Abstract
Purpose
To examine the association of baseline body mass index (BMI) with the risk of recurrence or death in postmenopausal women with early-stage breast cancer receiving adjuvant tamoxifen or letrozole in the Breast International Group (BIG) 1-98 trial at 8.7 years of median follow-up.
Patients and Methods
This report analyzes 4,760 patients with breast cancer randomly assigned to 5 years of monotherapy with letrozole or tamoxifen in the BIG 1-98 trial with available information on BMI at randomization. Multivariable Cox modeling assessed the association of BMI with disease-free survival, overall survival (OS), breast cancer–free interval, and distant recurrence-free interval and tested for treatment-by-BMI interaction. Median follow-up was 8.7 years.
Results
Seventeen percent of patients have died. Obese patients (BMI ≥ 30 kg/m2) had slightly poorer OS (hazard ratio [HR] = 1.19; 95% CI, 0.99 to 1.44) than patients with normal BMI (< 25 kg/m2), whereas no trend in OS was observed in overweight (BMI 25 to < 30 kg/m2) versus normal-weight patients (HR = 1.02; 95% CI, 0.86 to 1.20). Treatment-by-BMI interactions were not statistically significant. The HRs for OS comparing obese versus normal BMI were HR = 1.22 (95% CI, 0.93 to 1.60) and HR = 1.18 (95% CI, 0.91 to 1.52) in the letrozole and tamoxifen groups, respectively.
Conclusion
There was no evidence that the benefit of letrozole over tamoxifen differed according to patients' BMI.
INTRODUCTION
Obesity is a well-documented adverse prognostic factor in early-stage breast cancer.1–3 The causal mechanism by which obesity influences prognosis remains to be elucidated, but it has been suggested that it relates to the biology of the disease or to treatment being less effective in obese patients.1,4 The mechanism whereby obesity might affect adjuvant cytotoxic therapy could differ from any such effect on adjuvant endocrine therapy. Thus some studies have shown that obese patients received reduced doses of adjuvant chemotherapy and that this was associated with a worse outcome.5,6 However, in a retrospective multivariate analysis of 2,887 patients with node-positive breast cancer enrolled onto the Breast International Group (BIG) 02-98 trial, obesity remained an independent prognostic factor for disease-free survival as well as overall survival despite similar relative dose-intensities of chemotherapy with docetaxel and doxorubicin, or cyclophosphamide, methotrexate, and fluorouracil among obese and nonobese patients.7 In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 trial involving 3,385 women with lymph node-negative, estrogen receptor–positive breast cancer, the benefit of tamoxifen did not vary across body mass index (BMI) groups.8 Aromatase inhibitors depend on a reduction in peripheral formation of estrogen by aromatization, mainly in adipose tissue, in postmenopausal women. The investigators of the Arimidex, Tamoxifen Alone or in Combination (ATAC) Trial reported that although tamoxifen was equally effective across all BMI categories, anastrozole was significantly less effective in postmenopausal women with a BMI exceeding 30 kg/m2. They suggested that estrogen suppression with anastrozole may not be complete in obese women.9
The purpose of the present report is to examine the same questions in the context of the BIG 1-98 trial10 comparing letrozole, an aromatase inhibitor that suppresses estrogen more effectively than anastrozole,11,12 versus tamoxifen in postmenopausal women with early breast cancer.
PATIENTS AND METHODS
Patients
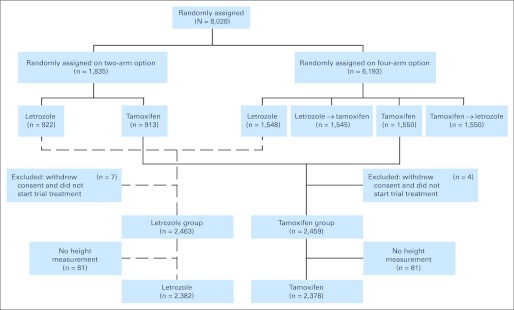
The BIG 1-98 study is a randomized, phase III, double-blind trial comparing 5 years of monotherapy with tamoxifen or with letrozole, or with sequences of 2 years of one followed by 3 years of the other for postmenopausal women with endocrine-responsive early invasive breast cancer.10,13–15 From 1998 to 2003, BIG 1-98 enrolled 8,028 women into one of two randomization options (Fig 1).The letrozole (Femara; Novartis, Basel, Switzerland) dose was 2.5 mg daily, and the tamoxifen dose was 20 mg daily. The ethics committees and required health authorities of each participating institution approved the study protocol, and all patients gave written informed consent. Details of eligibility, design, and protocol requirements were published in the first report of the overall study results.13 A total of 4,922 patients were randomly assigned to letrozole or tamoxifen monotherapy for 5 years. The results of the monotherapy comparison were first published in 200714 at 4.3 years of median follow-up and were most recently updated at 8.7 years of median follow-up (range, 0 to 12.4 years).15 This report is based on the most recent update15 and includes 4,760 patients after excluding 162 who did not have a height measurement recorded to allow the calculation of baseline BMI (Fig 1).
Fig 1.
CONSORT diagram showing the analytic cohort of 4,760 patients enrolled in the BIG 1-98 clinical trial.
Height and weight were recorded at randomization, before the start of adjuvant endocrine treatment, and were used to calculate baseline BMI, which was classified according to defined groups: normal (< 25 kg/m2), overweight (25 to < 30 kg/m2), and obese (≥ 30 kg/m2). The primary trial end point was disease-free survival (DFS), defined as the time from randomization to the first of the following events: invasive recurrence in local, regional, or distant sites; a new invasive cancer in the contralateral breast; any second (nonbreast) primary cancer; or death without a prior cancer event. In the absence of an event, DFS is censored at the last follow-up visit. Secondary end points included breast cancer–free interval, defined as the time from randomization to the first breast cancer event, and distant recurrence–free interval, defined as the time from randomization to the first invasive recurrence in a distant site: each ignored second (nonbreast) primary cancers and were censored at death without a prior cancer event or at last follow-up visit. Overall survival (OS) was defined as the time from randomization to death resulting from any cause or was censored at date last known alive. After the initial trial results were released in 2005, patients assigned to tamoxifen monotherapy were so informed and offered the chance to cross-over to letrozole for the remainder of their adjuvant therapy, and 619 (25.2%) did so; the follow-up of these patients is censored at the date of selective cross-over.16
Statistical Analysis
The associations between the BMI groups and other patient and disease characteristics were evaluated using the χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables. The Kaplan-Meier method was used to estimate distributions of disease outcomes according to BMI groups.
Multivariable Cox proportional hazards models17 were used to test heterogeneity of disease outcomes according to BMI categories (2-df likelihood ratio test) and to estimate hazard ratios (HR) and 95% CIs comparing obese or overweight versus normal weight. The proportional hazards assumption was assessed using Schoenfeld residuals. Because the hypothesis focuses on the obese versus normal BMI groups, the pairwise comparisons are reported regardless of the result of the global test. The multivariable models adjusted for age at randomization (continuous variable), geographic region, nodal status, tumor grade, tumor size, radiotherapy, mastectomy, centrally determined estrogen receptor/progesterone receptor status, centrally determined HER2 status, prior hormone replacement therapy, history of diabetes, smoking status, and history of hypertension and were stratified by randomization option (two- or four-arm option) and prior chemotherapy use (yes or no).
Treatment-by-BMI (pairwise) interaction was tested in Cox models using 1-df Wald tests. With our BMI and outcome event distributions and assuming exponential distribution, there was at least 71% power to detect an interaction ratio of 1.67 using large sample partial likelihood tests for treatment-by-BMI groups interaction in a Cox model (eg, treatment HR for BMI ≥ 30 kg/m2 relative to treatment HR for BMI < 25 kg/m2), which is similar magnitude to the interaction ratio for distant recurrence in ATAC.9 Subpopulation treatment effects pattern plots were used to summarize the 8-year OS according to treatment group and the HRs comparing letrozole versus tamoxifen across the continuum of BMI values with tests for treatment-by-BMI interaction.18,19
Cumulative incidence functions for breast cancer recurrence (accounting for competing risks of second [nonbreast] malignancies and deaths without a prior cancer event) and for distant recurrence (accounting for competing risks of death without prior cancer event) were estimated and compared among BMI groups using 2-df Gray's test20 with adjustment of stratification factors of randomization option (two- or four-arm option) and prior chemotherapy use (yes or no).
