Abstract
Social fears emerging in adolescence can have negative effects on emotional well-being. Yet the mechanisms by which these risks occur are unknown. One possibility is that associative learning results in fears to previously neutral social stimuli. Such conditioned responses may alter subsequent processing of social stimuli. We used a novel conditioning task to examine how associative processes influence social fear and attention orienting in adolescents. Neutral photographs were paired with socially rewarding or aversive stimuli during conditioning; a dot-probe task then assessed biases in attention orienting. The social conditioning task modified subjective ratings of the neutral stimuli. Moreover, for the neutral stimulus that was paired with the aversive stimulus, the strength of conditioning showed a relationship with subsequent attentional vigilance. The findings elucidate mechanisms by which negative peer experiences during adolescence may affect emotional processing.
Keywords: Adolescence, Social anxiety, Attentional biases, Fear conditioning
Negative social relationships during development adversely affect well-being. Peer victimisation, or repeated subjection to negative peer social experiences, predicts later psychological problems, particularly social anxiety (Hawker & Boulton, 2000; McCabe, Antony, Summerfeldt, Liss, & Swinson, 2003; Sourander et al., 2007). However, the mechanisms by which negative peer interactions shape maladaptive emotional responses to future social situations are poorly understood.
Social fears may emerge through repeated pairing of an initially neutral stimulus with a socially threatening stimulus, such that the neutral stimulus comes to elicit fear (Lissek et al., 2008). The notion that dysfunctional anxiety arises through such associative learning has a long history (Lissek et al., 2005; Watson & Rayner, 1920). For example, talking to peers might begin as a neutral or even a positive experience; however, if repeatedly paired with humiliation, social encounters may become feared experiences. Retrospective self-report data from individuals with non-clinical social fears support this possibility (Ishiyama, 1983, cited in Ishiyama, 1984; Mulkens & Bogels, 1999); moreover, patients frequently attribute the onset or worsening of social phobia to conditioning experiences (Ost, 1985; Stemberger, Turner, Beidel, & Calhoun, 1995).
Most research on social-conditioning experiences in emergent clinical and non-clinical social fears, however, relies on retrospective self-report data, which have many limitations, including biases in recall of aversive events (Esteves, Parra, Dimberg, & Öhman, 1994; Knight, Nguyen, & Bandettini, 2003; Olsson & Phelps, 2004). Data from experimental conditioning studies could further inform theories implicating associative learning in the development of maladaptive emotional responses to social situations. Using an experimental task to produce social conditioning, one study presented adults with and without social phobia with three photographs of neutral facial expressions (conditioned stimuli, CS), each paired with socially rewarding, aversive, or neutral experiences (unconditioned stimuli, UCS; Lissek et al., 2008). Following conditioning, subjective ratings of fear and pleasantness and fear-potentiated startle to each (neutral) CS photograph were modified in those with social phobia. This suggests that “learned fears” acquired through conditioning may contribute to maladaptive social responses.
How, then, might conditioning influence subsequent emotional responses? Several studies in adults have shown that fear conditioning alters subsequent information processing of the CS (Kelly & Forsyth, 2007; Lee, Lim, Lee, Kim, & Choi, 2009; Pischek-Simpson, Boschen, Neumann, & Waters, 2009; Van Damme, Crombez, Hermans, Koster, & Eccleston, 2006). Specifically, previous conditioning experiences produce increased vigilance for the CS. Attentional biases for threat are well documented in adolescents with anxiety. Compared to their non-anxious peers, anxious children and adolescents preferentially attend to threat-relevant stimuli as demonstrated by emotional Stroop and dot-probe tasks (Muris & Field, 2008). In emotional Stroop tasks participants name the colour of words; slower colour naming for fear-relevant words relative to neutral words generally indicates greater vigilance for threatening material although alternative explanations have been proposed (Williams, Watts, MacLeod, & Mathews, 1997). Dot-probe tasks present a valenced stimulus and a neutral stimulus simultaneously; one is followed by a probe, which participants must detect as quickly and accurately as possible. Faster probe detection on “congruent” trials (where probes follow valenced stimuli) relative to “incongruent” trials (where probes follow neutral stimuli) suggests an attentional bias towards the valenced stimuli (vigilance), whereas slowed probe detection on congruent versus incongruent trials suggests an attentional bias away from the valenced stimuli (avoidance; MacLeod, Mathews, & Tata, 1986).
