Abstract
Calcium is relevant for several vital functions in apicomplexan parasites including host cell invasion, parasite motility, and differentiation. The endoplasmic reticulum (ER) and calcium-rich acidocalcisomes have been identified as major calcium stores. Other potential calcium storage organelles include the Golgi, the mitochondrion, the apicoplast, and the recently described plant-like vacuole in T. gondii. Compared to most eukaryotic systems, apicomplexan parasites contain a reduced number of calcium-related genes, a vast majority of which remain uncharacterized. Several Ca2+-ATPases have been described in apicomplexans, several of which are annotated in the different genomes. There is experimental evidence for an inositol 1,4,5-trisphosphate (IP3)-dependent calcium response in Plasmodium spp. and T. gondii although no IP3 or ryanodine receptors have been identified. Genes encoding potential calcium channels are present in T. gondi but not in Plasmodium spp. and Cryptosporidium spp. Effector calcium binding proteins including calmodulins and calcium-dependent protein kinase (CDPK) genes mainly found in plants have also been described. The characterized CDPKs were found to play important roles in protein secretion, host cell invasion and parasite differentiation. Together, the available information on calcium storage and function in apicomplexans, although fragmented, suggest the existence of unique calcium-mediated pathways in these parasites. An in-depth functional characterization of the apicomplexan calcium-related genes could lead to the identification of novel therapeutic targets, and will improve our understanding of the role of calcium in parasite development and virulence.
Introduction
Fluctuations of cytosolic free Ca2+ concentrations ([Ca2+]i) regulate a variety of cellular functions in all eukaryotes. The [Ca2+]i is maintained at very low levels (of the order of 10−7 M) compared with that in the extracellular medium (about 10−3 M). A variety of mechanisms, enzyme transporters, channels and calcium binding proteins contribute to maintaining the [Ca2+]i at 10−7 M. The total calcium inside the cell is much higher than 10−7 M, but the bulk of this calcium is either bound to proteins, polyphosphate, membranes and/or other cellular constituents, or is sequestered inside intracellular organelles such as mitochondria, endoplasmic reticulum (ER), Golgi apparatus, and nuclei [1]. A key event in calcium signaling is the influx of calcium across the plasma membrane. Ca2+ storage organelles capable of both high affinity uptake and rapid triggered release of Ca2+ are believed to be ubiquitous among eukaryotes [2].
Ca2+ homeostasis and storage in apicomplexans
Apicomplexan parasites are a large group of protists, which include a number of pathogens of medical and agricultural relevance. These parasites are named for their peculiar apical end, which contains a number of unique organelles and structures.
Calcium homeostasis and storage has been studied mainly in two groups of apicomplexan parasites: T. gondii and Plasmodium spp. T. gondii is an important cause of congenital disease and infection in immunocompromised patients. Plasmodium spp. represent the causative agents of malaria, one of the most devastating human infectious diseases. Intracellular calcium measurements have been performed in apicomplexan parasites mainly using the fluorescent calcium indicator fura 2-AM (fura 2-acetoxymethylester). This reagent, crosses the plasma membrane and is converted inside the cell to fura 2 by endogenous cytosolic esterases. This de-esterified form of the reagent is unable to cross membranes and becomes trapped in the cytoplasm of the cell where it is able to monitor changes in the cytosolic calcium concentration. Using this methodology, the [Ca2+]i in T. gondii tachyzoites was measured at 70 ± 6 nM in the absence of extracellular Ca2+ (with the Ca2+ chelator EGTA added to the medium) and 100 ± 9 nM in the presence of 1 mM extracellular Ca2+ [3]. In Plasmodium chabaudi, and P. falciparum the [Ca2+]i was also measured using fura 2-loaded free parasites and the values obtained were also at nanomolar levels in single-cell imaging experiments [4] or in parasite suspensions [5]. When imaging P. falciparum-infected erythrocytes loaded with fura red, a ratiometric calcium indicator with a low sensitivity to pH, much higher levels of [Ca2+]i (289–352 nM), were measured [6]. This high value may be the result of superposition of Ca2+ signals from the cytosol and the extensive ER compartment within these cells [6]. The [Ca2+]i in the parasite is likely within the range of concentrations observed in other eukaryotic cells (i.e. 90–100 nM).
In addition to known eukaryotic calcium stores including acidocalcisomes, the ER, Golgi apparatus and mitochondria, apicomplexan parasites contain several unique organellar compartments that potentially could contribute to diverse calcium transients necessary for vital functions within the parasites. These include the apicoplast, a remnant plastid derived from a secondary endosymbiotic event, and various acidic organelles including a recently described plant-like vacuole.
