Abstract
Background
Vein grafts fail due to wall mal-adaptations to surgical injury and hemodynamic perturbations. Interleukin-1 signaling has emerged as an important mediator of the vascular response to trauma and hemodynamically induced vascular lesions. We therefore hypothesized that interleukin-1 signaling drives early vein graft wall adaptations.
Methods
Using interleukin-1 type I receptor knockout (IL-1RI−/−) and wild-type (B6129SF2/J) mice, we investigated morphologic changes 28 days after interposition isograft from donor inferior vena cava to recipient carotid artery, without (n=19) or with (n=13) outflow restriction. The impact of mouse strain on the response to vein arterialization was also evaluated between B6129SF2/J (n=18) and C57BL/6J (n=19) mice.
Results
No significant differences were observed in the traditional endpoints of intimal thickness and calculated luminal area, yet media+adventitia thickness of the vein graft wall of IL-1RI−/− mice was 44-52% smaller than wild-type mice, at the both proximal (P<.01, P<.01) and distal (P=.054, P<.01) portions of vein grafts, for both normal flow and low flow respectively. Compared with C57BL/6J strain, B6129SF2/J mice exhibited no difference in vein graft intimal thickness, but 2-fold higher media+adventitia thickness (P<.01).
Conclusion
When lacking interleukin-1 signaling, the vein graft wall adapts differently compared to the injured artery, showing typical intima hyperplasia though attenuated media+adventitia thickening. B6129SF2/J mice exhibit more media+adventitia response than C57BL/6J mice. The inflammatory networks that underlie the vein response to arterialization hold many roles in the adaptation of the total wall, thus the utility of anti-inflammatory approaches to extend the durability of vein grafts comes into question.
Keywords: interleukin-1, vein graft, wall adaptation, adventitia, mouse model
Autologous vein bypass grafting for coronary or low extremity arterial occlusive diseases stands as one of the most common major procedures performed, though a high incidence of vein graft failure remains.1-3 Vessel wall mal-adaptations, mainly intimal hyperplasia4, 5 and wall remodeling,6, 7 are the primary culprits of this critical clinical issue. All layers (intima/endothelium, media and adventitia) of the vein graft wall and elements of circulatory system may be the repository for the factors which drive wall mal-adaptation.8
Interleukin-1 (IL-1) α and β are major proinflammatory cytokines, functioning in immuno-modulatory and inflammatory processes, which occur mainly via interactions with the IL-1 type I receptor (IL-1RI).9 There is the increasing recognition of the effects of IL-1/IL-1RI axis on cardiovascular events, such as atherosclerosis, myocardial infarction, vascular wall remodeling, and the response to vascular injury.10-12 We previously reported13 that IL-1RI knockout (KO) mice (IL-1RI−/−) undergoing carotid artery ligation (low flow) manipulation14 tended to have 7-fold less intimal hyperplasia than B6129 wild-type (WT) control mice. Other groups15-18 subsequently showed that several factors in IL-1 signaling pathway impact neointimal lesions after arterial injury.
Arterialization of a vein massively upregulates IL-1β gene expression in the vessel (over 1,000 fold at post-operative day 1, detected by quantitative RT-PCR).19 In our pilot genome-wide microarray studies this upregulation ranks very high among other mediators: IL-1β gene ranks the 7th out of ~40,000 genes in upregulation in the mouse vein graft wall (day 7), and 2nd out of over 4,000 genes in rabbit vein grafts two and twenty-four hours after implantation.
In the context of these revelations and founded on the common inflammatory driven mechanisms between arterial and venous intimal hyperplasia, we hypothesized that the disruption of IL-1 signaling pathway would attenuate intimal hyperplasia in the early vein graft. We directly tested this hypothesis employing murine vein graft models combined with flow perturbation and gene KO strategies.