All reported P values are two-sided. The analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC) and R 2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patients
The analytic cohort of 4,760 patients were randomly assigned to tamoxifen (n = 2,378) or letrozole (n = 2,382) for 5 years as monotherapy. Of these, 1,097 patients (23%) were obese (BMI ≥ 30 kg/m2) and 1,734 (36%) were overweight (BMI 25 to < 30 kg/m2) at randomization. The overall median BMI at randomization was 26.1 kg/m2 (mean = 26.8 kg/m2; standard deviation = 5.1 kg/m2). Table 1 shows the characteristics of the study population according to BMI categories. The median time from diagnosis to randomization was 1.3 months, which did not differ between treatment groups. Obese patients had larger tumors (P < .001) and more positive lymph nodes (P < .001), and tumors were more often progesterone receptor positive (P < .001). Obese patients were older (age ≥ 62 years, P < .001) and had more comorbidities, such as diabetes, and history of hypertension and any cardiac morbidity. No significant differences were detected in tumor grade, estrogen receptor status, peritumoral vascular invasion, HER2 status, or Ki67 status. Prior treatment varied by BMI, with obese patients being more likely to have had a mastectomy and prior chemotherapy.
Table 1.
Characteristics of 4,760 of 4,922 Patients Randomly Assigned in BIG 1-98 Trial Monotherapy Population Who Had Weight and Height Data Reported at Randomization, According to BMI at Randomization
| Characteristic | BMI (kg/m2) at Randomization |
P* | |||||
|---|---|---|---|---|---|---|---|
| < 25 (normal)(n = 1,929) |
25 to < 30 (overweight)(n = 1,734) |
≥ 30 (obese)(n = 1,097) |
|||||
| No. | % | No. | % | No. | % | ||
| Randomized treatment assignment | |||||||
| Letrozole | 958 | 50 | 880 | 51 | 544 | 50 | .760* |
| Tamoxifen | 971 | 50 | 854 | 49 | 553 | 50 | |
| Age, years | |||||||
| Median | 60 | 62 | 62 | < .001† | |||
| Range | 38-90 | 39-88 | 42-85 | ||||
| < 56 | 655 | 34 | 414 | 24 | 258 | 24 | |
| 57-61 | 435 | 23 | 395 | 23 | 253 | 23 | |
| 62-67 | 413 | 21 | 470 | 27 | 291 | 27 | |
| ≥ 68 | 426 | 22 | 455 | 26 | 295 | 27 | |
| Diagnosis to randomization, months | |||||||
| Median | 1.2 | 1.2 | 1.4 | .179† | |||
| Quartile 1 to quartile 3 | 0.9-3.0 | 0.9-3.0 | 0.9-3.4 | ||||
| Geographic regions‡ | |||||||
| AUS/NZ | 161 | 8 | 161 | 9 | 158 | 14 | < .001* |
| South America | 59 | 3 | 63 | 4 | 43 | 4 | |
| Eastern Europe | 229 | 12 | 327 | 19 | 265 | 24 | |
| Western Europe/other | 1,480 | 77 | 1,183 | 68 | 631 | 58 | |
| Prior chemotherapy | |||||||
| Yes | 465 | 24 | 433 | 25 | 317 | 29 | .010* |
| No | 1,464 | 76 | 1,301 | 75 | 780 | 71 | |
| Local therapy | |||||||
| BCS+RT | 1,016 | 53 | 856 | 49 | 505 | 46 | .013* |
| BCS without RT | 61 | 3 | 65 | 4 | 49 | 4 | |
| Mastectomy+RT | 334 | 17 | 320 | 18 | 231 | 21 | |
| Mastectomy without RT | 513 | 27 | 492 | 28 | 308 | 28 | |
| Other | 5 | 0 | 1 | 0 | 4 | 0 | |
| Nodal status | |||||||
| Negative/Nx | 1,161 | 60 | 1011 | 58 | 578 | 53 | < .001* |
| Positive | 767 | 40 | 722 | 42 | 516 | 47 | |
| Unknown | 1 | 1 | 3 | ||||
| Tumor grade | |||||||
| 1 | 530 | 31 | 444 | 29 | 261 | 28 | .701* |
| 2 | 913 | 53 | 813 | 53 | 496 | 54 | |
| 3 | 290 | 17 | 274 | 18 | 165 | 18 | |
| Unknown | 196 | 203 | 175 | ||||
| Tumor size, cm | |||||||
| Median | 1.7 | 1.9 | 2.0 | < .001† | |||
| Quartile 1 to quartile 3 | 1.2-2.4 | 1.3-2.5 | 1.5-2.8 | ||||
| ≤ 2 | 1,299 | 68 | 1,037 | 60 | 584 | 54 | |
| 2-5 | 554 | 29 | 606 | 35 | 444 | 41 | |
| ≥ 5 | 63 | 3 | 76 | 4 | 54 | 5 | |
| Unknown | 13 | 15 | 15 | ||||
| Peritumoral vascular invasion | |||||||
| No | 1,439 | 82 | 1,264 | 81 | 775 | 79 | .322* |
| Yes | 321 | 18 | 301 | 19 | 201 | 21 | |
| Unknown | 169 | 169 | 121 | ||||
| Centrally assessed ER status | |||||||
| Absent | 21 | 1 | 28 | 2 | 12 | 2 | .331* |
| Present | 1,390 | 99 | 1,251 | 98 | 772 | 98 | |
| Unknown | 518 | 455 | 313 | ||||
| Centrally assessed PgR status | |||||||
| Absent | 202 | 14 | 155 | 12 | 65 | 8 | < .001* |
| Present | 1,204 | 86 | 1,127 | 88 | 720 | 92 | |
| Unknown | 523 | 452 | 312 | ||||
| Negative | 1,323 | 92 | 1,218 | 94 | 734 | 93 | .155* |
| Positive | 113 | 8 | 78 | 6 | 59 | 7 | |
| Unknown | 493 | 438 | 304 | ||||
| Centrally assessed Ki67 LI | |||||||
| Median | 12 | 12 | 12 | .903† | |||
| Quartile 1 to quartile 3 | 6-18 | 7-19 | 7-18 | ||||
| < 14% | 1,259 | 68 | 1,137 | 68 | 743 | 69 | |
| ≥ 14% | 606 | 32 | 543 | 32 | 334 | 31 | |
| Unknown | 64 | 54 | 20 | ||||
| History of diabetes | |||||||
| Yes | 47 | 2 | 87 | 5 | 125 | 11 | < .001* |
| No | 1,882 | 98 | 1,645 | 95 | 971 | 89 | |
| Unknown | 0 | 2 | 1 | ||||
| Smoking history | |||||||
| Yes | 751 | 39 | 587 | 34 | 327 | 30 | < .001* |
| No | 1,178 | 61 | 1,147 | 66 | 770 | 70 | |
| HRT before randomization | |||||||
| No | 1,074 | 56 | 1,156 | 67 | 826 | 75 | < .001* |
| Within the last 3 months | 437 | 23 | 269 | 16 | 118 | 11 | |
| > 3 months ago | 418 | 22 | 309 | 18 | 153 | 14 | |
| History of hypercholesterolemia | |||||||
| Yes | 146 | 8 | 149 | 9 | 101 | 9 | .256* |
| No | 1,783 | 92 | 1,585 | 91 | 996 | 91 | |
| History of hypertension | |||||||
| Yes | 396 | 21 | 576 | 33 | 531 | 48 | < .001* |
| No | 1,533 | 79 | 1,158 | 67 | 566 | 52 | |
| History of CVA/TIA | |||||||
| Yes | 29 | 2 | 36 | 2 | 18 | 2 | .399* |
| No | 1,900 | 98 | 1,698 | 98 | 1,079 | 98 | |
| History of any ischemia | |||||||
| Yes | 47 | 2 | 53 | 3 | 73 | 7 | < .001* |
| No | 1,882 | 98 | 1,681 | 97 | 1,024 | 93 | |
| History of any cardiac morbidity | |||||||
| Yes | 146 | 8 | 136 | 8 | 135 | 12 | < .001* |
| No | 1,783 | 92 | 1,598 | 92 | 962 | 88 | |
Abbreviations: BCS, breast-conserving surgery; BMI, body mass index; ER, estrogen receptor; LI, labeling index; Nx, nodes not assessed; PgR, progesterone receptor; RT, radiotherapy.
