scholar]
- 62. Seccombe LM, Giddings HL, Rogers PG, et al. Abnormal ventilatory control in Parkinson's disease: further evidence for non-motor dysfunction. Respir Physiol Neurobiol. 2011;179:300–304 [DOI] [PubMed] [Google Scholar]
- 63. Stevens-Lapsley JE, Kluger BM, Schenkman M. Quadriceps muscle weakness, activation deficits, and fatigue with Parkinson's disease. Neurorehabil Neural Repair. 2012;26:533–541 [DOI] [PubMed] [Google Scholar]
- 64. Friedman JH, Brown RG, Comella C, et al. Fatigue in Parkinson's disease: a review. Mov Disord. 2007;22:297–308 [DOI] [PubMed] [Google Scholar]
- 65. Ene H, McRae C, Schenkman M. Attitudes toward exercise following participation in an exercise intervention study. J Neurol Phys Ther. 2011;35:34–40 [DOI] [PubMed] [Google Scholar]
- 66. Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP. Progression of motor impairment and disability in Parkinson disease: a population-based study. Neurology. 2005;65:1436–1441 [DOI] [PubMed] [Google Scholar]
- 67. de la Fuente-Femandez R, Stoessl AJ. The placebo effect in Parkinson's disease. Trends Neurosci. 2002;25:302–306 [DOI] [PubMed] [Google Scholar]
- 68. Skidmore FM, Patterson SL, Shulman LM, et al. Pilot safety and feasibility study of treadmill aerobic exercise in Parkinson disease with gait impairment. J Rehabil Res Dev. 2008;45:117–124 [DOI] [PubMed] [Google Scholar]
- 69. Dibble LE, Christensen J, Ballard DJ, Foreman KB. Diagnosis of fall risk in Parkinson disease: an analysis of individual and collective clinical balance test interpretation. Phys Ther. 2008;88:323–332 [DOI] [PubMed] [Google Scholar]
- 70. Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(suppl 11): S512–S522 [DOI] [PubMed] [Google Scholar]
- 71. Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.