Adult data demonstrate longer colour-naming latencies for previously reinforced CSs relative to previously non-reinforced CSs during the emotional Stroop task (Kelly & Forsyth, 2007; Lee et al., 2009). Similarly spatial attention is biased towards the location of the previously reinforced CS in dot-probe tasks (i.e., vigilance; Pischek-Simpson et al., 2009; Van Damme et al., 2006). To date most of the studies investigating the link between fear conditioning and attentional biases have been conducted in adults and have tended to use non-social UCSs. Thus, their relevance for understanding adolescent social fears is limited.
The current study used a new task, based on Lissek et al. (2008), to examine the degree to which associative processes influence appraisals of and attention to social stimuli. During the task, photographs of three children with neutral expressions were each paired with one of three social unconditioned stimuli (UCS): an angry facial expression and a critical comment; a happy facial expression and a compliment; and a neutral facial expression and a neutral comment. To enhance ecological validity of the task, participants were led to believe that these face–comment pairings represented online interactions between the three individuals. The neutral photographs thus became conditioned stimuli (CS) with negative (CSneg), positive (CSpos), and neutral (CSneutr) emotional value respectively. The strength of the acquired fear response to the CSneg relative to the CSpos and CSneutr was assessed using subjective fear and pleasantness ratings of each CS. Subsequently, the relationship between conditioning and attentional biases was examined using a dot-probe task, which assessed attentional bias for the CSneg and the CSpos relative to the CSneutr.
METHODS
Participants
Participants were an unselected sample of 42 12- to 15-year-old pupils (mean age = 13.45, SD = 1.33, 23 males) recruited from local mainstream schools. A parent of each child gave written informed consent to their participation and the study was approved by the local ethics committee.
Procedure
Anxiety symptoms
Self-reported anxiety symptoms were assessed with the Screen for Childhood Anxiety-Related Disorders (SCARED; Birmaher et al., 1999).
Social conditioning task
The task comprised three phases: baseline, acquisition, and extinction. During baseline, participants viewed the three CSs, each of which appeared for 3 s. Each photo was presented twice, in random order. During acquisition, each CS was paired with one of three categories of unconditioned stimuli (UCS) as follows: (i) the CSpos was paired on approximately 75% of trials1 with a photograph of the same child with a happy facial expression and a written compliment (e.g., “You look nice today”), and on remaining trials with a neutral photo and neutral comment; (ii) the CSneg was paired on approximately 75% of trials (see Footnote 1) with a photo of the same child with an angry facial expression and a written insult (e.g., “I don’t like you”) and on remaining trials with a neutral photograph and neutral comment; and (iii) the CSneutr was always paired with a neutral (i.e., identical) photograph and neutral comment (e.g., “I live in Bristol”). There were 9 trials of each type, resulting in 27 acquisition trials in random order. The CS appeared for 1 s and the UCS (photo plus comment) appeared for 2 s. During extinction, the neutral photographs were again presented alone for 3 s. Each CS was presented eight times in random order, resulting in 24 extinction trials. The inter-trial interval (ITI) was 1 s throughout.
Following each phase of the task, participants rated the scariness and pleasantness of each CS on a 9-point Likert scale (anchored with the labels very scary/very unpleasant and unscary/very pleasant). The task was presented using E-prime on a laptop computer.
Participants were told that they were viewing photographs of other students their own age, along with extracts of online conversations between them; they were debriefed as to the true nature of the task at the end of the experiment. Photographs were taken from a set of headshots of adolescent actors posing different expressions (Guyer, McClure-Tone, Shiffrin, Pine, & Nelson, 2009). Four sets of three pictures were selected to create four versions (older boys, older girls, younger boys, younger girls) of the task. Participants were assigned the version that most closely matched their own gender and age group (12–13 or 14–15). Allocation of the three identities within each version to the CS valences was counterbalanced between participants.
