Abstract
Campylobacter jejuni has been isolated previously from market produce and has caused gastroenteritis outbreaks linked to produce. We have tested the ability of this human pathogen to utilize organic compounds that are present in leaf and root exudates and to survive in the plant environment under various conditions. Carbon utilization profiles revealed that C. jejuni can utilize many organic acids and amino acids available on leaves and roots. Despite the presence of suitable substrates in the phyllosphere and the rhizosphere, C. jejuni was unable to grow on lettuce and spinach leaves and on spinach and radish roots of plants incubated at 33°C, a temperature that is conducive to its growth in vitro. However, C. jejuni was cultured from radish roots and from the spinach rhizosphere for at least 23 and 28 days, respectively, at 10°C. This enteric pathogen also persisted in the rhizosphere of spinach for prolonged periods of time at 16°C, a temperature at which many cool-season crops are grown. The decline rate constants of C. jejuni populations in the spinach and radish rhizosphere were 10- and 6-fold lower, respectively, than on healthy spinach leaves at 10°C. The enhanced survival of C. jejuni in soil and in the rhizosphere may be a significant factor in its contamination cycle in the environment and may be associated with the sporadic C. jejuni incidence and campylobacteriosis outbreaks linked to produce.
Thermophilic Campylobacter species, Campylobacter coli, Campylobacter lari, and Campylobacter jejuni, cause more cases of human gastrointestinal illness in the United States, the United Kingdom, and other Western countries than any other bacteria (2, 3, 7, 19). C. jejuni alone is responsible for more than 90% of these cases (18). In contrast to Salmonella enterica and pathogenic Escherichia coli, which cause frequent foodborne outbreaks, Campylobacter infections appear to occur mainly as sporadic illnesses (18). Due to the sporadic nature of campylobacteriosis and the microaerophilic and thermophilic lifestyles of Campylobacter spp., which make this organism difficult to recover from environments outside of its hosts, the point sources of Campylobacter contamination often remain unidentified.
Campylobacter species are highly prevalent on poultry and are present also at various levels of incidence in other animals (24). While poultry products and contaminated water are considered to be major sources of Campylobacter illness, outbreaks of campylobacteriosis linked to contaminated fruits, vegetables, or other produce-related products have been reported (4, 5, 24). Specific produce items implicated in these outbreaks are lettuce, sweet potatoes, cucumber, and melon or strawberries. Surprisingly, a review of the epidemiology of campylobacteriosis outbreaks and/or cases reported between 1990 and 1999 in the United States revealed that produce was associated with more cases of Campylobacter illness than any other food source during this period and was second only to dairy products in the total number of outbreaks (30). In a retrospective cohort study conducted in the United Kingdom, Evans et al. (16) observed that salad vegetables were the second-highest risk factor after chicken in cases of Campylobacter infection. A common hypothesis for campylobacteriosis outbreaks or cases associated with produce is that fruits and vegetables, which often are consumed raw, become cross-contaminated during food preparation by another contaminated food, such as raw poultry. This scenario is highly probable and would explain the unexpectedly high number of produce-related outbreaks compared to those related to other food sources. Nevertheless, it is noteworthy that several studies have reported the presence of Campylobacter species on produce sampled at the marketplace, including spinach, lettuce, radish, green onion, parsley, and potatoes (34), ready-to-eat grated carrots and cabbage (17), modified-atmosphere packaged mixed salad vegetables (35), mushrooms (14), and spinach and fenugreek (28).
The significance of the occurrence of Campylobacter species on fresh fruits and vegetables for the epidemiology of food-borne Campylobacter infections is unclear. Other human enteric pathogens that have caused large epidemics of food-borne illness linked to contaminated produce have also been reported to be relatively fit on various plant species under conditions favorable for their growth in the laboratory (10, 44). In this study, we have explored the ability of C. jejuni to grow and survive in the plant environment in order to gain a better understanding of the epidemiological importance and risks associated with the contamination of fruits and vegetables with Campylobacter species.
MATERIALS AND METHODS
Bacterial strains and inoculum preparation.
C. jejuni strain RM1221 was isolated from a retail chicken in California and tagged with green fluorescent protein (GFP) by transformation with plasmid pWM1007; GFP was stable in this strain over multiple generations in vitro (31). C. jejuni strain RM1221 was used for our studies due to the scarcity of available produce isolates. In addition, the genome sequence of this strain has been obtained in our laboratory (unpublished data) and will be used to further investigate the biology of C. jejuni in the environment. The inoculum for all plant experiments was prepared by growing C. jejuni pWM1007 in Brucella broth (Difco Laboratories, Detroit, Mich.) supplemented with sodium metabisulfite, pyruvic acid (Na salt), and FeSO4 · 7H2O at 0.25, 0.25, and 0.46 mg/ml, respectively, and kanamycin at 200 μg/ml. The cultures were incubated on a rotary shaker at 40 rpm and 42°C under a microaerophilic atmosphere composed of 83% N2, 7% CO2, 6.5% O2, and 3.5% H2. The bacteria were cultured to early stationary phase of growth, washed twice in 1× phosphate-buffered saline (PBS) (10 mM Na phosphate, 0.15 mM NaCl [pH 7.2]), and suspended in 0.1× PBS at a final concentration of 108 cells/ml for inoculation.
Leaf inoculations.
Spinach (Spinacia oleracea cv. Bolero) and lettuce (Lactuca sativa var. Grand Rapids) plants were grown in Supersoil (Rod McLellan Co., San Mateo, Calif.) in controlled-environment growth chambers to the 6-8 true-leaf stage. In order to test the effect of plant tissue damage on the survival of C. jejuni on spinach, each leaf was wounded manually with tweezers over 25% of its surface. Healthy and damaged leaves were inoculated by immersion of the upper plant part in the inoculum suspension for 2 s. Six replicate pots with two plants each were used per treatment. Immediately after inoculation, five leaves were removed at random from each pot for each treatment and assessed for initial inoculum population as described below.
