Abstract
Fifty analogues of batzelladine K were synthesized and evaluated for in vitro antimalarial (Plasmodium falciparum), antileishmanial (Leishmania donovani), antimicrobial (panel of bacteria and fungi), antiviral (HIV-1) activities. Analogues 14h and 20l exhibited potential antimalarial activity against chloroquine-sensitive D6 strain with IC50 1.25 and 0.88 μM and chloroquine-resistant W2 strain with IC50 1.64 and 1.07 μM, respectively. Analogues 12c and 14c having nonyl substitution showed the most potent antileishmanial activity with IC50 2.39 and 2.78 μM and IC90 11.27 and 12.76 μM respectively. Three analogues 12c, 14c and 14i were the most active against various pathogenic bacteria and fungi with IC50 <3.02 μM and MIC/MBC/MFC <6 μM. Analogue 20l having pentyl and methyl substituents on tricycle showed promising activities against all pathogens. However, none was found active against HIV-1. Our study demonstrated that the tricyclic guanidine compounds provide new structral class for broad spectrum activity.
Keywords: Batzelladine, tricyclic guanidine, antimalarial, antileishmanial, antimicrobial, anti-HIV
Introduction
Among the parasitic diseases, leishmaniasis and malaria have a high mortality rate affecting millions of people worldwide particularly in developing countries. Malaria ranks third among the major infectious diseases in causing deaths after pneumococcal acute respiratory infections and tuberculosis. Approximately, 1.5-2.5 million people die of malaria every year, accounting for about 5% of all fatalities in the world [1]. Leishmaniasis caused by protozoa of the genus Leishmania remains a significant health issue in large part due to the lack of effective and affordable drugs and increasing resistance against existing drugs. More than 2 million new cases of leishmaniasis occur each year, while 15 million people are infected [2-3].
HIV and parasitic infections interact and affect each other mutually. HIV infection may alter the natural history of parasitic diseases, impede rapid diagnosis or reduce the efficacy of antiparasitic treatment, whereas parasitoses may facilitate the infection with HIV and the progression from asymptomatic infection to AIDS. Recently, cause-effect interactions between HIV and malaria were also shown to occur in pregnant women [4]. Leishmania/HIV co-infection has emerged as a result of the increasing overlap of areas in which HIV or leishmaniasis occur, particularly in eastern Africa, India, Brazil and Europe [2]. Multiple immunological mechanisms mediate the impact of HIV infection on visceral leishmaniasis (VL) and vice versa. Both pathogens infect monocytes/macrophages and may establish a latent infection or may accelerate their intracellular multiplication. An increasing number of multidrug-resistant microbial pathogens have become a serious problem particularly during the last decade [5-6]. Consequently, these conditions demand the rapid search and discovery of new broad spectrum antiparasitic agents with novel structural backbone.
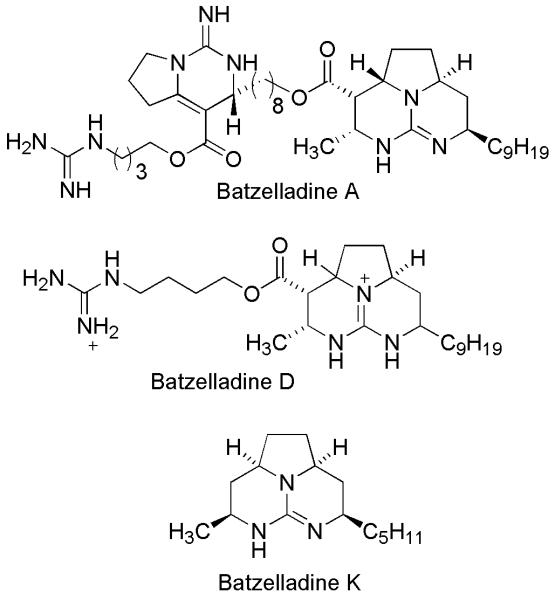
The batzelladines, a class of polycyclic marine alkaloids containing a guanidine group, have been isolated from various Batzella species. Batzelladines A-N, isolated from various sponges of the genus Batzella are of biological interests [7-11]. Batzelladines A and B were found to inhibit the binding of HIV glycoprotein gp-120 to CD4 receptors [7]. Batzelladines F, G and a mixture of H and I were active in the p56lck-CD4 dissociation assay [9]. Few selected batzelladine alkaloids are shown in Figure 1. It has been identified that the tricyclic core of batzelladines is essential for anti-HIV activity [12]. However, antiparasitic potential of this class of compounds has never been explored which led us to synthesize a series of compounds with tricyclic core of batzelladines. Total synthesis of complex batzelladines with many numbers of stereocentres is one of the challenging endeavor for synthetic chemists. We have previously reported the total synthesis of batzelladine K using a biomimetic approach [13]. We report herein, the synthesis of tricyclic guanidine analogues of batzelladines and evaluation of their in vitro antimalarial, antileishmanial, antimicrobial and anti-HIV activities.
