Abstract
Recombinant cellulose-binding domain (CBD) derived from the cellulolytic bacterium Clostridium cellulovorans was found to modulate the elongation of different plant cells in vitro. In peach (Prunus persica L.) pollen tubes, maximum elongation was observed at 50 μg mL−1 CBD. Pollen tube staining with calcofluor showed a loss of crystallinity in the tip zone of CBD-treated pollen tubes. At low concentrations CBD enhanced elongation of Arabidopsis roots. At high concentrations CBD dramatically inhibited root elongation in a dose-responsive manner. Maximum effect on root hair elongation was at 100 μg mL−1, whereas root elongation was inhibited at that concentration. CBD was found to compete with xyloglucan for binding to cellulose when CBD was added first to the cellulose, before the addition of xyloglucan. When Acetobacter xylinum L. was used as a model system, CBD was found to increase the rate of cellulose synthase in a dose-responsive manner, up to 5-fold compared with the control. Electron microscopy examination of the cellulose ribbons produced by A. xylinum showed that CBD treatment resulted in a splayed ribbon composed of separate fibrillar subunits, compared with a thin, uniform ribbon in the control.
Endogenous regulation of cell elongation appears to be dominated by cell wall mechanics. This process is a result of the interaction between internal turgor pressure and the mechanical strength of the cell wall (Steer and Steer, 1989). Unlike most plant cells, the growth of pollen tubes and root hairs is restricted to the tip zone (Cresti and Tiezzi, 1992). The growing region of pollen tubes consists of two distinct layers when fully mature. The inner layer consists mostly of callose-related molecules, and the outer layer contains pectin, XG, cellulose (at low levels and poor crystallinity), and other polysaccharides (Steer and Steer, 1989). XG is bound to cellulose microfibrils in the cell walls of all dicotyledons and some monocotyledons (Roberts, 1994). The XG bound to the cellulose microfibrils cross-links the cell wall framework. Plant cell expansion requires the integration of local wall loosening and the controlled deposition of new wall materials. Fry et al. (1992) and Nishitani and Tominaga (1992) purified XG endo-transglycosylase and endo-XG transferase, respectively. These two enzymes were shown to be responsible for the transfer of intermicrofibrillar XG segments to another XG molecule and were therefore suggested to be wall-loosening enzymes. However, McQueen-Mason et al. (1993) showed that XG endo-transglycosylase activity did not correlate with in vitro cell wall extension in cucumber hypocotyls. Another type of cell wall-loosening protein, expansin, was isolated by McQueen-Mason et al. (1992). Expansin does not exhibit hydrolytic activity with any of the cell wall components. Instead, it binds at the interface between cellulose microfibrils and matrix polysaccharides in the cell wall and is suggested to induce cell wall expansion by reversibly disrupting noncovalent bonds within this polymeric network (McQueen-Mason and Cosgrove, 1995).
XGs are linear chains of β-(1→4)-d-glucan, but unlike cellulose, they possess numerous xylosyl units added at regular sites to the O-6 position of the glucosyl units of the chain (Carpita and Gibeaut, 1993). XG can be extracted by alkaline treatment and then bound again in vitro to cellulose (Hayashi et al., 1994). The effect of XG on growing tissues has been investigated extensively. XG oligosaccharides, produced by partial digestion with β-(1 → 4)-d-glucanase and referred to as “oligosaccharins,” alter plant cell growth (Aldington and Fry, 1993). In pea stem segments one such oligosaccharin, XXFG (XG9), antagonizes the auxin-induced growth promotion at a concentration of about 1 nm (York et al., 1984; McDougall and Fry, 1988). On the other hand, in etiolated pea stem segments high concentrations (100 μm) of oligosaccharins promote the elongation process (McDougall and Fry, 1990). The mode of action of such oligosaccharins is still unknown.
The gram-negative bacterium Acetobacter xylinum has long been regarded as a model of cellulose synthesis, mainly because it separates between the cellulose microfibril synthesis and cell wall formation (Ross et al., 1991). Cellulose synthesized by A. xylinum is produced as separate ribbons composed of microfibrils; thus, potential interactions with other polysaccharides do not exist as in the plant cell wall. Since polymerization and crystallization are coupled processes in cellulose synthesis in A. xylinum, interference with the crystallization results in the acceleration of polymerization (Benziman et al., 1980). Some cellulose-binding organic substances can also alter cell growth and cellulose microfibril assembly in vivo. Direct dyes, CMC, and fluorescent brightening agents (e.g. calcofluor white ST) prevent microfibril crystallization in A. xylinum, thereby enhancing polymerization. These molecules bind to the polysaccharide chains immediately after their extrusion from the cell surface, preventing normal assembly of microfibrils and cell walls (Haigler, 1991).
Shoseyov and Doi (1990) isolated a unique cellulose-binding protein from the cellulolytic bacterium Clostridium cellulovorans L. This major subunit of the cellulase complex was found to bind to cellulose but had no hydrolytic activity and was essential for the degradation of crystalline cellulose. The cbpA gene has been cloned and sequenced (Shoseyov et al., 1992). When PCR primers flanking the CBD gene were used, the latter was successfully cloned into an overexpression vector that enabled us to overproduce the 17-kD CBD in Escherichia coli. The recombinant CBD exhibits very strong affinity to cellulose (Goldstein et al., 1993). In recent years, several CBDs have been isolated from different sources, but most of them have been isolated from proteins that have separate catalytic cellulase and CBDs, and only two have been isolated from proteins that have no apparent hydrolytic activity but exhibit cellulose-binding activity (Goldstein et al., 1993; Morag et al., 1995).
In this study we investigated the effect of CBD on the elongation of growing plant tissues and its interaction with XG on cellulose binding. We show that CBD modulates the elongation of plant cells and tissues, competes with XG for cellulose binding, and increases the rate of cellulose synthase of A. xylinum.