When the effect of warm temperatures, such as 28 and 33°C, on the survival of C. jejuni was tested, the pots were placed under fluorescent lights in a humid chamber that allowed the presence of visible free water on the leaves immediately after inoculation. For incubation of the inoculated plants at 10°C, the pots were placed in a plant growth chamber with a 20-s period of fine mist every 15 min to allow the presence of free water on part of the leaf surface and to avoid overdrying of the plant surface caused by the heavy airflow in the growth chamber. All pots were incubated in the chambers in a randomized design.
For inoculation of spinach leaves that were contaminated with soil, moist autoclaved Supersoil was sprinkled at ca. 400 mg of wet weight/leaf onto each leaf prior to spray inoculation with a bacterial suspension of 108 cells per ml in 0.1× PBS. Control plants in these experiments were spray inoculated without prior application of soil to the leaves. The potted plants were then placed in a growth chamber at 10°C with periodic misting, as described above.
Root inoculations.
Radish (Rhafinus sativus cv. Comet) plants were grown in Supersoil for 6 weeks in a growth chamber at 16°C until fully mature. Each pot contained two radish plants. The temperature of the potted plants and soil was equilibrated at 10°C for 24 h prior to inoculation. The radishes of 28 replicate pots were inoculated by application of 20 ml of a 108-cells-per-ml, 0.1×-PBS bacterial suspension onto the soil surface at the center of the pot and around the radish roots. Immediately after inoculation, two radishes from two pots each were processed for estimation of initial C. jejuni populations. The plants were then placed in growth chambers at 10 or 33°C, under the same conditions as described above.
Prior to inoculation of C. jejuni into the spinach rhizosphere, the potted plants, which were grown to the 8 to 10 true-leaf stage for 2 months under the same conditions as described above, were placed in a container in 2 cm of water for 24 h at 10°C to equilibrate the water content of the soil. The pots were then transferred to a growth chamber for 24 h before inoculation with C. jejuni, at the temperature at which they would be incubated for the duration of the experiment, i.e., 10, 16, 28, and 37°C. Each pot contained two spinach plants. The spinach roots and soil were inoculated by application of 20 ml of a 108-cells-per-ml, 0.1×-PBS bacterial suspension onto the soil surface between the two plants in each pot. A core of ca. 100 g of soil and roots was sampled from the zone of inoculation from each of two pots immediately after inoculation and processed as described below for estimation of the C. jejuni population in the rhizosphere at the start of the experiment. The pots were placed in a container with 2 cm of water at the bottom to allow for the water content of the soil to remain constant throughout the experiment and across incubation temperatures. The container with the pots was placed in a growth chamber maintained at 10, 16, 28, or 37°C under a day-and-night cycle of 12 h.
Bacterial cell recovery and estimation of population sizes.
Bacterial suspensions obtained from plant tissue and/or soil were plated for determination of C. jejuni populations onto Campy-Cefex agar (40) with supplements as listed above and with kanamycin and cycloheximide at 75 and 100 μg/ml, respectively (CCAS). The plates were equilibrated in the microaerophilic atmosphere described above prior to use. After dilution plating of the bacterial suspensions, the plates were incubated immediately at 37°C for 12 h and then at 42°C for 36 h in an anaerobic jar (MART Microbiology BV, Lichtenvoorde, The Netherlands) containing the microaerophilic atmosphere described above and generated by a MART Anaxomat WS9000 (MART Microbiology BV, Lichtenvoorde, The Netherlands).
When enrichment for C. jejuni from plant tissue or soil was attempted, plant washings, whole tissue, or soil was incubated in Preston enrichment broth (8) containing 100 μg of cycloheximide ml−1 and the supplements described above for Brucella broth (PEBS). The enrichment culture was incubated in a tissue culture flask on a rotary shaker at 40 rpm and 42°C in the microaerophilic gas mixture. Samples from the enrichment culture were plated after 24 and 48 h of incubation onto CCAS plates as described above. Specific details regarding the enrichment procedure for each type of sample are given below.
Determination of C. jejuni population sizes was performed manually by enumerating green fluorescent colonies on culture plates under a Leica MZ-FLIII fluorescence stereoscope (Leica Microsystems, Wetzlar, Germany) equipped with a GFP filter (Chroma Technology Corp., Battleboro, Vt.). GFP fluorescence was maintained in at least 95% of the cells recovered from plant samples. The few colonies that appeared like C. jejuni colonies, but did not have green fluorescence, were confirmed under a phase-contrast microscope to be C. jejuni colonies by observation of the small spiral-shaped cells that are typical of this species.
(i) Spinach leaves.
At regular time intervals after inoculation, 10 leaves were sampled at random from all replicate pots within a treatment. Each leaf was placed in 10 ml of 1× PBS in a Ziplock bag and sonicated for 75 s in an FS-30 sonicator bath (Fisher Scientific, Pittsburgh, Pa.). The bagged leaves were then rubbed gently for 30 s, and the resulting suspension was hand plated and dilution plated with an Autoplate 4000 automated plater (Spiral Biotech Inc., Norwood, Mass.) onto CCAS plates. For enrichment, the leaves were placed directly in 10 ml of PEBS, the bacterial cells were washed off as described above, the leaves were then removed, and the resulting suspension was incubated as described above.
(ii) Radish roots.
At regular time intervals after inoculation, two radishes per pot were sampled at random from each of three replicate pots. The soil adhering to the radishes was either removed by wiping the roots gently with the fingers (covered with latex gloves) or by immersing the roots in 150 ml of water. Each radish pair was then placed in a Ziplock bag in 25 ml of 1× PBS, sonicated, rubbed, and processed for enumeration of C. jejuni populations as described above for the spinach leaves. For enrichment, two radishes per replicate pot were washed in PEBS, the radishes were then removed, and the resulting suspension was incubated as described above.
(iii) Spinach rhizosphere.
