Abstract
Objective
To examine the neural basis of cognitive complaints in healthy older adults in the absence of memory impairment and to determine whether there are medial temporal lobe (MTL) gray matter (GM) changes as reported in Alzheimer disease (AD) and amnestic mild cognitive impairment (MCI).
Methods
Participants were 40 euthymic individuals with cognitive complaints (CCs) who had normal neuropsychological test performance. The authors compared their structural brain MRI scans to those of 40 patients with amnestic MCI and 40 healthy controls (HCs) using voxel-based morphometry and hippocampal volume analysis.
Results
The CC and MCI groups showed similar patterns of decreased GM relative to the HC group on whole brain analysis, with differences evident in the MTL, frontotemporal, and other neocortical regions. The degree of GM loss was associated with extent of both memory complaints and performance deficits. Manually segmented hippocampal volumes, adjusted for age and intracranial volume, were significantly reduced only in the MCI group, with the CC group showing an intermediate level.
Conclusions
Cognitive complaints in older adults may indicate underlying neurodegenerative changes even when unaccompanied by deficits on formal testing. The cognitive complaint group may represent a pre–mild cognitive impairment stage and may provide an earlier therapeutic opportunity than mild cognitive impairment. MRI analysis approaches incorporating signal intensity may have greater sensitivity in early preclinical stages than volumetric methods.
Memory complaints, a cardinal feature of mild cognitive impairment (MCI)1 that confers a high risk of Alzheimer disease (AD),2,3 are reported in 25 to 50% of the older adult population.4 Longitudinal research on older adults with cognitive complaints (CCs) has yielded inconsistent findings,5–12 although a range of associated factors including apolipoprotein E (APOE) genotype, depression, somatic concerns, female sex, and older age have been identified.4,13–19
Normal aging, MCI, and AD have been associated with loss of gray matter (GM).20,21 Many studies have used manual tracing of regions of interest (ROIs) to assess medial temporal lobe (MTL) structures in AD and MCI.22–25 Voxel-based morphometry (VBM) assesses tissue compartments on a voxel-by-voxel basis and has the advantages of automation, reliability, and unbiased comprehensive sampling across the brain.26 Regional decline in GM volume has been reported in healthy adults, as a function of age,27–29 with more pronounced reduction in patients with MCI30,31 or AD.22,30,32–36 Regions reported most frequently include MTL structures, cingulate, and diffuse cortical association regions.22,30,32–36 Prior studies had not quantitatively examined the severity of CCs in preclinical AD and directly assessed the relationship to GM.
We used ROI and VBM analyses to examine the structural underpinnings of memory complaints in older adults with normal memory test performance compared to individuals with MCI and healthy controls (HCs). We hypothesized that individuals with CCs would show decreased GM density in MTL and other cortical regions, as well as an intermediate level of hippocampal volume reduction between MCI and controls. We also hypothesized that subjective and objective measures of memory would be related to GM density.
Methods
Participants were 120 older adults consecutively enrolled into the Dartmouth Memory and Aging Study. The sample included 40 individuals with significant CCs despite normal cognitive test performance (CC group), 40 patients with MCI (MCI group), and 40 HCs (HC group) with no significant CCs or deficits. Two additional participants, one with MCI and one HC, were excluded from the present analyses due to suboptimal image quality. Participants were recruited through use of flyers, public lectures, newspaper advertisements, and referrals from our medical center’s General Internal Medicine, Community Health, and Geropsychiatry Clinics. The sample was predominantly white, with one Asian and one Hispanic participant, consistent with the demographic composition of the surrounding northern New England region. Participants provided written informed consent according to procedures approved by the Institutional Committee for the Protection of Human Subjects.
Screening for eligibility included standardized phone interview with a memory screen,37,38 in-person interview, and review of medical records. Inclusion criteria were at least 60 years of age, right-handed, fluent in English, and at least 12 years of formal education or a GED. Participants were required to have an informant who knew them well and could answer questions about their cognition and general health. The relationships of the informants to the participants were spouse or significant other (70%), adult child (14%), and friend or other family member (16%); this distribution did not differ between groups. Exclusion criteria included any medical, psychiatric, or neurologic condition (other than MCI) that could significantly affect brain structure or cognition, history of head trauma with loss of consciousness lasting more than 5 minutes, history of substance dependence, and factors contraindicating MRI. Nonamnestic forms of MCI39,40 were excluded. One MCI patient was taking a cholinesterase inhibitor, and no participant was taking any other psychoactive medication.
Methods of assessment
Participants underwent a detailed neuropsychological evaluation, including measures of memory, attention, executive function, language, spatial ability, general intellectual ability, and psychomotor speed as well as standard dementia screens. Tests included Mini-Mental State Examination,41 Mattis Dementia Rating Scale-2,42 California Verbal Learning Test (CVLT-I or CVLT-II),43,44 Boston Naming Test,45 Trail Making Test (original or DKEFS),46,47 Wechsler Adult Intelligence Scale III (Digit Symbol, Digit Span, Block Design, Vocabulary, and Information subtests),48 Wechsler Memory Scale (WMS-III: Logical Memory [LM] and Visual Reproduction subtests),49 and Wisconsin Card Sorting Test (short form).50,51 Estimates of baseline intellectual functioning included the American National Adult Reading Test52 and the Barona Index.53
Multiple inventories were employed including the Memory Self-Rating Questionnaire,54 self and informant versions of the Neurobehavioral Function and Activities of Daily Living Rating Scale,55,56 self and informant versions of the Informant Questionnaire on Cognitive Decline in the Elderly,57 the four cognitive items from the Geriatric Depression Scale (GDS),58 10 cognitive items from a telephone-based screening for MCI,37 and 23 items from the Memory Assessment Questionnaire,59 adapted in part from the Functional Activities Questionnaire.60 A Cognitive Complaint Index was calculated as the percentage of all items endorsed.
A board-certified geropsychiatrist (R.B.S.) ruled out depression, dementia, and other Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) Axis I psychiatric disorders based on a semistructured evaluation that included the Hamilton Rating Scale for Depression (HAM-D)61 and GDS.58 A neurologic examination and the original and revised Hachinski Ischemia Scale62,63 were also completed.