Dot-probe task
Following conditioning, participants completed a dot-probe task. We were interested in biases arising from the acquired emotional value of the CSneg and CSpos, so we used the neutral photographs that had served as CSs during conditioning.
Each trial began with a 500 ms central fixation point. Pairs of photographs were then presented to the left and right of the central fixation position for 500 ms. The pair of photographs was immediately followed by a probe (an asterisk) appearing in the same position as one of the photographs. Participants reported the location of the probe (left or right) as quickly and accurately as possible by pressing one of two keys. The probe remained on the screen for 1100 ms. The ITI was 1900 ms. There were two blocks of 32 trials (CSneutr paired with CSneg and CSneutr paired with CSpos). The order of the blocks and the positions of the photographs and probes were fully counterbalanced.
Trials where the participant’s response was incorrect (~ 1.1% of trials) or where their reaction time (RT) was more than three standard deviations from their mean RT (~ 1.6% of trials) were excluded from the analysis. Attentional bias scores were calculated for each participant by subtracting the mean RT for congruent trials from the mean RT for incongruent trials. Positive values reflect attention towards the valenced photo (vigilance) and negative values reflect attention away from the valenced photo (avoidance).
Data analysis
For the social conditioning task, data on fear and pleasantness ratings were each analysed using a 3 × 3 repeated-measures analysis of variance (ANOVA) with two within-subjects factors: Phase (baseline, acquisition, extinction) and CS (CSneg, CSpos, CSneutr). Significant interactions were examined using simple main-effect analyses. The Greenhouse–Geisser correction for non-sphericity was used where necessary. For the dot-probe task, attentional bias scores for the CSneg and for the CSpos were first analysed using one-sample t-tests to examine whether scores differed significantly from zero. Next, we explored links between ratings following social conditioning and dot-probe attentional bias scores. SCARED scores (including scores on the social anxiety subscale) were subsequently included as a covariate in the analyses to see whether anxiety symptoms explained any of the observed effects.
Age and gender were initially each included as a covariate in the main analyses; however, no significant main effects or interactions involving these variables emerged and so they were omitted from the analyses reported here. Similarly, we repeated the analyses including total SCARED and social anxiety subscale scores as covariates but this did not substantively change the pattern of results.
RESULTS
Social conditioning task
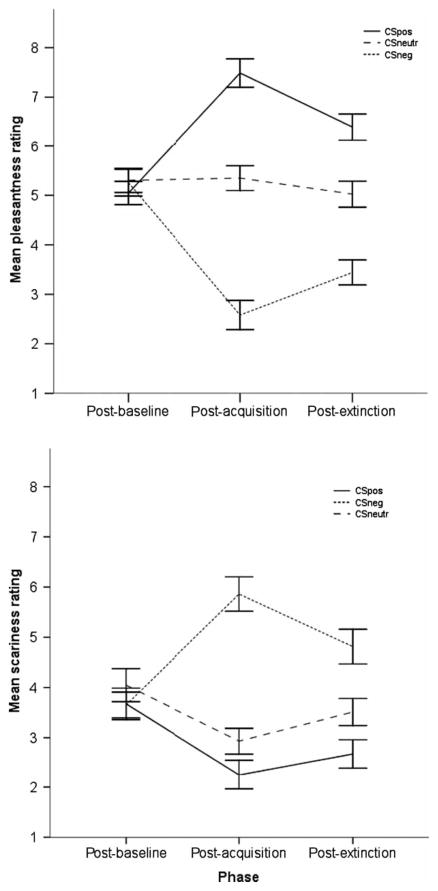
Scariness ratings for each CS at each phase are presented in Figure 1. Results showed a significant Phase × CS-type interaction, F(4, 164) = 21.37, MSE = 2.07, p< .001, and a significant main effect of CS-type, F(2, 82) = 27.23, MSE = 5.01, p < .001. The interaction was decomposed by considering differences in scariness ratings between CS-types at each phase separately. No differences in ratings between CS-types were apparent for baseline trials, F(2, 82) = 1.11, MSE = 2.66, ns. Instead divergences emerged following conditioning, F(1.6, 65.8) = 49.26, MSE = 4.14, p < .001. Post hoc t-tests revealed higher scariness ratings for the CSneg (M = 5.88, SD = 2.25) relative to the CSneutr (M = 2.86, SD = 1.63), t(41) = 7.89, p < .001, and to the CSpos (M = 2.17, SD = 1.85), t(41) = 7.72, p < .001, and higher CSneutr ratings than for CSpos, t(41) = 2.22, p = .032. Differences across CSs persisted after extinction, F(1.74, 71.2) = 18.35, MSE = 3.65, p < .001, and post hoc paired t-tests revealed significant post-extinction differences between all three CS-types (p-values ≤ .005).