A core sample containing soil and spinach roots was obtained from each pot by cutting a 12-cm2 area through the entire 13-cm depth of the pot and digging the core out with a spoon. Two pots were sampled at random per treatment per time point. Each core was weighed and then placed in 75 ml of 1× PBS in a Ziplock bag. This root-soil suspension was homogenized by hand and sonicated as described above. The suspension was homogenized by hand again and dilution plated by hand onto CCAS plates. To enrich for C. jejuni from rhizosphere samples, 500 μl of the root-soil suspension was added to 5 ml of PEBS and processed as described above.
Microscopy.
C. jejuni RM1221 was grown overnight in Brucella broth as described above. The cells were then washed twice in 1× PBS and resuspended in 1× PBS at a concentration of 100 cells ml−1. This suspension was mixed with autoclaved Supersoil at 0.8 g of soil ml−1. The soil was then deposited onto water agar plates, which were incubated at 37°C in the microaerophilic atmosphere described above. The soil and organic debris on the agar plates were examined after 7 days of incubation under a fluorescence stereomicroscope for the presence of green fluorescent C. jejuni colonies. The images were captured with a Sony DKC5000 digital color camera (Sony Electronics, Inc., Tokyo, Japan).
Carbon source utilization.
C. jejuni strains were assayed for respiration induced by carbon substrates present in GN2 and ECOI plates (Biolog Inc., Hayward, Calif.). C. jejuni was grown on the medium recommended by the manufacturer and modified by addition of 5% sheep blood. C. jejuni cells were then collected from the plates, suspended in Biolog GN/GP inoculation fluid to an absorbance of 0.75, measured by densitometry (Biolog Absorbance Meter), and added to microtiter wells containing substrates. The microtiter plates were incubated for 24 h at 37°C in a microaerophilic atmosphere composed of 85% N2, 10% CO2, and 5% O2. At least two replicate assays were performed for each strain. Utilization of l-cysteine was tested by addition of this substrate to MT plates (Biolog Inc., Hayward, Calif.), which contain only the reactive tetrazolium violet dye. The color change resulting from the reduction of tetrazolium violet by bacterial activity was measured in a Biolog spectrophotometer according to the manufacturer (Biolog Inc., Hayward, Calif.).
Statistical methods.
Statistical calculations were performed with the programs Instat version 3.0 and Prism version 3.0 (GraphPad Software, Inc., San Diego, Calif.). The population size data were log transformed to obtain a normal distribution of the data, which is a requirement to compare means statistically and perform regression analysis. The log-transformed values of the C. jejuni population size of all individual samples were plotted over time after inoculation, and analyzed by regression analysis. The trend of all data sets was appropriately described by linear regression, as confirmed with the runs test. The linear regression model for the population size of C. jejuni on plant surfaces over time is the following: log CFUt = log CFU0 − kt, where CFU0 and CFUt is the population size per sample at the time of inoculation and at time t (days) after inoculation, respectively, and k is the rate constant. Thus, k equals the slope of the regression line and represents the decline rate constant for C. jejuni populations under various conditions. All reported slope values were significantly different from zero with a confidence level of at least 95%, as determined with Fisher's statistical test. All experiments were repeated independently at least twice with bacterial cultures and plants grown at different times.
RESULTS
Carbon source utilization.
The carbon utilization profile of C. jejuni was determined with GN2 Biolog plates. C. jejuni was positive for a wide range of compounds present on the plates (data not shown). Table 1 provides a list of organic acids, amino acids, and carbohydrates that induced respiration in C. jejuni in the Biolog assay and that have been reported to be present in root and/or leaf exudates or in plant cells. l-Cysteine and α-hydroxybutyric acid, which have been shown to occur in root but not in leaf exudates, induced the highest levels of activity. The carbohydrates l-arabinose and l-fucose induced a significant rate of respiration in C. jejuni. In contrast, α-d-glucose induced relatively negligible activity.
TABLE 1.
Carbon substrates utilized by C. jejuni on Biolog plates and reported to be present on root or leaf surfaces
| Substrate | Activity in biolog platesa | Root exudatesb | Leaf exudatesb |
|---|---|---|---|
| l-Arabinose | 382 ± 33 | + | + |
| l-Fucose | 445 ± 41 | +c | +c |
| α-d-Glucose | 74 ± 22 | + | + |
| α-Hydroxybutyric acid | 871 ± 47 | + | NRd |
| Succinic acid | 196 ± 20 | + | + |
| l-Asparagine | 300 ± 33 | + | + |
| l-Aspartic acid | 231 ± 27 | + | + |
| l-Proline | 68 ± 11 | + | + |
| l-Serine | 62 ± 21 | + | + |
| l-Cysteinee | 1,564 ± 90 | + | − |
C. jejuni strains were assayed for respiration induced by carbon substrates present in GN2 Biolog plates (Biolog, Inc., Hayward, Calif.). The data are presented as the averages ± standard errors of the means of the normalized optical densities for 38 strains of C. jejuni. These strains included isolates from retail chicken liver and carcass samples (n = 14), human clinical isolates representing different heat-labile (Lior; n = 8) and heat-stable (Penner: n = 6) serotypes, and one calf isolate. Each strain was tested at least twice.
+ or − indicates whether the compound was detected in soluble root exudates (12, 27) or leaf exudates (13, 33).
Present in plant cell walls (47).
NR, not reported to be present.
l-Cysteine was tested separately as described in Material and Methods.
Survival on leaves.
