Abstract
Background
Diabetic peripheral neuropathy affects nearly half of individuals with diabetes and leads to increased fall risk. Evidence addressing fall risk assessment for these individuals is lacking.
Objective
The purpose of this study was to identify which of 4 functional mobility fall risk assessment tools best discriminates, in people with diabetic peripheral neuropathy, between recurrent “fallers” and those who are not recurrent fallers.
Design
A cross-sectional study was conducted.
Setting
The study was conducted in a medical research university setting.
Participants
The participants were a convenience sample of 36 individuals between 40 and 65 years of age with diabetic peripheral neuropathy.
Measurements
Fall history was assessed retrospectively and was the criterion standard. Fall risk was assessed using the Functional Reach Test, the Timed “Up & Go” Test, the Berg Balance Scale, and the Dynamic Gait Index. Sensitivity, specificity, positive and negative likelihood ratios, and overall diagnostic accuracy were calculated for each fall risk assessment tool. Receiver operating characteristic curves were used to estimate modified cutoff scores for each fall risk assessment tool; indexes then were recalculated.
Results
Ten of the 36 participants were classified as recurrent fallers. When traditional cutoff scores were used, the Dynamic Gait Index and Functional Reach Test demonstrated the highest sensitivity at only 30%; the Dynamic Gait Index also demonstrated the highest overall diagnostic accuracy. When modified cutoff scores were used, all tools demonstrated improved sensitivity (80% or 90%). Overall diagnostic accuracy improved for all tests except the Functional Reach Test; the Timed “Up & Go” Test demonstrated the highest diagnostic accuracy at 88.9%.
Limitations
The small sample size and retrospective fall history assessment were limitations of the study.
Conclusions
Modified cutoff scores improved diagnostic accuracy for 3 of 4 fall risk assessment tools when testing people with diabetic peripheral neuropathy.
Diabetes is a disease that affects nearly 25.6 million people in the United States,1 with a prevalence rate of nearly 50% for diabetic peripheral neuropathy (DPN).2 Distal symmetric neuropathy, the most common form of DPN,3 is a serious complication of diabetes that, irrespective of age, leads to physical impairments, such as slower reaction times, decreased ankle strength and mobility, greater postural instability, and altered walking patterns.4–7 For all adults, balance and gait problems alone are responsible for a 3-fold increase in risk of falling.8 Powell et al9 found that 29% of older people who had been diagnosed with DPN had fallen in the previous year, with 73% of those having fallen 2 or more times during that period. This identified fall risk is true not only of older people with DPN. Richardson et al4 demonstrated that neuropathy affecting the lower extremities was a significant risk factor for and significantly associated with falling and repetitive falling, independent of age. In 2008, a report by the Quality Standards Subcommittee of the American Academy of Neurology indicated that people with peripheral neuropathy have probable (level B evidence) risk of falling and that those with disorders of balance and gait have established (level A evidence) risk of falling.10
Given this information, it is important that fall risk as it relates to balance and gait dysfunction be accurately assessed in people with DPN. Physical therapists often use functional mobility tools to assess balance and gait as part of a more comprehensive fall risk assessment. A recent systematic review of literature related to balance interventions for people with DPN indicated a significant need for additional research in this area, including research that uses more reliable and valid outcomes measures than have been used previously.11
Fortunately, a number of different functional mobility tools are available to assess fall risk as it relates to balance and gait dysfunction; however, the usefulness of these tools for identifying fall risk in specific populations has been questioned.12–14 Some investigators have determined that traditional cutoff scores used to classify a person's fall risk using functional mobility tools need to be modified for people with specific diagnoses in order to accurately assess fall risk. For example, Dibble and Lange13 found that when using the Timed “Up & Go” Test (TUG) in people with Parkinson disease, a cutoff score of 8.5 seconds, as opposed to the traditionally suggested 13.5 seconds, increased the validity of this tool for this population. Similarly, other investigators have found that cutoff scores for the Berg Balance Scale (BBS) of 49 in older adults,15 52 in people with chronic stroke,16 and 54 in people with Parkinson disease,13,17 instead of the traditional cutoff score of 45, have improved the accuracy of this fall risk assessment tool. To our knowledge, no studies have been conducted to compare the accuracy of clinically used, functional mobility fall risk assessment tools in people who have DPN.
The 4 functional mobility tools that were selected to assess fall risk in this study were the Functional Reach Test (FRT), the TUG, the BBS, and the Dynamic Gait Index (DGI). These tools were selected because each measures some degree of static or dynamic balance and gait and is easily administered in a clinical setting. Of the 4 tools, the DGI presents the greatest level of dynamic challenge in that not only do all test items require ambulation, but some also require ambulation with visual and vestibular modifications (head turns). We expected this altered visual and vestibular input to increase the overall accuracy of the DGI compared with the other tools because people with impaired sensory input from the periphery, such as those with DPN, may rely more on visual and vestibular inputs to maintain balance. All of these functional mobility assessment tools appear to be valid for use with older people18–23 and some for diagnoses that are associated with increased fall risk, such as Parkinson disease,24 vestibular disorders,25 and stroke.26
The primary purpose of this study was to identify which 1 of these 4 commonly used functional mobility tools used to assess fall risk is best able to accurately discriminate, in people with DPN, between recurrent “fallers” and those who are not recurrent fallers. A secondary purpose was to determine whether modified cutoff scores would improve the diagnostic accuracy of these tools. We hypothesized that of the 4 functional mobility fall risk assessment tools, the DGI would demonstrate the greatest diagnostic accuracy.