MATERIALS AND METHODS
Mouse Vein Graft Models
Nine-week-old male C57BL/6J mice (#000664), IL-1RI−/− mice (KO; #003018) and their WT B6129SF2/J controls (WT; #101045), weighing 20 to 24 grams, were purchased from the Jackson Laboratory (Bar Harbor, ME). The animals were maintained on a 12-hour light-dark cycle, and received water and standard chow ad libitum. All animal experiments were performed according to protocols approved by our local Institutional Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, Revised 1996). All recipient mice underwent vein bypass grafting, without (normal flow groups; WT n = 10, KO n = 9, C57BL/6J n = 8) or with (low flow groups; WT n = 8, KO n = 5, C57BL/6J n = 11) outflow restriction, and donor mice were of the same age, gender, and genetic background with their recipients.
All operative procedures were performed aseptically, with general continuous isoflurane inhalant anesthesia (2% during painful stimuli, and 1% at latent periods). Mouse vein graft model was created via interposition of a supradiaphragmatic inferior vena cava from a donor mouse into the right common carotid artery of a recipient mouse, combining with outflow restriction manipulation or not as described before.20, 21 Briefly, the mouse right common carotid artery was dissected and ligated with 9-0 nylon sutures at the midpoint and divided. After clamping, the ligatures were removed and the proximal and distal artery ends were everted over pre-made polyetheretherketone cuffs (Zeus, Orangeburg, SC). The IVC was then sleeved over the cuffed arterial ends and secured into position with another 9-0 suture. Increased outflow resistance was achieved in the low flow groups via partially ligating the outflow common carotid artery (at the location 1 mm beyond the distal cuff body) with a 33 gauge blunt needle (outside diameter 0.21 mm, Hamilton, Reno, NV) by a 9-0 nylon ligature, and then removing the mandrel needle to restore blood flow.
Flowmetry
Blood flow rate of vein graft was measured at least 20 minutes after bypass grafting completion ± outflow restriction (day 0), and immediately before harvest (day 28), via a Transonic TS420 flowmeter with 1PRB flowprobe (Transonic Systems, Ithaca, NY) and data acquisition system (PowerLab 4/30 with LabChart v7.0.2; ADInstruments, Colorado Springs, CO).
Vein Graft Duplex Imaging
Utilizing a VisualSonics Vevo 2100 Imaging System (VisualSonics, Toronto, Canada), ultrasonography on mouse vein grafts was completed at post-operative days 4 and 28 to monitor graft patency.
Tissue Harvest and Processing
At post-operative day 28, the recipient mice were whole-body perfusion fixed by 10% formalin under physiologic pressure; the vein grafts were harvested, tissue processed and embedded into paraffin for sectioning. The cross-sections at 200 μm, 400 μm and 600 μm distances from the proximal/distal cuff edge were selected to represent the proximal/distal portion of that vein graft.
Morphometry
Masson’s trichrome staining was utilized on selected cross-sections. Digital microscopic images were captured using a Zeiss Axio A1 microscope (Carl Zeiss, Germany). Lumen circumference, internal elastic lamina (IEL) and outside boundary of the vein graft were determined as we described before,20 and planimetry was completed by AxioVision Rel 4.7 software (Carl Zeiss). Each morphologic parameter from the adjacent 200 μm, 400 μm and 600 μm cross-sections was averaged to represent that vein graft portion’s remodeling status. The calculation methods were previously described.20, 21
Statistical analysis
Data are showed as means ± SEM. Differences among more than two groups were analyzed by one-way analysis of variance. Comparison for two groups was performed via unpaired two-tailed Student’s t-test. P < .05 was considered statistically significant.
RESULTS
Two of eight WT low flow vein grafts (at days 4 and 28, respectively) and two of eleven C57BL/6J low flow vein grafts (at day 28) were found occluded by ultrasonography. Both were excluded from all analyses. All other mice survived until harvest, and all these vein grafts were patent.
Outflow restriction produced an overall 43.89% ± 1.03% decrease in vein graft mean blood flow at day 0, and a 60.48% ± 10.20% decrease at day 28 before harvest, without significant differential between the WT group vs. KO group, and the WT (B6129SF2/J) group vs. our previous data in C57BL/6J mice.21 These C57BL/6J mice underwent vein grafting exactly as described in the current protocol.