MATERIALS AND METHODS
Plant Material
Peach (Prunus persica L. cv Texas) flowers were obtained from a plot near Rehovot, Israel. Anthers collected from the flowers on the 1st d of anthesis were excised from the filaments and dehydrated at 30°C for at least 24 h. The released pollen was used fresh or stored at −20°C. Seeds of Arabidopsis cv Columbia were obtained from the Hebrew University of Jerusalem stock. Pea (Pisum sativum L.) seeds were purchased from HAZERA (Mevchor, Israel).
Expression and Purification of CBD
Overexpression of CBD was obtained in Escherichia coli BL21 (DE3) harboring the pET-CBD plasmid (Goldstein et al., 1993). Inoculum was prepared by growing the cells overnight in M9 minimal medium (0.6% Na2HPO4, 0.3% KH2PO4, 0.25% NaCl, 0.5% NH4Cl, 0.2% Glc, 2 mm MgSO4, 0.1 mm CaCl2, and 1 mm thiamine-HCl) containing 50 μg mL−1 ampicillin. The percentages of salt concentrations were expressed as weight per volume. After dilution to a ratio of 1:50 in TB medium (1.2% tryptone, 2.4% yeast extract, 0.4% [v/v] glycerol, 0.17 m KH2PO4, and 0.72 m K2HPO4) containing 100 μg mL−1 ampicillin, cells were grown in shaking flasks at 250 rpm, 37°C, to an A600 of 1.7, after which 0.5 mm isopropyl β-d-thiogalactopyranoside was added. After 4 h of incubation the cells were harvested by centrifugation at 2,000g, resuspended in 20 mm Tris-HCl buffer, pH 7.0, and sonicated. Inclusion bodies were isolated by centrifugation at 10,000g and washed with water to remove the slimy part of the pellet. The white pellet was dissolved in urea buffer (4.5 m urea, 40 mm Tris base, and 1 mm Cys, pH 11.3) at a protein concentration of 1 mg mL−1 and stirred at 4°C for 2 to 4 h to solubilize the inclusion bodies. The denatured proteins were dialyzed twice against 20 mm Tris, pH 8.6, containing 10 mm β-mercaptoethanol, and once against 20 mm Tris-HCl buffer, pH 7.0, at 4°C. At this stage CBD was already more than 95% pure as determined by 12.5% SDS-PAGE (Laemmli, 1970). To remove bacterial components that might have been carried along during the CBD preparation, CBD was further affinity purified on a cellulose column and refolded as described above. The binding capacity to cellulose was determined according to the work of Goldstein et al. (1993).
Pollen Germination in Vitro
Pollen grains were germinated in liquid cultures, each containing 100 μL of 15% Suc, 100 μg mL−1 H3BO3, 200 μg mL−1 MgSO4 · 7H2O, and 200 μg mL−1 Ca(NO3)2 · 4H2O in 1.5-mL microtubes. Different concentrations of CBD or BSA were added to the growth medium. The number of pollen grains in each tube was approximately 1000, as determined by a hemocytometer. After an overnight incubation at 25°C in a dark chamber, the pollen tubes were fixed and stained according to the method of Alexander (1980). The pollen was examined in three populations of at least 100 grains per specimen (300 grains per treatment).
Seed Germination
Arabidopsis seeds were washed in 70% ethanol for 1 min and then five times in distilled water. About 100 seeds per treatment were soaked in 1 mL of distilled water containing different concentrations of CBD or BSA in 2-cm-diameter, 10-cm-long, glass culture tubes. The tubes were placed in a growth chamber at 25°C under a 16-/8-h light/dark photoperiod. At different intervals or after 3 d the lengths of the shoot, root, and longest representative root hair were measured in each seedling. The examinations were conducted in three populations of 30 seedlings per treatment.
Histochemical Observation
Peach pollen tubes, grown with or without CBD, were separated from the growth medium by pelleting for 1 min at 10,000g and fixed overnight at 4°C using 4% (v/v) glutaraldehyde in 0.1 m phosphate buffer, pH 7.4. The pollen tubes were repelleted, thoroughly washed with distilled water, and stained with white fluorescent brightener (0.1% [w/v] calcofluor in 0.1 m K3PO3) to reveal crystalline cell wall components under a fluorescent light microscope (Zeiss). Arabidopsis seedlings were treated similarly, except that the solutions were replaced without pelleting.
Peach pollen tubes and Arabidopsis seedlings were examined by IGSS to detect CBD attachment to cellulose. The plant material was first fixed overnight at 4°C in 4% glutaraldehyde in PBST (15 mm phosphate buffer, 150 mm NaCl, 3 mm KCl, pH 7.4, and 0.1% [v/v] Tween 20). The specimens were washed with PBST for 1 h, soaked for 1 h in 1% skim milk, and then incubated for 1 h with polyclonal rabbit anti-CBD antibodies or preimmune serum, both diluted 1:500 in PBST. The specimens were then washed three times, for 10 min each time, in PBST and then incubated for 1 h with goat anti-rabbit IgG conjugated with 5-nm gold particles diluted 1:100 in PBST. The specimens were washed twice, for 10 min each time, with PBST and once with water. A silver-stain kit (BioCell Research Laboratories, Cardiff, UK) was used for the final development of the reaction. The specimens were soaked in the combined kit solutions for about 10 min, washed in excess distilled water, and observed under a light microscope (BX40, Olympus).