The endoplasmic reticulum
The largest store of Ca2+ in cells is usually found in the ER (Fig. 1), with local concentration reaching millimolar levels. The ER possesses independent pathways for calcium influx and efflux. The influx is catalyzed by the very well known sarco-endoplasmic reticulum Ca2+-ATPase referred to as SERCA-type Ca2+-ATPase, that actively translocates 2 Ca2+ for the hydrolysis of 1 ATP molecule. SERCA-type Ca2+-ATPases have been characterized in T. gondii (Fig. 1, b) [7] and in Plasmodium falciparum [8]. Thapsigargin, a specific inhibitor of SERCA-type Ca2+-ATPases of other eukaryotes produces an increase in the cytosolic Ca2+ levels of T. gondii and, of Plasmodium spp. at high concentrations [4] [9]. Release of Ca2+ from the ER occurs through an inositol 1,4,5-trisphosphate (IP3)-stimulated calcium channel. An IP3/ryanodine-sensitive store has been postulated to be present in T. gondii on the basis of pharmacological studies (Fig. 1, f) [10] and the release of Ca2+ from intracellular stores of malaria parasites was also shown to respond to IP3 [9, 11]. The enzyme that catalyzes the production of IP3, the phosphoinositide phospholipase C (PI-PLC) has been characterized in T. gondii (TgPI-PLC) [12]. However, there is no genetic evidence to indicate the presence of an IP3R in any of the apicomplexan parasites. This means that the parasite may use a different mechanism (probably responsive to IP3) to release calcium from the ER.
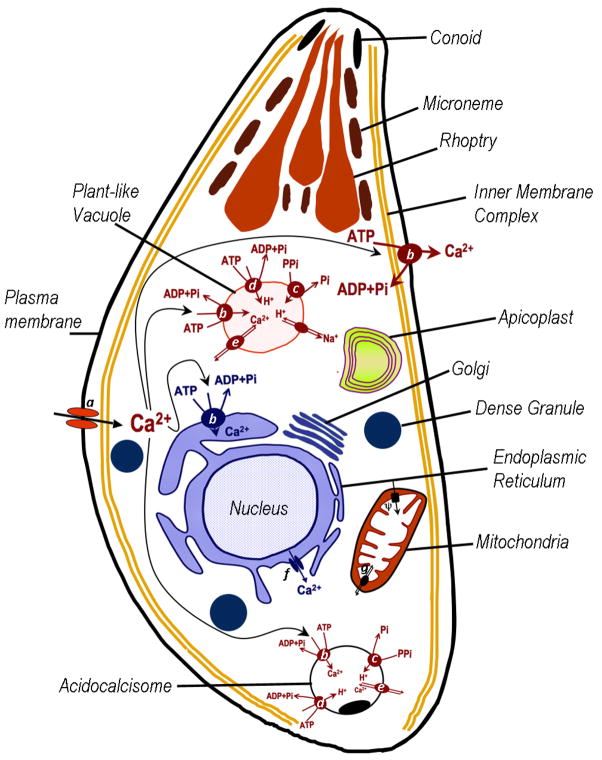
Figure 1.
Schematic representation of the distribution of Ca2+ in a T. gondii tachyzoite. Abbreviations: Ca2+ entry is probably through Ca2+ channels (a). Once inside the cells, Ca2+ can be translocated back to the extracellular environment, primarily by the action of the PMCA (b). In addition, Ca2+ will interact with Ca2+-binding proteins or become sequestered by the ER by the action of the SERCA-Ca2+-ATPase (b), sequestered by the mitochondrion through a postulated uniport driven by their membrane potential (ΔΨ), sequestered by the acidocalcisome or the plant-like vacuole by the action of a Ca2+-ATPase (TgA1) (b). Ca2+ appears to diffuse freely into the nucleus. Calcium could also be released into the cytoplasm from the internal stores as the ER through an uncharacterized channel, which appears to respond to ryanodine and caffeine (f). It may also be released from the PLV and the acidocalcisome through a Ca2+/H+ exchanger (e). Acidic compartments as the acidocalcisome and the PLV contain enzymes involved in their acidification as the H+-ATPase (d) and the V-H+-pyrophosphatase (c). Mitochondrial Ca2+ release is through a Ca2+-H+ exchanger (g).
Acidic organelles
Acidocalcisomes are organelles that contain large amounts of calcium in an acidic environment. This calcium is probably not free but bound to other molecules as short- and long-chain polyphosphate [13]. Acidocalcisomes were first studied in trypanosomes and apicomplexan parasites and later found to be similar to the previously described “volutin granules” in bacteria or polyphosphate bodies in algae [13]. These organelles were initially found because the stored Ca2+ could be released into the cell cytoplasm with nigericin (a K+/H+ exchanger) or the weak base NH4Cl [3]. This compartment was further characterized and named acidocalcisomes (Fig. 1). More recent work in Leishmania suggests that acidocalcisomes are lysosome-related organelles [14]. Acidocalcisomes have also been found in Plasmodium spp. [13] and more recently in Eimeria parasites [15].
The calcium inside the acidocalcisome is probably pumped in by a Ca2+-ATPase, which in T. gondii has been named as TgA1 (Fig. 1, b in acidocalcisome) [16]. Recent work with a purified acidocalcisome fraction from T. gondii tachyzoites shows that calcium uptake is sensitive to vanadate, (a Ca2+-ATPase inhibitor) supporting the idea of calcium being stored in this organelle [17].