Structural brain MRI scans, described below, were reviewed by a board-certified neuroradiologist (A.C.M.), blinded to clinical status, to rule out incidental pathology. White matter changes were rated on a scale adapted from the Age-Related White Matter Changes Scale.64,65 In an effort to enhance sensitivity to subthreshold microvascular or other white matter changes, we added intermediate scores for subtle but detectable white matter changes that were judged within (0.5) or beyond (0.75) those typical for age. The resulting scale included the following designations: 0 = no lesion (including symmetric, well-defined caps or bands); 0.5 = white matter changes noted but age appropriate or less than expected for age; 0.75 = white matter changes noted are more than expected for age; 1 = focal lesions; 2 = beginning confluence of lesions; 3 = diffuse involvement of the entire region/with or without involvement of U fibers.
Group classification and characterization
Group classifications (HC, CC, MCI) were based on results of the neuropsychological assessment, self and informant report indices, and the geropsychiatric and neurologic evaluation. A multidisciplinary clinical consensus panel reviewed each case according to the criteria outlined in table 1. The decision to characterize a participant as having significant CCs was determined by a consensus evaluation of self and informant responses; those considered to have significant CCs typically endorsed 20% or more of the items on the Cognitive Complaint Index.
Table 1.
Criteria used to classify study participants
| HC group | CC group | MCI group | |
|---|---|---|---|
| 1. Abnormal memory performance* | + | ||
| 2. Significant memory complaints, corroborated by an informant† | + | + | |
| 3. Relatively preserved general cognitive functioning | + | + | + |
| 4. Generally normal activities of daily living | + | + | + |
| 5. No dementia | + | + | + |
| 6. No depression or other major psychiatric disorder | + | + | + |
At least 1.5 SDs below the mean established for age- and education-matched controls on standardized tests of episodic memory.
Endorsed at least 20% of possible cognitive complaints across all inventories or complaints deemed significant by clinical consensus.
HC = healthy control; CC = cognitive complaint; MCI = mild cognitive impairment.
The Cognitive Complaint Index and its component scores are presented in table 2. Based on the study classification criteria, the Cognitive Complaint Index was by definition elevated in both the MCI and CC groups relative to the HC group (p < 0.001; figure 1). The CC and MCI groups did not differ and endorsed approximately three times as many complaints as the HC group. Assessment of memory performance was based on age, education, and gender-adjusted scores. The adjustment was made using the mean, SD, and β coefficients obtained from an expanded healthy demographically balanced control group. The MCI participants performed 1.5 SDs below the adjusted mean of HCs on at least one verbal memory test score (CVLT Total 1–5, Short Delay, Long Delay, WMS-III LM I or LM-II; table 2). On average, the MCI group was below the −1.5-SD level on 3.58 (1.39) of the five scores. By contrast, the CC group was below the −1.5-SD level on 0.85 (1.05) of the five scores, similar to the HC group, which had 0.35 (0.74) scores below the cutoff. A composite verbal memory Z score was calculated as the mean of the Z scores of the above five measures and results are shown in table 2 and figure 1. The MCI group differed from the CC and HC groups on both composite memory Z score and the number of tests below cutoff. The CC and HC groups did not differ from each other after adjustment for multiple comparisons.
Table 2.
Participant characteristics
| HC group, n = 40 | CC group, n = 40 | MCI group, n = 40 | p (post hoc differences) | |
|---|---|---|---|---|
| Age, y | 71.0 (5.1) | 73.3 (6.0) | 72.9 (7.1) | NS |
| Education, y | 16.6 (2.7) | 16.4 (2.8) | 16.3 (3.3) | NS |
| Sex (M/F) | 12/28 | 16/24 | 23/17 | 0.042 |
| APOE E4 (−/+)* | 24/16 | 31/9 | 20/18 | 0.063 |
| ANART, est. of verbal IQ | 122.8 (4.7) | 120.7 (6.3) | 120.7 (6.6) | NS |
| Barona FSIQ est.† | 116.1 (5.6) | 116.2 (5.4) | 115.8 (6.1) | NS |
| MMSE (max. 30) | 29.1 (1.0) | 28.9 (1.2) | 27.2 (2.2) | <0.001** |
| DRS total score (max. 144) | 141.0 (2.1) | 141.3 (2.4) | 137.0 (4.6) | <0.001** |
| CVLT Total 1–5 (max. 80)‡ | 48.6 (6.8) | 47.1 (8.6) | 32.6 (6.0) | <0.001** |
| Short delay (max. 16) | 11.2 (1.9) | 10.4 (2.4) | 5.4 (2.7) | <0.001** |
| Long delay (max. 16) | 11.8 (2.0) | 10.5 (2.8) | 5.6 (2.7) | <0.001** |
| WMS-III LM immediate (max. 75) | 48.0 (7.4) | 45.6 (8.2) | 33.6 (8.5) | <0.001** |
| LM delay (max. 50) | 31.6 (6.1) | 28.4 (6.8) | 18.8 (7.9) | <0.001** |
| Verbal memory§ | ||||
| Composite (Z) | 0.01 (0.62) | −0.36 (0.79) | −2.24 (0.76) | <0.001** |
| Tests below cutoff | 0.35 (0.74) | 0.85 (1.05) | 3.58 (1.39) | <0.001** |
| CC scales | ||||
| Overall CCI (%)¶ | 9.8 (6.4) | 30.0 (9.5) | 34.8 (13.9) | <0.001§§ |
| Squire Memory (max. 18) | 5.3 (3.5) | 11.0 (3.8) | 11.3 (4.4) | <0.001§§ |
| ADL self (max. 54) | 3.2 (3.0) | 12.7 (6.7) | 14.4 (9.6) | <0.001§§ |
| ADL informant (max. 54) | 1.7 (2.2) | 8.0 (7.6) | 13.2 (10.6) | <0.001†† |
| IQCODE self (max. 16) | 1.6 (2.3) | 5.5 (3.0) | 5.8 (3.5) | <0.001§§ |
| IQCODE informant (max. 16) | 2.2 (1.2) | 3.9 (2.4) | 5.3 (3.2) | <0.002‡‡ |
| GDS cognitive items (max. 4) | 0.4 (0.8) | 2.0 (1.1) | 2.1 (1.3) | <0.001§§ |
| Memory interview (max. 10) | 2.2 (1.7) | 4.4 (1.9) | 5.2 (2.5) | <0.001§§ |
| MAQ (max. pace 23) | 2.0 (1.6) | 4.0 (2.0) | 4.8 (1.9) | <0.001§§ |
| GDS-NC (max. 26) | 1.8 (2.5) | 3.6 (3.1) | 3.5 (3.2) | <0.01§§ |
| HAM-D61# | 0.1 (0.3) | 1.1 (1.8) | 1.2 (2.0) | NS |
| Hachinski Ischemia score62 (max. 18) | 1.4 (0.5) | 1.3 (0.5) | 1.3 (0.5) | NS |
| Revised Ischemia score63 (max. 52)|| | 4.6 (0.9) | 4.7 (1.1) | 4.7 (1.3) | NS |
APOE data missing for two participants in MCI group.