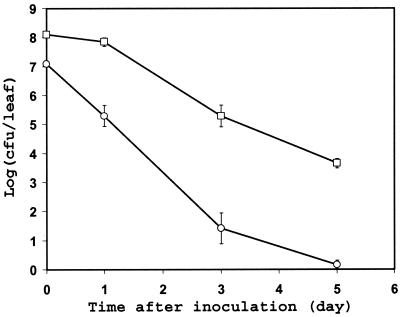
The survival of C. jejuni on leaves was assessed with both lettuce and spinach plants. In all experiments conducted at temperatures of 28 and 33°C, C. jejuni populations on lettuce and spinach leaves decreased more than 100-fold within the first 24 h of incubation; at that time, the C. jejuni populations on most lettuce and spinach leaves were below our detection threshold by direct plating, which was 100 and 15 cells per lettuce and spinach leaf, respectively (data not shown). Additionally, C. jejuni cells were not recovered from these leaves by enrichment in PEBS at 48 h after inoculation, indicating that C. jejuni cells had become nonculturable or had died. In contrast, the population size of C. jejuni on the spinach plants incubated at 10°C averaged 81 CFU per leaf, 48 h after inoculation (Fig. 1). Although the population size of C. jejuni decreased over time on spinach incubated at 10°C also, the pathogen remained culturable, albeit from a low percentage of leaf samples, by direct plating of leaf washings for 6 days after inoculation and by enrichment for 9 days postinoculation. The survival of the GFP-labeled C. jejuni strain on spinach plants and its carbon utilization profile in the BIOLOG assay were not different from those of the non-GFP strain.
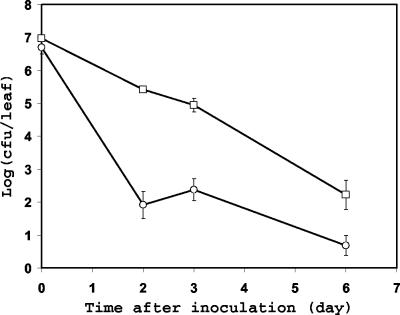
FIG. 1.
Population dynamics of C. jejuni strain RM1221 after its inoculation onto spinach plants and incubation at 10°C. The spinach leaves were healthy (○) or mechanically wounded in the laboratory before inoculation (□). Each data point represents the mean of the log-transformed bacterial population size on 10 leaves. The error bars represent ±1 standard error of the mean.
A clinical isolate of C. jejuni, strain TGH9011 (26), was also tested and compared to strain 1221 for its ability to grow and survive on spinach plants. Strain TGH6011 was unable to grow or persist for prolonged periods of time in the spinach phyllosphere (data not shown). Thus, its behavior was very similar to that of strain 1221, with the exception that it survived on spinach leaves at a slightly higher rate than strain 1221 in two replicate experiments.
In order to test the hypothesis that C. jejuni survives best on leaves in microsites where microaerophilic conditions prevail (e.g., in wounded tissue or depressions where water accumulates), we compared C. jejuni populations over time after inoculation onto healthy spinach plants versus spinach plants damaged physically in the laboratory. C. jejuni was undetectable 48 h after inoculation on both healthy and wounded plants incubated at 33°C (data not shown). While a decrease in the population of culturable C. jejuni cells on leaves was apparent at 10°C also, the C. jejuni population decline rate constant was lower on wounded leaves (−0.66) than on healthy leaves (−1.28) (Fig. 1). Indeed, a comparison of the rate constants (slope of the regression line) suggests that C. jejuni survived slightly better on injured leaf tissue than on intact leaf tissue (F = 3.66; P < 0.063). In addition, C. jejuni was detectable by direct plating onto CCAS from a higher proportion of leaf samples (Fisher's exact test; P < 0.048) and for a longer time on wounded plants than on healthy plants (Table 2).
TABLE 2.
Effect of leaf tissue damage on the recovery of C. jejuni strain 1221 from inoculated spinach plants
| No. of days after inoculation | % of leaves with detectable C. jejunia
|
|
|---|---|---|
| Healthy leaves | Wounded leaves | |
| 0 | 100 | 100 |
| 2 | 80 | 100 |
| 3 | 80 | 100 |
| 6 | 40 | 90 |
| 9 | 0 | 20 |
Values represent percentages of leaves from the total sampled leaves on which C. jejuni was detected by plating onto CCAS. The spinach plants were incubated at 10°C after inoculation.
Survival in soil.
(i) Radish roots.
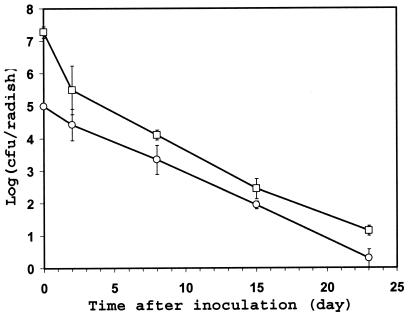
The behavior of C. jejuni on underground plant parts was investigated initially using radishes growing in pots in the laboratory. C. jejuni was culturable from radish roots by direct plating for at least 23 days after inoculation into soil in the vicinity of mature radish roots and incubation at 10°C under humid conditions (Fig. 2). Thus, the survival rate of C. jejuni was much higher on radish roots than on spinach leaves. In order to determine whether the C. jejuni cells had survived mainly in the soil attached to the radish root or to the radish tissue per se, the soil was either partially removed by wiping the radishes or more thoroughly removed by swirling the radishes gently in water. Two days after inoculation, wiped radishes harbored on average ca. 10-fold more C. jejuni cells than washed radishes, but the majority of the C. jejuni population was not removed from the radishes by the wash treatment (Fig. 2). Furthermore, the C. jejuni population decline rate constant was not significantly different on wiped radishes versus washed radishes in the period during 2 to 23 days postinoculation (F = 0.006; P < 0.938); the pooled decline rate constant calculated by regression analysis of the entire data equals −0.208.
FIG. 2.
Population sizes of C. jejuni strain RM1221 over time after its inoculation onto radish roots growing in pots in the laboratory and incubation at 10°C. The soil remaining on the radishes after sampling was wiped (□) or gently washed off in water (○). Each data point represents the mean of the log-transformed bacterial population size on six radishes from a total of three replicate pots. The error bars represent ±1 standard error of the mean.
(ii) Spinach rhizosphere.