Method
Participants
Prior to recruitment of study participants from the surrounding metropolitan area via advertisement at local community centers and in-person recruitment through diabetes education classes, the study was approved by the Human Subjects Committee of University of Kansas Medical Center. Consecutive recruitment and data collection were conducted for approximately 2 years (2009–2011) in a research medical center setting. Interested people could participate if they: (1) had signs and symptoms of DPN, (2) were between the ages of 40 and 65 years, and (3) were able to ambulate without the assistance of another person. People older than 65 years were not eligible to participate in an effort to minimize the potential confound associated with increased fall risk in older adults. Individuals also were excluded if they had untreated major medical depression, open wounds on the weight-bearing surfaces of either foot, or any uncorrected visual deficits or musculoskeletal, vestibular, or neurological conditions that could significantly alter gait or balance.
Procedure
After written informed consent was obtained, participants underwent a single testing session that included nerve conduction studies, screening for neuropathy using the Michigan Neuropathy Screening Instrument (MNSI),27 4 functional mobility fall risk assessments, and a fall history assessment as part of this cross-sectional study. All assessments, except the nerve conduction studies, were conducted by the same physical therapist with 10 years of experience, including experience with all of the assessment tools. Nerve conduction studies were conducted by an experienced technician. The fall risk assessments for every participant were conducted in a randomized order (SPSS version 16.0, SPSS Inc, Chicago, Illinois) and always preceded the fall history assessment to avoid tester bias. Rest was allowed as needed during testing, and a gait belt was used during the fall risk assessments.
Confirmation of diabetic peripheral neuropathy.
Nerve conduction studies were conducted for the peroneal, tibial, and sural nerves of the right lower extremity to confirm participant-reported symptoms or diagnosis of DPN. Nerve conduction velocity, amplitude, and latency were measured for the tibial and peroneal nerves. Nerve conduction amplitude and latency were assessed for the sural nerve. The MNSI is a screening tool for diabetic neuropathy and comprises 2 parts: a history portion and a physical examination portion.27 The history portion consists of 15 questions that address neuropathy-related symptoms in the legs or feet, with a maximum score of 13. The physical examination portion includes foot inspection, monofilament sensation, vibration sensation, and reflex testing, with a maximum score of 10. Higher scores indicate increased presence of neuropathic signs or symptoms. A physical examination score of 2 or greater indicates neuropathy.27,28 If the results of the nerve conduction studies or the MNSI raised questions about the presence of DPN, a collaborating neurologist was consulted to determine the absence or presence of DPN.
Fall risk assessment.
The 4 functional mobility fall risk assessments were the FRT, the TUG, the BBS, and the DGI. The FRT29 is a test of anterior and posterior dynamic stability29 and assesses the furthest distance one can reach forward beyond arm's length while standing with a fixed base of support.20 The test was performed 5 times (2 practice trials and 3 test trials), which is consistent with the procedure used by the creator of this tool.20
The TUG22 has been described as a test of balance and functional mobility30 and is a timed test. While completing the TUG, the individual stands up from a seated position, walks 3 m, turns around, walks back to the chair, and sits down. The TUG was performed 3 times (1 practice trial and 2 test trials). The TUG procedure used was consistent with that used by Shumway-Cook and colleagues30 in their assessment of fall risk in older adults.
The BBS31 is a tool used to rate a person's ability to maintain balance while performing movements associated with different activities encountered during daily living.31 This test is a multiple item (14-item) test and was conducted once with each participant. Each item was scored on a 4-point scale, with a total possible score of 56.
The DGI was developed to measure a person's ability to perform movement tasks while walking and to determine fall risk in community-living older adults.32 Like the BBS, it is a multiple item (8-item) test and was conducted once with each participant. Each item was scored on a 3-point scale, with a total possible score of 24.
Both the BBS and DGI were conducted according to standard procedure as outlined by each test. A lower test score indicates greater impairment for all of these fall risk assessment tools, except for the TUG, for which the opposite is true. Fall risk was determined based on participant performance relative to cutoff scores established by the developers of these fall risk assessment tools or reported in the literature (FRT<25.4 cm,13,29,33 TUG ≥13.5 seconds,13,30 BBS<45/56,18,31 and DGI≤19/2413,15,34).
Fall history assessment.
Fall history over the previous 12 months was assessed to categorize participants as recurrent fallers (≥2 falls)11 or “nonfallers” (0 or 1 fall), and was used as the reference standard. Similar grouping of participants was used by Faulkner and colleagues35 because recurrent falling is thought to be more associated with an intrinsic predisposition to falling than with an isolated fall. For this study, a fall was defined, as in other studies,36–38 as an event that resulted in a person coming to rest unintentionally on the ground or other level, not as the result of a major intrinsic event or overwhelming hazard. This definition is more specific than those used by other investigators,4,13,24,39–41 leaving less room for subjective interpretation of what constitutes a fall. If a participant reported falling based on the aforementioned definition, the individual then was asked if he or she could recall the exact or approximate dates of any falls and any circumstances surrounding the falls.4,38,41–43 For a fall to count toward the fall status categorization, the participant had to recall at least an approximate date and specific circumstances surrounding the fall.4
Data Analysis
All data were analyzed using Microsoft Office Excel 2007 (Microsoft Corp, Redmond, Washington) and PASW Statistics version 18.0 (SPSS Inc). Descriptive statistics were calculated. Recurrent fall history and functional mobility fall risk assessment results were used to calculate 5 indexes for each fall risk assessment tool44: (1) sensitivity, (2) specificity, (3) positive likelihood ratio, (4) negative likelihood ratio, and (5) overall accuracy.