χ2 test.
Kruskal-Wallis test.
The geographic regions were defined as: AUS/NZ: Australia, New Zealand; South America: Brazil, Peru, Argentina, Chile; Eastern Europe: Czech Republic, Russia, Hungary, Poland, Slovenia, and Turkey; Western Europe/Other: the rest of countries in the BIG 1-98 trial (including Canada, South Africa).
Outcomes
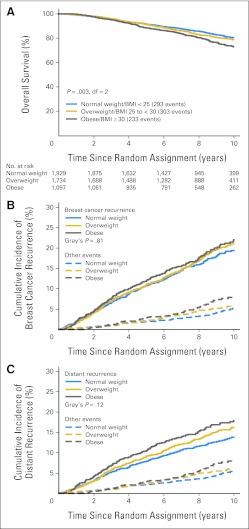
At 8.7 years median follow-up, DFS events were observed in 1,272 patients, and 829 died. The proportions of patients with DFS events were 24%, 27%, and 30% among normal, overweight, and obese patients, respectively, and the proportions who died were 15%, 17%, and 21%, respectively. Obese patients had more bone and visceral metastases as site of first DFS event and had more deaths without a prior cancer event (Table 2). Among all patients, there was evidence of heterogeneity in OS according to the three BMI categories (likelihood ratio test P = .003, df = 2; Fig 2A), and some indication of heterogeneity in distant recurrence–free interval according to BMI categories (P = .11, df = 2, data not shown). Analyses of the cumulative incidence of distant recurrence and breast cancer recurrence accounting for the competing risks of second (nonbreast) primaries and deaths without a prior cancer event illustrate that the observed results for OS were not entirely due to breast cancer (Fig 2B,2C).
Table 2.
Disease Outcomes at 8.7 Years of Median Follow-Up in BIG 1-98Trial Monotherapy Population, According to BMI at Randomization
| Disease Outcome | BMI (kg/m2) at Randomization |
|||||
|---|---|---|---|---|---|---|
| < 25, Normal |
25 to< 30, Overweight |
≥ 30, Obese |
||||
| No. | % | No. | % | No. | % | |
| Patients | 1,929 | 1,734 | 1,097 | |||
| Death | 293 | 15 | 303 | 17 | 233 | 21 |
| Any distant recurrence | 222 | 12 | 236 | 14 | 167 | 15 |
| Any breast cancer recurrence | 311 | 16 | 303 | 17 | 201 | 18 |
| DFS event | 471 | 24 | 471 | 27 | 330 | 30 |
| Site of first of DFS event | ||||||
| Local | 38 | 8 | 34 | 7 | 15 | 5 |
| Contralateral breast | 46 | 10 | 35 | 7 | 12 | 4 |
| Regional | 22 | 5 | 11 | 2 | 9 | 3 |
| Distant: soft tissue/nodes | 12 | 3 | 19 | 4 | 10 | 3 |
| Distant: bone | 79 | 17 | 84 | 18 | 71 | 22 |
| Distant: viscera | 100 | 21 | 103 | 22 | 75 | 23 |
| Other breast cancer | 9 | 2 | 7 | 1 | 6 | 2 |
| Second (nonbreast) primary | 93 | 20 | 97 | 21 | 63 | 19 |
| Death without prior cancer event | 72 | 15 | 81 | 17 | 69 | 21 |
Abbreviations: BMI, body mass index; DFS, disease-free survival.
Fig 2.
(A) Overall survival according the three body mass index (BMI) groups is shown in the Kaplan-Meier curve; P value tests for heterogeneity among the three groups with 2 df. (B) Cumulative incidence of breast cancer recurrence and (C) distant recurrence comparing normal, overweight, and obese BMI groups. Comparisons of BMI groups using Gray's test P value stratified by chemotherapy use and random assignment option.
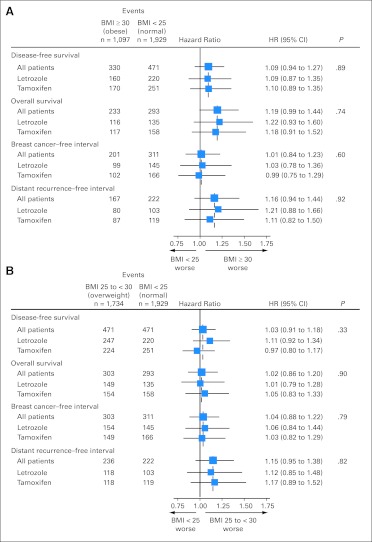
Obese patients tended to have a greater hazard of death (HR = 1.19; 95% CI, 0.99 to 1.44) compared with patients with normal weight (Fig 3A), although no difference in OS was observed among overweight (BMI 25 to < 30 kg/m2) versus normal weight patients (HR = 1.02; 95% CI, 0.86 to 1.20; Fig 3B). These effects were not observed for breast cancer–free interval, distant disease–free interval, or DFS (Figs 3A and 3B).
Fig 3.
Hazard ratios (HRs), 95% CIs, and P values from multivariate Cox models comparing (A) obese, body mass index (BMI) ≥ 30 kg/m2 and (B) overweight, BMI 25 to less than 30 kg/m2, with normal-weight patients, BMI less than 25 kg/m2, adjusted for patient/disease characteristics and stratified by chemotherapy use and randomization option. P values are for 1-df tests of treatment-by-BMI interaction from multivariate Cox model that adjusted for prognostic factors and stratified by chemotherapy use and random assignment option. The size of the boxes is inversely proportional to the SE of the HR.
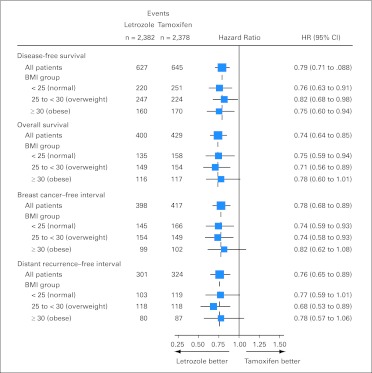
There was no indication that the relative hazard of obesity versus normal weight on OS was different between treatment arms (P = .74 for interaction; Fig 3A). The estimated HR for OS was 1.22 (95% CI, 0.93 to 1.60) comparing obese versus normal weight patients in the letrozole arm and 1.18 (95% CI, 0.91 to 1.52) in the tamoxifen arm. For distant recurrence–free interval, similar estimates of HR comparing obese versus normal weight were observed for letrozole (HR = 1.21; 95% CI, 0.88 to 1.66) and for tamoxifen (HR = 1.11; 95% CI, 0.82 to 1.50; P = .92 for interaction; Fig 3A). Figure 4 summarizes treatment comparisons within BMI groups, showing that letrozole is superior to tamoxifen in all BMI groups for all end points.
Fig 4.
Hazard ratios (HRs) and 95% CIs from multivariate Cox models comparing letrozole versus tamoxifen according to body mass index (BMI) groups, adjusted for patient/disease characteristics and stratified by chemotherapy use and random assignment option. The size of the boxes is inversely proportional to the SE of the hazard ratio.
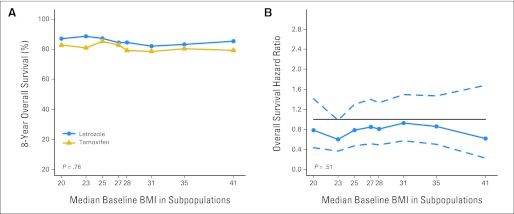
The subpopulation treatment effects pattern plots analysis of 8-year OS according to treatment, looking at BMI as a continuum rather than categorically (Fig 5A), also showed no evidence of treatment-by-BMI interaction (P = .76). The similar benefits of letrozole over tamoxifen across the continuum of BMI levels is illustrated by relatively constant HRs over all BMI levels (Fig 5B).
Fig 5.
Subpopulation treatment effect pattern plots showing (A) 8-year overall survival according to randomly assigned treatment group across a continuum of baseline body mass index (BMI) subpopulations and (B) overall survival hazard ratio (solid line) with 95% confidence bands (dashed lines) comparing letrozole versus tamoxifen across a continuum of baseline BMI subpopulations.