Two enzymes with proton pumping activity have been found to localize to acidocalcisomes. The vacuolar-H+-pyrophosphatase is an enzyme that hydrolyses pyrophosphate and the energy released is used to pump protons toward the lumen of the organelle where it localizes. This enzyme was described and characterized in Toxoplasma gondii (TgVP1) (Fig. 1, c) and also in Plasmodium falciparum (PfVP1) [13]. The second enzyme is the vacuolar-H+-ATPase (Fig. 1, d), which pumps protons and uses the energy from the hydrolysis of ATP. Acidification of the acidocalcisome by these pumps is important to maintain organellar calcium, as alkalinizing agents such as NH4Cl, release calcium to the cytoplasm. This is postulated to occur through a calcium-proton exchanger (see the diagram of the acidocalcisome in Fig. 1, e).
The vacuolar-H+-pyrophosphatase also localizes to other compartments, as for example the plant-like vacuole in T. gondii, the food vacuole in Plasmodium spp., the plasma membrane in both parasites and also to other endocytic compartments. This is unique because in mammalian cells, acidic compartments are maintained by the V-H+-ATPase. One possible explanation for this difference is that the V-H+-pyrophosphatase uses pyrophosphate as an energy source, which is quite abundant in these parasites. Some of the developmental stages of the parasites might run short in ATP supply because of their suboptimal mitochondrial function as for example in the blood stages of Plasmodium spp. and extracellular tachyzoites of T. gondii [18].
The plant-like vacuole (PLV), recently described in T. gondii, was also found to contain calcium. This was demonstrated by adding the compound glycyl-L-phenylalanine-naphthylamide (GPN) to intact parasites loaded with the Ca2+ indicator fura 2-AM. GPN is specifically hydrolyzed in the lysosome of a variety of different cell types by a cathepsin C protease, and the product of its hydrolysis has a swelling effect in the lysosome leading to calcium leaking out to the cytoplasm [19]. The presence of a cathepsin C (CPC) inside the PLV was demonstrated by proteomic data of enriched fractions and IFA analysis of cells expressing a C-terminal tagged CPC gene (Moreno and Carruthers, unpublished). This GPN-dependent calcium release was shown to be independent of other calcium stores such as the ER [19].
Further evidence supporting the presence of Ca2+ inside the PLV is the detection of a vacuolar PMCA-type calcium ATPase (TgA1). This was shown by proteomic analysis of subcellular fractions and by immunofluorescence assays. TgA1 has been characterized previously and shown to have a role in intracellular Ca2+ homeostasis as well as in parasite virulence. The specific role of the PLV in Ca2+ homeostasis or Ca2+ related function is still not known.
Mitochondria
Mitochondria possess a high capacity to sequester Ca2+ although under physiologic conditions, total mitochondrial Ca2+ levels and free Ca2+ reflect and parallel cytosolic Ca2+. The inner mitochondrial membrane possesses a uniport carrier for Ca2+, which allows the electrogenic entry of the cation driven by the electrochemical gradient generated by respiration or ATP hydrolysis (Fig. 1, Ψ in the mitochondrion cartoon). Ca2+ efflux, on the other hand, takes place by a different pathway, which appears to catalyze the electroneutral exchange of internal Ca2+ by external sodium or protons.
Experiments performed with malaria parasites using digitonin to measure mitochondrial activity in situ, showed Ca2+ uptake from the incubation medium by a mechanism associated with depolarization of the membrane potential. These results support the presence of a Ca2+ uniport similar to that of mammalian mitochondria [20]. A Ca2+/H+ antiporter was recently localized to P. falciparum mitochondria. Sensitivity to ruthenium red and ruthenium 360 suggested the presence of a Ca2+ uniport in these mitochondria [21]. Unlike the mammalian mitochondria, where intracellular Ca2+ regulates the activity of several dehydrogenases no such Ca2+-regulated dehydrogenases have been reported in apicomplexan parasites.
Calcium signaling and functions in apicomplexan parasites
Ca2+ signaling involves the mobilization of Ca2+ from two sources: intracellular stores and the extracellular medium. Mechanisms to introduce Ca2+ into the cytoplasm are compensated by a coordinated set of removal mechanisms consisting of buffers, pumps and exchangers. Free Ca2+ binds a number of effectors, which are responsible for stimulating numerous Ca2+-dependent processes. Each cell type expresses a unique set of mechanisms and effectors, which create a Ca2+-signaling system with the appropriate spatial and temporal properties.
In excitable mammalian cells, such as in muscle cells, the primary signal (membrane depolarization) activates Ca2+ entry across the plasma membrane, and this Ca2+ signal is amplified and propagated by a mechanism of Ca2+-induced Ca2+ release (CICR) from the sarcoplasmic reticulum [22]. In non-excitable mammalian cells, such as endothelial cells, activation by a hormone or growth factor receptor coupled to a phospholipase C results in the hydrolysis of phosphatidylinositol 4,5-disphosphate (PIP2) to generate inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 diffuses to the intracellular stores (ER) and causes the release of Ca2+ into the cytoplasm through IP3 receptors [23]. Some mammalian cells, such as smooth muscle and neuroendocrine cells, can utilize both of these pathways.