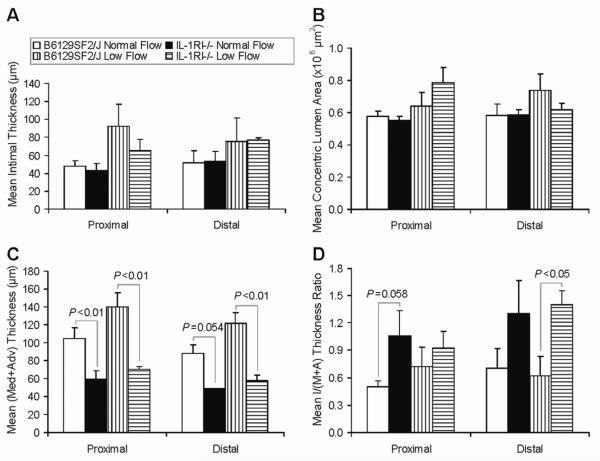
Under a B6129 genetic background, IL-1RI−/− mice did not show a change in mean intimal thickness (Fig 1, A) or mean (calculated) concentric lumen area21 (Fig 1, B). However, the mean thickness of media+adventitia was quite different between WT and KO mice consistently, with significantly less (44-52% decrease) in KO mice, in both the normal flow and the low flow models, and in both the proximal and the distal portions of the vein grafts (Fig 1, C and Fig 2). Consequently, KO mice tended to have a higher intima/(media+adventitia) thickness ratio (Fig 1, D).
Fig 1.
Morphologic analysis of B6129 background mouse vein graft wall adaptation. (A) Mean intimal thickness. (B) Mean concentric lumen area. (C) Mean media+adventitia thickness. (D) Mean intima/(media+adventitia) thickness ratio. Values are shown as mean ± SEM (error bars).
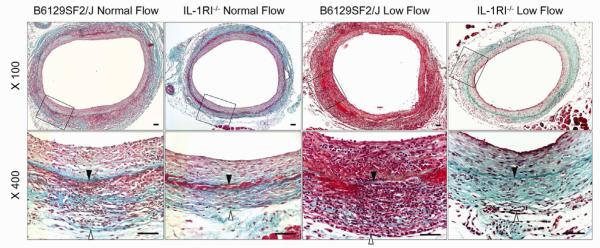
Fig 2.
Representative Masson’s trichrome staining of vein graft models on B6129SF2/J wild type or IL-1R1−/− mice. Lower panel shows the enlargements of area defined by the black boxes in the upper panel. Solid arrows indicate internal elastic lamina; blank arrows indicate outside boundary of the vein graft wall. Scale bars = 50 μm.
Finally, to evaluate the mouse strain differential vein graft response, we compared the day-28 vein graft morphologic parameters of B6129SF2/J mice with those of C57BL/6J mice (Table I).21 Under the same blood flow manipulation (normal flow or low flow condition), B6129SF2/J mice showed a similar level of intimal thickness with C57BL/6J mice, though the hybrid strain B6129SF2/J tended to have a higher variation. Meanwhile, B6129SF2/J vein grafts exhibited an approximate 2-fold thicker media+adventitia layer (which we define as the area between IEL and vein graft outside boundary20), and a lower intima/(media+adventitia) thickness ratio, when compared to C57BL/6J vein grafts. No significant difference in lumen area was found among all mouse vein grafts.
Table I.