Demographically based estimate of full-scale IQ.53
California Verbal Learning Test-I/II, Total Learning Trials 1 through 5 (maximum 80), short and long delay free recall (maximum 16 per trial).
Composite is the mean age, education and sex adjusted Z score for the five verbal memory measures; tests below cutoff is the number of verbal memory tests out of five that fell ≥1.5 SDs below control group mean.
Cognitive Complaint Index (CCI), percentage of all complaint items endorsed in a positive (i.e., symptomatic) direction. See text for references for the component scales.
Available for 43 participants (HCs, 15; CCs 16; MCI, 12).
Available for 96 participants (HCs, 29, CCs, 34, MCI, 33).
For analyses of variance post hoc group contrasts:
MCI vs HCs, CCs;
MCI vs CC vs HC;
MCI vs HCs;
MCI, CC vs HC with direction indicated by the table values. For sex, χ2 differed for HCs vs MCI only. For APOE, MCI had a higher frequency of the E4 allele than CCs.
HC = healthy control; CC = cognitive complaint; MCI = mild cognitive impairment; NS = not significant; ANART = American National Adult Reading Test; FSIQ = full-scale IQ; MMSE = Mini-Mental State Examination; DRS = Dementia Rating Scale-2; CVLT = California Verbal Learning Test-I/II; WMS-III = Wechsler Memory Scale III; LM = logical memory; CCI = Cognitive Complaint Index; ADL = activities of daily living; IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly; GDS = Geriatric Depression Scale; MAQ = Memory Assessment Questionnaire; GDS-NC = Geriatric Depression Scale noncognitive items; HAM-D = Hamilton Rating Scale for Depression.
Figure 1.
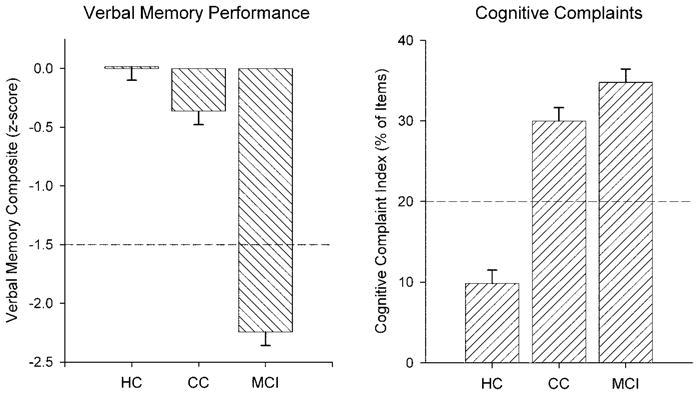
Characterization of the healthy control (HC, n = 40), cognitive complaint (CC, n = 40), and mild cognitive impairment (MCI, n = 40) groups on verbal memory performance composite domain score and the Cognitive Complaint Index indicating the percentage of possible complaints. By definition, the HC group had normal memory performance and a low level of complaints, whereas the MCI group had significant complaints and deficits. The CC group had normal performance but was nearly as elevated in complaints as the MCI group.
On depression measures, there were no significant elevations or between-group differences on the HAM-D. Although the CC and MCI groups scored an average of 2 points higher than the HC group on the adjusted GDS (four cognitive items deleted), all three group means were well within normal limits (table 2). No participant showed depression on the comprehensive geropsychiatric evaluation.
With the exception of a sex difference, there were no significant group differences in demographics (table 2). There was a group difference in APOE genotype, with the CC group showing a preponderance of E4-negative individuals (table 2). Sex and APOE genotype were used in secondary analyses to clarify their potential relationship with GM density.
Imaging
Scan acquisition
Scans were obtained on a GE Signa 1.5-T Horizon LX magnet with echo speed gradients using a standard head RF coil. A T1-weighted three-dimensional spoiled gradient echo (SPGR) coronal volume was acquired. Parameters were TR = 25, TE = 3 or min, flip angle = 40 degrees, 1 NEX, and slice thickness = 1.5 mm (no skip), yielding 124 contiguous slices with a 24-cm field of view and a 256 × 256 matrix with 0.9375 mm in-plane resolution. We also acquired a fast spin echo T2-weighted scan as a screen for focal lesions or other incidental findings (TR = 3000, TE = 96, 3 mm contiguous axial slices).
Preprocessing and VBM
Scans were reconstructed from slice data using scripts written in Matlab (Mathworks, Inc.). Data were then resampled to isotropic 1-mm3 voxels, aligned visually to the AC-PC plane using BRAINS software,66,67 and reformatted to the axial plane. VBM was performed using locally developed automation scripts to implement the optimized methods described by Good et al.33 and Ashburner and Friston68 Briefly, the T1-weighted AC-PC–aligned SPGR volumes were resampled to 1.5-mm3 voxels and segmented to extract GM maps. A custom age-appropriate brain template was used for automated removal of extracerebral tissue including the skull and meninges. GM maps were then spatially normalized to the GM prior probability template using a 12-parameter model including nonlinear basis functions as implemented in the Statistical Parametric Mapping package (Wellcome Department of Imaging Neuroscience, London, UK, http://www.fil.ion.ucl.ac.uk/spm/). The normalized scans were then smoothed using an isotropic spatial filter with full width half maximum of 12 mm to help account for individual differences in gyral anatomy. The smoothed normalized GM maps were used for subsequent analyses.