Ca2+ release from the ER is followed by the entry of Ca2+ ions across the plasma membrane. This process is known as capacitative Ca2+ entry or store-operated Ca2+ entry (SOCE) [24]. The connection between the ER and the plasma membrane was discovered only in recent years and it is orchestrated mainly by two protein families: Stim (Stim 1 and 2), which appear to function as Ca2+ sensors within the ER, and Orai proteins (Orai 1, 2, and 3) which function as SOCE channels in the plasma membrane [25]. In addition, a number of newly discovered second messengers, such as cyclic adenosine diphosphate ribose (cADPr), sphingosine-1-phosphate, and nicotinic acid adenine dinucleotide phosphate (NAADP) have been observed to release or modulate the release of intracellular Ca2+ from different cells. The plasma membrane of eukaryotic cells contains a number of channels through which calcium gains access into the cytoplasm. Some of these channels respond to changes in the membrane potential (voltage-gated Ca2+ channels), others are under the control of receptors (receptor-operated Ca2+ channels), and others are controlled by the content of intracellular stores (store-operated Ca2+ channels). The active export of calcium from eukaryotic cells is accomplished by the action of a Na+/Ca2+ exchanger or the plasma membrane Ca2+-ATPase (PMCA).
The information available on calcium signaling components in apicomplexan parasites is still fragmentary although important features of their life cycle such as motility, host cell invasion, and egress from infected cells, are linked to calcium. A sequence with general similarity to a voltage dependent Ca2+ channel has been found in the T. gondii genome (TGME49_005260). The demonstration that this gene product is functional as a Ca2+ channel awaits further work (Fig. 1, a). There is no direct evidence for receptor-operated Ca2+ influx and none of the genes that correspond to the SOCE machinery, Stim and Orai, [26] are present in any of the apicomplexan genomes. A Ca2+ channel has been postulated to be inserted in the plasma membrane of erythrocytes infected with P. falciparum [27]. There are several reports of PMCA-type-Ca2+-ATPases in apicomplexan parasites [28]. Biochemical evidence for calmodulin (CaM) stimulation of this pump has been reported [29] although TgA1 appears to lack a typical CaM-binding domain. This might suggest the presence of a different domain able to bind CaM. A gene encoding for a second putative PMCA has been found in the T. gondii genome (TGME29_033770) [28]. The deduced amino acid sequence (1200 aa) shows 45% identity with TgA1 [16]. No homologues to this enzyme are found in the Plasmodium genomes.
Calcium binding proteins in apicomplexan parasites
Once inside the cell, Ca2+ can either interact with so-called soluble Ca2+-binding proteins or become sequestered into intracellular organelles.
Calcium binding proteins (CBP) are characterized by the presence of a highly conserved helix-loop-helix structural motif known as an EF-hand. Typically, EF hand motifs occur in pairs (also called EF hand domain) and facilitate the cooperative binding of two Ca2+ ions per domain. However, CBPs with single or odd number of EF hand motifs have been reported in both bacteria and eukaryotes and are believed to function via dimerization mechanisms. At least 69 EF hand domain-containing proteins are encoded by the P. falciparum genome (PlasmoDB), 55 in T. gondii (ToxoDB), and 45 in C. parvum (CryptoDB). As with most apicomplexan protein families, a majority of these EF hand domain-containing proteins are hypothetical with no known functions.
CBPs are generally classified into three main families: the calmodulin (CaM) family, the calcineurin B-like (CBL) family, and the calcium-dependent protein kinase (CDPK) family. The CaM family includes classical calmodulins (sequence identity with human CaM > 75%), calmodulin-like (CML) proteins (sequence identity with human CaM < 75%), and all other CaM-related proteins (presence of at least one non-CaM domain structure). Structurally, calmodulins are acidic proteins comprised of two globular domains (each with a pair of EF hands) linked by a flexible helical region. Most apicomplexan genomes encode single prototype CaMs and a variable number of CMLs/CaM-related proteins. Of these proteins, only the T. gondii CaM has been cloned and shown to bind Ca2+ in vitro [30]. Additional evidence in support of a functional CaM in T. gondii has been provided by in vitro experiments using the CaM inhibitors calmidazolium and trifluoperazine [31]. These drugs significantly reduce host cell entry by T. gondii tachyzoites, suggesting a role of TgCaM in host cell invasion. CaM inhibitors are equally toxic to P. falciparum, affecting parasite development and erythrocyte invasion by merozoites [32, 33]. Unlike CaM proteins, CBLs have been identified only in higher plants and, to a limited extent, in some protist genomes. Inspection of representative apicomplexan genomes reveals single gene sequences that potentially may encode functional CBLs in T. gondii and P. falciparum.
The third class of CBPs comprises several enzymes that are modulated through direct interactions with Ca2+. These include a variety of kinases (e.g. CDPKs and CCaMKs), proteases, phosphatases, synthases, and nucleoside triphosphatases (NTPases). Most apicomplexan genomes encode several CDPKs, Ser/Thr protein phosphatases, tRNA synthases, ubiquitin C-terminal hydrolases and cathepsins.