Sensitivity was calculated as the proportion of participants with a recurrent fall history who were correctly identified by a fall risk assessment tool as having fall risk. Specificity was calculated as the proportion of participants who did not have a recurrent fall history and were correctly identified by a fall risk assessment tool as not having fall risk. In the context of this study, the positive likelihood ratio indicates how much the odds of being a recurrent faller increase when a fall risk assessment tool indicates that someone has fall risk. It was calculated by taking the probability of a recurrent faller being identified as having fall risk divided by the probability of a nonfaller being identified as having fall risk, or sensitivity divided by 1 − specificity. The negative likelihood ratio indicates how much the odds of being a recurrent faller decrease when a fall risk assessment tool indicates that someone does not have fall risk. It was calculated by taking the probability of a recurrent faller being identified as not having fall risk divided by the probability of a nonfaller being identified as not having fall risk, or 1 − sensitivity divided by specificity. Overall accuracy was calculated as the total number of true positives and negatives divided by the total number of true and false positives and negatives. Wald confidence intervals were calculated for all binomial proportions45(pp9–15) (sensitivity, specificity, and overall accuracy) except for the BBS 100% specificity using the traditional cutoff score, for which the exact Clopper-Pearson confidence interval was calculated.45(pp18–20) Confidence intervals also were calculated for the ratios of binomial proportions (positive and negative likelihood ratios).45(p73) Receiver operating characteristic (ROC) curves44 were used to determine modified cutoff scores that maximized sensitivity and specificity for each fall risk assessment tool. The participants' scores on each tool then were compared with the modified cutoff scores to recalculate the aforementioned 5 indexes.
Role of the Funding Source
This work was supported, in part, by University of Kansas Medical Center General Clinical Research Center grant M01 RR 02394, the National Center for Research Resources/National Institute of Health, Frontiers: The Heartland Institute for Clinical and Translational Research (University of Kansas Medical Center's CTSA [UL1RR033179]), and Kansas Partners in Progress.
Results
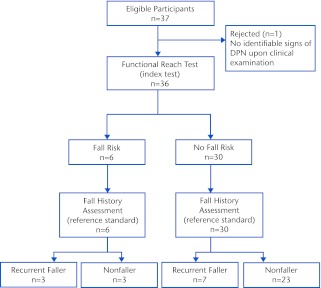
Of the 106 people screened for eligibility, 69 were excluded from participation due to no symptoms of DPN (n=21), age (n=13), schedule conflict (n=10), significant musculoskeletal issues (n=7), no interest after further information (n=6), significant neurological issues (n=4), wound on foot (n=4), uncorrected visual deficits (n=2), vestibular dysfunction (n=1), and severe, untreated depression (n=1). Of the 37 participants who were enrolled in the study, 36 completed the study. The excluded participant, upon clinical examination, did not have signs and symptoms consistent with DPN. Descriptive statistics of all participants, including sex, age, body mass index, years with DPN, peroneal and tibial nerve conduction velocities, MNSI scores, and functional mobility fall risk assessment scores for each tool, are listed in Table 1. Of the 36 participants, 10 (27.8%) were classified as recurrent fallers (≥2 falls) and 26 (72.2%) as nonfallers (<2 falls). Of the 26 nonfallers, 12 (46.2%) had fallen 1 time and 14 (53.8%) had fallen 0 times. The recurrent faller and nonfaller groups' descriptive statistics also are listed in Table 1. Differences between the 2 groups were assessed using an independent samples t test for parametric data and Fischer exact test for categorical data, with the alpha level set at .05. Significant differences were noted only for tibial nerve conduction velocity (P=.003) and all fall risk assessment tool scores (FRT, P=.042; TUG, P=.001; BBS, P<.001; and DGI, P<.001).
Table 1.