Hippocampal volume and ROI analysis
Methods for manual segmentation of the hippocampus have been described elsewhere,69,70 and our protocol71 is summarized briefly here. Images were reformatted into isotropic 1-mm voxels and resampled into the plane perpendicular to the long axis of the hippocampus using BRAINS.66,67 Manual traces were performed in the coronal plane with reference to markings placed in the orthogonal views to guide boundary determination. The anterior boundary, visualized in the sagittal plane, included the point where the alveus, a thin band of white matter, was observed between the hippocampus and amygdala. Additional anterior boundary landmarks in the axial plane included the uncal notch indicating the beginning of the coronal nucleus of the amygdala. The posterior boundary was defined in the sagittal plane where the tail of the hippocampus was surrounded by white matter on three sides.72 The lateral border of the hippocampus was the CSF of the temporal horn of the lateral ventricle. On the inferior bank, the subicular complex was included. The boundary with the entorhinal cortex was defined by outlining the subiculum in the sagittal plane superiorly, adjacent to the lateral ventricle, as well as inferiorly, adjacent to the uncinate fasciculus.69,73 The medial edge was bounded by the CSF in the uncal and ambient cisterns, and the dorsomedial boundary included the choroidal fissure.
Hippocampal volume and ROI template procedure
Left and right hippocampal volumes were calculated for each participant by summing the coronal slice areas and then adjusted for age and total intracranial volume using a regression model. Inter- and intrarater reproducibility assessed by intraclass correlation coefficients were >0.94 for both the left and right hippocampi. Templates for the left and right hippocampi were constructed by averaging the ROIs derived from the HC group on a voxel-by-voxel basis in Montreal Neurological Institute atlas space. Individual ROIs were smoothed using an isotropic 3-mm FWHM filter prior to averaging. These ROI templates were then used to extract hippocampal GM signal intensity values from VBM for all participants.
Statistical analyses
The GM maps derived from VBM were analyzed on a voxel-by-voxel basis using general linear model and random effects methods. Analysis of variance (ANOVA) was used to assess group differences in GM density using a two-stage approach. First, we examined the hypothesized MTL region of interest. Second, we performed an unbiased whole brain analysis of GM using a more stringent spatial threshold. For the hypothesized MTL region, we employed a small volume search area with a family-wise error threshold of 0.05 and a minimum cluster size (k) of seven contiguous voxels (24 mm3). For the whole brain analysis, we used a threshold of 0.001 at the voxel level and k of 75 contiguous voxels (253 mm3). Major clusters identified on the whole brain analyses by the omnibus F test were further analyzed using the following ROI approach. Spherical ROIs were centered at the cluster local maxima. Because brain structures of varying sizes were included in the analyses, we used a diameter of 6 mm for the ROI to standardize GM sampling. For each ROI, the first eigen-variate of signal intensity was subjected to further analysis using the Tukey honestly significant difference test to assess pairwise group differences. A series of covariance analyses was used to assess the relationships between GM reduction and memory in the combined sample (n = 120). For episodic memory performance and memory complaints, we analyzed the composite verbal memory Z score and the Cognitive Complaint Index. Hippocampal volume data were analyzed by ANOVA with planned comparisons after adjustment for age and total intracranial volume (ICV) with regression-based estimates of these covariates derived from the HC group.
Results
Group differences on VBM
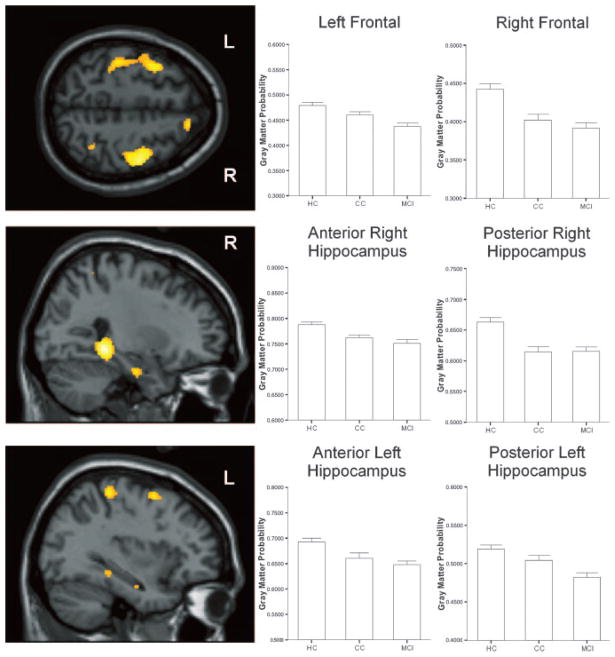
Group differences in GM density were found in bilateral MTL and distributed cortical regions (table 3). As expected, the MCI group showed reduced GM density relative to the HC group in distributed brain regions, including bilateral medial temporal, frontotemporal, and other neocortical areas (figure 2, table 3). The CC group showed a similar, although slightly more circumscribed, pattern of reduced GM density relative to the HC group (figure 2, table 3). There were no regions in which the MCI or CC group showed higher GM density than the HC group.
Table 3.
Regions showing reduced gray matter density in MCI and CC groups relative to the HC group: brain region,* Montreal Neurological Institute atlas coordinates (mm), and individual group effects
| Brain region | x, y, z | HC > MCI | HC > CC | CC > MCI |
|---|---|---|---|---|
| Hippocampal region of interest† | ||||
| Right subgyral, hippocampus | 27, −39, −4 | <0.001 | <0.001 | NS |
| Left parahippocampal gyrus, BA27 | −26, −32, −6 | <0.001 | 0.024 | NS |
| Right parahippocampal gyrus, hippocampus | 28, −12, −27 | <0.001 | 0.015 | NS |
| Left parahippocampal gyrus, BA28 | −20, −18, −18 | 0.009 | 0.001 | NS |
| Left parahippocampal gyrus, hippocampus | −32, −12, −22 | 0.001 | 0.022 | NS |
| Whole brain‡ | ||||
| Right precentral gyrus, BA4 | 48, −9, 52 | <0.001 | 0.001 | NS |
| Left middle frontal gyrus, BA6 | −36, 4, 56 | <0.001 | NS | 0.041 |
| Right inferior frontal gyrus, BA47 | 14, 18, −21 | <0.001 | NS | 0.015 |
| Right middle temporal gyrus, BA21 | 51, −30, −2 | <0.001 | 0.001 | NS |
| Left insula, BA13 | −38, −26, 15 | <0.001 | 0.012 | NS |
| Left medial frontal gyrus, BA11 | −3, 52, −20 | 0.003 | <0.001 | NS |
| Right inferior temporal gyrus, BA20 | 48, 0, −45 | <0.001 | 0.003 | NS |
| Left angular gyrus, BA39 | −44, −76, 27 | <0.001 | NS | NS |
| Left superior temporal gyrus, BA42 | −63, −32, 18 | 0.002 | <0.001 | NS |
| Right cuneus, BA30 | 15, −70, 9 | 0.001 | 0.001 | NS |
Spherical region of interest (diameter 6 mm) centered on representative voxel.