Morphologic differences of vein graft models between B6129SF2/J and C57BL/6J mice
|
Morphologic
parameters |
Portion of
vein graft |
B6129SF2/J
normal flow |
C57BL/6J
normal flow21 |
B6129SF2/J
low flow |
C57BL/6J
low flow21 |
|---|---|---|---|---|---|
| Intimal thickness (μm) |
Proximal | 47.7 ±6.5 | 49.6 ±4.7 | 92.1 ±24.6 | 88.9 ±8.4 |
| Distal | 51.8 ± 13.4 | 42.6 ±3.4 | 75.8 ±25.9 | 77.5 ±12.7 | |
| Media+adventitia thickness (μm) |
Proximal | 104.7± 11.9 | 49.5 ±3.9** | 139.6 ± 16.7 | 61.6 ±2.5** |
| Distal | 88.1 ±9.5 | 57.2±7.0† | 121.4 ± 12.1 | 65.0 ±7.4** | |
| Intima/(media+ adventitia) thickness |
Proximal | 0.5 ±0.1 | 1.1 ±0.1** | 0.7 ±0.2 | 1.5 ±0.2* |
| Distal | 0.7 ±0.2 | 0.8 ±0.1 | 0.6 ±0.2 | 1.3 ±0.2‡ | |
| Concentric lumen area (×lO6 μm2) |
Proximal | 0.6 ±0.0 | 0.5 ±0.0 | 0.6±0.1 | 0.5 ±0.1 |
| Distal | 0.6 ±0.1 | 0.5 ±0.0 | 0.7±0.1 | 0.6 ±0.1 |
P < .05
P < .01, vs B6129SF2/J mice with the same flow situation
P = .07 vs B6129SF2/J normal flow
P = .08 vs B6129SF2/J low flow.
DISCUSSION
IL-1 pathway stands proximally in many fundamental inflammatory signaling networks12 that have been linked to arterial adaptations such as flow induced arterial remodeling10 and intimal hyperplasia.11, 13 The factors which have been targeted in this pathway include IL-1α, IL-1β, IL-1RI, IL-1 receptor antagonist (IL-1Ra), P2X7-receptor (mediator of intercellular IL-1Ra release), caspase-1 (cleaves the precursor of IL-1β), myeloid differentiation primary response gene 88 (MyD88), and Toll-like receptor 4 (TLR4). Researchers have found that at least IL-1β, IL-1RI, IL-1Ra and MyD88 play important roles in arterial intimal hyperplasia or total wall adaptations (Table II).
Table II.
Previous reports on the effects of IL-1 signaling on vascular wall adaptations
|
Targeted
gene |
Mouse
background/ WT control |
Mouse model | Effects on arterial wall adaptations (vs. WT) | ||||
|---|---|---|---|---|---|---|---|
| Intima area |
Intima/media
area |
Media |
Lumen
area |
Total
vessel area |
|||
| IL-1RI−/− | B6x129 | Carotid artery ligation13 |
7-fold less (NS) |
8.6-fold less | |||
| IL-1Ra−/− | C57BL/6J | Femoral artery injury via external vascular cuff15 |
249% increase (on intimal thickness) |
257% increase |
|||
| IL-1RI−/− | C57BL/6J | Carotid artery ligation16 |
16.5-fold less | 19-fold less | 3.4-fold increase |
1.6-fold increase |
|
| Exogenous IL-1Ra |
C57BL/6J | 16.5-fold less | 7-fold less | 2.9-fold increase |
|||
| IL-1 β−/− | C57BL/6 | 3-fold less | 4-fold less | 4-fold increase |
NS | ||
| IL-1 α−/− | C57BL/6 | NS | NS | NS | |||
| P2X7− receptor−/− |
C57BL/6X DBA |
~1.6-fold less (NS) |
NS | NS | NS | ||
| Caspase-1−/− | C57/129Sv | NS | NS | NS | NS | ||
| IL-1RI−/− | C57BL/6J | Abdominal aorta stenting17 | 64% less | NS | NS | ||
| MyD88−/− | C57BL/6 | Carotid artery non- occlusive plastic collar18 |
~80% less | ~70% less | |||
| IL-1RI blocking antibody |
C57BL/6 with IgG1 control antibody |
80% less | ~80% less | ||||
| TLR4 blocking antibody |
NS | NS | |||||
NS: not significant
Furthermore, broad based discovery approaches have identified IL-1 signaling mediators as some of the most upregulated genes in the setting of vein graft construction.19, 22, 23 For instance unpublished pilot genome-wide microarray analysis data from C57BL/6J mouse vein grafts (post-operative day 7) showed that IL-1β gene ranks 7th out of ~40,000 genes in upregulation with vein graft arterialization, a pattern which was also confirmed with quantitative RT-PCR and rabbit vein graft microarray genomic analysis.