Pea XG Extraction and Cellulose Pretreatment
Pea XG was extracted as described by Hayashi et al. (1987). Pea XG concentrations were determined by the iodine-sodium sulfate method (Kooiman, 1960). Cellulose (Sigma Cell 20) was extracted five times with 4% KOH containing 0.1% NaBH4 in an ultrasonic bath set at a temperature below 30°C for 3 h to remove polymeric contamination material. The cellulose was neutralized with 2 m acetic acid and washed five times with 20 mm Tris-HCl, pH 7.0.
Binding Capacity of XG to Cellulose
Pea XG (10 μg) was mixed with an elevated amount of pretreated cellulose in sodium acetate buffer (25 mm sodium acetate and 0.01% NaN3, pH 5.0) and incubated at 37°C for 4 h with constant mixing to resuspend the cellulose. The cellulose was then centrifuged and the amount of unbound XG was determined by the iodine-sodium sulfate method. In a preliminary experiment we found that 15 μg of pea XG binds to 1 mg of pretreated cellulose (data not shown).
Competition Assay
All three of the experiments were conducted in a final volume of 400 μL in 1.5-mL microtubes at 37°C in sodium acetate buffer (25 mm sodium acetate and 0.01% NaN3, pH 5.0) with constant mixing: Different amounts of CBD or BSA were first added to 1 mg of cellulose and allowed to bind for 1 h. Only then was 15 μg of XG added and binding allowed for 4 h. XG (15 μg) was added to 1 mg of cellulose and allowed to bind for 4 h. Then different amounts of CBD were added and allowed to bind for 1 h. Different amounts of CBD together with 15 μg of XG were added to 1 mg of cellulose. Binding was allowed for 4 h.
The cellulose mixtures were centrifuged at 10,000g and the amount of unbound XG was determined by the iodine-sodium sulfate method. The amount of unbound CBD was determined with a Bio-Rad protein assay kit (Bradford, 1976).
All of the experiments were repeated at least three times. Representative data are presented.
The Effect of CBD on Cellulose Synthesis in A. xylinum
A. xylinum strain ATCC 23769 was kindly donated by the laboratory of Prof. Moshe Benziman at The Hebrew University of Jerusalem. Cells were grown for 24 h under constant shaking at 30°C in a medium consisting of 0.5% peptone, 0.5% yeast extract, 2% Glc, and 0.3% K2HPO4, pH 6.0, containing 1.5 units/mL Trichoderma viride L. cellulase (Fluka). The cells were harvested by centrifugation and washed twice with precooled phosphate buffer (50 mm NaH2PO4, pH 6.0). The bacterial pellet was resuspended in phosphate buffer to a concentration of 2 mg mL−1 dry weight (2.5 A600 = 1 mg mL−1). One-milliliter reaction mixtures were placed in 20-mL scintillation vials containing 0.8 mg cells mL−1 phosphate buffer. Cellulose synthesis was initiated by the addition of 40 mm Glc (d-[U-14C]Glc; Amersham) at a specific activity of 40,000 cpm μmol−1 and was conducted for 1 to 2 h at 30°C with constant shaking. 14CO2 formed was trapped in coverless 1.5-mL tubes containing 0.2 mL of 1 m NaOH placed in the reaction vial. The reaction was stopped by the addition of 0.1 mL of 0.5 m HCl to the bacterial suspension and was further incubated for 15 min. One-hundred-fifty microliters of the NaOH solution containing the trapped 14CO2 was transferred to scintillation tubes. The cells and the cellulose were transferred to 1.5-mL tubes, centrifuged, and washed three times with water. The cells were lysed by mixing with 0.2 n NaOH and 1% SDS; cellulose was recovered on a GF/A filter (Whatman), washed with 15 mL of water to remove radioactive background, and dried in an oven at 60°C. Filters and NaOH containing trapped 14CO2 were counted in a scintillation counter using Opti-fluor (Packard, Meriden, CT) scintillation liquid for Glc incorporation (cellulose synthase activity) and respiration, respectively.
Electron microscopy was conducted by placing a copper grid on top of a drop of the appropriate solution at room temperature. The cellulose synthesis reaction contained 0.5 mg mL−1 dry weight cells in phosphate buffer and 40 mm Glc with or without CBD, at a concentration of 300 μg mL−1. The reaction was incubated for 30 min and then stopped with 2.5% glutardialdehyde for 30 min, washed three times with water, and dried. The grids were negatively stained with 1.5% phosphotungstic acid and examined with a 100 CX electron microscope (JEOL) operating at 80 kV.