Figure 1.
Selected batzelladine alkaloids
Materials and Methods
General
All commercial chemicals and solvents were reagent grade and were used without further treatment unless otherwise noted. Nuclear magnetic resonance spectra were recorded on Brukers avance (400 MHz) with tetramethyl silane (TMS) as internal standard. Chemical shifts were recorded in parts per million (ppm, δ) and were reported relative to TMS. Mass spectra were recorded on GCMS-QS Shimadzu (QP-500) and LCMS waters (Micromass ZQ). IR spectra were recorded on Nicolet spectrometer. HRMS was recorded on LCMS (Bruker Maxis). TLC was performed on Merck 0.25 mm Kieselgel 60 F254 plates. Column chromatography was performed using either silica gel-60 (60-120 mesh).
Antimalarial activity
In vitro antimalarial activity of all synthesized compounds was evaluated against chloroquine-sensitive (D6) and chloroquine-resistant (W2) clones of P. falciparum based on the determination of plasmodial LDH activity [14]. All the analogues were evaluated for in vitro cytotoxicity against mammalian kidney cell line (Vero) up to a highest concentration of 4.76 μg/mL by neutral red assay [15-16]. Selectivity index was calculated for all analogues (Data not shown). S.I. is calculated as the ratio of IC50 for cytotoxicity and IC50 for antimalarial activity and measures the therapeutic index of the compound under investigation to malaria parasites in comparison to its toxicity to the mammalian cells (if there is any).
Antileishmanial activity
The antileishmanial activity of all analogues was evaluated in vitro against Leishmania donovani promastigotes by Alamar Blue assay [17-18]. The activity is reported in terms of IC50 and IC90 values. Pentamidine and Amphotericin B are used as standards.
Antimicrobial activity
Synthesized analogues were evaluated for their antibacterial properties against Staphylococcus aureus ATCC 29213, Methicillin-resistant S. aureus ATCC 33591 (MRSA), Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, and Mycobacterium intracellulare ATCC 23068. Susceptibility testing was performed using a modified version of the CLSI (formerly NCCLS) methods [19-23]. M. intracellulare was tested using a modified method of Franzblau et al [24]. Ciprofloxacin was used as standard.
Antifungal activity
The antifungal activities of all analogues against the opportunistic fungi Candida albicans ATCC 90028 (Ca), Cryptococcus neoformans ATCC 90113 (Cn), Aspergillus fumigatus ATCC 204305 (Afu), Candida glabrata ATCC 90030 (Cg) and Candida krusei ATCC 6258 (Ck) were determined. Amphotericin B was used as positive control.
Anti-HIV activity
Selected synthesized analogues were tested for anti-HIV potential. They were first evaluated for their cytotoxicity in MTT based cell viability assay in CEM-GFP cells [25]. Further non-cytotoxic concentration of each analogue was used for determination of anti-HIV activity. They were evaluated for anti-HIV potential in human CD4+ T cell line CEM-GFP, infected with HIV-1NL4.3 virus by p24 antigen capture ELISA assay [26-28]. Zidovudine (AZT) was used as standard.