The fourth group of CBPs encoded by the apicomplexan genomes includes several hypothetical proteins, heat-shock proteins, centrin/troponin C-like proteins, and variants of the Plasmodium-specific virulence factor PfEMP1. These proteins, which have barely been characterized in some apicomplexans, are likely to exhibit vital functions including a role in buffering and species-specific signaling processes.
Functional studies of calcium and CBPs in apicomplexan parasites
Microneme secretion, invasion, and egress
T. gondii and Plasmodium spp. contain micronemes (Fig. 1), specialized apical secretory organelles, which appear to play an important role in the early phase of the invasion process. These organelles contain protein complexes or adhesins, which are discharged and participate in the interaction with host cell surface. A large number of studies support the relevance of microneme secretion during invasion of the host cell.
The secretion of micronemes can be induced artificially by treating parasites with calcium ionophores [34]. This effect can be blocked with the intracellular Ca2+ chelator BAPTA-AM demonstrating that the secretion of micronemes is triggered by an increase in intracellular Ca2+. BAPTA-AM was also used to demonstrate the role of calcium in conoid extrusion [35], gliding motility [36] and invasion [37]. The stimulation of microneme secretion by Ca2+ has also been demonstrated in C. parvum [38] and P. berghei [39].
The relevance of intracellular Ca2+ homeostasis was also highlighted by the phenotype of the TgA1 null mutant parasites. These cells have their intracellular calcium levels altered, are unable to maintain it at physiological level under the experimental conditions tested, and are deficient in microneme secretion. In addition, these cells have an invasion defect and reduced virulence in vivo [40].
T. gondii replicates inside its host cell but at some point needs to exit to be able to infect other cells and this egress process is still poorly understood. T. gondii egress is rapid and results in lysis of the infected host cell. It is also known that calcium ionophores like A23187 can stimulate this process [41]. Parasite mutants with a defect in egress (delayed egress) have been isolated and found to have their intracellular calcium levels elevated [42] and in addition have reduced pathogenicity [43].
Calcium and motility
Secretion of microneme proteins is also important for motility of T. gondii. Microneme secretion is triggered by an increase in intracellular calcium (see above) meaning calcium may have a role in motility. Several pieces of information available in the literature support this statement. T. gondii parasites were loaded with the Ca2+ indicator fluo 4 and analyzed by live imaging and it was observed that periodic oscillations in the intracellular Ca2+ levels were linked to gliding of the parasites [36].
The exit from the host cell is also a process dependent in motility of the parasite. The effect of Ca2+ ionophores on egress could be linked to the effect on the motility of the parasite. Changes in extracellular Ca2+ have not been evaluated as a possible trigger of microneme secretion and the natural agonist responsible for stimulating intracellular Ca2+ increase and subsequent microneme release is yet to be identified.
Role of CDPK
The Ca2+-dependent protein kinase (CDPK) family constitutes a group of kinases that are only found in plants and protists. In plants, CDPKs are a required response mechanism that allows external Ca2+ signals to regulate a diverse number of pathways including cell cycle progression and stress responses. The typical CDPK is composed of an amino-terminal serine/threonine kinase domain, followed by a junction domain (also known as the autoinhibitory domain) that connects to the carboxy-terminal calmodulin-like domain. The calmodulin-like domain typically consists of multiple calcium binding domains (i.e., 4 EF hand domains).
Phylogenetic analyses of CDPKs in apicomplexans show that there is a large diversity of these kinases present in Plasmodium, Toxoplasma, and Cryptosporidium genera. In addition to the typical CDPKs described above and found in plants, apicomplexan parasites contain 4 additional structural variants. The primary source of this variation is the number of EF hand domains and the length of the amino-terminus preceding the kinase domain. P. falciparum possesses 7 annotated CDPKs while T. gondii possesses 12 CDPKs [44].
Ca2+ is known to have a key role in critical features of apicomplexan parasites as a second messenger system, and CDPKs have been implicated as a mechanistic link between Ca2+ signaling and differentiation, motility, invasion, and egress. The rodent malarial parasite, P. berghei, requires CDPKs for developmental differentiation. Genetic disruption of CDPK4 in P. berghei gametocytes (sexual stages located in mammalian blood stream) inhibits calcium dependent pathways that are required for microgamete differentiation [44]. Additionally, knockout of a different kinase, CDPK3, in P. berghei severely inhibits the ability of ookinetes to traverse the peritrophic membrane in the mosquito gut, thereby stopping oocysts production in the mosquito gut and short-circuiting the vector pathway of this parasite [45].
Essential attributes of Ca2+-dependent protein kinases have also been observed in the related apicomplexan T. gondii. The protein kinase inhibitor, KT5926, which inhibits microneme secretion required for host cell attachment of the parasite, has been shown to target a CDPK in T. gondii [46]. More specifically, down regulation of T. gondii CDPK1, resulted in loss of parasite motility and host cell invasion and egress abilities, further demonstrating the essential nature of CDPKs in this important apicomplexan parasite [47].