RESULTS
Pollen Tube Elongation
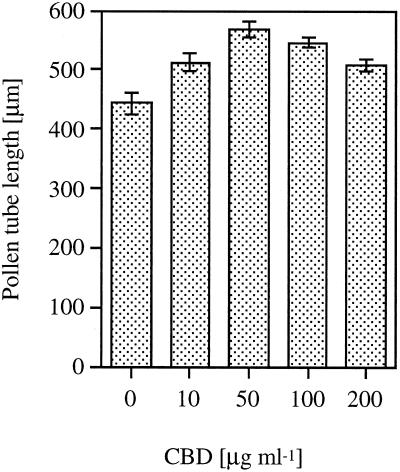
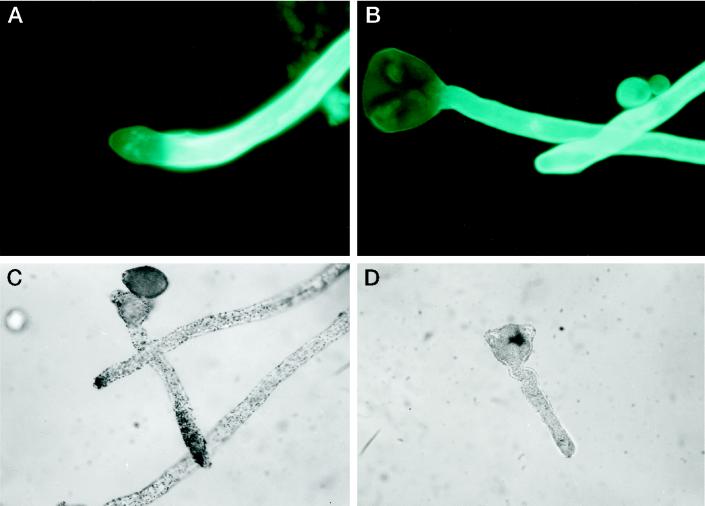
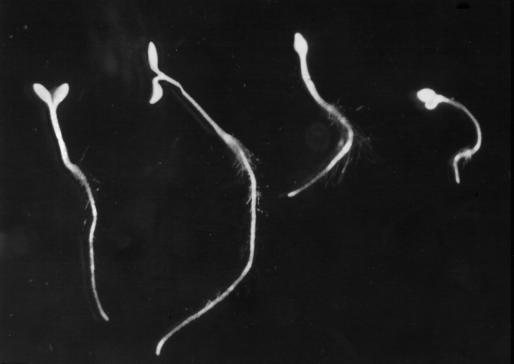
Peach pollen was germinated in the presence of different concentrations of CBD, and its effect on pollen-tube elongation is illustrated in Figure 1. At low concentrations CBD caused an increase in tube length as compared with the control without CBD, with an optimum at 50 μg mL−1. Increasing concentrations of BSA as a control had no effect on pollen-tube elongation. Figure 2 shows the effect of CBD on peach pollen tubes stained with calcofluor. In the tip region of CBD-treated pollen tubes (Fig. 2A), no fluorescence could be detected. In contrast, the control pollen tubes showed continuous bright color, suggesting crystalline cell wall structures along the length of the pollen tube (Fig. 2B). IGSS of CBD in pollen tubes grown in the presence of CBD revealed the protein along the pollen tube. Intensive staining was observed predominantly in the tip zone (Fig. 2C). In the control pollen tubes, no CBD staining was observed (Fig. 2D)
Figure 1.
Pollen tube elongation in liquid culture containing different concentrations of CBD. Vertical bars represent se.
Figure 2.
Calcofluor staining of pollen tubes grown with (A) or without (B) CBD. IGSS of pollen tubes grown in the presence of CBD reacted with anti-CBD antibodies (C) or with preimmune serum (D).
Arabidopsis Seedlings
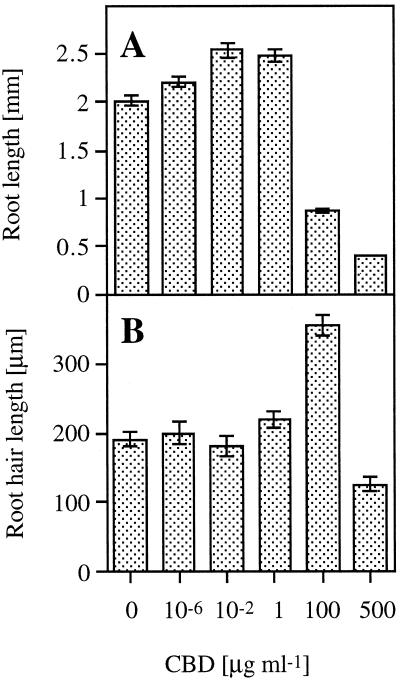
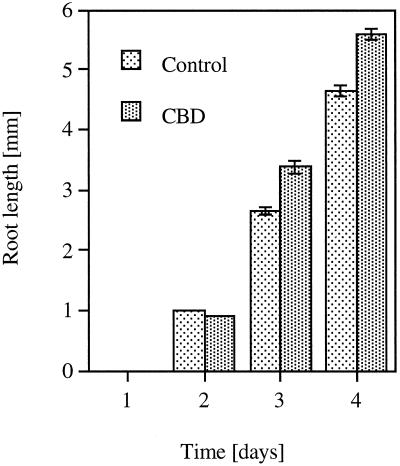
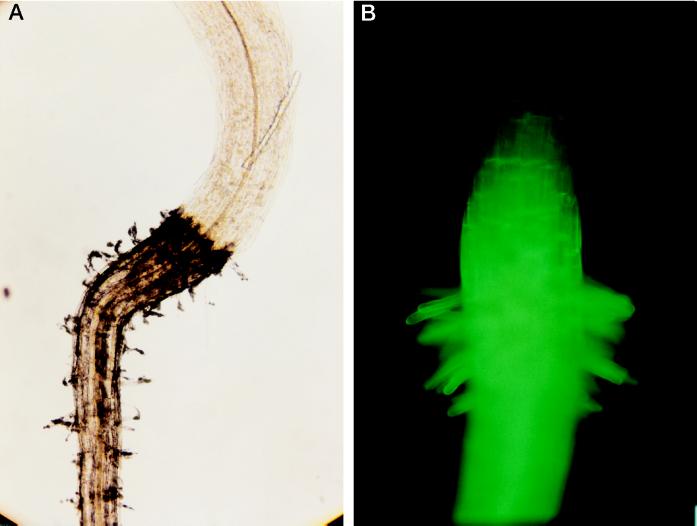
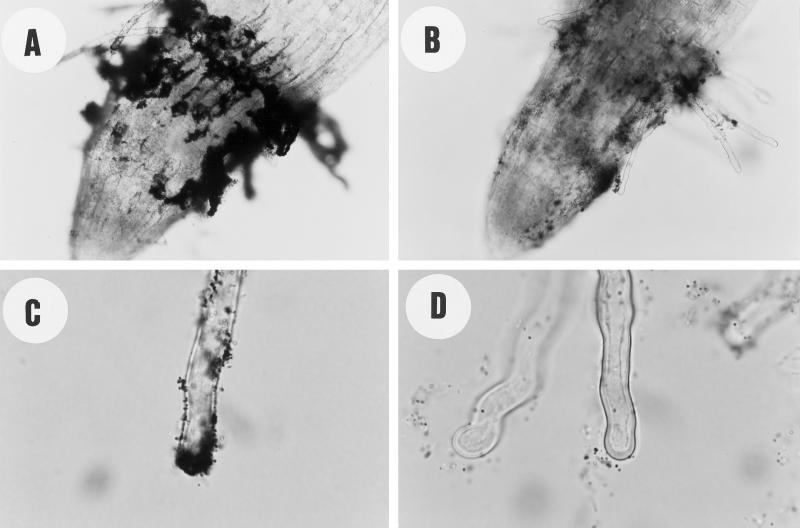
Arabidopsis seedlings were grown in the presence of different concentrations of CBD. Figure 3A shows that low concentrations (0.01–1 μg mL−1) of CBD caused an approximately 30% increase in root elongation relative to the control. Higher CBD concentrations caused a significant inhibition of root elongation in a dose-responsive manner. At lower CBD concentrations no significant effect on root elongation was observed. However, at 100 μg mL−1, CBD had the opposite effect on root hair elongation compared with its effect on root elongation. An almost 2-fold increase in root hair elongation was observed at this CBD concentration (Fig. 3B). Only at the highest concentration (500 μg mL−1) did the effect of CBD on root and root hair elongation show a similar dramatic inhibition (Fig. 3). Figure 4 shows the time course of the effect of 1 μg mL−1 CBD on the length of the roots. Two days after the beginning of the experiment, no significant difference was observed between the control and the CBD-treated seedlings, indicating that CBD did not affect the germination process. The CBD effect on root elongation was first observed after 3 d. BSA