The left and right hippocampal regions of interest templates were based on the mean of smoothed individual regions of interest from the 40 healthy controls. After thresholding, these regions of interest templates included portions of parahippocampal gyrus. Small volume search area with family-wise error threshold of 0.05 and minimum cluster extent (k) of seven.
p uncorr < 0.001, k = 75.
HC = healthy control; MCI = mild cognitive impairment; CC = cognitive complaint; NS = not significant.
Figure 2.
Regions showing significant GM atrophy in the MCI and the CC groups compared to HC group. Displayed at the left of each panel are images showing selected regions with group differences in the overall analysis, including bilateral frontal (top), right hippocampus (middle), and left hippocampus (bottom, p < 0.001). Also displayed are graphs of group differences in signal intensity from spherical regions of interest in each of the corresponding brain areas. See text for full description of results of statistical analyses.
Relationship between GM and memory
The composite verbal memory Z score was lower in those with reduced GM density, predominantly in bilateral medial temporal and distributed cortical regions (figure 3 and table E-1 on the Neurology Web site at www.neurology.org). No regions showed increased GM density with a reduction in verbal memory. A higher Cognitive Complaint Index indicated a reduction in GM density, predominantly in bilateral medial temporal and other cortical and subcortical regions (table E-1; figure 4). No regions showed an increase in GM density as complaints increased, nor did GM density diminish with fewer complaints.
Figure 3.
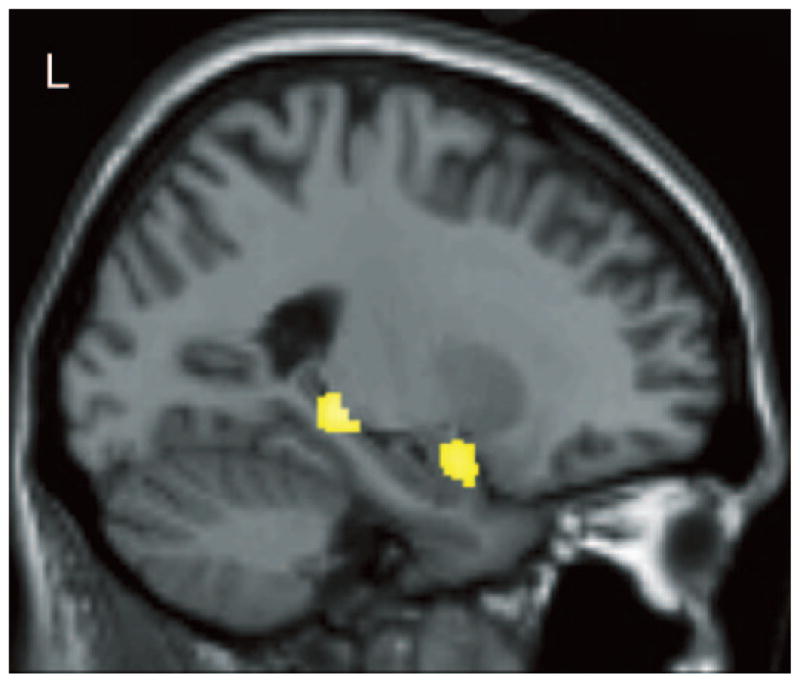
Verbal learning performance was positively related to gray matter density in left medial temporal regions across the entire sample (N = 120, p < 0.001). See text for a detailed description of the statistical analyses and results.
Figure 4.
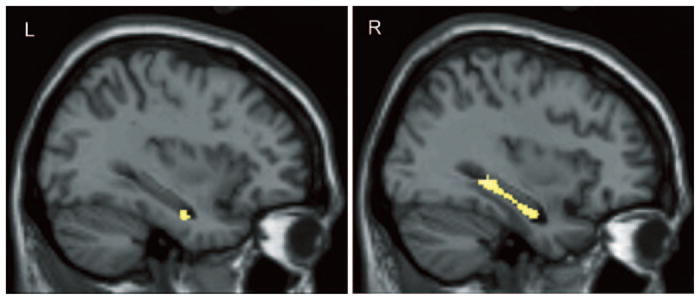
Higher levels of cognitive complaints were associated with decreased gray matter density in the left and right hippocampi across the entire sample (N = 120, p < 0.001). See text for a detailed description of the statistical analyses and results.
Additional analyses
We examined several relevant covariates including sex, APOE genotype, and total ICV. Although each covariate slightly attenuated the regional effect sizes, all areas showing group differences in GM density in the original analysis remained significant. Using the white matter ratings scale described above, three HCs, three patients with MCI, and no CC group members had subtle white matter hyperintensities greater than expected for age. No participant had diffuse hyperintensities. We repeated the analysis of group differences after excluding these participants with subtle white matter changes of presumed microvascular etiology and one additional MCI patient with enlargement of the Sylvian fissure; regions showing group differences in the original analysis remained significant again with a slight reduction of effect size.
Hippocampal volume and GM density
Age- and ICV-adjusted hippocampal volumes are shown in figure 5 (top row). As expected, there were between group differences (left: F(2,117) = 7.55, p = 0.0008; right: F(2,117) = 8.67, p = 0.0003). The MCI group showed hippocampal volume reduction compared to both the HC (left and right both p < 0.0005) and CC (left and right both p < 0.005) groups. The HC and CC groups did not differ. Although all groups showed larger right than left volumes, there was no group by hemisphere interaction. Age-adjusted GM density for the left and right hippocampal template ROIs are shown in figure 5 (bottom row). There were between-group differences (left: F(3,116) = 25.06, p < 0.0001; right: F(3,116) = 22.77, p < 0.0001). Both the CC and MCI groups showed reduction of GM density vs HC (CC < HC, left p = 0.024 and right p = 0.008; MCI < HC, left and right p < 0.001). The CC and MCI groups did not differ. There was a trend toward higher GM values for the left than right hemisphere across groups (p = 0.08) but no group x hemisphere interaction.
Figure 5.