Results and discussion
Chemistry
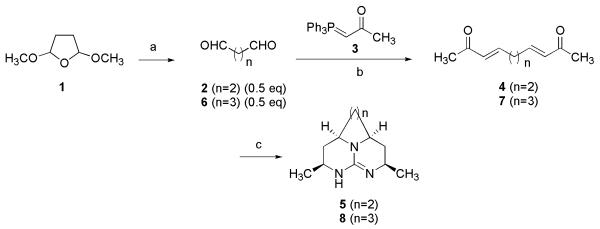
Four series of tricyclic guanidine analogues were synthesized using the protocol developed in our laboratory [13]. As shown in Scheme 1, succinaldehyde (2) was freshly prepared by hydrolysis of commercially available 2,5-dimethoxytetrahydrofuran (1) with 0.6 N HCl [29]. A Wittig reaction of phosphorane 3 with succinaldehyde (0.5 equiv.) at room temperature for 24 hours afforded E-isomer of ketone 4 in 60% yield. The Michael addition of guanidine to 4 at 0 °C followed by a reductive imination with sodium borohydride gave 5.
Scheme 1.
Reagents and conditions. a) HCL, Na2CO3, 2 h (repeated 5 times), 2: 70%; b) DCM, 24 h, 4: 60%, 7: 65%; c) Guanidine, DMF/0°C-RT/5h, 3:1:3 DMG/H2O/MeOH, NaBH4/16 h, 5: 30 % 8: 25%.
To identify the role of tricyclic guanidinium core in biological activity, the pyrrolidine ring of tricycle was replaced by piperidine ring. Glutaraldehyde was employed to construct the piperidine ring in previously described synthetic route. As shown in Scheme 1, Wittig reaction of 3 with glutaraldehyde (0.5 equiv.) gave ketone 7. Condensation of 7 with guanidine in DMF via sequential double Michael addition, followed by sodium borohydride reduction afforded the 8.
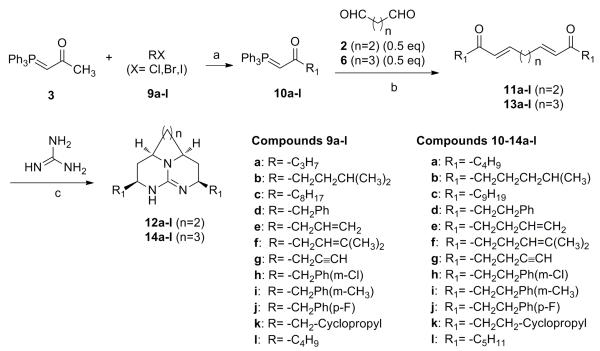
A series of compounds 12a-l were synthesized following a strategy shown in Scheme 2 (Series 1). The synthesis was started with alkylation of phosphorane 3 with alkyl halides (9a-l) employing butyllithium as a base to obtain phosphorane 10a-l. Wittig reaction of 10a-l with succinaldehyde afforded 11a-l which were identified by NMR experiment. The NMR data showed coupling constants in range of 15-18 Hz for trans protons indicating synthesis of E-isomeric form of 11a-l. Condensing guanidine with 11a-l at 0 °C via Michael addition, followed by a reduction with sodium borohydride accomplished synthesis of compounds 12a-l. Using the similar synthetic route, compounds 14a-l (Series 2) were synthesized where glutaraldehyde was employed instead of succinaldehyde as depicted in Scheme 2. In Series 1 and 2, compounds were substituted with identical alkyl chains on both side of tricycle.
Scheme 2.
Reagents and condition: a) BuLi/−78 °C, RT/16 h, 95-98%; b) DCM/24 h 60-70%; c) DMF, 0 °C-RT/5 h, 3:1:3 DMG/H2O/MeOH, NaBH4/16 h, 25-40%.