had no significant effect on the Arabidopsis seedlings. Except for the highest concentration, CBD was effective in the roots rather than in the hypocotyls, as illustrated in Figure 5. IGSS of CBD-treated seedlings using anti-CBD antibodies revealed the protein to be bound primarily to the root but not to the hypocotyl (Fig. 6A). The hypocotyl of these seedlings was not permeable to calcofluor, and therefore no fluorescence could be observed above the root (Fig. 6B). The IGSS shown in Figure 7 also revealed CBD to be primarily in the root hair-zone, especially at the tips (Fig. 7C).
Figure 3.
The effect of CBD on root (A) and root hair (B) length. Vertical bars represent se.
Figure 4.
Time-course analysis of Arabidopsis root length as affected by 1 μg mL−1 CBD. Vertical bars represent se.
Figure 5.
The effect of CBD on Arabidopsis seedlings. Representative seedlings from left to right are: control (no CBD), 10−2, 100, and 500 μg mL−1 CBD.
Figure 6.
A, IGSS of CBD-treated Arabidopsis seedlings using anti-CBD antibodies. B, Calcofluor staining of the seedlings.
Figure 7.
IGSS of CBD-treated Arabidopsis seedlings. Root zone of a 500 μg mL−1 CBD-treated seedling using anti-CBD antibodies (A) or preimmune serum (B). Root hair of 1 μg mL−1 CBD-treated seedling using anti-CBD antibodies (C) or preimmune serum (D).
CBD-XG Competition
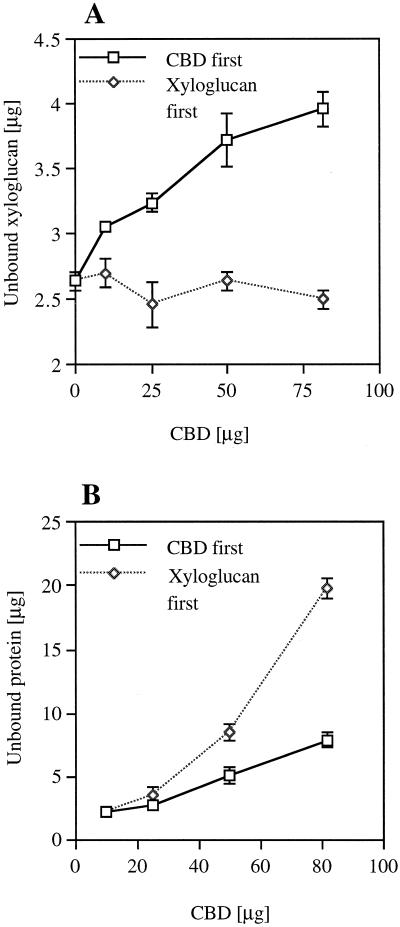
Cellulose-binding competition between CBD and XG was assayed in three different ways: (a) Different amounts of CBD were first added to a fixed amount of cellulose and allowed to bind, and only then was a saturating amount of XG (as determined in an earlier experiment) added. (b) A saturating amount of XG was added and allowed to bind to the cellulose, and only then were different amounts of CBD added. (c) Different amounts of CBD together with a saturating amount of XG were added together to a fixed amount of cellulose.
Figure 8 shows that when CBD was added first, as described in method (a) above, increasing concentrations of CBD resulted in increasing amounts of unbound XG (Fig. 8A). However, when XG was added first, as described in method (b) above, increasing concentrations of CBD did not affect the level of unbound XG (Fig. 8A), whereas the level of unbound CBD was higher (Fig. 8B). When CBD and XG were added together, as described for method (c) above, the results were similar to those observed when the CBD was added first as in method (a) (data not shown). BSA had no effect on the binding of XG to cellulose (data not shown).
Figure 8.
CBD-XG competition. The effect of CBD concentration on the amount of unbound XG (A) and unbound CBD (B) when CBD was added first to the cellulose or when XG was added first. Vertical bars represent se.