Descriptive Statisticsa
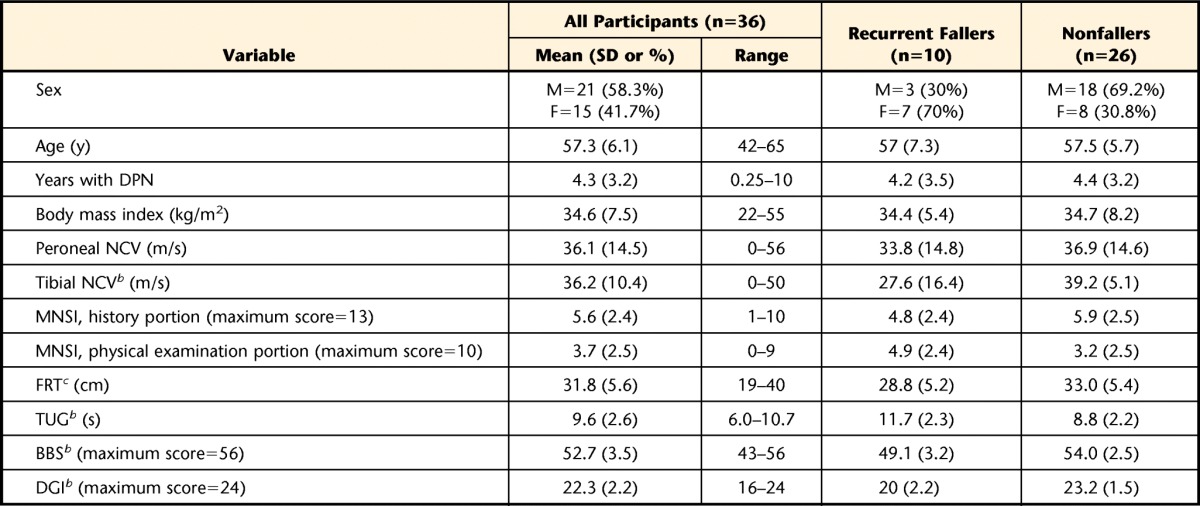
All values are listed as means (standard deviations) except for sex, for which frequencies and percentages are listed. M=male, F=female, DPN=diabetic peripheral neuropathy, NCV=nerve conduction velocity, MNSI=Michigan Neuropathy Screening Instrument, FRT=Functional Reach Test, TUG=Timed “Up & Go” Test, BBS=Berg Balance Scale, DGI=Dynamic Gait Index.
b Significant difference (P<.01) between recurrent fallers and nonfallers.
c Significant difference (P<.05) between recurrent fallers and nonfallers.
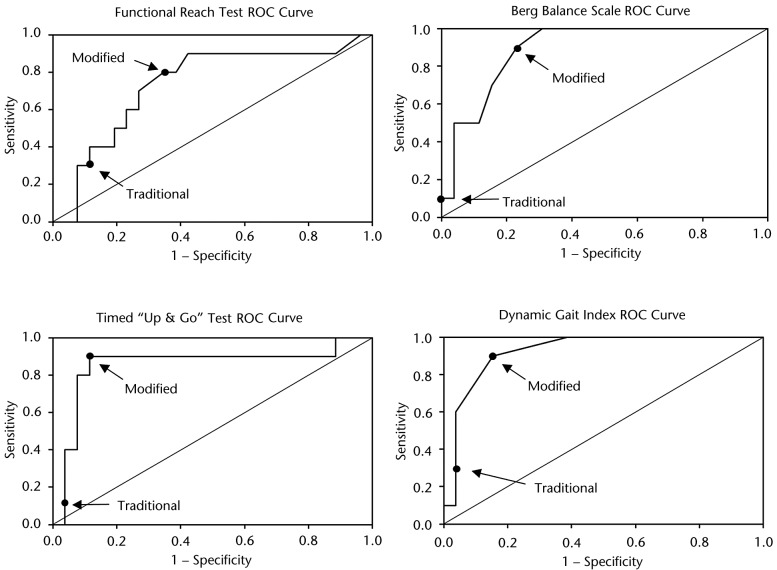
The calculated indexes used to compare the diagnostic accuracy of each fall risk assessment tool using the traditional and modified cutoff scores are listed in Table 2. Using the traditional cutoff scores, the DGI and the FRT demonstrated the highest sensitivities at 30%, but the DGI had higher specificity (96.2% versus 88.5%) and had the highest overall accuracy (77.8%). The DGI also demonstrated the second highest positive likelihood ratio and the lowest negative likelihood ratio. When using the modified cutoff scores, the TUG, BBS, and DGI all demonstrated the highest sensitivity at 90%, with the TUG demonstrating the highest specificity of the 3 assessment tools (88.5% versus 76.9% and 84.6%); therefore, the TUG had the highest overall accuracy (88.9%). The TUG also demonstrated the highest positive and lowest negative likelihood ratios. Table 3 displays the cross-tabulation outcomes when comparing the fall risk assessment tools' (index test) results with the fall history results (reference standard) using the traditional cutoff scores, from which all the aforementioned indexes were calculated. Figure 1 is a flow diagram depicting how the cross-tabulation results listed in Table 3 were determined for the FRT. Table 4 displays similar information but for modified rather than traditional cutoff scores.
Table 2.