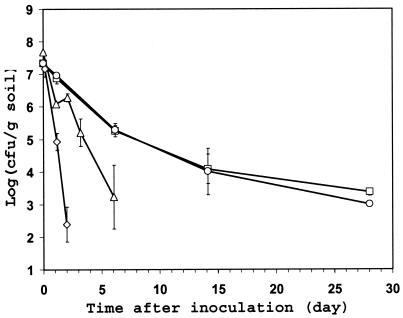
The spinach rhizosphere provided an alternative environment for investigating the survival of C. jejuni in the soil and the underground part of plants. Since the roots of 2-month-old spinach plants were dense, thin, and fragile, the soil core that was removed from the center of the pots in which the spinach plants were growing was comprised mostly of both roots and soil attached to them. Because of its high root density, the sampled core represented mainly the spinach rhizosphere. The population size of C. jejuni in the spinach rhizosphere decreased over time after inoculation at all temperatures tested (Fig. 3). However, significant differences in the population decline rate were observed at the various test temperatures (Fisher's test on difference of slopes: F = 40.46; P < 0.0001); C. jejuni survived best and remained culturable for the longest time in the rhizosphere of plants incubated at 10 and 16°C (Fig. 3). C. jejuni was culturable by direct plating from the rhizosphere of plants incubated at 37°C for only 2 days after inoculation but for at least 28 days at 10 and 16°C. At the latter incubation temperatures, the population sizes of C. jejuni in the rhizosphere of spinach appeared to have stabilized after 14 days following inoculation. It is noteworthy that the C. jejuni decline rate constant at 10°C was lower in the spinach rhizosphere and on radish roots (−0.13 and −0.21, respectively) than on healthy and wounded spinach leaves (−1.28 and −0.66, respectively) (Fig. 1 to 3).
FIG. 3.
Effect of temperature on the survival of C. jejuni strain RM1221 in the rhizosphere of spinach plants growing in organic soil in the laboratory. After inoculation of C. jejuni into the soil, the plants were incubated at 10°C (□), 16°C (○), 28°C (▵), or 37°C (◊). Each data point represents the mean of the log-transformed bacterial population sizes in two cores comprised of soil and roots that were sampled from two replicate pots at each temperature. The error bars represent ± 1 standard error of the mean.
Microscopy.
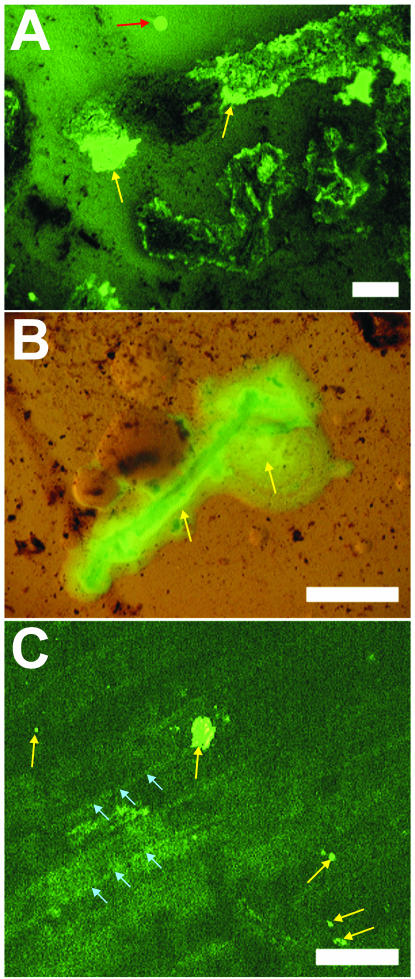
The plating of undiluted rhizosphere samples suspended in 1× PBS onto CCAS plates resulted in a high concentration of root and soil debris on the agar surface. A close examination by fluorescence stereomicroscopy of the C. jejuni colonies growing from cells isolated from the rhizosphere on these plates revealed their different morphology when growing in association with soil and organic debris compared to colonies growing on agar alone. As shown in Fig. 4A and B, C. jejuni cells appear to coat the soil particles and the plant debris on the plate rather than forming distinct round colonies such as those present in the clean areas of the same plate. In addition, C. jejuni formed microcolonies on the surface of organic debris present in soil that was inoculated with the pathogen and then incubated on water agar at 37°C (Fig. 4C). The microcolonies were picked from the debris with a sterile needle and confirmed to be composed of green fluorescent C. jejuni cells under the phase-contrast microscope at 1,000× magnification.
FIG. 4.
Fluorescence stereomicroscope images of GFP-labeled C. jejuni colonies (light yellow-green fluorescent signal, yellow arrows) present on soil and plant material after incubation at 37°C on agar plates. (A) C. jejuni cells that were recovered from the spinach rhizosphere formed colonies that spread through the soil and on organic debris (black material) but not on the clean CCAS agar surface (red arrow). (B) Colonies of C. jejuni recovered from spinach and transferred onto agar plates also spread on roots, which appear as the dark material below the yellow-green fluorescent bacteria. (C) Microcolonies of GFP-labeled C. jejuni (yellow arrows) were observed on woody plant material present in soil that was inoculated with the pathogen and deposited onto water agar plates; the plant cells are delineated by lighter striations in the dark green background (blue arrows). Bars, 5 mm (A and B) and 1 mm (C).
Survival on leaves contaminated with soil.
The surface of spinach plants was contaminated intentionally with soil in the laboratory in order to determine the effect of the presence of soil on the survival of C. jejuni on aerial plant parts. Sterile soil was used to test specifically the hypothesis that soil could provide a physicochemical microenvironment on leaves that would benefit C. jejuni. The C. jejuni population decline rate was −0.98 and −1.39 on leaves with and without soil, respectively. Thus, the population size of C. jejuni decreased at a significantly lower rate on leaves that were contaminated with soil at the time of inoculation than on leaves without soil (F = 8.85; P < 0.004) (Fig. 5). In addition, C. jejuni was culturable over time from a higher proportion of leaves exposed to soil than from leaves not exposed to soil (Fisher's exact test; P < 0.0001) (Table 3).
FIG. 5.
Effect of the presence of soil on the survival of C. jejuni strain RM1221 on spinach leaves during incubation of the plants at 10°C. C. jejuni was inoculated onto clean leaves (○) or leaves that were contaminated with soil in the laboratory (□). Each data point represents the mean of the log-transformed bacterial population size on 10 leaves. The error bars represent ±1 standard error of the mean.