The Effect of CBD on Cellulose Synthesis in A. xylinum
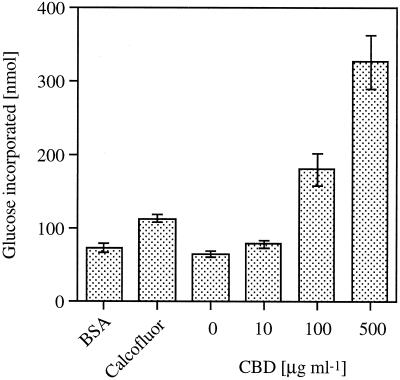
Resting cells of A. xylinum were allowed to synthesize cellulose in phosphate buffer containing radioactive Glc and different concentrations of CBD or calcofluor (as a positive control) and BSA (as a negative control) for 1 h or for the indicated time. Cellulose synthase activity was determined as Glc incorporation. Figure 9 shows the effect of CBD at different concentrations (10–500 μg mL−1, 0.6–30 μm) compared with 1 mm calcofluor and 100 μg mL−1 BSA (1.5 μm). CBD increased Glc incorporation in a dose-responsive manner by up to 5-fold at 500 μg mL−1. Calcofluor increased the rate by 2-fold, whereas BSA had no effect. Since CO2 production was stimulated by only 10% in the CBD treatment (at the highest concentration), the effect of CBD on cellulose synthesis appears to be direct and not related to general processes such as Glc uptake or respiration.
Figure 9.
The effect of different concentrations of CBD, 1 mm calcofluor (as a positive control), and 100 μg mL−1 BSA (as a negative control) on cellulose synthase activity in A. xylinum. Vertical bars represent se.
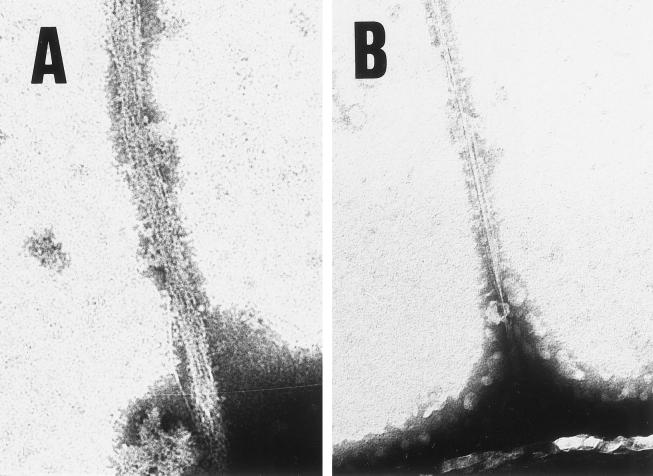
Electron microscopy examination of the cellulose ribbons produced by A. xylinum showed that CBD treatment resulted in a splayed ribbon composed of separate fibrillar subunits, as compared with a thin, uniform ribbon in the control (Fig. 10).
Figure 10.
The effect of CBD on cellulose ribbon produced by A. xylinum (A) or control without CBD (B). The magnification in both panels is ×26,000.
DISCUSSION
CBD is shown to modulate the elongation of various plant tissues. At low concentrations it enhances elongation; at high concentrations, however, it inhibits it. Cell walls of pollen tubes have been shown to contain exposed cellulose fibrils in the tip zone (Steer and Steer, 1989). Gold immunolabeling of CBD in pollen tubes revealed that CBD was present primarily at the tip zone. Pollen tube elongation is known to be apical (Cresti and Tiezzi, 1992). Moreover, the lack of calcofluor staining in the tip zone of CBD-treated pollen tubes suggested the absence of a crystalline structure. We propose that the elongation effect of CBD is driven by its ability to bind to cellulose and prevent the normal assembly of microfibrils and, consequently, the cell wall.
At low concentrations CBD enhanced elongation of Arabidopsis roots; at high concentrations it dramatically inhibited root elongation in a dose-responsive manner. The maximum effect on root hair elongation was at 100 μg mL−1, whereas at that concentration root elongation was inhibited. IGSS of CBD-treated seedlings revealed CBD to be bound primarily to the root but not to the hypocotyl (Fig. 6). Accordingly, most of the effect of CBD was observed in the root and not in the hypocotyl (Fig. 5). Once again, the effect of CBD was seen to coincide with its location. The absence of CBD in the hypocotyl could be explained by its inability to penetrate the cuticle, as seen with the smaller molecule calcofluor (Fig. 6B). A closer observation of IGSS-treated seedlings revealed that CBD was present primarily in the root hair zone (Fig. 7A) but also on the other parts of the root. Similar to pollen tubes, CBD was predominantly present at the tip of the root hairs (Fig. 7C).
There were some differences between the effect of CBD on Arabidopsis root and the effect on root hairs. Maximum elongation of the roots was achieved at 0.01 to 1 μg mL−1 CBD, whereas in root hairs maximum elongation was achieved only at 100 μg mL−1. Nevertheless, the effect of CBD on root hairs seems to be similar to its effect on the pollen tube. In both, maximum elongation was achieved at about 100 μg mL−1 CBD and the inhibitory effect was achieved at the highest concentration, although it was not as outstanding as on the root. These findings concur with the knowledge that pollen tubes and root hairs have the same elongation pattern, which is called “tip growth” (Cresti and Tiezzi, 1992; Peterson and Farquhar, 1996).
The inhibitory effect of CBD can be explained by steric hindrance of the cellulose fibrils by excess amounts of CBD, which block the access of enzymes and other proteins that modulate cell elongation via loosening of the rigid cellulose-fibril network. This hypothesis is supported by the work of Nevins, who prevented auxin-induced elongation with anti-β-d-glucan antibodies (Hoson and Nevins, 1989) or with antibodies specific to cell wall glucanases (Inouhe and Nevins, 1991).