The essential nature of CDPKs in regulating critical pathways of apicomplexan parasites has been clearly established. However, further efforts in determining the specific substrates of CDPKs will provide a more mechanistic understanding of their control of invasion and differentiation. Additionally, Ca2+-dependent protein kinases exhibit a significant level of crosstalk with other protein kinases, most notably the cyclic nucleotide-dependent kinases. Taking this into account along with the large number and structural diversity of Ca2+-dependent kinases, it is apparent that further research on the CDPKs in apicomplexans is likely to be very rewarding in terms of cell biology and development of potential drug targets for clinical use.
Conclusion
Ca2+ has important roles in secretion, motility, cell invasion and differentiation of apicomplexan parasites. Ca2+ regulation is controlled by a variety of systems for uptake and release that differ in several aspects from the processes that occur in other eukaryotic cells, providing excellent opportunities for targeting them for new therapies. Apicomplexan parasites contain several P-type Ca2+-ATPases including a SERCA-type important for Ca2+ uptake in the endoplasmic reticulum, as well as Ca2+/H+ antiporters. However, a PMCA-type Ca2+-ATPase and voltage-dependent Ca2+ channels are only present in T. gondii. There is evidence for mechanisms of Ca2+ release stimulated by IP3 and cADP ribose in some of these parasites but no receptors for these second messengers have been identified. A number of Ca2+-binding proteins including calmodulins, calmodulin-like proteins, and an array of Ca2+-dependent protein kinases are present in these parasites. Acidocalcisomes are present in Plasmodium spp., T. gondii, and Eimeria spp., but absent in Cryptosporidium spp. Other acidic organelles containing Ca2+ include the digestive vacuole of malaria parasites and the plant-like vacuole of T. gondii. Because of their situation at an early branch point in eukaryotic evolution studies of Ca2+ storage and signaling in these parasites could shed light about the origins of complex signaling networks in eukaryotes. Several apicomplexan genomes are completed, making it possible to look for conserved pathways through sequence-based phylogenetic comparisons. Additionally, the continuing availability and enhancement of experimental tools for genetic manipulation and molecular investigation of apicomplexan parasites (especially T. gondii and Plasmodium spp.) should allow for significant advances in deciphering calcium signaling pathways in these important eukaryotes.
Summary.
Ca2+ is important for motility, secretion, invasion, egress, and differentiation of apicomplexan parasites
There is insufficient evidence for the presence of some mechanisms for Ca2+ uptake and release, as for example IP3 and cADP ribose receptors, PMCA-type Ca2+-ATPases or voltage dependent Ca2+ channels in many of these organisms.
Acidic calcium stores, such as the acidocalcisome, plant-like vacuole, and digestive vacuole appear to play important roles as Ca2+ stores.
Apicomplexans contain a diversity of calcium-dependent kinases, which are commonly found in plants.
Acknowledgments
S.N.J.M. is supported by a grant from the U.S. National Institutes of Health (AI-079625) D.A.P. is supported in part by NIH T32 training grant AI-060546 to the Center for Tropical and Emerging Global Diseases
Biographies
Silvia N.J. Moreno obtained her PhD in Biochemistry from the University of Buenos Aires, Argentina, and did postdoctoral work at the NIH and the Rockefeller University. After several years as Professor of Parasitology at the University of Illinois at Urbana-Champaign, IL, she became Professor of Cellular Biology at the University of Georgia, Athens, GA, U.S.A. Her laboratory identified the acidocalcisome, and more recently the plant-like vacuole of Toxoplasma gondii. Her research team focuses on the role of these organelles in Apicomplexan parasites and in cell signaling in Trypanosoma cruzi.
Lawrence S. Ayong earned his Ph.D in Biomolecular Science from the University of Central Florida, USA, and is currently a post-doctoral fellow at the Center for Tropical and Emerging Global Diseases, University of Georgia, Athens. His research focus is on membrane biogenesis and vesicle trafficking in Apicomplexan parasites. His background includes five years undergraduate teaching experience, a Fulbright Fellowship and authorship of several peer-reviewed articles.
Douglas Pace completed his Ph.D. in Biological Sciences at the University of Southern California, USA. He is currently an NIH post-doctoral fellow at the Center for Tropical and Emerging Global Diseases, University of Georgia, Athens. He studies the molecular physiology of Apicomplexan parasites. Specific research interests include characterization of aquaporin water channels and ion regulation in Toxoplasma gondii.