TABLE 3.
Effect of the presence of soil on the recovery of C. jejuni strain RM1221 from inoculated spinach plants
| No. of days after inoculation | % of leaves with detectable C. jejunia
|
|
|---|---|---|
| Leaves without soil | Leaves with soil | |
| 0 | 100 | 100 |
| 2 | 90 | 100 |
| 4 | 70 | 100 |
| 8 | 20 | 40 |
Values represent the percentages of the total number of leaves sampled on which C. jejuni was detected by plating onto CCAS. The spinach plants were incubated at 10°C after inoculation.
DISCUSSION
The ability of C. jejuni to grow and survive on plants was tested by inoculation of this pathogen onto spinach and lettuce leaves and onto spinach and radish roots of live plants. C. jejuni has been isolated previously from these plant species at outdoor markets (28, 34). Plant surfaces are hostile environments for most microorganisms because of prevailing rapid and repeated fluctuations in temperature, water availability, and radiation (22). Nutrients on leaves are sparse compared to the animal and human enteric environments, but oases occur where microbes can thrive and form large aggregates (29). Roots release 25% or more of their organic matter (12), and the nutrients exuded at the junction of lateral roots and at the root tip may form energy-rich microenvironments where bacteria can multiply (9, 25). The ability of C. jejuni to survive on plant surfaces and consequently present a potential food safety hazard will depend upon the physicochemical conditions that prevail in the plant environment and upon its tolerance to wide fluctuations of these conditions over short periods of time.
Campylobacter species are reported to obtain their energy from amino acids and tricarboxylic acid cycle intermediates, not from carbohydrates (15). Our carbon utilization studies indicate that many compounds detected commonly in leaf and root exudates are also utilized by C. jejuni on Biolog plates, either metabolically or for respiration, and thus could serve as potential nutrient sources for C. jejuni cells in the plant environment. Since Campylobacter species are unable to ferment or oxidize sugars (15), it was surprising that C. jejuni was active in l-arabinose and l-fucose on Biolog plates. l-Arabinose is a major root exudate (27), and l-fucose is an important component of plant cell walls (47) that is likely to be present on plants, particularly when injury of plant tissue occurs. It is unclear whether, and how, C. jejuni would utilize these specific sugars upon encountering them on plants.
C. jejuni is thermophilic; its minimal growth temperature ranges between 31 and 36°C, and growth ceases between 30 and 31°C (21). In our studies, the population sizes of C. jejuni on plants never increased after inoculation, even when the plants were inoculated at temperatures that are conducive to its growth in vitro. On the other hand, survival rates of C. jejuni on leaves and on roots were higher at low temperatures, such as 10 and 16°C, than at 33 and 37°C. It is likely that the growth of C. jejuni is inhibited by the presumably high oxygen levels in the phyllosphere. However, C. jejuni may find niches on plants where oxygen tension is low, such as within bacterial aggregates, in depressions where free water accumulates, or in the broken tissue of plant lesions. In addition, lesions may contain higher osmolyte concentrations; conditions of high osmolality have been shown to increase the survival of Campylobacter spp. at low temperatures (36). Although C. jejuni did not grow on wounded leaves, even at 33°C, a positive effect of mechanical wounds on the survival of C. jejuni on leaves was observed at 10°C. This observation may have implications not only for the preharvest contamination of crops with C. jejuni but also for its survival on cut produce after cross-contamination from meat products during food preparation or processing.
The biological cycle of C. jejuni in the environment is still poorly understood. There is strong evidence that C. jejuni survives well in farm animal feces (20, 32, 41, 46), in water (39, 42), and in sand from the runs of chicken farms (41). It is possible that manure-contaminated soil acts as a reservoir for C. jejuni in the field. To our knowledge, the survival of C. jejuni in agricultural soils and in association with the underground part of plants has not been investigated. Our studies indicate that the survival rate of C. jejuni at 10°C was enhanced significantly on roots and in soil compared to that on leaves. Because the rhizosphere is comprised of both roots and soil and the two components cannot be analyzed separately without difficulty, it is unclear whether C. jejuni survived in association with root tissue or in the soil attached to roots. However, the similarity in survival rates and the small difference between the C. jejuni population sizes on radishes that were wiped and those that were washed to remove most of the soil suggest that C. jejuni was located mainly at the radish root-soil interface.
The survival rate of C. jejuni in the spinach rhizosphere was dependent on the temperature at which the plants were incubated, with the highest persistence at 10 and 16°C. This enhanced ability of C. jejuni to survive at temperatures that are nonpermissive for growth in vitro has been observed in many environments (6, 11). Furthermore, there is evidence that C. jejuni cells are metabolically active and motile in vitro at temperatures as low as 4°C (21). Based on this observation, it is noteworthy that the population sizes of C. jejuni in the spinach rhizosphere appear to have stabilized after 14 days of incubation at 10 and 16°C, indicating that the cell death rate is extremely low. One could hypothesize that after a period of adaptation, a subpopulation of C. jejuni cells has found a niche in the rhizosphere that allows the cells to be sufficiently physiologically active to be culturable in the laboratory. This niche may be composed of microsites that have low oxygen tension due to microbial and root respiration (38) or to high water content and in which organic compounds that are suitable as nutrients or electron acceptors exist (Table 1). Humic acids, which are abundant in soil, have been reported to increase the isolation of Campylobacter spp. in the laboratory (45). The lower decline rate of C. jejuni population sizes on spinach leaves that were coinoculated with organic soil to simulate the contamination of crops by water and soil splashing during rain or irrigation indicates that organic soil has a protective effect.
The spread of C. jejuni in soil and on plant debris on CCAS plates and the microcolonies that formed on organic material deposited on water agar are intriguing. Chemotactic effectors of C. jejuni, such as fucose, cysteine, serine, aspartate, glutamate, succinate, citrate, and fumarate (23), most of which also up-regulate transcription of the flagellar gene flaA (1), all have been detected in root and/or leaf exudates (13, 27, 33, 47). We hypothesize that the physicochemical conditions generated by organic material in the soil, including nutrients that may induce chemotaxis, allow C. jejuni to spread to and colonize favorable niches and enhance its survival in the environment.