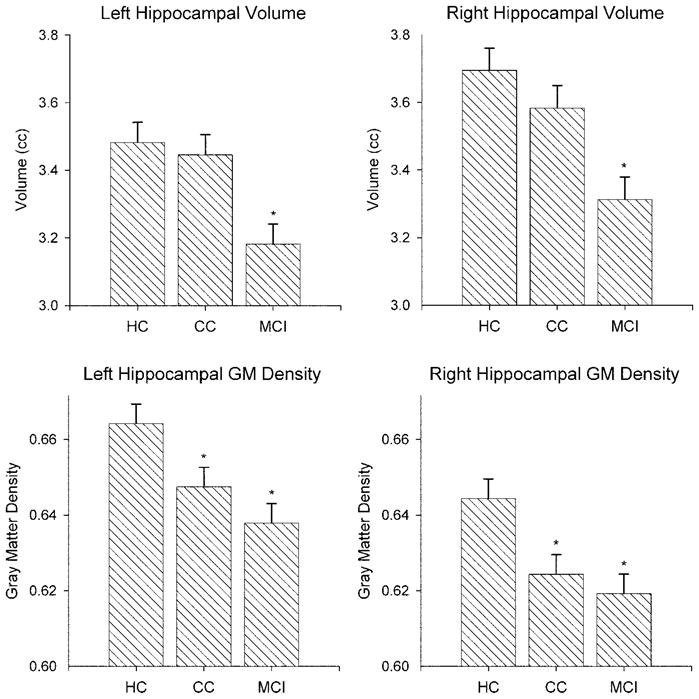
Hippocampal volume and gray matter density by group. Age- and intracranial volume–adjusted means (± SE) for manually segmented left and right hippocampi are shown in the top row. Age-adjusted gray matter densities for the hippocampi are shown in the bottom row.
Discussion
This study characterized the pattern of regional GM loss in older adults with marked CCs but normal test performance. The MCI and CC groups showed a similar pattern of reduced GM density in bilateral medial temporal, frontal, and other distributed brain regions. This pattern of findings indicates that structural brain changes similar to those seen in MCI are present even in cognitively intact, nondepressed older adults with significant memory complaints. The changes were slightly more extensive in the MCI group than the CC group compared to the HC group, suggesting that the CC group may represent a point on a continuum between normal aging and MCI. Significant CCs may signify a very early stage of the dementing process for some individuals and may constitute a pre-MCI stage in these cases.
Models suggesting a continuum from normal aging to AD, however, have been questioned based on neuropathologic evidence of changes characteristic of AD in some individuals with MCI.74 Such data are not presently available for CC cohorts. Prior studies75 have reported elevated incidence of conversion to dementia in individuals with CCs meeting criteria for Clinical Dementia Rating 0.5 (“questionable dementia”).76 The results of our ongoing longitudinal study will ultimately help to clarify the relative rates of conversion to dementia from CCs and MCI. Follow-up with neuropsychological and neuroimaging methods will help determine the cognitive trajectory, progression of structural brain changes, and diagnostic outcomes within each group.
Across the entire combined sample of older adults, reduction of GM density in medial temporal and other regions was correlated with both subjective memory complaints and verbal learning performance. This indicates that the structural brain changes seen in the CC and MCI groups have functional significance in terms of memory ability. Together with prior research relating frontal metabolism to subjective memory ratings in older adults,77 these findings highlight the importance of CCs in the clinical evaluation of older adults and suggest that those who present with significant CCs warrant evaluation and close monitoring over time. Although subtle cognitive anomalies may be present many years before dementia onset,78–80 incorporating information on cognitive complaints81 and structural changes may be important for prognosis. As new treatments and preventive strategies for MCI and AD are developed and refined, the earliest possible accurate detection of people at increased risk of dementia will take on critical importance.
The design of this study enabled us to rule out factors commonly associated with memory complaints in older adults, including depression, other DSM-IV Axis I disorders, psychoactive medication, and significant white matter pathology.6,82–84 Therefore, our data indicate that GM atrophy and associated cognitive changes can occur independently of these factors. Other notable strengths of this study are the comprehensive nature of the assessment of CCs including both self and informant perception as well as the combination of VBM GM density and hippocampal volume ROI analyses in the same cohort.
A potential limitation in terms of the generalizability of our results is that most participants had high education and estimated baseline intellect. High baseline functioning or cognitive reserve may buffer the effects of brain pathology on cognition.85,86 High baseline individuals may be more likely to express subjective complaints before objective measures can detect decline. Our study warrants replication in cohorts with lower levels of baseline functioning given the potential implications for early diagnosis. Our results can not be generalized to nonamnestic subtypes of MCI,39 which may show a different profile on neuroimaging. Within amnestic MCI, we did not attempt to assess whether single and multiple domain subtypes can be distinguished using structural MRI.
VBM may be sensitive to the earliest stages of dementia, before the onset of cognitive changes measurable on comprehensive neuropsychological evaluation. It has advantages and limitations as compared to an ROI-based approach involving manual tracing of specific brain structures.33,87,88 VBM is largely automated and is therefore highly reproducible and much less labor-intensive than manual tracing. In addition, VBM may be ideal for application in the earliest stages of disease, when brain structural changes are so subtle that they cannot readily be detected visually. Our observation that the CC group showed significantly reduced hippocampal GM density but not volume reduction suggests that voxel-based approaches incorporating signal intensity may have greater sensitivity in early preclinical stages than volumetric methods. This finding warrants replication in view of the implications for early detection of those at elevated risk of dementia. VBM will likely be useful to aid in early detection, selection of participants in clinical trials, and treatment monitoring. However, VBM analyses are computationally demanding, and some processing steps, such as the use of age-specific templates, have yet to be fully standardized. These methodologic issues are discussed in detail by Ashburner et al.89 Overall, VBM- and ROI-based approaches are likely to provide complementary information.
Supplementary Material
Acknowledgments
Supported in part by grants from the National Institute on Aging (R01 AG19771), Alzheimer’s Association (Hedco Foundation), Hitchcock Foundation, Ira DeCamp Foundation, National Science Foundation, New Hampshire Hospital, and NAMIC (U54 EB005149).