Figure 1.
Subjective pleasantness (top) and scariness (bottom) ratings (mean±SEM) for each CS following baseline, acquisition, and extinction trials showing that conditioning modified perceptions of the neutral stimuli.
Pleasantness ratings mirrored scariness ratings (Figure 1). A significant Phase × CS-type interaction, F(3.27, 133.99) = 38.84, MSE = 2.27, p < .001, and a main effect of CS-type, F(2, 82) = 52.01, MSE = 4.02, p < .001, emerged. The interaction was driven by differences between CS-types following conditioning, F(2, 82) = 87.03, MSE = 2.95, p < .001, and extinction, F(2, 82) = 36.68, MSE = 2.62, p < .001, but not baseline, F(2, 82) = 0.37, MSE = 2.17, ns. Post hoc paired t-tests revealed higher pleasantness ratings following conditioning for the CSpos (M = 7.50, SD = 1.82) relative to both the CSneutr (M = 5.35, SD = 1.54), t(41) = 6.15, p < .001, and the CSneg (M = 2.57, SD = 1.95), t(41) = 11.56, p < .001, and higher scores for the CSneutr relative to the CSneg, t(41) = 8.11, p < .001. Again, these differences persisted after extinction, post hoc paired t-test p-values < .001.
Dot-probe task
One participant with extremely long reaction times on the dot-probe task (~ 1000 ms) was excluded from further analyses. For the sample as a whole, irrespective of other variables, attentional bias scores for the CSneg and the CSpos were not significantly different from zero (p-values > .70). Thus, no overall vigilance (or avoidance) for either of the valenced CSs relative to the CSneutr emerged. Moreover, there was no relationship between anxiety symptoms (SCARED total score or social anxiety subscale) and attentional bias scores (p-values > .29).
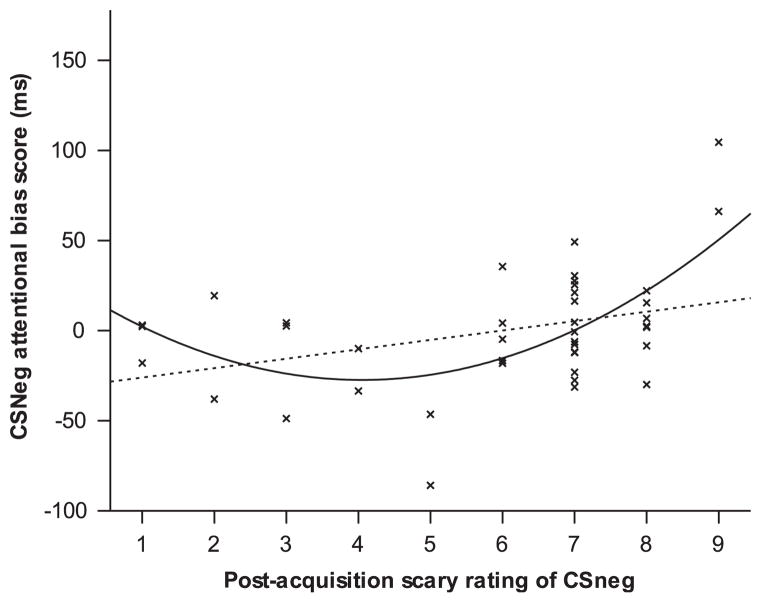
We next examined the relationship between degree of conditioning, as measured by subjective ratings to the CSpos and CSneg, and attentional bias scores. Inspection of the data suggested nonlinearity (Figure 2). A hierarchical regression analysis was performed with CSneg attentional bias scores as the dependent variable, including a quadratic term in the model. Post-acquisition CSneg scariness ratings were entered in step 1, and the quadratic term added in step 2. At step 1, the regression model was significant, R2 = .362, F(1, 39) = 5.90, p = .020. However, adding the quadratic term at step 2 significantly improved the fit of the model, ΔR2 = .211, ΔF(1, 38) = 12.16, p= .001, with the overall model at step 2 being highly significant, F(2, 38) = 9.87, p < .001, and accounting for around 34% of the variance in attentional bias scores. Both the linear and the quadratic trend lines are shown in Figure 2; as can be seen, there were three possible outliers (two participants with a post-acquisition scariness rating of 9 and one with a rating of 5). However, none of these participants’ scores were identified as multivariate outliers using Mahalanobis or Cook’s distances, and repeating the analysis after deletion of each of these data points did not substantively change the results. Thus, participants reporting greater conditioned fear to the CSneg showed greater attentional bias for the CSneg in the dot-probe task, whereas for those who showed very little conditioning to the CSneg, there was little or no effect on attentional bias scores. Including SCARED or social anxiety subscale scores did not change this pattern of results.