Indexes of Diagnostic Accuracy for Fall Risk Assessment Tools: Traditional Versus Modified Cutoff Scoresa
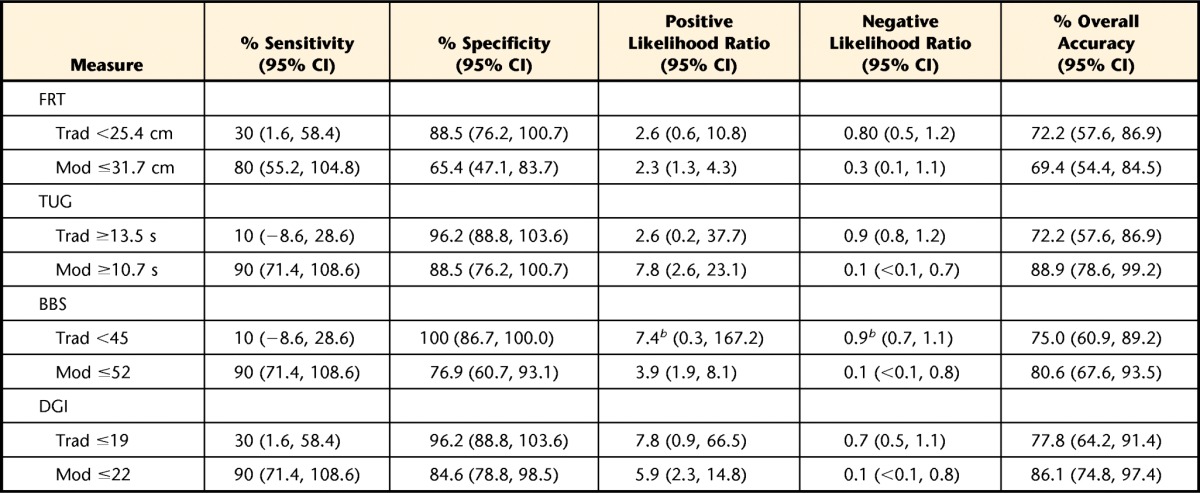
95% CI=95% confidence interval, Trad=traditional cutoff score, Mod=modified cutoff score, FRT=Functional Reach Test, TUG=Timed “Up & Go” Test, BBS=Berg Balance Scale, DGI=Dynamic Gait Index.
b Added 0.5 to each cell in the cross tabulation to avoid the occurrence of infinities when calculating positive and negative likelihood ratios.
Table 3.
Cross Tabulation of Fall Risk Assessment Results Using the Traditional Fall Risk Assessment Tool Cutoff Scorea
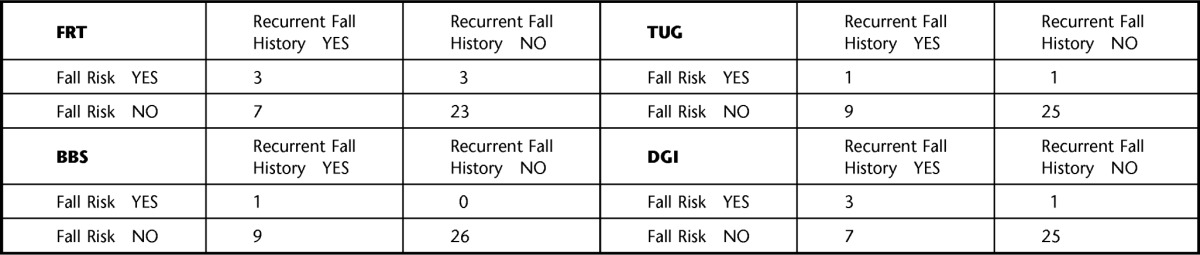
FRT=Functional Reach Test, TUG=Timed “Up & Go” Test, BBS=Berg Balance Scale, DGI=Dynamic Gait Index. Recurrent fall history is considered the reference standard.
Figure 1.
Flow diagram depicting the relationship between the functional reach test results and the fall history assessment results. DPN=diabetic peripheral neuropathy.
Table 4.
Cross Tabulation of Fall Risk Assessment Results Using the Modified Fall Risk Assessment Tool Cutoff Scoresa
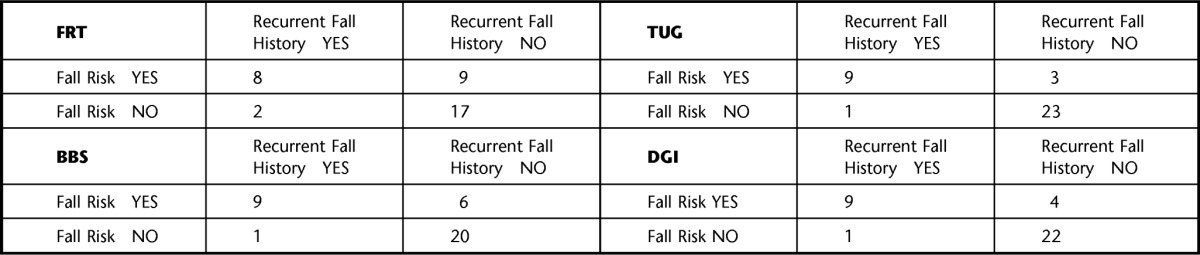
FRT=Functional Reach Test, TUG=Timed “Up & Go” Test, BBS=Berg Balance Scale, DGI=Dynamic Gait Index. Recurrent fall history is considered the reference standard.
A ROC curve for each fall risk assessment tool is depicted in Figure 2; the points on each curve that correspond to the sensitivity and 1 − specificity values associated with the traditional and modified cutoff scores are labeled. When comparing the indexes associated with the modified cutoff scores with the indexes associated with the traditional cutoff scores, sensitivity increased by between 50% and 80%, and specificity decreased by between 7.7% and 23.1%. Positive likelihood ratios increased for the TUG but decreased for the FRT, BBS, and DGI. Negative likelihood ratios decreased for all of the assessments, with the TUG demonstrating the greatest decrease. Overall accuracy increased the most for the TUG (16.7%), followed by the DGI (8.3%) and BBS (5.6%), whereas the overall accuracy of the FRT decreased by 2.8%.
Figure 2.
Fall risk assessment tool receiver operating characteristic (ROC) curves for traditional versus modified cutoff scores.