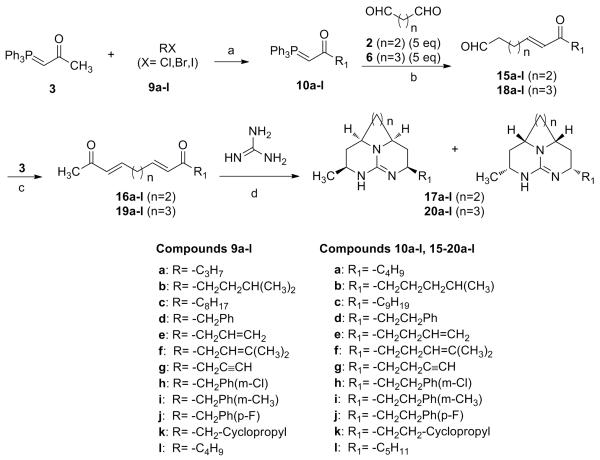
Compounds of Series 3 (17a-l) were synthesized as shown in Scheme 3, with two different alkyl groups on each side of tricyclic core. Synthesis of phosphorane 10a-l was carried out as shown in Scheme 2. Wittig reaction of 10a-l with excess of succinaldehyde afforded ketone 15a-l. It is noteworthy that excess of succinaldehyde (>3 equiv.) is essential to obtain the ketone (15a-l), to avoid the reaction of the phosphorane (3) with free aldehyde group of 15a-l to form di-α, β-unsaturated ketone (symmetrical). Further, 15a-l were treated with 3 at room temperature to provide 16a-l, which were reacted with guanidine followed by a reduction with sodium borohydride to accomplish synthesis of compounds 17a-l as single diastereo-isomers. Similarly, compounds 20a-l (Series 4) were synthesized using glutaraldehyde in the synthetic Scheme 3. The relative stereochemistry of all derivatives was established by NOE experiments as shown previously [13].
Scheme 3.
Reagents and conditions. a) BuLi/−78 °C, RT/16 h, 95-98%; b) DCM/24 h, 60-70%; c) DCM/24 h, 70-80%; d) DMG/0 °C-RT/5 h, 3:1:3 DMF/H2O/MeOH, NaBH4/16 h, 25-45%.
Antimalarial activity
The antimalarial activities of all the compounds are reported as IC50 values against D6 and W2 strains of P. falciparum. Activity data is shown only for active compounds in Table 1. Compounds having methyl substituents, 5 and 8 did not show any antimalarial activity. In Series 1 and 2 (compounds 12a-l and 14a-l), all analogues were active against the P. falcifarum. Amongst, compound 14h was the most active and showed IC50 of 1.25 and 1.64 μM against D6 and W2 strains, respectively. Analogue 14b exhibited IC50 of 1.52 and 1.96 μM against D6 and W2 strains, respectively, while 14i displayed IC50 of 1.37 and 2.26 μM against D6 and W2 strains, respectively. Whereas in Series 3 and 4 (compounds 17a-l and 20a-l), the most potent analogue, 20l displayed IC50 of 0.88 μM for D6 clone and 1.07 μM for W2 clone. The activity of this compound was much higher in comparison to the natural product, batzelladine K (17l) which showed IC50 of 14.85 μM for D6 clone and 13.65 μM for W2 clone.
Table 1.