Figure 2.
Relationship between post-acquisition scariness ratings and attentional bias scores for the CSneg; both the linear and quadratic models are displayed, but the quadratic model showed a significantly improved fit over the linear model.
Similar analyses using post-extinction scariness ratings and post-acquisition and post-extinction pleasantness ratings for the CSneg did not show any significant effects on attentional bias scores. Predicting CSpos attentional bias from post-acquisition or post-extinction CSpos pleasantness or scariness ratings did not yield significant relationships either.
DISCUSSION
Two main findings emerged from the current study. First, repeated pairing with socially relevant unconditioned stimuli induced changes in perceived scariness and pleasantness of neutral, but socially relevant, CSs. The CSneg, which was paired with a socially aversive experience, came to be perceived as scarier and less pleasant than the CSpos and CSneutr, whereas the CSpos, paired with a socially rewarding experience, was perceived as less scary and more pleasant than the CSneg and CSneutr. The subjective attributions persisted despite extinction. Second, the relationship between the strength of conditioning to the CSneg and attentional bias in the subsequent dot-probe was non-linear: Those participants who showed little or no conditioning to the CSneg showed no subsequent attentional bias for it, whereas as conditioned responses to the CSneg escalated, vigilance for the CSneg increased.
These findings extend data from healthy adults (Lissek et al., 2008), demonstrating that emotional responses to conditioned social stimuli can occur in typically developing adolescents. These data show that young people can develop negative and positive perceptions of social relationships through learning mechanisms based on past experiences. Interestingly, a small number of participants in the current study rated the CSpos and CSneutr as being more scary after the acquisition phase than at baseline (n = 4 and n = 8 participants for the CSpos and CSneutr, respectively), even though these stimuli were never paired with a negative outcome. This suggests some degree of generalisation of the fear response from the CSneg to the CSpos and CSneutr; however, not enough participants displayed this pattern of responses for us to examine it further. Relatedly, social fear is often associated with encountering new people or situations and further investigation would also establish whether negative and/or positive perceptions following conditioning generalise to new social stimuli or situations. The present task provides an excellent experimental basis for such an investigation. Additional work is also needed on the developmental course of social conditioning; that is, to explore at what age social UCSs begin to shape behaviour and the degree to which early emerging conditioned responses predict lifelong pathology. One might expect greater social conditioning during adolescence, a period of heightened salience of peer relationships. This prediction awaits further empirical examination.
Our findings also inform work on the links between social conditioning and attentional biases in adolescence. Our results are consistent with the model suggested by Mogg and Bradley (1998), that attentional biases are related not primarily to anxiety level, but rather to level of subjective threat. Mogg and Bradley (1998) proposed that the relationship between perceived threat and attention is non-linear: stimuli perceived as mildly threatening may be avoided in the interests of pursuing current goals and preventing distraction by trivial aversive stimuli; on the other hand, as perceived threat value of the stimulus increases, attention is more likely to be allocated towards it. All individuals will avoid stimuli that they perceive as only mildly threatening, and, similarly, all individuals will attend towards stimuli that are perceived as extremely threatening. However, individual differences in threat evaluation thresholds give rise to different tendencies to attend to or avoid a given stimulus.