Discussion
To our knowledge, this is the first study to address functional mobility fall risk assessment tool utility for people who have DPN. Clinically, one of the more important measures of the utility of fall risk assessment tool is sensitivity. In particular, high sensitivity is preferred because it corresponds to more true positives and fewer false negatives,44 whereas the opposite is true for low sensitivity. It is important that everyone who has fall risk be correctly identified as such (true positives) and that no one who has fall risk is identified as not having fall risk (false negative) so that appropriate clinical fall prevention measures can be taken.
In this study, using traditional cutoff scores resulted in the accurate identification of only 1 (10%) to 3 (30%) out of the 10 recurrent fallers. If these tools and traditional cutoff scores were used clinically as the sole guidance for fall prevention intervention, up to 9 of the 10 recurrent fallers would not receive much-needed fall risk–related intervention. When traditional cutoff scores have been used previously, similar low sensitivities of between 26% and 42% have been found for the BBS14 and the TUG12,13 in older adults. Dibble and Lange13 found only moderate sensitivity for the FRT (54%) and DGI (57%) in people with Parkinson disease, and Shumway-Cook et al15 found 59% sensitivity for the DGI in community-dwelling older adults. Some authors have postulated that these lower sensitivities may be due to differences in populations tested or test procedures,12 specific characteristics of the samples tested,13 and because other factors, not just balance impairment, contribute to increased fall risk.14 Clearly, the participants in this study differed from those in other studies based on diagnosis alone. The majority of these participants had relatively mild DPN, which could influence sensitivity, especially if the tools' traditional cutoff scores were established for populations with relatively lower levels of function. Lastly, these fall risk assessment tools assess only functional mobility and, therefore, do not fully assess the array of possible reasons for increased fall risk; it is well known that falling is a complex and multifaceted issue.
The modified cutoff scores that improved the fall risk assessment tool accuracy in this study are similar to those reported in the literature. The FRT cutoff score of 31.7 cm is similar to those suggested by Dibble and Lange (31.75 cm)13 and Behrman and colleagues (30.1 cm).33 The TUG cutoff score of 10.7 seconds falls between the traditional cutoff score (13.5 seconds) and that suggested by Dibble and Lange (7.95 seconds).13 The BBS cutoff score of 52 is comparable to those suggested by Dibble and Lange (54),13 Alzayer et al (52),16 and Belgen et al (52).17 The DGI cutoff score of 22 is the same as that suggested by Dibble and Lange.13 Even though all of the aforementioned studies were conducted with participants diagnosed with something other than DPN (ie, stroke and Parkinson disease), it should be noted that most of the participants had relatively high levels of function (unlimited community ambulation,17 ranging from no balance impairment to some impairment but physically independent13). This relatively high level of function also was characteristic of the participants in our study in that all were able to ambulate in the community without assistance of another person and only 4 used an assistive device (2 used single-tipped canes, 2 used unilateral ankle-foot orthoses). If the participants in this study had a wider range of function and severity of DPN, outcomes likely would have been different. Regardless, these tools did not accurately identify between 70% and 90% of the recurrent fallers as having fall risk when using traditional cutoff scores, and this accuracy was improved substantially with the use of modified cutoff scores.
The primary purpose of this study was to identify which of 4 commonly used fall risk assessment tools is best able to accurately discriminate between recurrent fallers and nonfallers who have DPN. When using the modified cutoff scores, the TUG shares the highest sensitivity with the BBS and DGI, has the lowest negative likelihood ratio, and has the highest specificity, positive likelihood ratio, and overall accuracy. Descriptively, our hypothesis that the DGI would best discriminate between recurrent fallers and nonfallers was true using the traditional cutoff scores; however, when the modified cutoff scores were used, the TUG demonstrated greater diagnostic accuracy compared with the DGI, but only by a small margin. Although the hypothesis was based on the potential difficulty people with DPN might experience on certain DGI items, the difference in type of scoring between these 2 tests may have influenced the results. Each item of the DGI is scored on an ordinal scale, whereas scoring of the TUG is continuous (ratio scale); ratio scale scoring may be more sensitive to performance differences. Statistically, however, the relatively wide 95% confidence intervals surrounding the calculated indexes preclude the designation of one tool as significantly better than the others. Although a single fall risk assessment tool, such as those used in this study, can provide a therapist with useful information related to patient fall risk, it should not be assumed that a single tool, designed to assess only balance and gait dysfunction or functional mobility, adequately assesses fall risk; these tools should be used as part of a more comprehensive and multifactorial fall risk evaluation.
Limitations of this study include the small sample size and the retrospective falls assessment (fall history). The sample of participants in this study was small and relatively homogenous; therefore, external validity is limited. Although retrospective fall assessment studies are not uncommon, a prospective assessment would allow for validation of the predictive ability of these fall risk assessment tools. Lastly, the categorical distinction between people who fell only once and recurrent fallers may be perceived as a limitation; however, using people with a history of multiple falls to assess tool validity is supported in the literature,13,15,24,46 perhaps because it is the more conservative approach. Although a history of falls is the second-most common risk factor for falls,47 some authors suggest that a single isolated fall over a 12-month period may not justify fall risk designation, whereas multiple falls would. Ashburn and colleagues46 suggested that people who have fallen 2 or more times in the previous 12 months should be considered for fall prevention programs. Had all fallers (≥1 fall) been used to assess tool accuracy, rather than just recurrent fallers, confidence in the results would be lessened. It is clear from this study that the modified cutoff scores allowed for better diagnostic accuracy between those with a recurrent fall history (≥2 falls) and those who fell ≤1 time than traditional cutoff scores.