DISCUSSION
On the basis of the patients enrolled in the BIG 1-98 trial, this report confirms the results of numerous other studies showing that obese (BMI ≥ 30 kg/m2) patients with breast cancer are diagnosed with a generally poorer prognostic profile. However, after taking prognostic factors into account, obese patients in our study still have a trend to poorer OS than normal-weight patients (BMI < 25 kg/m2).1,2 The results of this study with an HR of 1.19 (95% CI, 0.99 to 1.44) for OS are consistent with those of the Danish study reporting an HR of 1.09 (95% CI, 1.00 to 1.18) for OS for the first 10 years of follow-up.2 The present results also show that poorer OS is mediated by more distant recurrences as well as deaths without a prior cancer event.
The investigators of the ATAC Trial sought to determine “if anastrozole is relatively more effective than tamoxifen in preventing recurrences in postmenopausal women with early-stage breast cancer and a high BMI” among 4,939 estrogen receptor–positive women randomly assigned in their trial.9 Contrary to this hypothesis, they found that although tamoxifen was equally effective across all BMI categories, anastrozole was significantly less effective in postmenopausal women with a BMI exceeding 30 kg/m2. They suggested that estrogen suppression with anastrozole may not be complete in obese women.9 This possibility is supported by data from the Austrian Breast and Colorectal Cancer Study Group (ABCSG) 12 Trial reporting that overweight (BMI > 25 kg/m2) premenopausal patients treated with goserelin plus anastrozole had a poorer DFS and OS than normal-weight patients.21 Several studies have reported that estrogen suppression is more complete with letrozole than with anastrozole.11,12 One possible inference from the contrast between the findings of ATAC and the present study may therefore be that letrozole is sufficiently active to overcome any incomplete suppression seen with anastrozole. This explanation is reassuring because it indicates that a dose of 2.5 mg of letrozole is sufficient to inhibit the larger amount of estrogens that obese women produce from peripheral aromatization of androstenedione.21a
Several models have been suggested to explain the biologic mechanisms underlying the poorer prognosis in obese patients with breast cancer. These involve complex relationships between estrogen synthesis, insulin resistance, and altered adipokine and cytokine production.22,23 Obese patients have higher levels of estrone, estradiol, and free circulating estradiol and reduced levels of sex hormone binding globulin.24 In addition, cytokines secreted by the adipocytes can upregulate the aromatase enzyme to further increase the estrogen production, which may stimulate tumor cell growth.25 Obesity is a component of the metabolic syndrome that also includes hyperglycemia, hyperinsulinemia, and insulin resistance. Insulin has mitogenic, antiapoptotic, and proangiogenic properties, and breast cancer cells have been shown to express the insulin receptor.26 Several studies have demonstrated that hyperinsulinemia is an independent adverse prognostic factor in breast cancer27–30 and that adiponectin is also related to breast cancer prognosis.31 Finally, obesity causes subclinical inflammation, increasing the levels of proinflammatory mediators, which may parallel increasing levels of aromatase.32
In summary, this report with a median of 8.7 years of follow-up is in broad agreement that obesity is an independent adverse prognostic factor for death after breast cancer, although the statistical significance is marginal after allowance for multiple other patient characteristics. Letrozole was more effective than tamoxifen in reducing disease-free survival events, overall deaths, breast cancer recurrences, and distant metastases across all BMI categories.
Appendix
BIG 1-98 Collaborative Group Participants
Steering Committee: B. Thürlimann (Chair), S. Aebi, L. Blacher, H. Bonnefoi, A. S. Coates, T. Cufer, B. Ejlertsen, J. F. Forbes, R. D. Gelber, A. Giobbie-Hurder, A. Goldhirsch, A. Hiltbrunner, S. B. Holmberg, R. Maibach, A. Martoni, L. Mauriac, G. MacGrogan, H. T. Mouridsen, R. Paridaens, D. Phuong, K. N. Price, M. Rabaglio, B.B. Rasmussen, M.M. Regan, A. Santoro, I. E. Smith, A. Wardley, G. Viale. Novartis: H. A. Chaudri-Ross, S. Segal.
IBCSG Foundation Council (members from 1998 to 2010): S. Aebi, A. S. Coates, M. Colleoni, J. P. Collins, H. Cortés Funes, R. D. Gelber, A. Goldhirsch, M. Green, A. Hiltbrunner, S. B. Holmberg, P. Karlsson, I Kössler, I. Láng, J. Lindtner, F Paganetti, M. de Stoppani, C.-M. Rudenstam, H.-J. Senn, R. Stahel, B. Thürlimann, A. Veronesi.
Coordinating Center (Berne, Switzerland): M. Castiglione (Chief Executive Officer 1998-2007), A. Hiltbrunner (Director), M. Rabaglio, G. Egli, H. Hawle, B. Cliffe, S. Ribeli-Hofmann, F. Munarini, R. Kammler, R. Studer, B. Ruepp, R. Maibach, N. Munarini.
Statistical Center (Dana-Farber Cancer Institute, Boston, MA, USA): R. D. Gelber (Director), M.M. Regan (Group Statistician), K.N. Price (Director of Scientific Administration), A. Giobbie-Hurder (Trial Statistician), A. Keshaviah, H. Litman, B.F. Cole, Z. Sun, P.K. Gray, H. Huang, L. J. Somos, B. Timmers, L. Nickerson.
Data Management Center (Frontier Science & Technology Research Foundation, Amherst, NY, USA): L. Blacher (Director of Data Management), T. Heckman Scolese (Coordinating Data Manager), M. Belisle, M. Caporale, J. Celano, L. Dalfonso, L. Dooley, S. Fischer, K. Galloway, J. Gould, R. Hinkle, M. Holody, G. Jones, R. Krall, S. Lippert, J. Meshulam, L. Mundy, A. Pavlov-Shapiro, K. Scott, M. Scott, S. Shepard, J. Swick, L. Uhteg, D. Weinbaum, C. Westby, T. Zielinski.
Central Pathology Review Office (University of Glasgow, Glasgow, United Kingdom): B. A. Gusterson, E. Mallon; (European Institute of Oncology, Division of Pathology, Milano, Italy): G. Viale, P. Dell'Orto, M. Mastropasqua, B. Del Curto.
Study Support (Novartis Corp. Basel, Switzerland): E. Waldie, I. van Hoomissen, M. De Smet, U. Trostmann, W. Schmidt, A. Bolton, W. Hackl.
Breast International Group (BIG)
International Breast Cancer Study Group (IBCSG).
Australian New Zealand Breast Cancer Trials Group (ANZ BCTG): R. D. Snyder, J. Chirgwin, J. F. Forbes, A. S. Coates, F. Boyle, D. Lindsay, D. Preece, J. Cowell, D. Talbot, A. Whipp.