The authors thank the following for help with this study: Leslie Baxter, Marlana Borgos, Cheryl Brown, Katherine Nutter-Upham, Nadia Paré, Heather Pixley, Jennifer Randolph, Li Shen, and Paul Wang of the Department of Psychiatry and Kelli Clifford, Alice Davison, Robert Ferranti, Bob Shaffer, Shreve Soule, and colleagues in the Department of Radiology.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 3.Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand. 2002;106:403–414. doi: 10.1034/j.1600-0447.2002.01417.x. [DOI] [PubMed] [Google Scholar]
- 4.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Taylor JL, Miller TP, Tinklenberg JR. Correlates of memory decline: a 4-year longitudinal study of older adults with memory complaints. Psychol Aging. 1992;7:185–193. doi: 10.1037//0882-7974.7.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7– 8 years. Psychol Med. 2001;31:441–449. [PubMed] [Google Scholar]
- 7.Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46:121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- 8.Schmand B, Jonker C, Geerlings MI, Lindeboom J. Subjective memory complaints in the elderly: depressive symptoms and future dementia. Br J Psychiatry. 1997;171:373–376. doi: 10.1192/bjp.171.4.373. [DOI] [PubMed] [Google Scholar]
- 9.Tobiansky R, Blizard R, Livingston G, Mann A. The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med. 1995;25:779–786. doi: 10.1017/s0033291700035029. [DOI] [PubMed] [Google Scholar]
- 10.Jorm A, Masaki K, Davis D, et al. Memory complaints in nondemented men predict future pathologic diagnosis of Alzheimer disease. Neurology. 2004;63:1960–1961. doi: 10.1212/01.wnl.0000144348.70643.f2. [DOI] [PubMed] [Google Scholar]
- 11.Flicker C, Ferris SH, Reisberg B. A longitudinal study of cognitive function in elderly persons with subjective memory complaints. J Am Geriatr Soc. 1993;41:1029–1032. doi: 10.1111/j.1532-5415.1993.tb06448.x. [DOI] [PubMed] [Google Scholar]
- 12.Jorm AF. Alzheimer’s disease: risk and protection. Med J Aust. 1997;167:443–446. doi: 10.5694/j.1326-5377.1997.tb126660.x. [DOI] [PubMed] [Google Scholar]
- 13.Derouesne C, Lacomblez L, Thibault S, LePoncin M. Memory complaints in young and elderly subjects. Int J Geriatr Psychiatry. 1999;14:291–301. [PubMed] [Google Scholar]
- 14.Smith GE, Petersen RC, Ivnik RJ, Malec JF, Tangalos EG. Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychol Aging. 1996;11:272–279. doi: 10.1037//0882-7974.11.2.272. [DOI] [PubMed] [Google Scholar]
- 15.Stewart R. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;57:2149. doi: 10.1212/wnl.57.11.2149. Correspondence. [DOI] [PubMed] [Google Scholar]
- 16.Stewart R, Russ C, Richards M, Brayne C, Lovestone S, Mann A. Depression, APoE genotype and subjective memory impairment: a cross-sectional study in an African-Caribbean population. Psychol Med. 2001;31:431–440. [PubMed] [Google Scholar]
- 17.Saykin AJ, Wishart HA. Mild cognitive impairment: conceptual issues and structural and functional brain correlates. Semin Clin Neuropsychiatry. 2003;8:12–30. doi: 10.1053/scnp.2003.50002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baxter LC, Caselli RJ, Johnson SC, Reiman EM, Osborne D. Apolipoprotein e e4 affects new learning in cognitively normal individuals at risk for Alzheimer’s disease. Neurobiol Aging. 2003;24:947–952. doi: 10.1016/s0197-4580(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 19.Jorm AF, Butterworth P, Anstey KJ, et al. Memory complaints in a community sample aged 60 – 64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, ApoE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol Med. 2004;34:1495–1506. doi: 10.1017/s0033291704003162. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 21.Raz N, Gunning FM, Head D, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the pre-frontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 22.Frisoni G, Testa C, Zorzan A, et al. Detection of grey matter loss in mild Alzheimer’s disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2003;73:657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zakzanis KK. Quantitative evidence for neuroanatomic and neuropsychological markers in dementia of the Alzheimer’s type. J Clin Exp Neuropsychol. 1998;20:259–269. doi: 10.1076/jcen.20.2.259.1174. [DOI] [PubMed] [Google Scholar]
- 24.Kantarci K, Jack CR., Jr Neuroimaging in Alzheimer disease: an evidence-based review. Neuroimaging Clin N Am. 2003;13:197–209. doi: 10.1016/s1052-5149(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 25.Anstey KJ, Maller JJ. The role of volumetric MRI in understanding mild cognitive impairment and similar classifications. Aging Ment Health. 2003;7:238–250. doi: 10.1080/1360786031000120732. [DOI] [PubMed] [Google Scholar]
- 26.Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- 27.Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 28.Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14:966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- 29.Taki Y, Goto R, Evans A, et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol Aging. 2004;25:455–463. doi: 10.1016/j.neurobiolaging.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron J-C. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13:1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- 31.Pennanen C, Testa C, Laakso MP, et al. A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2005;76:11–14. doi: 10.1136/jnnp.2004.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron JC, Chetelat G, Desgranges B, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 33.Good CD, Scahill RI, Fox NC, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- 34.Karas GB, Burton EJ, Rombouts SA, et al. A comprehensive study of gray matter loss in patients with Alzheimer’s disease using optimized voxel-based morphometry. Neuroimage. 2003;18:895–907. doi: 10.1016/s1053-8119(03)00041-7. [DOI] [PubMed] [Google Scholar]
- 35.Grossman M, McMillan C, Moore P, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- 36.Busatto GF, Garrido GE, Almeida OP, et al. A voxel-based morphometry study of temporal lobe gray matter reductions in Alzheimer’s disease. Neurobiol Aging. 2003;24:221–231. doi: 10.1016/s0197-4580(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 37.Rabin LA, Saykin AJ, Wishart HA, Copenhaver BR, Flashman LA, Santulli RB. Telephone-based screening for MCI and cognitive complaints: preliminary validation by comprehensive assessment. Presented at the 2004 International Neuropsychological Society Meeting; 2004. [Google Scholar]
- 38.Rabin LA, Saykin AJ, Wishart HA, Wang PJ, Nutter-Upham KE, Flashman LA. Telephone-based screening for MCI and cognitive complaints: preliminary validation of alternate test forms. Presented at the 24th Annual Conference of the National Academy of Neuropsychology; November 17–20, 2004; Seattle, WA. [Google Scholar]