Our data showed a relationship very similar to the curvilinear one suggested by Mogg and Bradley (1998, Figure 3, p. 819); that is, for those who perceived little or no threat from the CSneg, there were no observable effects on attentional biases, whereas with increasing perceived threat, increasing attentional vigilance was seen. Other data investigating such a relationship also support this (Wilson & MacLeod, 2003), suggesting that the group of participants who showed a conditioning effect (and subsequent modification of attentional biases depending on the strength of that effect) may represent a subset of individuals especially vulnerable to harmful downstream effects of peer victimisation. Interestingly, although both negative and positive conditioning were successfully demonstrated using this paradigm, conditioning to the CSpos was not associated with attentional bias. This suggests some specificity between negative conditioning and social fear. Moreover, the relationship with attentional bias scores was only evident for post-acquisition ratings of the CSneg and not post-extinction ratings. This was somewhat surprising as post-extinction ratings reflected attributions at the start of the dot-probe task whereas the post-acquisition ratings were taken earlier. This may have arisen because of the subjective nature of the ratings. For example, among those participants who gave high post-acquisition scariness ratings, although reported fear of the CSneg may have abated by the end of the extinction phase, longer-lasting basic affective responses may have increased vigilance for the CSneg during the dot-probe task. Future research examining objective emotional responses, such as measured through physiological recordings, at each phase could clarify this point.
No relationship characterised anxiety symptoms and conditioned subjective responses to the CSs, or to attentional vigilance for the CS. The task may not be sensitive enough to discriminate individual differences in anxiety. However, other factors may have influenced the strength of responses to the CS, such as variability in responses to the UCSs (Davey, 1989), which in turn may be influenced by previous experiences of peer victimisation. Future research should examine these factors in the acquisition of adolescent social fears.
Limitations
This study is subject to a number of limitations. First, we did not measure attentional bias after acquisition as well as extinction. This was because we were also interested in the persistence of conditioned responses. Moreover, measuring post-extinction attentional biases might have better ecological validity as adolescents are likely to experience episodes of “extinction” interspersed with “acquisition” in real social situations. Second, due to ethical concerns, the face–comment pairings were described as being part of an interaction between the three children depicted on screen rather than as being directed towards the participant. Non-self-referential UCSs may be milder in their anxiety-eliciting properties. On the other hand, the fact that these stimuli served as effective UCSs suggests that even simply observing negative peer interactions may shape emotional processing in adolescents. A subtle difference from the typical “observational learning” paradigm should be noted here: our task did not involve observing a conspecific displaying an unconditioned response (e.g., Kelly & Forsyth, 2007; Olsson & Phelps, 2004) but rather involved observing a UCS directed towards another person—no unconditioned responses were ever observed by the participants. Finally, as with many studies investigating the affective properties of complex social stimuli, we assumed rather than tested the emotional salience and valence of the UCSs.
In summary, we have shown that socially aversive and rewarding UCSs can modulate perceptions of previously neutral stimuli in typically developing adolescents, and, moreover, that in a subset of adolescents in whom the acquired negative value of these neutral stimuli is particularly strong, attentional vigilance emerges. These findings help to elucidate the mechanisms by which negative peer experiences in adolescence increase risk for psychopathology.
Acknowledgments
This research was supported by a grant from the British Academy to JYFL.
We thank Harvey Iwamoto and Michelle Goldwin for their help with the programming of the tasks; Carolyn Plateau and Phoebe Sanders for their help with data collection; and the schools and participants for their cooperation.
Footnotes
The intended 75% reinforcement schedule was not obtained because the task was inadvertently programmed to present 9 trials of each CS type during acquisition instead of 8; thus there was no way to reinforce exactly 75% of trials. For both the CSpos and the CSneg, the reinforcement schedule was therefore either 66.7% (i.e., 6/9 trials reinforced) or 77.8% (i.e., 7/9 trials reinforced). However, including reinforcement schedule as a categorical variable in the analyses did not change the results.