Additional research that uses prospective methods with a larger and more heterogeneous sample of people with DPN is needed to validate these findings and improve generalizability. If functional mobility fall risk assessment tools are used as part of a more comprehensive fall risk assessment, research that investigates which other factors, when combined with the functional mobility tool scores, lead to improved diagnostic accuracy would be useful. It also would be interesting to investigate the additive effect of DPN for fall risk in people older than 65 years. Lastly, if sensitive functional mobility fall risk assessment tools are identified for people with DPN, the effect of fall prevention programs on fall risk could be examined. Until further validation research is conducted, clinicians should be careful when interpreting the results of these functional mobility fall risk assessment tools in people with DPN, especially if used in isolation and not as part of a more comprehensive evaluation of fall risk.
Footnotes
All authors provided concept/idea/research design. Dr Jernigan, Dr Kluding, and Dr Mahnken provided writing. Dr Jernigan provided data collection, fund procurement, and clerical/secretarial support. Dr Jernigan and Dr Mahnken provided data analysis. Dr Jernigan and Dr Kluding provided project management. Dr Kluding provided study participants, facilities/equipment, and institutional liaisons. Dr Kluding, Dr Mahnken, and Dr Pohl provided consultation (including review of manuscript before submission). The authors thank Dr Mamatha Pasnoor and Dr Wen Liu, who provided guidance and consultation; Laura Herbelin and Jason Rucker, PT, who provided data collection assistance; and Mary Beth Fisher, ANP-BC, MSN, RN, CDE, and Kim Wernel, RD, LD, CDE, who provided participant recruitment assistance.
This study was approved by the Human Subjects Committee, University of Kansas Medical Center.
A poster presentation of some of the data was given at the Combined Sections Meeting of the American Physical Therapy Association; February 9–12, 2011; New Orleans, Louisiana. A platform presentation of some of the data also was given at the Kansas Physical Therapy Association Spring Conference; April 8–9, 2011; Wichita, Kansas.
This work was supported, in part, by University of Kansas Medical Center General Clinical Research Center grant M01 RR 02394, the National Center for Research Resources/National Institutes of Health, Frontiers: The Heartland Institute for Clinical and Translational Research (University of Kansas Medical Center's CTSA [UL1RR033179]), and Kansas Partners in Progress.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention; 2011 [Google Scholar]
- 2. Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43:817–824 [DOI] [PubMed] [Google Scholar]
- 3. Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43:957–973 [DOI] [PubMed] [Google Scholar]
- 4. Richardson JK, Ching C, Hurvitz EA. The relationship between electromyographically documented peripheral neuropathy and falls. J Am Geriatr Soc. 1992;40:1008–1012 [DOI] [PubMed] [Google Scholar]
- 5. Mueller MJ, Minor SD, Sahrmann SA, et al. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74:299–308; discussion 309–213. [DOI] [PubMed] [Google Scholar]
- 6. Boucher P, Teasdale N, Courtemanche R, et al. Postural stability in diabetic polyneuropathy. Diabetes Care. 1995;18:638–645 [DOI] [PubMed] [Google Scholar]
- 7. Courtemanche R, Teasdale N, Boucher P, et al. Gait problems in diabetic neuropathic patients. Arch Phys Med Rehabil. 1996;77:849–855 [DOI] [PubMed] [Google Scholar]
- 8. Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18:141–158 [DOI] [PubMed] [Google Scholar]
- 9. Powell MW, Carnegie DH, Burke TJ. Reversal of diabetic peripheral neuropathy with phototherapy (MIRE) decreases falls and the fear of falling and improves activities of daily living in seniors. Age Ageing. 2006;35:11–16 [DOI] [PubMed] [Google Scholar]
- 10. Thurman DJ, Stevens JA, Rao JK. Practice parameter: assessing patients in a neurology practice for risk of falls (an evidence-based review)—report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70:473–479 [DOI] [PubMed] [Google Scholar]
- 11. Ites KI, Anderson EJ, Cahill ML, et al. Balance interventions for diabetic peripheral neuropathy: a systematic review. J Geriatr Phys Ther. 2011;34:109–116 [DOI] [PubMed] [Google Scholar]
- 12. Thrane G, Joakimsen RM, Thornquist E. The association between Timed Up and Go Test and history of falls: the Tromso study. BMC Geriatr. 2007;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dibble LE, Lange M. Predicting falls in individuals with Parkinson disease: a reconsideration of clinical balance measures. J Neurol Phys Ther. 2006;30:60–67 [DOI] [PubMed] [Google Scholar]
- 14. Muir SW, Berg K, Chesworth B, Speechley M. Use of the Berg Balance Scale for predicting multiple falls in community-dwelling elderly people: a prospective study. Phys Ther. 2008;88:449–459; discussion 460–441. [DOI] [PubMed] [Google Scholar]