It has already been established that XG chains cross-link the cellulosic network in the cell wall (Roberts, 1994). It is accepted that a prerequisite for cell elongation is a loosening of the cross-linked cellulose network by hydrolysis, as demonstrated by Inouhe and Nevins (1991), by transglycosylation (Fry et al., 1992; Nishitani and Tominaga, 1992), or by expansins that interact with the XG-cellulose bond (McQueen-Mason et al., 1992). CBD competes with XG on binding to cellulose (Fig. 8). Maximum CBD binding to cellulose has been achieved after 1 h (Goldstein et al., 1993), compared with XG binding to cellulose, which has been achieved only after 4 h (Hayashi et al., 1987). CBD was unable to elute XG from cellulose when it was already bound (Fig. 8A); note that the highest concentration of CBD tested in this experiment prevented only about 12% of the amount of XG that bound to the cellulose in the absence of CBD. It should be noted that CBD does not have expansin activity, as assayed in the cucumber hypocotyl wall by Dan Cosgrove (personal communication; Cosgrove, 1997). It suggested that during the elongation process cellulose microfibrils become exposed and CBD competes with XG on binding to the exposed cellulose microfibrils. It is therefore possible that this competition results in a temporary loosening of the cell wall and, consequently, enhanced elongation.
It is evident that polymerization and crystallization are coupled reactions in cellulose synthesis in A. xylinum bacteria (Benziman et al., 1980). CBD enhances incorporation of radioactive Glc in A. xylinum by interference with the crystallization process. Our hypothesis is supported by the review by Haigler (1991), in which dyes and fluorescent brightening agents that bind to cellulose alter cellulose microfibril assembly in vivo. Modifications in cell shape were observed when red alga (Waaland and Waaland, 1975) and plant root tips (Hughes and McCully, 1975) were grown in the presence of dyes. It is now evident that these molecules can bind to the cellulose chains immediately upon their extrusion from the cell surface of prokaryotes and eukaryotes (Haigler and Brown, 1979; Benziman et al., 1980; Haigler et al., 1980; Brown et al., 1982) and prevent crystal-structure formation (Haigler and Chanzy, 1988).
In addition, the rate of cellulose polymerization has been shown to increase up to 4-fold in the presence of dye (Benziman et al., 1980). It has been proposed that crystallization is the bottleneck in this coupled reaction, and its prevention results in accelerated polymerization. The suitability of A. xylinum as a model system for higher plants has long been controversial. Nevertheless, it remains fundamentally an important model organism in cellulose research. The effect of CBD as observed by electron microscopy is comparable to the effect of CMC rather than to the effect of calcofluor (Haigler, 1991); in both cases the cellulose ribbon only splayed. The effect of CBD on cellulose synthase activity was higher than the effect of CMC and was comparable and even higher than that of calcofluor (Fig. 9).
The different effects of CBD, CMC, and calcofluor can be attributed to the differences in their Mr and their affinities to cellulose. CMC (90 kD) can prevent only the normal association of larger fibrillar subunits and, therefore, hardly alter crystallization, whereas the small molecule calcofluor prevents the glucan chain association immediately after its initiation. CBD is somewhere between the two molecules: it is not small enough to prevent the association of very small fibrils as done by calcofluor, but its high affinity to cellulose makes it an efficient cellulose-intercalating agent, which leads to as much as a 5-fold increase in cellulose synthesis rate.
Two hypotheses explaining the effect of CBD on plant cell elongation were examined in this study: (a) CBD competes with XG on binding to cellulose, thus causing loosening of the cell wall network, which results in turgor-driven elongation. (b) When A. xylinum is used as a model system, CBD enhances cellulose synthesis, which is a limiting factor in plant cell elongation. At this time, our results show that none of the above mechanisms can be ruled out.
CBD is a bacterial protein. Its mode of action in modulating plant cell wall elongation is probably different from that of the natural process. However, its effect is relevant in that it may shed more light on this controversial process. In addition, its gene may be useful for biotechnological applications in modulating cell wall elongation and cell wall architecture of transgenic plants expressing cbd under the control of various tissue-specific promoters. Construction of transgenic plants expressing the CBD protein under different promoters is under way.
ACKNOWLEDGMENTS
The authors are indebted to Prof. Takahisa Hayashi for his useful suggestions in XG-cellulose interaction assays, to Prof. Deborah P. Delmer for a fruitful discussion in the early days of this work, to Prof. Dan Cosgrove for conducting stress relaxation experiments with CBD, and to Prof. Moshe Benziman and Dr. Hayim Weinhouse for their kind help with the A. xylinum experiments.
Abbreviations:
- CBD
cellulose-binding domain
- CMC
carboxymethyl-cellulose
- IGSS
immunogold silver stain
- XG
xyloglucan
Footnotes
This study was supported in part by an Eshkol fellowship (to E.S.).