Therefore IL-1 signaling recently emerged as a target to enhance the durability of vascular interventions such as vein grafts by proximally blocking the IL-1 driven inflammatory cascades that lead to myofibroblast migration and proliferation. Several anti-IL-1 therapeutic approaches have emerged.24 We evaluated the effects of IL-1 signaling on vein graft wall adaptations directly utilizing normal and low flow murine models and gene KO strategies based on B6129 genetic background which is consistent with our previous study on mouse arterial system.13
Contrary to our initial hypothesis, the pattern of intimal hyperplasia (the traditional target for anti-vein graft failure therapies) inhibition in the mouse arterial system was not observed in normal flow mouse vein grafts, though the media+adventitia thickness decreases by ~44% in IL-1RI−/− mice. Considering the relatively higher variation in this hybrid mouse strain, and the limited baseline neointima formation in this normal flow/normal lipid-profile/non-aged mouse vein graft model,20 we repeated testing of the hypothesis utilizing a validated low flow model that yields more intimal hyperplasia.21 Consistent with the initial findings, the same pattern of vessel wall adaptations was observed in day-28 low-flow vein grafts. In totality, one can conclude that IL-1 signaling significantly impacts vein graft wall adaptation to arterialization.
In this study we also report the differential morphologic response to vein graft arterialization between the commonly used hybrid strain B6129SF2/J mice and the most popular inbred strain C57BL/6J mice. With the same age and gender (male), and similar body weight, these experimental mice underwent vein interposition grafting (under normal flow or low flow condition) completed by the same microsurgeon under exactly the same protocol. The B6129SF2/J strain showed similar amounts of intimal hyperplasia and lumen area compared to its relative inbred C57BL/6J strain, but it did have a 2-fold higher thickness of the vein graft media+adventitia layer, leading to a significantly thicker overall vein graft wall. Strain differentials in the vascular response to injury have also been previously reported by others.25-27
The potential clinical translation implications of our findings are murky since the relevance of the difference in media+adventitia thickness is unknown. This is a relatively sort-term model, so it is possible that if assayed at later time points the luminal anatomy could be affected by these adaptations, including enhanced outward remodeling in the setting of no IL-1 signaling. It appears that the inflammatory IL-1 signaling networks that participate in the early vein graft response to arterialization hold many roles in the adaptation of the total wall, thus the utility of broad anti-IL-1 approaches to extend the durability of vein grafts comes into question. Researchers have increasingly recognized the vein graft tunica adventitia as an important repository of progenitor cells, which can subsequently migrate and proliferate; and as a source of vascular wall inflammatory cells, cytokines, and chemokines.28, 29 However, its anatomical morphologic changes have not been the focus of prior reports. The vascular responses after vessel dissection and bypass implantation involve common pathologic stages (such as inflammation, granulation, fibroplasia and contraction) with regular wound healing. Thus some researchers have described the early vein graft wall adaptation as a wound healing process.30-32 This less organized tissue repair process is more typically seen in the area outside IEL, which is the zone termed the “media+adventitia” area of the mouse vein graft.20 IL-1 signaling links to wound healing biology are recognized.33-35 Thus, our findings indirectly support the theory relating the response to vein grafting/arterialization to wound healing.30
Our research strategy holds some limitations. By utilizing the IL-1RI−/− mice, we are unable to separate IL-1α and IL-1β effects. It is also possible that with time there might be aneurysmal degeneration owing to the thinner vein graft wall in IL-1RI−/− mice, but we examined only a single time point. However, the 28-day time point is most commonly reported in mouse models of vein graft failure.20 Finally, differences in the biochemical qualities of the media+adventitia between the test groups is not described, but such experiments might provide additional insights into the role of this cytokine in early vascular wall adaptations after hemodynamic perturbations.
In summary, we find that lack of IL-1 signaling in the setting of the early vein graft leads to overall thinning of the conduit wall, due to less media+adventitia volume. While anti-IL-1 strategies do not appear to impact intimal hyperplasia in vein grafts, the results confirm the role of pro-inflammatory cytokines in modulation of the vein graft wall adaptation to arterialization.19, 36 However, translation of the perturbed morphologic adaptations observed toward enhanced vein graft durability is unclear.