Australia: The Cancer Council Victoria, Melbourne, VIC: F. Abell, R. Basser, R. Bell, B. Brady, D. Blakey, P. Briggs, I. Burns, P. Campbell, M. Chao, J. Chirgwin, B. Chua, K. Clarke, J. Collins, R. De Boer, J. C. Din, R. Doig, A. Dowling, R. Drummond, N. Efe, S. T. Fan, M. Francis, P. Francis, V. Ganju, P. Gibbs, G. Goss, M. Green, P. Gregory, J. Griffiths, I. Haines, M. Henderson, R. Holmes, P. James, J. Kiffler, M. Lehman, M. Leyden, L. Lim, G. Lindeman, R. Lynch, B. Mann, J. McKendrick, S. McLachlan, R. McLennan, G. Mitchell, S. Mitra, C. Murphy, I. Parker, K. Phillips, I. Porter, G. Richardson, J. Scarlet, S. Sewak, J. Shapiro, R. Snyder, R. Stanley, C. Steer, D. Stoney, A. Strickland, G. Toner, C. Underhill, K. White, M. White, A. Wirth, S. Wong; W P Holman Clinic, Launceston General Hospital, Launceston, Tasmania: D. Byram, I. Byard; Liverpool Hospital, Sydney, NSW: S. Della-Fiorentina, A. Goldrick, E. Hovey, E. Moylan, E. Segelov; Mount Hospital, Perth, WA: A. Chan, M. Buck, D. Hastrich, D. Ingram, G. Van Hazel, P. Willsher; Nepean Cancer Care Centre, Sydney, NSW: N. Wilcken, C. Crombie; Calvary Mater Newcastle, Newcastle, NSW: J. F. Forbes, F. Abell, S. Ackland, A. Bonaventura, S. Cox, J. Denham, R. Gourlay, D. Jackson, R. Sillar, J. Stewart; Prince of Wales Hospital, Sydney, NSW: C. Lewis, B. Brigham, D. Goldstein, M. Friedlander; Princess Alexandra Hospital, Woollongabba, QLD: E. Walpole, D. Thompson; Royal Adelaide Hospital, Adelaide, SA: P. G. Gill, M. Bochner, J. Coventry, J. Kollias, P. Malycha, I. Olver; Royal Brisbane and Women's Hospital, Brisbane, QLD: M. Colosimo, R. Cheuk, L. Kenny, N. McCarthy, D. Wyld; Royal Hobart Hospital, Hobart, Tasmania: R. Young, R. Harrup, R. Kimber, R. Lowenthal; Royal Perth Hospital, Perth, WA: J. Trotter, E. Bayliss, A. Chan, D. Ransom; Sir Charles Gairdner Hospital, Perth, WA: M. Byrne, M. Buck, J. Dewar, A. Nowak, A. Powell, G. Van Hazel; Toowoomba Hospital, Toowoomba, QLD: E. A. Abdi, R. Brodribb, Z. Volobueva; Westmead Hospital, Sydney, NSW: P. Harnett, V. Ahern, H. Gurney, N. Wilcken.
New Zealand: Auckland Hospital, Auckland: V. J. Harvey, B. Evans, W. Jones, M. McCrystal, D. Porter, P. Thompson, M. Vaughan; Christchurch Hospital, Christchurch: D. Gibbs, C. Atkinson, R. Burcombe, B. Fitzharris, B. Hickey, M. Jeffery, B. Robinson; Dunedin Hospital, Dunedin: B. McLaren, S. Costello, J. North, D. Perez; Waikato Hospital, Hamilton: I. D. Campbell, L. Gilbert, R. Gannaway, M. Jameson, I. Kennedy, J. Long, G. Round, L. Spellman, D. Whittle, D. Woolerton.
Brazil: Hospital de Clinicas de Porto Alegre, Porto Alegre: C. Menke, J. Biazús, R. Cericatto, J. Cavalheiro, N. Xavier, A. Bittelbrunn, E. Rabin.
Chile: Chilean Cooperative Group for Oncologic Research, GOCCHI: J. Gutiérrez (Chairman), R. Arriagada (Scientific Adviser), L. Bronfman (Principal Investigator), M. Zuñiga (Data Manager); Clinica Las Condes, Santiago: J. Gutiérrez, J. C. Acevedo, S. Torres, A. León, E. Salazar; Hospital DIPRECA, Las Condes, Santiago: L. Soto Diaz, R. Duval, N. Oddeshede, M. C. Venti; Hospital San Juan de Dios, Santiago: K. Peña, L. Puente, V. Maidana; IRAM/Instituto de Radiomedicina, Vitacura, Santiago: R. Baeza, R. Arriagada, P. Olfos, J. Solé, E. Vinés, C. Mariani.
Hungary: National Institute of Oncology, Budapest: I. Láng, E. Hitre, E. Szabó, Z. Horváth, E. Ganofszky, E. Juhos.
Italy: Centro di Riferimento Oncologico, Aviano: A. Veronesi, D. Crivellari, M. D. Magri, A. Buonadonna, F. Coran, E. Borsatti, E. Candiani, S. Massarut, M. Roncadin, M. Arcicasa, A. Carbone, T. Perin, A. Gloghini; Ospedali Riuniti di Bergamo, Bergamo: C. Tondini, R. Labianca, P. Poletti, A. Bettini; Ospedale degli Infermi, Biella: M. Clerico, M. Vincenti, A. Malossi, E. Seles, E. Perfetti, B. Sartorello; Spedali Civili, Brescia: E. Simoncini, G. Marini, P. Marpicati, R. Farfaglia, A. M. Bianchi, P. Grigolato, L. Lucini, P. Frata, A. Huscher, E. Micheletti, C. Fogazzi; U. O. Medicina Oncologica, Ospedale Carpi, Ospedale Mirandola: F. Artioli, K. Cagossi, L. Scaltriti, E. Bandieri, L. Botticelli, G. Giovanardi; Ospedale di Cattolica “Cervesi,” Cattolica: A. Ravaioli, E. Pasquini, B. Rudnas; Ospedale Civile, Gorizia: L. Foghin; Ospedale “A. Manzoni” Lecco, Lecco: M. Visini, L. Zavallone, G. Ucci; Istituto Europeo di Oncologia, Milano: M. Colleoni, G. Viale, G. Renne, F. Pruneri, M. Mastropasqua, S. Dellapasqua, A. Balduzzi, M. Iorfida, G. Cancello, E. Montagna, A. Cardillo, P. Veronesi, G. Peruzzotti, R. Gisini, A. Luini, V. Galimberti, S. Zurrida, M. Intra, O. Gentilini, F. Nolé, R. Orecchia, C. Leonardi, A. Goldhirsch; Ospedale Infermi, Rimini: A. Ravaioli, L. Gianni.
Peru: Instituto de Enfermedades Neoplásicas, Lima: H. Gome.
Slovenia: Institute of Oncology, Ljubljana: T. Cufer, B. Pajk, J. Cervek.
South Africa: Groote Schuur Hospital and University of Cape Town, Cape Town: I. D. Werner, E. Murray, D. Govender, S. Dalvie, T. Erasmus, B. Robertson, B. Read, E. Nel, J. Toop, N. Nedeva, E. Panieri; Sandton Oncology Centre, Johannesburg: D. Vorobiof, M. Chasen, G. McMichael, C. Mohammed. Local funding provided by the Cancer Association of South Africa.
Sweden: West Swedish Breast Cancer Study Group: S. B. Holmberg; Sahlgrenska U Hospital, Moelndal: S. B. Holmberg, J. Mattsson; Boras Hospital, Boras; Karlstads Hospital, Karlstads: H. Sellström; Kungalvs Hospital, Kungalvs: B. Lindberg.
Switzerland: Swiss Group for Clinical Cancer Research (SAKK): A. Goldhirsch (up to January 2004), R. Herrmann (June 2004 to June 2010), B. Thürlimann (from July 2010): Kantonsspital Aarau, Zentrum f. Onkologie, Aarau: A. Schönenberger, W. Mingrone, Ch. Honegger, E. Bärtschi, M. Neter, M. Rederer, G. Schär; University Hospital Basel, Basel: C. Rochlitz, R. Herrmann, D. Oertli, E. Wight, H. Moch; Institute of Oncology of Southern Switzerland: Ospedale San Giovanni, Bellinzona: J. Bernier, L. Bronz, F. Cavalli, E. Gallerani, A. Richetti, A. Franzetti; Ospedale Regionale di Lugano (Civico & Italiano), Lugano: M. Conti-Beltraminelli, M. Ghielmini, T. Gyr, S. Mauri, P.C. Saletti; Ospedale Regionale Beata Vergine, Mendrisio: A. Goldhirsch, O. Pagani, R. Graffeo, M. Locatelli, S. Longhi, P.C. Rey, M. Ruggeri; Ospedale Regionale La Carità, Locarno: E. Zucca, D. Wyss; Istituto Cantonale di Patologia, Locarno: L. Mazzucchelli, E. Pedrinis, T. Rusca; Inselspital, Berne: S. Aebi, M.F. Fey, M. Castiglione, M. Rabaglio; Kantonsspital Olten, Olten: S. Aebi, M.F. Fey, M. Zuber, G. Beck; Bürgerspital, Solothurn: S. Aebi, M.F. Fey, R. Schönenberger; Spital Thun-Simmental AG Thun: J.M. Lüthi, D. Rauch; Hôpital Cantonal Universitaire HCUG, Geneva: H. Bonnefoi; Rätisches Kantons- und Regionalspital, Chur: F. Egli, R. Steiner, P. Fehr; Centre Pluridisciplinaire d'Oncologie, Lausanne: L. Perey, P. de Grandi, W. Jeanneret, S. Leyvraz, J.-F. Delaloye; Kantonsspital St. Gallen, St. Gallen: B. Thürlimann, D. Köberle, F. Weisser, S., Mattmann, A. Müller, T. Cerny, B. Späti, M. Höfliger, G. Fürstenberger, B. Bolliger, C. Öhlschlegel, U. Lorenz, M. Bamert, J. Kehl-Blank, E. Vogel; Kantonales Spital Herisau, Herisau: B. Thürlimann, D. Hess, I. Senn, D. Köberle, A. Ehrsam, C. Nauer, C. Öhlschlegel, J. Kehl-Blank, E. Vogel; Stadtspital Triemli, Zürich: L. Widmer, M. Häfner; Universitätsspital Zürich, Zürich: B. C. Pestalozzi, M. Fehr, R. Caduff, Z. Varga, R. Trüb, D. Fink.