LITERATURE CITED
- Aldington S, Fry SC. Oligosaccharins. Adv Bot Res. 1993;19:1–101. [Google Scholar]
- Alexander MP. A versatile stain for pollen fungi, yeast and bacteria. Stain Technol. 1980;55:13–18. doi: 10.3109/10520298009067890. [DOI] [PubMed] [Google Scholar]
- Benziman M, Haigler CH, Brown RM, Jr, White AR, Cooper KM. Cellulose biogenesis: polymerization and crystallization are coupled processes in Acetobacter xylinum. Proc Natl Acad Sci USA. 1980;77:6678–6682. doi: 10.1073/pnas.77.11.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown RMJ, Haigler C, Cooper K. Experimental induction of altered nonmicrofibrillar cellulose. Science. 1982;218:1141–1142. doi: 10.1126/science.218.4577.1141. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Relaxation in a high-stress environment: the molecular bases of extensible cell walls and cell enlargement. Plant Cell. 1997;9:1031–1041. doi: 10.1105/tpc.9.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresti M, Tiezzi A. Pollen tube emission organization and tip growth. In: Cresti M, Tiezzi A, editors. Sexual Plant Reproduction. Berlin: Springer-Verlag; 1992. pp. 89–97. [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J. 1992;282:821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MA, Takagi M, Hashida S, Shoseyov O, Doi RH, Segel IH. Characterization of the cellulose-binding domain of the Clostridium cellulovorans cellulose-binding protein A. J Bacteriol. 1993;175:5762–5768. doi: 10.1128/jb.175.18.5762-5768.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler C, Brown RM., Jr The fluorescent brightener, calcofluor white, alters cellulose synthesis in Acetobacter xylinum (abstract) J Cell Biol. 1979;83:70a. [Google Scholar]
- Haigler C, Brown RM, Jr, Benziman M. Calcofluor white ST alters the in vivo assembly of cellulose microfibrils. Science. 1980;210:903–906. doi: 10.1126/science.7434003. [DOI] [PubMed] [Google Scholar]
- Haigler CH (1991) Relationship between polymerization and crystallization in microfibril biogenesis. In CH Haigler, PJ Weimer, eds, Biosynthesis and Biodegradation of Cellulose. Marcel Dekker, New York, pp 99–124
- Haigler CH, Chanzy H. Electron diffraction analysis of the altered cellulose synthesized by Acetobacter xylinum in the presence of fluorescent brightening agents and direct dyes. J Ultrastruct Mol Struct Res. 1988;98:299–311. [Google Scholar]
- Hayashi T, Marsden MPF, Delmer D. Plant Physiol. 1987;83:384–389. doi: 10.1104/pp.83.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Ogawa K, Mitsuishi Y. Characterization of the adsorption of xyloglucan to cellulose. Plant Cell Physiol. 1994;35:1199–1205. [PubMed] [Google Scholar]
- Hoson T, Nevins DJ. β-d-Glucan antibodies inhibit auxin-induced cell elongation and changes in the cell wall of Zea coleoptile segments. Plant Physiol. 1989;90:1353–1358. doi: 10.1104/pp.90.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, McCully ME. The use of an optical brightener in the study of plant structure. Stain Technol. 1975;50:319–329. doi: 10.3109/10520297509117082. [DOI] [PubMed] [Google Scholar]
- Inouhe M, Nevins DJ. Inhibition of auxin-induced cell elongation of maize coleoptiles by antibodies specific for cell wall glucanases. Plant Physiol. 1991;96:426–431. doi: 10.1104/pp.96.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooiman P. A method for the determination of amyloid in plant seeds. Recl Trav Chim Pays-Bas Bel. 1960;79:675–678. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDougall GJ, Fry SC. Inhibition of auxin-stimulated growth of pea stem segments by a specific nonasaccharide of xyloglucan. Planta. 1988;175:412–416. doi: 10.1007/BF00396348. [DOI] [PubMed] [Google Scholar]
- McDougall GJ, Fry SC. Xyloglucan oligosaccharides promote growth and activate cellulase: evidence for a role of cellulase in cell expansion. Plant Physiol. 1990;93:1042–1048. doi: 10.1104/pp.93.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ. Expansin mode of action on cell walls—analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol. 1995;107:87–100. doi: 10.1104/pp.107.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Fry SC, Durachko DM, Cosgrove DJ. The relationship between xyloglucan endotransglycosylase and in-vitro cell wall extension in cucumber hypocotyls. Planta. 1993;190:327–331. doi: 10.1007/BF00196961. [DOI] [PubMed] [Google Scholar]
- Morag E, Lapidot A, Govorko D, Lamed R, Wilchek M, Bayer EA, Shoham Y. Expression, purification, and characterization of the cellulose-binding domain of the scaffold in subunit from the cellulosome of Clostridium thermocellum. Appl Environ Microbiol. 1995;61:1980–1986. doi: 10.1128/aem.61.5.1980-1986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani K, Tominaga R. Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. J Biol Chem. 1992;267:21058–21064. [PubMed] [Google Scholar]
- Peterson RL, Farquhar ML. Root hairs: specialized tubular cells extending root surfaces. Bot Rev. 1996;62:1–40. [Google Scholar]
- Roberts K. The plant extracellular matrix: in a new expansive mood. Curr Opin Cell Biol. 1994;6:688–694. doi: 10.1016/0955-0674(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Ross P, Mayer R, Benziman M. Cellulose biosynthesis and function bacteria. Microbiol Rev. 1991;55:35–58. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoseyov O, Doi RH. Essential 170-kDa subunit for degradation of crystalline cellulose by Clostridium cellulovorans cellulase. Proc Natl Acad Sci USA. 1990;87:2192–2195. doi: 10.1073/pnas.87.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoseyov O, Takagi M, Goldstein MA, Doi RH. Primary sequence analysis of Clostridium cellulovorans cellulose binding protein A. Proc Natl Acad Sci USA. 1992;89:3483–3487. doi: 10.1073/pnas.89.8.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer MW, Steer JM. Pollen tube tip growth. New Phytol. 1989;111:323–358. doi: 10.1111/j.1469-8137.1989.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Waaland SD, Waaland JR. Analysis of cell elongation in red algae by fluorescent labeling. Planta. 1975;126:127–138. doi: 10.1007/BF00380616. [DOI] [PubMed] [Google Scholar]
- York WS, Darvill AG, Albersheim P. Inhibition of 2,4-dichlorophenoxyacetic acid-stimulated elongation of pea stem segments by a xyloglucan oligosaccharide. Plant Physiol. 1984;75:295–297. doi: 10.1104/pp.75.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]