Acknowledgments
Sources of funding: Supported by the National Heart, Lung, and Blood Institute R01HL079135, 1R01HL079135-06S1, T32HL007734, and the Carl and Ruth Shapiro Family Foundation.
Non-standard Abbreviations and Acronyms
- KO
Knockout
- IEL
Internal elastic lamina
- IL-1
Interleukin-1
- IL-1Ra
Interleukin-1 receptor antagonist
- IL-1RI
Interleukin-1 type I receptor
- MyD88
Myeloid differentiation primary response gene 88
- NS
Not significant
- TLR4
Toll-like receptor 4
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr., Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 3.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–51. doi: 10.1016/j.jvs.2005.12.058. discussion 51. [DOI] [PubMed] [Google Scholar]
- 4.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Wallitt EJ, Jevon M, Hornick PI. Therapeutics of vein graft intimal hyperplasia: 100 years on. Ann Thorac Surg. 2007;84:317–23. doi: 10.1016/j.athoracsur.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Lau GT, Ridley LJ, Bannon PG, Wong LA, Trieu J, Brieger DB, et al. Lumen loss in the first year in saphenous vein grafts is predominantly a result of negative remodeling of the whole vessel rather than a result of changes in wall thickness. Circulation. 2006;114:I435–40. doi: 10.1161/CIRCULATIONAHA.105.001008. [DOI] [PubMed] [Google Scholar]
- 7.Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: Clinical implications. J Vasc Surg. 2010;51:736–46. doi: 10.1016/j.jvs.2009.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muto A, Model L, Ziegler K, Eghbalieh SD, Dardik A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ J. 2010;74:1501–12. doi: 10.1253/circj.cj-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Z, Berceli SA, Pfahnl CL, Wu L, Killingsworth CD, Vieira FG, et al. Impact of IL-1beta on flow-induced outward arterial remodeling. Surgery. 2004;136:478–82. doi: 10.1016/j.surg.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Kusuhara M, Isoda K, Ohsuzu F. Interleukin-1 and occlusive arterial diseases. Cardiovasc Hematol Agents Med Chem. 2006;4:229–35. doi: 10.2174/187152506777698335. [DOI] [PubMed] [Google Scholar]
- 12.Vicenova B, Vopalensky V, Burysek L, Pospisek M. Emerging role of interleukin-1 in cardiovascular diseases. Physiol Res. 2009;58:481–98. doi: 10.33549/physiolres.931673. [DOI] [PubMed] [Google Scholar]
- 13.Rectenwald JE, Moldawer LL, Huber TS, Seeger JM, Ozaki CK. Direct evidence for cytokine involvement in neointimal hyperplasia. Circulation. 2000;102:1697–702. doi: 10.1161/01.cir.102.14.1697. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–44. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 15.Isoda K, Shiigai M, Ishigami N, Matsuki T, Horai R, Nishikawa K, et al. Deficiency of interleukin-1 receptor antagonist promotes neointimal formation after injury. Circulation. 2003;108:516–8. doi: 10.1161/01.CIR.0000085567.18648.21. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain J, Evans D, King A, Dewberry R, Dower S, Crossman D, et al. Interleukin-1beta and signaling of interleukin-1 in vascular wall and circulating cells modulates the extent of neointima formation in mice. Am J Pathol. 2006;168:1396–403. doi: 10.2353/ajpath.2006.051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamberlain J, Wheatcroft M, Arnold N, Lupton H, Crossman DC, Gunn J, et al. A novel mouse model of in situ stenting. Cardiovasc Res. 2010;85:38–44. doi: 10.1093/cvr/cvp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxena A, Rauch U, Berg KE, Andersson L, Hollender L, Carlsson AM, et al. The vascular repair process after injury of the carotid artery is regulated by IL-1RI and MyD88 signalling. Cardiovasc Res. 2011;91:350–7. doi: 10.1093/cvr/cvr075. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Berceli SA, Pfahnl CL, Wu L, Goldman D, Tao M, et al. Wall shear modulation of cytokines in early vein grafts. J Vasc Surg. 2004;40:345–50. doi: 10.1016/j.jvs.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 20.Yu P, Nguyen BT, Tao M, Campagna C, Ozaki CK. Rationale and practical techniques for mouse models of early vein graft adaptations. J Vasc Surg. 2010;52:444–52. doi: 10.1016/j.jvs.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu P, Nguyen BT, Tao M, Bai Y, Ozaki CK. Mouse vein graft hemodynamic manipulations to enhance experimental utility. Am J Pathol. 2011;178:2910–9. doi: 10.1016/j.ajpath.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christiansen JF, Hartwig D, Bechtel JF, Kluter H, Sievers H, Schonbeck U, et al. Diseased vein grafts express elevated inflammatory cytokine levels compared with atherosclerotic coronary arteries. Ann Thorac Surg. 2004;77:1575–9. doi: 10.1016/j.athoracsur.2003.10.107. [DOI] [PubMed] [Google Scholar]
- 23.Hinokiyama K, Valen G, Tokuno S, Vedin JB, Vaage J. Vein graft harvesting induces inflammation and impairs vessel reactivity. Ann Thorac Surg. 2006;82:1458–64. doi: 10.1016/j.athoracsur.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler-Stec EM, Hartnett C, et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med. 2003;9:47–52. doi: 10.1038/nm811. [DOI] [PubMed] [Google Scholar]
- 25.Harmon KJ, Couper LL, Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol. 2000;156:1741–8. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: the “Glagov phenomenon” is genetically determined. Circulation. 2004;110:220–6. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- 27.Cooley BC. Mouse strain differential neointimal response in vein grafts and wire-injured arteries. Circ J. 2007;71:1649–52. doi: 10.1253/circj.71.1649. [DOI] [PubMed] [Google Scholar]
- 28.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–8. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Havelka GE, Kibbe MR. The vascular adventitia: its role in the arterial injury response. Vasc Endovascular Surg. 2011;45:381–90. doi: 10.1177/1538574411407698. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien JE, Jr., Shi Y, Fard A, Bauer T, Zalewski A, Mannion JD. Wound healing around and within saphenous vein bypass grafts. J Thorac Cardiovasc Surg. 1997;114:38–45. doi: 10.1016/S0022-5223(97)70115-6. [DOI] [PubMed] [Google Scholar]
- 31.Tomas JJ, Stark VE, Kim JL, Wolff RA, Hullett DA, Warner TF, et al. Beta-galactosidase-tagged adventitial myofibroblasts tracked to the neointima in healing rat vein grafts. J Vasc Res. 2003;40:266–75. doi: 10.1159/000071890. [DOI] [PubMed] [Google Scholar]
- 32.Forte A, Della Corte A, De Feo M, Cerasuolo F, Cipollaro M. Role of myofibroblasts in vascular remodelling: focus on restenosis and aneurysm. Cardiovasc Res. 2010;88:395–405. doi: 10.1093/cvr/cvq224. [DOI] [PubMed] [Google Scholar]
- 33.Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW, et al. Disruption of interleukin-1 signaling improves the quality of wound healing. Am J Pathol. 2009;174:2129–36. doi: 10.2353/ajpath.2009.080765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Y, Liang D, Li X, Liu HH, Zhang X, Zheng M, et al. The role of interleukin-1 in wound biology. Part I: Murine in silico and in vitro experimental analysis. Anesth Analg. 2010;111:1525–33. doi: 10.1213/ANE.0b013e3181f5ef5a. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y, Liang D, Li X, Liu HH, Zhang X, Zheng M, et al. The role of interleukin-1 in wound biology. Part II: In vivo and human translational studies. Anesth Analg. 2010;111:1534–42. doi: 10.1213/ANE.0b013e3181f691eb. [DOI] [PubMed] [Google Scholar]
- 36.Ozaki CK. Cytokines and the early vein graft: strategies to enhance durability. J Vasc Surg. 2007;45:A92–8. doi: 10.1016/j.jvs.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]