Swiss Private MDs: Private Praxis, Zürich: B. A. Bättig; Sonnenhof-Klinik Engeried, Berne: K. Buser; Frauenklinik Limmattalspital, Schlieren: N. Bürki; Private Praxis, Birsfelden: A. Dieterle; Private Praxis, Biel: L. Hasler; Private Praxis, Baar: M. Mannhart-Harms; Brust-Zentrum, Zürich: C. Rageth; Private Praxis, Berne: J. Richner; Private Praxis, Bellinzona: V. Spataro; Private Praxis, Winterthur: M. Umbricht.
United Kingdom: King's College Hospital/Breast Unit, London: P. Ellis, S. Harris, N. Akbar, H. McVicars, C. Lees, R. Raman, G. Crane.
Danish Group (DBCG).
B. Ejlertsen, H.T. Mouridsen; Rigshospitalet, Copenhagen: H.T. Mouridsen; Vejle Hospital, Vejle: E. Jakobsen; Odense University Hospital, Odense: S. Cold; KAS Herlev/Herlev University Hospital, Herlev: C. Kamby; Aalborg Sygehus Syd, Aalborg: M. Ewertz; Hilleroed Hospital, Hilleroed: P.M. Vestlev; Aarhus University Hospital, Aarhus: J. Andersen; Roskilde County Hospital, Roskilde: P. Grundtvig; Esbjerg Central Hospital, Esbjerg: E. Sandberg; Naestved Central Hospital, Naestved: P. Philip; Soenderborg Sygehus, Soenderborg: E.L. Madsen; Herning Central Hospital, Herning: K.A. Moeller; Viborg Sygehus, Viborg: V. Haahr; Landspitali University Hospital, Reykjavik, Iceland: J. Johansson.
French Group (FNCLCC).
Institut Bergonié, Bordeaux: L. Mauriac, M. Debled, H. Bonnefoi, P. Campo; Centre Hospitalier de la Côte Basque, Bayonne D. Larregain-Fournier, S. Remy, Centre Jean Perrin, Clermont-Ferrand: H. Auvray; Centre Georges François Leclerc, Dijon: C. De Gislain, F. Delille, M.-C. Porteret; Centre Oscar Lambret, Lille: V. Servent, M. Chapoutier; CHRU, Limoges: N. Tubiana-Mathieu, S. Lavau-Denes, P. Bosc; Centre Léon Bérard, Lyon: J. P. Guastalla, Th. Bachelot, C. Arbault; Centre Hospitalier Meaux, Meaux: G. Netter-Pinon; C.H.G. André Boulloche, Montbéliard: V. Perrin, A. Monnier, Y. Hammoud; Centre Paul Lamarque, Montpellier: G. Romieu, L. Culine, V. Pinosa; Clinique Francheville, Périgueux: L. Cany, C. Maguire; Hôpital de la Milétrie, Poitiers: A. Daban, M. Le Saux, C. Grandon; Centre Eugène Marquis, Rennes: P. Kerbrat, C. Catheline; Centre Henri Becquerel, Rouen: C. Veyret, E. Jugieau, V. Talon; Centre René Gauducheau, Saint-Herblain: A. Le Mevel, S. Maury; Centre Claudius Régaud, Toulouse: L. Gladieff, N. Lignon.
North Yorkshire Group.
D. Dodwell; Harrogate District Hospital, Harrogate, North Yorkshire: D. Dodwell; Huddersfield Royal Infirmary, Huddersfield: J. Joffe; Castlehill Hospital, Hull: P. Drew; Airedale General Hospital, Keighley,W. Yorkshire: A. Nejim; Leeds General Infirmary, Leeds: D. Dodwell, K. Horgan; St. James's University Hospital, Leeds: M. Lansdown, T. Perren; Weston Park Hospital, Sheffield: R.E. Coleman.
Independent Centers/Groups
Argentina: Centro Oncológico Confidence, Buenos Aires: D. Campos; Hospital Allemán, Buenos Aires: F. Cóppola; Hospital Británico, Buenos Aires: J. Martinez; Hospital Evita, Buenos Aires: M. Freue; Hospital Posadas, Buenos Aires: C. Wainstein; Hospital Zubizarreta, Buenos Aires: A. Zori Comba; Instituto Dr. Estevez, Buenos Aires: E. Cazap; Instituto Oncológico Dr. Angel H. Roffo, Buenos Aires: E. Mickiewicz; Sanatorio Municipal Julio A. Mendez, Buenos Aires: L. Balbiani; Centro Privado de Ginecología, Córdoba: A. Osuna; Hospital Privado de Córdoba, Córdoba: E. Palazzo; Instituto Modelo de Ginecología y Obstetricia, Córdoba: M. de Romedis; Fundación Mainetti-Centro Oncológico de Excelencia, La Pllata: S. Cagnolati; Hospital Privado de la Comunidad, Mar del Plata: C.A. Delfino, G. Caccia; Escuela de Medicina Nuclear (COIR), Mendoza: R.L. de Angelis; Centro Oncológico de Rosario, Rosario: L. Fein, R. Sala; Hospital Provincial de Rosario, Rosario: C. Nassurdi, A. Colombo Berra; Clínica Especializada ISIS, Santa Fe: R. Viroglio, C. Blajman; Hospital Regional de Concepción, Tucumán: H. Requejo; Instituto de Maternidad y Ginecología Nuestra Señoras de las Mercedes, Tucumán: L. Silberman.
Australia: Flinders Medical Centre, Adelaide, SA: S. Birrell, M. Eaton, C. Hoffman; Queen Elizabeth Hospital, Adelaide, SA: V. Humeniuk; The Canberra Hospital, Canberra, ACT; P. Craft, R. Stuart-Harris, D. Yip; The Geelong Hospital, Geelong, VIC: R. Bell, F. Abell, M. Francis, J. Kiffer, R. Lynch, R. McLennan, K. White; Royal Melbourne Hospital, Melbourne, VIC: M. Green, R. Basser, J. Collins, R. De Boer, J.C. Din, N. Efe, S.T. Fan, G. Lindeman, S. Wong; Western General Hospital, Melbourne, VIC: M. Green, R. Basser, J. Collins, R. De Boer, J.C. Din, N. Efe, S. T. Fan, G. Lindeman, S. Wong; Newcastle Mater Hospital, Newcastle, NSW: J. Stewart, F. Abell, S. Ackland, A. Bonaventura; Royal Perth Hospital, Perth, WA: J. Trotter, E. Bayliss, A. Chan, D. Ransom, A. Redfern; St. George Hospital, Sydney, NSW: P. de Souza, M. Links; St. Vincent's Hospital, Sydney, NSW: D. Dalley, J. Grygiel, R. Ward; Murray Valley Private Hospital, Wodonga, VIC: C. Underhill, K. Clarke, C. Steer; Princess Alexandra Hospital, Woolloongabba, QLD: E. Walpole, D. Thompson.
Belgium: Institut Jules Bordet, Bruxelles: J.M. Nogaret; University Hospitals Leuven, Leuven: M.R. Christiaens, P. Neven, R. Paridaens, A. Smeets, I. Vergote, C. Weltens, H. Wildiers; Les Cliniques Saint-Joseph ASBL, Liège: C. Focan; Clinique du Parc Léopold, Bruxelles: L. Marcelis; C. H. Etterbeek - Ixelles, Bruxelles: J.P. Kains; Service d'Oncologie Clinique Notre-Dame, Charleroi: J.-L. Canon; C. H. U. André Vèsale, Montigny-Le Tilleul: D. Brohèe.