References
- 1.Irvine RF. Calcium transients: mobilization of intracellular Ca2+ Br Med Bull. 1986;42:369–374. doi: 10.1093/oxfordjournals.bmb.a072154. [DOI] [PubMed] [Google Scholar]
- 2.Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 3.Moreno SN, Zhong L. Acidocalcisomes in Toxoplasma gondii tachyzoites. Biochem J. 1996;313(Pt 2):655–659. doi: 10.1042/bj3130655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia CR, Dluzewski AR, Catalani LH, Burting R, Hoyland J, Mason WT. Calcium homeostasis in intraerythrocytic malaria parasites. Eur J Cell Biol. 1996;71:409–413. [PubMed] [Google Scholar]
- 5.Alleva LM, Kirk K. Calcium regulation in the intraerythrocytic malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 2001;117:121–128. doi: 10.1016/s0166-6851(01)00338-3. [DOI] [PubMed] [Google Scholar]
- 6.Rohrbach P, Friedrich O, Hentschel J, Plattner H, Fink RH, Lanzer M. Quantitative calcium measurements in subcellular compartments of Plasmodium falciparum-infected erythrocytes. J Biol Chem. 2005;280:27960–27969. doi: 10.1074/jbc.M500777200. [DOI] [PubMed] [Google Scholar]
- 7.Nagamune K, Beatty WL, Sibley LD. Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot Cell. 2007;6:2147–2156. doi: 10.1128/EC.00262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M, O’Neill PM, Bray PG, Ward SA, Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 9.Alves E, Bartlett PJ, Garcia CR, Thomas AP. Melatonin and IP3-induced Ca2+ release from intracellular stores in the malaria parasite Plasmodium falciparum within infected red blood cells. J Biol Chem. 2011;286:5905–5912. doi: 10.1074/jbc.M110.188474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovett JL, Marchesini N, Moreno SN, Sibley LD. Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from inositol 1,4,5-triphosphate (IP(3))/ryanodine-sensitive stores. J Biol Chem. 2002;277:25870–25876. doi: 10.1074/jbc.M202553200. [DOI] [PubMed] [Google Scholar]
- 11.Passos AP, Garcia CR. Inositol 1,4,5-trisphosphate induced Ca2+ release from chloroquine-sensitive and -insensitive intracellular stores in the intraerythrocytic stage of the malaria parasite P. chabaudi. Biochem Biophys Res Commun. 1998;245:155–160. doi: 10.1006/bbrc.1998.8338. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Marchesini N, Moreno SN. A Toxoplasma gondii phosphoinositide phospholipase C (TgPI-PLC) with high affinity for phosphatidylinositol. Biochem J. 2006;394:417–425. doi: 10.1042/BJ20051393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN. Acidocalcisomes - conserved from bacteria to man. Nat Rev Microbiol. 2005;3:251–261. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 14.Besteiro S, Tonn D, Tetley L, Coombs GH, Mottram JC. The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J Cell Sci. 2008;121:561–570. doi: 10.1242/jcs.022574. [DOI] [PubMed] [Google Scholar]
- 15.Miranda K, de Souza W, Plattner H, Hentschel J, Kawazoe U, Fang J, Moreno SN. Acidocalcisomes in Apicomplexan parasites. Exp Parasitol. 2008;118:2–9. doi: 10.1016/j.exppara.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Luo S, Vieira M, Graves J, Zhong L, Moreno SN. A plasma membrane-type Ca2+-ATPase co-localizes with a vacuolar H+-pyrophosphatase to acidocalcisomes of Toxoplasma gondii. Embo J. 2001;20:55–64. doi: 10.1093/emboj/20.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohloff P, Miranda K, Rodrigues JCF, Fang J, Galizzi M, Plattner H, Hentschel J, Moreno SNJ. Calcium uptake and proton tyransport by acidocalcisomes of Toxoplasma gondii. Plos One. 2011 doi: 10.1371/journal.pone.0018390. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeber F, Limenitakis J, Soldati-Favre D. Apicomplexan mitochondrial metabolism: a story of gains, losses and retentions. Trends in parasitology. 2008;24:468–478. doi: 10.1016/j.pt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Miranda K, Pace DA, Cintron R, Rodrigues JC, Fang J, Smith A, Rohloff P, Coelho E, de Haas F, de Souza W, Coppens I, Sibley LD, Moreno SN. Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol Microbiol. 2010;76:1358–1375. doi: 10.1111/j.1365-2958.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uyemura SA, Luo S, Moreno SN, Docampo R. Oxidative phosphorylation, Ca2+ transport, and fatty acid-induced uncoupling in malaria parasites mitochondria. J Biol Chem. 2000;275:9709–9715. doi: 10.1074/jbc.275.13.9709. [DOI] [PubMed] [Google Scholar]
- 21.Rotmann A, Sanchez C, Guiguemde A, Rohrbach P, Dave A, Bakouh N, Planelles G, Lanzer M. PfCHA is a mitochondrial divalent cation/H+ antiporter in Plasmodium falciparum. Mol Microbiol. 76:1591–1606. doi: 10.1111/j.1365-2958.2010.07187.x. [DOI] [PubMed] [Google Scholar]
- 22.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 23.Berridge MJ, Lipp P, Bootman MD. Signal transduction. The calcium entry pas de deux. Science. 2000;287:1604–1605. doi: 10.1126/science.287.5458.1604. [DOI] [PubMed] [Google Scholar]