Although differences in behavior on plants may exist among strains, it is unlikely that a produce isolate would have a greater ability to grow on plants than a chicken or clinical isolate, based on the current knowledge of the biology of C. jejuni, particularly its thermophilic and microaerophilic requirements, and of the physicochemical conditions on plant surfaces. Rather, it is predicted that most C. jejuni strains would have a higher death rate than growth rate on plants but may survive better in the rhizosphere than in the phyllosphere due to the nature of these environments. The higher survival rate of C. jejuni in the rhizosphere than in the phyllosphere may be attributable also partly to differences in the microbial communities in these two habitats. The effect of the plant microflora on the survival of C. jejuni deserves further study. However, given our understanding of the physiology of C. jejuni, it is probable that the greater availability of organic substrates and lower oxygen tension in the rhizosphere than in the phyllosphere account for the enhanced survival of this organism on roots and surrounding soil.
It is noteworthy that the vegetables from which Campylobacter species have been isolated either are root crops or have a high probability of being contaminated with soil from the field (e.g., radish, spinach, lettuce, green onion, parsley, potatoes, carrots, cabbage, and mushrooms) (14, 17, 34). Additionally, all are crops grown under low temperatures, a condition that favors survival of C. jejuni in the environment. The enhanced persistence of C. jejuni on roots and in soil at low temperatures may explain the occasional outbreaks of campylobacteriosis and incidences of Campylobacter spp. associated with produce. It remains unclear, however, to what extent the occurrence of viable but nonculturable Campylobacter species (37, 43) and the present difficulty in recovering Campylobacter spp. from environmental samples affect our ability to fully assess the potential of this pathogen to colonize nonhost environments.
Acknowledgments
We thank Steven Huyhn and Felicidad Bautista for technical assistance. We are thankful to Ronald Amundson for useful discussions.
This work was supported by the U.S. Department of Agriculture, Agriculture Research Service CRIS project (201-5325-210-040 and 5325-42000-041), and is part of a U.S. collaboration with the European Commission Fifth Framework Project QLK1-CT-2002-0220, “CAMPYCHECK.”
REFERENCES
- 1.Allen, K. J., and M. W. Griffiths. 2001. Effect of environmental and chemotactic stimuli on the activity of the Campylobacter jejuni flaA σ28 promoter. FEMS Microbiol. Lett. 205:43-48. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. 1999. FoodNet 1998 annual report. Centers for Disease Control and Prevention, Atlanta, Ga.
- 4.Anonymous. 1998. Outbreak of Campylobacter enteritis associated with cross-contamination of food—Oklahoma, 1996. Morb. Mortal. Wkly. Rep. 47:129-131. [PubMed] [Google Scholar]
- 5.Beuchat, L. R. 1996. Pathogenic microorganisms associated with fresh produce. J. Food Prot. 59:204-216. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, M. J., H. L. Hardesty, B. Powers, and W. L. Wang. 1980. Survival of Campylobacter fetus subsp. jejuni in biological milieus. J. Clin. Microbiol. 11:309-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaser, M. J., J. G. Wells, R. A. Feldman, R. A. Pollard, and J. R. Allen. 1983. Campylobacter enteritis in the United States. A multicenter study. Ann. Intern. Med. 98:360-365. [DOI] [PubMed] [Google Scholar]
- 8.Bolton, F. J., and L. Robertson. 1982. A selective medium for isolating Campylobacter jejuni/coli. J. Clin. Pathol. 35:462-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolton, H., J. K. Fredrickson, and L. F. Elliot. 1993. Microbial ecology of the rhizosphere, p. 646. In F. B. Metting (ed.), Soil microbial ecology. Marcel Dekker, New York, N.Y.
- 10.Brandl, M. T., and R. E. Mandrell. 2002. Fitness of Salmonella enterica serovar Thompson in the cilantro phyllosphere. Appl. Environ. Microbiol. 68:3614-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buswell, C. M., Y. M. Herlihy, L. M. Lawrence, J. T. M. McGuiggan, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1998. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl. Environ. Microbiol. 64:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curl, E. A., and B. Truelove. 1986. Root exudates, p. 55-92. In The rhizosphere. Springer-Verlag, Berlin, Germany.
- 13.Derridj, S. 1996. Nutrients on the leaf surface, p. 25-42. In C. Nguyen-The (ed.), Aerial plant surface microbiology. Plenum Press, New York, N.Y.