Canada: Cambridge Memorial Hospital, Cambridge: J. Gowing; CHUM–Campus Notre-Dame, Montreal: L. Yelle; Hôpital Maisonneuve-Rosemont, Montreal: P. Dubé.
Chile: Fundacion Lopez Perez, Santiago: C. Vogel; Hospital Carlos Van Buren, Valparaiso: M. León Prieto.
Czech Republic: Institute of Oncology, Brno: K. Petrakova, M. Palacova, R. Demlova; Dept. of Clinical and Radiation Oncology, Ceske Budejovice: H. Siffnerova, J. Fischer, I. Bustova; Centre of Breast Diseases, Prague: H. Kankova, M. Pintova; Institute of Radiation Oncology, Prague: P. Vitek; University Hospital, Prague: J. Abrahamova, D. Kordikova; University Hospital Prague: L. Petruzelka, E. Sedlackova, H. Honova.
Germany: Onkologische Gemeinschaftspraxis, Augsburg: B. Heinrich; Zentralklinikum/Frauenklinik, Augsburg: A. Wischnik; Universitätsklinikum Essen, Essen: C. Oberhoff, A.E. Schindler; Universitäts-Frauenklinik d. JLU Giessen, Giessen: K. Münstedt; Onkologische Gemeinschaftspraxis, Göttingen: D. Meyer; Martin-Luther-Universität Halle-Wittenberg, Halle: R. Grosse, H. Kölbl; Universitätskliniken des Saarlandes, Homburg: W. Schmidt, D. Mink; Universitäts-Frauenklinik und Poliklinik Universitätskrankenhaus Eppendorf, Hamburg: F. Jänicke; Kliniken d. Med. Hochschule, Frauenklinik, Hannover: H.J. Lück; Krankenanstalt Mutterhaus der Borromäerinnen, Trier: W. Dornoff; Gynäkologische Abteilung des St. Josefshospital, Wiesbaden: G. Hoffmann; Gynäkologische Abteilung d. Marienhospitals, Universität Witten-Herdecke, Witten: J. Hackmann, W. Bader.
Hungary: SZOTE Onkoterápiás Klinika, Szeged: Z. Kahan; BM Központi Kórház, Budapest: G. Pajkos, K. Kristo; SOTE Radiológiai és Onkoterápiás Klinika, Budapest: M. Dank; Uzsoki Utcai Kórház, Budapest: T. Nagykalnai, L. Landherr; Almási Balogh Pál Kórház, Ózd: E. Kner; Területi Kórház Onkologia, Szentes: M. Kispál; Szent Borbála Kórház, Megyei Onkológiai Gondozó, Tatabánya: Á. Dani.
Italy: Policlinico S. Orsola-Malpighi, Bologna: A. Martoni, C. Zamagni, S. Giaquinta, E. Piana; Ospedale S. Croce, Fano: R. Mattioli, L. Imperatori; Istituto Clinica Humanitas, Milan/Rozzano: A. Santoro, C. Carnaghi, L. Rimassa; Azienda Ospedaliera San Filippo Neri, Rome: G. Gasparini, G. Sciarretta, A. Morabito; Az. Ospedaliera Treviglio-Caravaggio, Treviglio: S. Barni, M. Cazzaniga, M. Cabiddu; Policlinico Universitario (PUDG), Udine: F. Puglisi; Ospedale di Torrette, Ancona: R. Cellerino, S. Antognoli, F. Freddari; Universitiy of Cagliari, Policlinico Universitario, Cagliari: G. Mantovani, E. Massa, G. Astara; Ospedale Civile Feltre, Feltre: R. Segati; Istituto Nazionali Ricerca Cancro, Genova: R. Rosso, L. Del Mastro, M. Venturini, C. Bighin; Istituto Nazionale dei Tumori, Milano: E. Bajetta, N. Zilembo, D. Paleari, G. Procopio; Azienda Ospedaliera di Parma, Parma: S. Salvagni, M.A. Perrone, V. Franciosi; Azienda Ospedaliera “S. Salvatore,” Pesaro: G. Catalano, S. Luzi Fedeli; Azienda Ospedaliera “Ospedale di Circolo e Fondazione Macchi” Varese: G. Pinotti, G. Giardina, I. Vallini; Universitiy of Cagliari, Policlinico Universitario, Cagliari: B. Massidda, M.T. Ionta, M.C. Deidda; Ospedale Maggiore, Lodi: G. Nalli, G. Sita; Policlinico Universitario, Palermo: I. Carreca, S. Cucciarré, D. Burgio; Ospedale Civile dello Spirito Santo, Pescara: M. Lombardo, G. Pandoli, P. Di Stefano; Azienda Ospedaliera Santa Maria Nuova, Reggio Emilia: C. Boni, G. Bisagni, M. C. Banzi, P. Linarello; Azienda Ospedaliera Desenzano del Garda, Manerbio: G. Colosini, A. Spasiano, A. Caldonazzo; Ospedale Civile ASL 20, Tortona: M.G. Pacquola.
Netherlands: Ziekenhuis Leyenburg, Den Haag: H.P. Sleeboom; Catharina Ziekenhuis, Eindhoven: H.J.T. Rutten; St. Anna Ziekenhuis, Geldrop: E.J.T. Luiten; Tweesteden Ziekenhuis, Tilburg: H. Th. J. Roerdink; Maxima Medisch Centrum, Veldhoven: R.H.M. Roumen.
New Zealand: Dunedin Hospital, Dunedin: B. McLaren, S. Costello, J. North, D. Perez, K., Bayston, M. Pfieffer; Waikato Hospital, Hamilton: I. Kennedy, I.D. Campbell, L. Gilbert, R. Gannaway, M. Jameson, J. Long, G. Round, L. Spellman, D. Whittle, D. Woolerton.
Poland: Department of Oncology and Radiotherapy, Medical University of Gdansk, Gdansk: J. Jassem, M. Welnicka-Jaskiewicz, E. Senkus-Konefka, K. Matuszewska; Rydygier's Memorial Hospital, Krakow-Nova Huta: P. Koralewski, J. Pernal; Klinika Nowotworów Piersi i, Chirurgii Rekonstrukcyjnej-Warszawa, Warszawa: T. Pienkowski, E. Brewczynska, B. Bauer-Kosinska, R. Sienkiewicz-Kozlowska, A. Jagiello-Gruszfeld, K. Sudol.; Centrum Onkologii w Bydgoszczy, Oddzial Onkologii Klinicznej, Bydgoszcz: J. Tujakowski, B. Zurawski; Collegium Medicum Jagiellonian University, Krakow: J. Pawlega, E. Jablonska, A. Zygulska; Oddzial Kliniczny Onkologiczny, Centralnego Szpitala Klinicznego Wojskowej, Akademii Medycznej-Warszawa, Warszawa: M. Górnasiowa; Dolnoslaskie Centrum Onkologii, Wroclaw: E. Filypczyk-Cisarz, K. Pajak.
Portugal: Hospital de S. João, Porto: M. Damasceno; Instituto Português de Oncologia de Coimbra, Coimbra: J.Q. Albano; Hospital de Santa Maria, Lisboa: B. da Costa, L. Costa; Instituto Português de Oncologia de Lisboa, Lisboa: A. Henriques, H. Amaral; Hospital Geral de Santo António, Porto: F. Marques.
Russia: Cancer Research Centre, Moscow: D.V. Komov, S.B. Polikarpova; Moscow Municipal Hospital No. 62, Moscow: A.N. Makhson, N.V. Zabaznyi; Moscow Research Institute of Diagnostics and Surgery, Moscow: E.K. Vozny, N.Y. Dobrovolskaya, S. Bolshakova, O.V. Yurgina; N. M. Emmanuel Institute of Biochemical Physics, Moscow: D.B. Korman, I.A. Maslova; N.N. Petrov Research Institute of Oncology, St. Petersburg: V. Semiglazov, V. Ivanov; Saint-Petersburg City Oncological Dispensary, St. Petersburg: G. Manikhas, G. Dolmatov.