- 24.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 25.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 26.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW., Jr Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 27.Desai SA, McCleskey EW, Schlesinger PH, Krogstad DJ. A novel pathway for Ca2+ entry into Plasmodium falciparum-infected blood cells. Am J Trop Med Hyg. 1996;54:464–470. doi: 10.4269/ajtmh.1996.54.464. [DOI] [PubMed] [Google Scholar]
- 28.Nagamune K, Sibley LD. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the apicomplexa. Mol Biol Evol. 2006;23:1613–1627. doi: 10.1093/molbev/msl026. [DOI] [PubMed] [Google Scholar]
- 29.Bouchot A, Zierold K, Bonhomme A, Kilian L, Belloni A, Balossier G, Pinon JM, Bonhomme P. Tachyzoite calcium changes during cell invasion by Toxoplasma gondii. Parasitol Res. 1999;85:809–818. doi: 10.1007/s004360050637. [DOI] [PubMed] [Google Scholar]
- 30.Seeber F, Beuerle B, Schmidt HH. Cloning and functional expression of the calmodulin gene from Toxoplasma gondii. Mol Biochem Parasitol. 1999;99:295–299. doi: 10.1016/s0166-6851(99)00030-4. [DOI] [PubMed] [Google Scholar]
- 31.Pezzella-D’Alessandro N, Le Moal H, Bonhomme A, Valere A, Klein C, Gomez-Marin J, Pinon JM. Calmodulin distribution and the actomyosin cytoskeleton in Toxoplasma gondii. J Histochem Cytochem. 2001;49:445–454. doi: 10.1177/002215540104900404. [DOI] [PubMed] [Google Scholar]
- 32.Geary TG, Divo AA, Jensen JB. Effect of calmodulin inhibitors on viability and mitochondrial potential of Plasmodium falciparum in culture. Antimicrob Agents Chemother. 1986;30:785–788. doi: 10.1128/aac.30.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaid A, Thomas DC, Sharma P. Role of Ca2+/calmodulin-PfPKB signaling pathway in erythrocyte invasion by Plasmodium falciparum. J Biol Chem. 2008;283:5589–5597. doi: 10.1074/jbc.M708465200. [DOI] [PubMed] [Google Scholar]
- 34.Carruthers VB, Sibley LD. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol. 1999;31:421–428. doi: 10.1046/j.1365-2958.1999.01174.x. [DOI] [PubMed] [Google Scholar]
- 35.Mondragon R, Frixione E. Ca2+-dependence of conoid extrusion in Toxoplasma gondii tachyzoites. J Eukaryot Microbiol. 1996;43:120–127. doi: 10.1111/j.1550-7408.1996.tb04491.x. [DOI] [PubMed] [Google Scholar]
- 36.Wetzel DM, Chen LA, Ruiz FA, Moreno SN, Sibley LD. Calcium-mediated protein secretion potentiates motility in Toxoplasma gondii. J Cell Sci. 2004;117:5739–5748. doi: 10.1242/jcs.01495. [DOI] [PubMed] [Google Scholar]
- 37.Vieira MC, Moreno SN. Mobilization of intracellular calcium upon attachment of Toxoplasma gondii tachyzoites to human fibroblasts is required for invasion. Mol Biochem Parasitol. 2000;106:157–162. doi: 10.1016/s0166-6851(99)00182-6. [DOI] [PubMed] [Google Scholar]
- 38.Chen XM, O’Hara SP, Huang BQ, Nelson JB, Lin JJ, Zhu G, Ward HD, LaRusso NF. Apical organelle discharge by Cryptosporidium parvum is temperature, cytoskeleton, and intracellular calcium dependent and required for host cell invasion. Infect Immun. 2004;72:6806–6816. doi: 10.1128/IAI.72.12.6806-6816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gantt S, Persson C, Rose K, Birkett AJ, Abagyan R, Nussenzweig V. Antibodies against thrombospondin-related anonymous protein do not inhibit Plasmodium sporozoite infectivity in vivo. Infect Immun. 2000;68:3667–3673. doi: 10.1128/iai.68.6.3667-3673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo S, Ruiz FA, Moreno SN. The acidocalcisome Ca2+-ATPase (TgA1) of Toxoplasma gondii is required for polyphosphate storage, intracellular calcium homeostasis and virulence. Mol Microbiol. 2005;55:1034–1045. doi: 10.1111/j.1365-2958.2004.04464.x. [DOI] [PubMed] [Google Scholar]
- 41.Endo T, Sethi KK, Piekarski G. Toxoplasma gondii: calcium ionophore A23187-mediated exit of trophozoites from infected murine macrophages. Exp Parasitol. 1982;53:179–188. doi: 10.1016/0014-4894(82)90059-5. [DOI] [PubMed] [Google Scholar]
- 42.Arrizabalaga G, Ruiz F, Moreno S, Boothroyd JC. Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation. J Cell Biol. 2004;165:653–662. doi: 10.1083/jcb.200309097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavine MD, Knoll LJ, Rooney PJ, Arrizabalaga G. A Toxoplasma gondii mutant defective in responding to calcium fluxes shows reduced in vivo pathogenicity. Mol Biochem Parasitol. 2007;155:113–122. doi: 10.1016/j.molbiopara.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billker O, Lourido S, Sibley LD. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 2009;5:612–622. doi: 10.1016/j.chom.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol Microbiol. 2006;59:1175–1184. doi: 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- 46.Kieschnick H, Wakefield T, Narducci CA, Beckers C. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J Biol Chem. 2001;276:12369–12377. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- 47.Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]