References
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A replication study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Davey GC. UCS revaluation and conditioning models of acquired fears. Behaviour Research and Therapy. 1989;27:521–528. doi: 10.1016/0005-7967(89)90086-7. [DOI] [PubMed] [Google Scholar]
- Esteves F, Parra C, Dimberg U, Öhman A. Nonconscious associative learning: Pavlo-vian conditioning of skin conductance responses to masked fear-relevant facial stimuli. Psychophysiology. 1994;31:375–385. doi: 10.1111/j.1469-8986.1994.tb02446.x. [DOI] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker DS, Boulton MJ. Twenty years’ research on peer victimization and psychosocial maladjustment: A meta-analytic review of cross-sectional studies. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41:441–455. [PubMed] [Google Scholar]
- Ishiyama FI. Shyness: Anxious social sensitivity and self-isolating tendency. Adolescence. 1984;19:903–911. [PubMed] [Google Scholar]
- Kelly MM, Forsyth JP. Observational fear conditioning in the acquisition and extinction of attentional bias for threat: An experimental evaluation. Emotion. 2007;7:324–335. doi: 10.1037/1528-3542.7.2.324. [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences. 2003;100:15280–15283. doi: 10.1073/pnas.2535780100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Lim SL, Lee K, Kim HT, Choi JS. Conditioning-induced attentional bias for face stimuli measured with the emotional Stroop task. Emotion. 2009;9:134–139. doi: 10.1037/a0014590. [DOI] [PubMed] [Google Scholar]
- Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, Pine DS, et al. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. American Journal of Psychiatry. 2008;165:124–132. doi: 10.1176/appi.ajp.2007.06091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, et al. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- McCabe RE, Antony MM, Summerfeldt LJ, Liss A, Swinson RP. Preliminary examination of the relationship between anxiety disorders in adults and self-reported history of teasing or bullying experiences. Cognitive Behaviour Therapy. 2003;32:187–193. doi: 10.1080/16506070310005051. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mulkens S, Bogels SM. Learning history in fear of blushing. Behaviour Research and Therapy. 1999;37:1159–1167. doi: 10.1016/s0005-7967(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Muris P, Field AP. Distorted cognition and pathological anxiety in children and adolescents. Cognition and Emotion. 2008;22:395–421. [Google Scholar]
- Olsson A, Phelps EA. Learned fear of “unseen” faces after Pavlovian, observational, and instructed fear. Psychological Science. 2004;15:822–828. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- Ost LG. Ways of acquiring phobias and outcome of behavioral treatments. Behaviour Research and Therapy. 1985;23:683–689. doi: 10.1016/0005-7967(85)90066-x. [DOI] [PubMed] [Google Scholar]
- Pischek-Simpson LK, Boschen MJ, Neumann DL, Waters AM. The development of an attentional bias for angry faces following Pavlo-vian fear conditioning. Behaviour Research and Therapy. 2009;47:322–330. doi: 10.1016/j.brat.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Sourander A, Jensen P, Ronning JA, Niemela S, Helenius H, Sillanmaki L, et al. What is the early adulthood outcome of boys who bully or are bullied in childhood? The Finnish “From a Boy to a Man” study. Pediatrics. 2007;120:397–404. doi: 10.1542/peds.2006-2704. [DOI] [PubMed] [Google Scholar]
- Stemberger RT, Turner SM, Beidel DC, Calhoun KS. Social phobia: An analysis of possible developmental factors. Journal of Abnormal Psychology. 1995;104:526–531. doi: 10.1037//0021-843x.104.3.526. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Hermans D, Koster EH, Eccleston C. The role of extinction and reinstatement in attentional bias to threat: A conditioning approach. Behaviour Research and Therapy. 2006;44:1555–1563. doi: 10.1016/j.brat.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Watson JB, Rayner R. Conditioned emotional reactions. Journal of Experimental Psychology. 1920;3:1–14. [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. Chichester, UK: Wiley; 1997. [Google Scholar]
- Wilson E, MacLeod C. Contrasting two accounts of anxiety-linked attentional bias: Selective attention to varying levels of stimulus threat intensity. Journal of Abnormal Psychology. 2003;112:212–218. doi: 10.1037/0021-843x.112.2.212. [DOI] [PubMed] [Google Scholar]