- 14.Doyle, M. P., and J. L. Schoeni. 1986. Isolation of Campylobacter jejuni from retail mushrooms. Appl. Environ. Microbiol. 51:449-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elharrif, Z., and F. Megraud. 1986. Characterization of thermophilic Campylobacter: I. Carbon substrate utilization tests. Curr. Microbiol. 13:117-122. [Google Scholar]
- 16.Evans, M., C. Ribeiro, and R. Salmon. 2003. Hazards of healthy living: bottled water and salad vegetables as risk factors for Campylobacter infection. Emerg. Infect. Dis. 9:1219-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Federighi, M., C. Magras, M. F. Pilet, D. Woodward, W. Johnson, F. Jugiau, and J. L. Jouve. 1999. Incidence of thermotolerant Campylobacter in foods assessed by NF ISO 10272 standard: results of a two-year study. Food Microbiol. 16:195-204. [Google Scholar]
- 18.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized countries, p. 121-138. In M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 19.Frost, J. A., I. A. Gillespie, and S. J. O'Brien. 2002. Public health implications of campylobacter outbreaks in England and Wales, 1995-9: epidemiological and microbiological investigations. Epidemiol. Infect. 128:111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory, E., H. Barnhart, D. W. Dreesen, N. J. Stern, and J. L. Corn. 1997. Epidemiological study of Campylobacter spp. in broilers: source, time of colonization, and prevalence. Avian Dis. 41:890-898. [PubMed] [Google Scholar]
- 21.Hazeleger, W. C., J. A. Wouters, F. M. Rombouts, and T. Abee. 1998. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol. 64:3917-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano, S. S., and C. D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae: a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64:624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hugdahl, M. B., J. T. Beery, and M. P. Doyle. 1988. Chemotactic behavior of Campylobacter jejuni. Infect. Immun. 56:1560-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs-Reitsma, W. 2000. Campylobacter in the food supply, p. 467-481. In M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 25.Jaeger, C. H., S. E. Lindow, S. Miller, E. Clark, and M. K. Firestone. 1999. Mapping of sugar and amino acid availability in soil around roots with bacterial sensors of sucrose and tryptophan. Appl. Environ. Microbiol. 65:2685-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, N. W., H. Bingham, R. Khawaja, H. Louie, E. Hani, K. Neote, and V. L. Chan. 1992. Physical map of Campylobacter jejuni TGH9011 and localization of 10 genetic markers by use of pulsed-field gel electrophoresis. J. Bacteriol. 174:3494-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraffczyk, I., G. Trolldenier, and H. Beringer. 1984. Soluble root exudates of maize: influence of potassium supply and rhizosphere microorganisms. Soil Biol. Biochem. 16:315-322. [Google Scholar]
- 28.Kumar, A., R. K. Agarwal, K. N. Bhilegaonkar, B. R. Shome, and V. N. Bachhil. 2001. Occurrence of Campylobacter jejuni in vegetables. Int. J. Food Microbiol. 67:153-155. [DOI] [PubMed] [Google Scholar]
- 29.Leveau, J. H. J., and S. E. Lindow. 2001. Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc. Natl. Acad. Sci. USA 98:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandrell, R. E., and M. T. Brandl. Campylobacter species and fresh produce: outbreaks, incidence and biology. In R. Beier, R. Ziprin, S. Pillai, and T. Philips (ed.), Pre-harvest and post-harvest food safety: contemporary issues and future directions, in press. Iowa State Press, Ames.
- 31.Miller, W. G., A. H. Bates, S. T. Horn, M. T. Brandl, M. R. Wachtel, and R. E. Mandrell. 2000. Detection on surfaces and in Caco-2 cells of Campylobacter jejuni cells transformed with new gfp, yfp, and cfp marker plasmids. Appl. Environ. Microbiol. 66:5426-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montrose, M. S., S. M. Shane, and K. S. Harrington. 1985. Role of litter in the transmission of Campylobacter jejuni. Avian Dis. 29:392-399. [PubMed] [Google Scholar]
- 33.Morgan, J. V., and H. B. Tukey, Jr. 1964. Characterization of leachate from plant foliage. Plant Physiol. 39:590-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park, C. E., and G. W. Sanders. 1992. Occurrence of thermotolerant campylobacters in fresh vegetables sold at farmers' outdoor markets and supermarkets. Can. J. Microbiol. 38:313-316. [DOI] [PubMed] [Google Scholar]
- 35.Phillips, C. A. 1998. The isolation of Campylobacter spp. from modified atmosphere packaged foods. Int. J. Environ. Health Res. 8:215-221. [Google Scholar]
- 36.Reezal, A., B. McNeil, and J. G. Anderson. 1998. Effect of low-osmolality nutrient media on growth and culturability of Campylobacter species. Appl. Environ. Microbiol. 64:4643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rollins, D. M., and R. R. Colwell. 1986. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 52:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, M. S., and J. M. Tiedje. 1979. The effects of roots on soil denitrification. Soil Sci. Soc. Am. J. 43:951-955. [Google Scholar]
- 39.Stanley, K., R. Cunningham, and K. Jones. 1998. Isolation of Campylobacter jejuni from groundwater. J. Appl. Microbiol. 85:187-191. [DOI] [PubMed] [Google Scholar]
- 40.Stern, N. J., B. Wojton, and K. Kwiatek. 1992. A differential-selective medium and dry-ice generated atmosphere for recovery of Campylobacter jejuni. J. Food Prot. 55:514-517. [DOI] [PubMed] [Google Scholar]
- 41.Studer, E., J. Luthy, and P. Hubner. 1999. Study of the presence of Campylobacter jejuni and C. coli in sand samples from four Swiss chicken farms. Res. Microbiol. 150:213-219. [DOI] [PubMed] [Google Scholar]
- 42.Terzieva, S. I., and G. A. McFeters. 1991. Survival and injury of Escherichia coli, Campylobacter jejuni, and Yersinia enterocolitica in stream water. Can. J. Microbiol. 37:785-790. [DOI] [PubMed] [Google Scholar]
- 43.Tholozan, J. L., J. M. Cappelier, J. P. Tissier, G. Delattre, and M. Federighi. 1999. Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl. Environ. Microbiol. 65:1110-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wachtel, M. R., L. C. Whitehand, and R. E. Mandrell. 2002. Association of Escherichia coli O157:H7 with preharvest leaf lettuce upon exposure to contaminated irrigation water. J. Food Prot. 65:18-25. [DOI] [PubMed] [Google Scholar]
- 45.Weinrich, V. K., K. Winkler, and E. Heberer. 1990. Studies concerning the use of selected humic acid products in media for isolation of thermophilic Campylobacter species. Dtsch. Tieraerztl. Wochenschr. 97:511-515. [PubMed] [Google Scholar]
- 46.Wesley, I. V., S. J. Wells, K. M. Harmon, A. Green, L. Schroeder-Tucker, M. Glover, and I. Siddique. 2000. Fecal shedding of Campylobacter and Arcobacter spp. in dairy cattle. Appl. Environ. Microbiol. 66:1994-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zablackis, E., W. S. York, M. Pauly, S. Hantus, W. D. Reiter, C. C. S. Chapple, P. Albersheim, and A. Darvill. 1996. Substitution of l-fucose by l-galactose in cell walls of Arabidopsis mur1. Science 272:1804-1808. [DOI] [PubMed] [Google Scholar]