- 39.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 40.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 41.Folstein MF, Folstein SE, McHugh PR. “Mini mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 42.Jurica P, Leitten C, Mattis S. Dementia Rating Scale-2. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 43.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: adult version research edition manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 44.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-second edition: adult version manual. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 45.Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination. 3. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 46.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 47.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 48.Wechsler D. Wechsler Adult Intelligence Scale. San Antonio, TX: Harcourt, Brace; 1997. [Google Scholar]
- 49.Wechsler D. Wechsler Memory Scale-third edition WMS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 50.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test manual revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 51.Axelrod BN, Goldman RS, Heaton RK, et al. Discriminability of the Wisconsin Card Sorting Test using the standardization sample. J Clin Exp Neuropsychol. 1996;18:338–342. doi: 10.1080/01688639608408991. [DOI] [PubMed] [Google Scholar]
- 52.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 53.Barona A, Reynolds C, Chastain R. A demographically based index of pre-morbid intelligence for the WAIS-R. J Consult Clin Psychol. 1984;52:885–887. [Google Scholar]
- 54.Squire LR, Wetzel CD, Slater PC. Memory complaint after electroconvulsive therapy: assessment with a new self-rating instrument. Biol Psychiatry. 1979;14:791–801. [PubMed] [Google Scholar]
- 55.Saykin AJ, Janssen R, Sprehn G, Spira T, Kaplan J, O’Connor B. Longitudinal evaluation of neuropsychological function in homosexual men with hiv-1 infection; 18 month follow-up. J Neuropsychiatry Clin Neurosci. 1991;3:286–298. doi: 10.1176/jnp.3.3.286. [DOI] [PubMed] [Google Scholar]
- 56.Saykin AJ. Neurobehavioral function and activities of daily living rating scale (NBFADL-63 item version) Hanover: Dartmouth Medical School; 1992. [Google Scholar]
- 57.Jorm AF, Jacomb PA. An informant questionnaire on cognitive decline in the elderly (IQCODE): Socio-demographic correlates reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 58.Yesavage JA, Brink TL, Lose TL, et al. Development and validation of Geriatric Depression Rating Scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 59.Santulli R, Saykin A, Rabin L, et al. Differential sensitivity of cognitive complaints associated with amnestic MCI: analysis of patient and informant reports. Presented at the Alzheimer’s Association International Conference on the Prevention of Dementia; Washington, DC. June 18 –21, 2005. [Google Scholar]
- 60.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 61.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 63.Small GW. Revised ischemic score for diagnosing multi-infarct dementia. J Clin Psychiatry. 1985;46:514–517. [PubMed] [Google Scholar]
- 64.Fazekas F, Barkhof F, Wahlund LO, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002;13:31–36. doi: 10.1159/000049147. [DOI] [PubMed] [Google Scholar]
- 65.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 66.Andreasen NC, Cohen G, Harris G, et al. Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci. 1992;4:125–133. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- 67.Andreasen NC, Rajarethinam R, Cizadlo T, et al. Automatic atlas-based volume estimation of human brain regions from MR images. J Comput Assist Tomogr. 1996;20:98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 68.Ashburner J, Friston KF. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 69.Jack CR., Jr MRI-based hippocampal volume measurements in epilepsy. Epilepsia. 1994;35:S21–S29. doi: 10.1111/j.1528-1157.1994.tb05986.x. [DOI] [PubMed] [Google Scholar]
- 70.Hasboun D, Chantome M, Zouaoui A, et al. MR determination of hippocampal volume: comparison of three methods. AJNR Am J Neuroradiol. 1996;17:1091–1098. [PMC free article] [PubMed] [Google Scholar]
- 71.McHugh TL, Saykin AJ, Wishart HA, et al. Hippocampal volume and shape analysis in an older adult population. Clin Neuropsychol. doi: 10.1080/13854040601064534. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duvernoy H. The human hippocampus. New York: J.F. Bergmann Verlag Munchen; 1988. [Google Scholar]
- 73.Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 74.Morris JC. Mild cognitive impairment is early-stage Alzheimer disease: time to revise diagnostic criteria. Arch Neurol. 2006;63:15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- 75.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 76.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- 77.Small GW, Okonek A, Mandelkern MA, et al. Age-associated memory loss: initial neuropsychological and cerebral metabolic findings of a longitudinal study. Int Psychogeriatr. 1994;6:23–44. doi: 10.1017/s1041610294001596. [DOI] [PubMed] [Google Scholar]
- 78.Snowdon DA, Greiner LH, Markesbery WR. Linguistic ability in early life and the neuropathology of Alzheimer’s disease and cerebrovascular disease: findings from the nun study. Ann NY Acad Sci. 2000;903:34–38. doi: 10.1111/j.1749-6632.2000.tb06347.x. [DOI] [PubMed] [Google Scholar]
- 79.Amieva H, Jacqmin-Gadda H, Orgogozo J-M, et al. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain. 2005;128:1093–1101. doi: 10.1093/brain/awh451. [DOI] [PubMed] [Google Scholar]
- 80.Backman L, Jones S, Berger A-K, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 81.Fisk JD, Rockwood K. Outcomes of incident mild cognitive impairment in relation to case definition. J Neurol Neurosurg Psychiatry. 2005;76:1175–1177. doi: 10.1136/jnnp.2004.053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Groot JC, de Leeuw F-E, Oudkerk M, Hofman A, Jolles J, Breteler MMB. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56:1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- 83.Small GW, Chen ST, Komo S, et al. Memory self-appraisal and depressive symptoms in people at genetic risk for Alzheimer’s disease. Int J Geriatr Psychiatry. 2001;16:1071–1077. doi: 10.1002/gps.481. [DOI] [PubMed] [Google Scholar]
- 84.Jungwirth S, Fischer P, Weissgram S, Kirchmeyr W, Bauer P, Tragl K. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc. 2004;52:263–268. doi: 10.1111/j.1532-5415.2004.52066.x. [DOI] [PubMed] [Google Scholar]
- 85.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 86.Rentz DM, Huh TJ, Faust RR, et al. Use of IQ-adjusted norms to predict progressive cognitive decline in highly intelligent older individuals. Neuropsychology. 2004;18:38–49. doi: 10.1037/0894-4105.18.1.38. [DOI] [PubMed] [Google Scholar]
- 87.Good CD, Ashburner J, Frackowiak RS. Computational neuroanatomy: new perspectives for neuroradiology. Rev Neurol (Paris) 2001;157:797–806. [PubMed] [Google Scholar]
- 88.Tisserand DJ, Pruessner JC, Sanz Arigita EJ, et al. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- 89.Ashburner J, Csernansky JG, Davatzikos C, Fox NC, Frisoni GB, Thompson PM. Computer-assisted imaging to assess brain structure in healthy and diseased brains. Lancet Neurol. 2003;2:79–88. doi: 10.1016/s1474-4422(03)00304-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.