South Africa: Mamma Clinic, Tygerberg Hospital, Cape Town: J. Apffelstaedt; Southern Cross Hospital, Cape Town: D. Eedes; Pretoria Academic Hospital, Pretoria: C. Slabber; Pretoria East Hospital, Pretoria: M.A. Coccia-Portugal; Eastern Cape Oncology Centre, Port Elizabeth: K. Maart.
Spain: Hospital Ruber Internacional, Madrid: J.E. Alés Martinez, P. Aramburo, R. Sánchez; Hospital Son Dureta, Palma del Mallorca: J. Rifa, J. Martin; Centro Oncológico Integral de Madrid (CONIM), Madrid: R. Pérez-Carrión, J.L. González Larriba, A. Cubillo; Hospital Universitario San Carlos, Madrid: M. M. Jiménez, A. Casado; Hospital Central de Asturias, Oviedo: J. Fra, J. M. Vieitez, E. Esteban, A. J. Lacave.
Switzerland: Universitätsfrauenklinik, Basel: E. Wight, S. Bartens, R. Decio, U. Güth; Klinik am Park, Zürich: U. Breitenstein.
Turkey: Ankara University Ibni Sina Hospital, Ankara: F. Icli, D. Dincol; Hacettepe University Oncology Institute, Ankara: E. Baltali, Y. Ozisik; Istanbul University Oncology Institute, Istanbul: E. Topuz, M. Basaran, A. Aydiner; Ege University Medical School, Izmir: E. Ozdedeli; 9 Eylul University Medical School, Izmir: O. Harmancioglu, A.U. Yilmaz.
United Kingdom: The Royal Marsden Hospital, London, Royal Marsden NHS Trust, Surrey: I.E. Smith; University of Dundee, Dundee: A.M. Thompson; Christie Hospital NHS Trust, South Manchester University Hospital Trust, Manchester: A. Wardley; Royal Bournemouth Hospital, Bournemouth: T. Hickish; North Middlesex Hospital, London: F. Neave.
Uruguay: Hospital de Clinicas Dr. Manuel Quintela, Montevideo, Uruguay: G. Sabini.
Footnotes
Author affiliations appear at the end of this article.
Written on behalf of the Breast International Group 1-98 Collaborative and International Breast Cancer Study Groups.
The Breast International Group 1-98 trial was sponsored by Novartis and coordinated by International Breast Cancer Study Group (IBCSG). IBCSG is supported by Swedish Cancer Society, the Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group, Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research, National Cancer Institute Grant No. CA-75362, Cancer Research Switzerland/Oncosuisse, and the Foundation for Clinical Cancer Research of Eastern Switzerland.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00004205.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: Beat Thürlimann, Novartis Honoraria: John F. Forbes, Novartis; Aron Goldhirsch, Novartis Research Funding: Bent Ejlertsen, Novartis; Marco Colleoni, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Marianne Ewertz, Kathryn P. Gray, Meredith M. Regan, Bent Ejlertsen, Beat Thürlimann, Alan S. Coates,Henning T. Mouridsen
Administrative support: Bent Ejlertsen, Karen N. Price, Beat Thürlimann, John F. Forbes
Provision of study materials or patients: Bent Ejlertsen, Beat Thürlimann, Hervé Bonnefoi, John F. Forbes, Robert J. Paridaens, Manuela Rabaglio, Marco Colleoni, István Láng, Ian E. Smith, Alan S. Coates, Aron Goldhirsch, Henning T. Mouridsen
Collection and assembly of data: Marianne Ewertz, Bent Ejlertsen, Karen N. Price, Beat Thürlimann, Hervé Bonnefoi, Robert J. Paridaens, Manuela Rabaglio, Marco Colleoni, István Láng, Aron Goldhirsch
Data analysis and interpretation: Marianne Ewertz, Kathryn P. Gray, Meredith M. Regan, Beat Thürlimann, John F. Forbes, Richard D. Gelber, Ian E. Smith, Alan S. Coates, Henning T. Mouridsen
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res Treat . 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 2.Ewertz M, Jensen MB, Gunnarsdóttir KÁ, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol . 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol . 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 4.Rose DP, Vona-Davis L. Influence of obesity on breast cancer receptor status and prognosis. Expert Rev Anticancer Ther . 2009;9:1091–1101. doi: 10.1586/era.09.71. [DOI] [PubMed] [Google Scholar]
- 5.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med . 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 6.Colleoni M, Li S, Gelber R, et al. Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet . 2005;366:1108–1110. doi: 10.1016/S0140-6736(05)67110-3. [DOI] [PubMed] [Google Scholar]
- 7.de Azambuja E, McCaskill-Stevens W, Francis P, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: The experience of the BIG 02-98 trial. Breast Cancer Res Treat . 2010;119:145–153. doi: 10.1007/s10549-009-0512-0. [DOI] [PubMed] [Google Scholar]
- 8.Dignam JJ, Wieand K, Johnson KA, et al. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst . 2003;95:1467–1476. doi: 10.1093/jnci/djg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol . 2010;28:3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 10.BIG 1-98 Collaborative Group. Mouridsen H, Giobbie-Hurder A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med . 2009;361:766–776. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon JM, Renshaw L, Young O, et al. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol . 2008;26:1671–1676. doi: 10.1200/JCO.2007.13.9279. [DOI] [PubMed] [Google Scholar]
- 12.Geisler J, Helle H, Ekse D, et al. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res . 2008;14:6330–6335. doi: 10.1158/1078-0432.CCR-07-5221. [DOI] [PubMed] [Google Scholar]
- 13.Breast International Group (BIG) 1-98 Collaborative Group. Thürlimann B, Keshaviah A, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med . 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 14.Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol . 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 15.Regan MM, Neven P, Giobbie-Hurder A, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: The BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol . 2011;12:1101–1108. doi: 10.1016/S1470-2045(11)70270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colleoni M, Giobbie-Hurder A, Regan MM, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol . 2011;29:1117–1124. doi: 10.1200/JCO.2010.31.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life-tables. JR Stat Soc B . 1972;34:187–220. [Google Scholar]
- 18.Bonetti M, Gelber RD. Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics . 2004;5:465–481. doi: 10.1093/biostatistics/5.3.465. [DOI] [PubMed] [Google Scholar]
- 19.Lazar AA, Cole BF, Bonetti M, Gelber RD. Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: Subpopulation treatment effect pattern plot. J Clin Oncol . 2010;28:4539–4544. doi: 10.1200/JCO.2009.27.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat . 1988;16:1141–1154. [Google Scholar]
- 21.Pfeiler G, Königsberg R, Fesl C, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: An analysis of the prospective ABCSG-12 trial. J Clin Oncol . 2011;29:2653–2659. doi: 10.1200/JCO.2010.33.2585. [DOI] [PubMed] [Google Scholar]
- 21a.MacDonald PC, Edman CD, Hemsell DL, et al. Effect of obesity on conversion of plasma androstenedione to estrone in postmenopausal women with and without endometrial cancer. Am J Obstet Gynecol. 1978;130:448–455. doi: 10.1016/0002-9378(78)90287-9. [DOI] [PubMed] [Google Scholar]
- 22.Grossmann ME, Ray A, Nkhata KJ, et al. Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev . 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 23.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: Weight of the evidence. J Clin Oncol . 2011;29:4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 24.McTiernan A, Rajan KB, Tworoger SS, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21:1961–1966. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer—The estrogen connection. Endocrinology . 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev . 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol . 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin- and obesity-related variables in early-stage breast cancer: Correlations and time course of prognostic associations. J Clin Oncol . 2012;30:164–171. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- 29.Pasanisi P, Berrino F, De Petris M, et al. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer . 2006;119:236–238. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard KI, Shepherd LE, Chapman JA, et al. Randomized trial of tamoxifen versus combined tamoxifen and octreotide LAR therapy in the adjuvant treatment of early-stage breast cancer in postmenopausal women: NCIC CTG MA.14. J Clin Oncol . 2011;29:3869–3876. doi: 10.1200/JCO.2010.33.7006. [DOI] [PubMed] [Google Scholar]
- 31.Duggan C, Irwin ML, Xiao L, et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol . 2011;29:32–39. doi: 10.1200/JCO.2009.26.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris PG, Hudis C, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res . 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]