- 15. Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997;77:812–819 [DOI] [PubMed] [Google Scholar]
- 16. Alzayer L, Beninato M, Portney LG. The accuracy of individual Berg Balance Scale items compared with the total Berg score for classifying people with chronic stroke according to fall history. J Neurol Phys Ther. 2009;33:136–143 [DOI] [PubMed] [Google Scholar]
- 17. Belgen B, Beninato M, Sullivan PE, Narielwalla K. The association of balance capacity and falls self-efficacy with history of falling in community-dwelling people with chronic stroke. Arch Phys Med Rehabil. 2006;87:554–561 [DOI] [PubMed] [Google Scholar]
- 18. Bogle Thorbahn LD, Newton RA. Use of the Berg Balance Test to predict falls in elderly persons. Phys Ther. 1996;76:576–583; discussion 584–575 [DOI] [PubMed] [Google Scholar]
- 19. Berg K, Wood-Dauphinée S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27:27–36 [PubMed] [Google Scholar]
- 20. Duncan PW, Studenski S, Chandler J, Prescott B. Functional reach: predictive validity in a sample of elderly male veterans. J Gerontol. 1992;47:M93–M98 [DOI] [PubMed] [Google Scholar]
- 21. Weiner DK, Duncan PW, Chandler J, Studenski SA. Functional reach: a marker of physical frailty. J Am Geriatr Soc. 1992;40:203–207 [DOI] [PubMed] [Google Scholar]
- 22. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148 [DOI] [PubMed] [Google Scholar]
- 23. O'Sullivan S, Schmitz T. Physical Rehabilitation. 5th ed. Philadelphia, PA: FA Davis Co; 2005 [Google Scholar]
- 24. Dibble LE, Christensen J, Ballard DJ, Foreman KB. Diagnosis of fall risk in Parkinson disease: an analysis of individual and collective clinical balance test interpretation. Phys Ther. 2008;88:323–332 [DOI] [PubMed] [Google Scholar]
- 25. Marchetti GF, Whitney SL, Blatt PJ, et al. Temporal and spatial characteristics of gait during performance of the Dynamic Gait Index in people with and people without balance or vestibular disorders. Phys Ther. 2008;88:640–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88:559–566 [DOI] [PubMed] [Google Scholar]
- 27. Feldman EL, Stevens MJ. Clinical testing in diabetic peripheral neuropathy. Can J Neurol Sci. 1994;21:S3–S7 [DOI] [PubMed] [Google Scholar]
- 28. Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108:477–481 [DOI] [PubMed] [Google Scholar]
- 29. Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45:M192–M197 [DOI] [PubMed] [Google Scholar]
- 30. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903 [PubMed] [Google Scholar]
- 31. Berg KO, Wood-Dauphineé SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(suppl 2):S7–S11 [PubMed] [Google Scholar]
- 32. Shumway-Cook A, Woollacott MH. Motor Control: Theory and Practical Applications. Baltimore, MD: Williams & Wilkins; 1995 [Google Scholar]
- 33. Behrman AL, Light KE, Flynn SM, Thigpen MT. Is the Functional Reach Test useful for identifying falls risk among individuals with Parkinson's disease? Arch Phys Med Rehabil. 2002;83:538–542 [DOI] [PubMed] [Google Scholar]
- 34. Marchetti GF, Whitney SL. Construction and validation of the 4-item Dynamic Gait Index. Phys Ther. 2006;86:1651–1660 [DOI] [PubMed] [Google Scholar]
- 35. Faulkner KA, Redfern MS, Cauley JA, et al. Multitasking: association between poorer performance and a history of recurrent falls. J Am Geriatr Soc. 2007;55:570–576 [DOI] [PubMed] [Google Scholar]
- 36. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707 [DOI] [PubMed] [Google Scholar]
- 37. Tilling LM, Darawil K, Britton M. Falls as a complication of diabetes mellitus in older people. J Diabetes Complications. 2006;20:158–162 [DOI] [PubMed] [Google Scholar]
- 38. Visser M, Marinus J, Bloem BR, et al. Clinical tests for the evaluation of postural instability in patients with parkinson's disease. Arch Phys Med Rehabil. 2003;84:1669–1674 [DOI] [PubMed] [Google Scholar]
- 39. Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25:1749–1754 [DOI] [PubMed] [Google Scholar]
- 41. Wallace C, Reiber GE, LeMaster J, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25:1983–1986 [DOI] [PubMed] [Google Scholar]
- 42. Arnold CM, Faulkner RA. The history of falls and the association of the Timed Up and Go Test to falls and near-falls in older adults with hip osteoarthritis. BMC Geriatr. 2007;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tinetti ME, Speechley M. Prevention of falls among the elderly. N Engl J Med. 1989;320:1055–1059 [DOI] [PubMed] [Google Scholar]
- 44. Domholdt E. Rehabilitation Research: Principles and Applications. 3rd ed. Philadephia, PA: Elsevier Saunders; 2005 [Google Scholar]
- 45. Agresti A. An Introduction to Categorical Data Analysis. 2nd ed. Hoboken NJ: John Wiley & Sons Inc; 2007:9–15, 18–20, 73 [Google Scholar]
- 46. Ashburn A, Stack E, Pickering RM, Ward CD. Predicting fallers in a community-based sample of people with Parkinson's disease. Gerontology. 2001;47:277–281 [DOI] [PubMed] [Google Scholar]
- 47. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc. 2001;49:664–672 [PubMed] [Google Scholar]