In vitro antimalarial activity and cytotoxicity of tricyclic guanidine derivatives
| Compd No. |
P. falciparum (D6 Clone) IC50 (μM) |
P. falciparum (W2 Clone) IC50 (μM) |
Cytotoxicity (Vero) IC50 (μM) |
Compd No. |
P. falciparum (D6 Clone) IC50 (μM) |
P. falciparum (W2 Clone) IC50 (μM) |
Cytotoxicity (Vero) IC50 (μM) |
|---|---|---|---|---|---|---|---|
| 12a | 6.49 | 7.22 | NC | 14l | 2.25 | 2.82 | NC |
| 12b | 1.98 | 2.70 | NC | 17b | 3.04 | 3.53 | NC |
| 12c | 1.48 | 1.43 | 6.71 | 17c | 4.59 | 6.20 | NC |
| 12d | 6.16 | 8.04 | NC | 17d | 7.62 | 9.93 | NC |
| 12e | 10.62 | 10.98 | NC | 17f | 16.05 | - | NC |
| 12f | 7.29 | 7.59 | NC | 17g | 20.60 | 20.60 | NC |
| 12g | 9.66 | 5.57 | NC | 17h | 10.09 | 7.25 | NC |
| 12h | 6.34 | 7.71 | NC | 17i | 9.39 | 8.05 | NC |
| 12i | 4.23 | 4.98 | NC | 17j | 15.76 | 13.24 | NC |
| 12j | 5.62 | 7.33 | NC | 17l | 14.85 | 13.65 | NC |
| 12l | 3.01 | 3.60 | NC | 20a | 3.21 | 5.62 | NC |
| 14a | 2.99 | 4.81 | NC | 20b | 4.69 | 7.22 | NC |
| 14b | 1.52 | 1.96 | NC | 20c | 3.44 | 3.76 | NC |
| 14c | 1.39 | 1.76 | 6.96 | 20d | 9.24 | 12.45 | NC |
| 14d | 6.20 | 8.26 | NC | 20e | 19.27 | 19.27 | NC |
| 14e | 6.27 | 7.66 | NC | 20f | 6.54 | 9.45 | NC |
| 14f | 1.69 | 2.18 | NC | 20g | 17.14 | 19.24 | NC |
| 14g | 14.13 | - | NC | 20h | 2.41 | 3.02 | NC |
| 14h | 1.25 | 1.64 | NC | 20i | 5.78 | 7.71 | NC |
| 14i | 1.37 | 2.26 | NC | 20l * | 0.88 | 1.07 | NC |
IC50 = sample concentration that causes 50% cell growth inhibition compared to vehicle control; NC = No cytotoxicity upto 4.76 μg/mL; “-” = Not active;
S.I.: selectivity index [TC50 (Vero)/IC50 (P. falciparum)] > 17;
Chloroquine: IC50 = 0.052 μM (D6 clone); IC50 = 0.46 μM, (W2 clone). Artemisinin: IC50 = 0.018 μM (D6 clone); IC50 = 0.015 μM (W2 clone).
All the analogues were evaluated for in vitro cytotoxicity and Selectivity Index was calculated. S.I. ≥ 10 is generally considered significant, indicating that antimalarial activity is not due to cytotoxicity. None of the compounds were found cytotoxic with the exception of 12c and 14c, indicating their selectivity for antimalarial activity.
Antileishmanial activity
Activity data is shown only for active compounds in Table 2. From Series 1 and 2, analogues 12c and 14c were the most potent with IC50 of 2.39 and 2.78 μM and IC90 of 11.27 and 12.76 μM, respectively. Remaining compounds also displayed antileishmanial activity to various extents with the exception of 12g and 14g. Among Series 3 and 4, 20l was found be to the most active with IC50 of 9.52 μM and IC90 of 20.95 μM. Natural product, batzelladine K (17l) was weakly active with IC50 of 48.19 μM and IC90 of 124 μM.
Table 2.
In vitro antileishmanial activity (L. donovani) of tricyclic guanidine derivatives
| Compd. No. |
L. donovani
|
Compd. No |
L. donovani
|
||
|---|---|---|---|---|---|
| IC50 (μM) |
IC90 (μM) |
IC50 (μM) |
IC90 (μM) |
||
| 12a | 64.98 | 126 | 17b | 14.44 | 29.65 |
| 12b | 12.31 | 33.03 | 17c | 10.13 | 23.85 |
| 12c | 2.39 | 11.27 | 17d | 59.60 | 112.5 |
| 12d | 48.25 | 91.15 | 17e | 85.69 | >13 |
| 12e | 73.26 | >130 | 17f | 61.06 | 125 |
| 12f | 28.87 | 91.18 | 17h | 63.09 | >100 |
| 12h | 29.47 | 72.56 | 17i | 63.75 | >100 |
| 12i | 32.41 | 79.80 | 17j | 62.91 | >100 |
| 12j | 29.34 | 78.24 | 17l | 48.19 | 124.4 |
| 12l | 13.33 | 55.73 | 20a | 29.31 | 116 |
| 14a | 34.02 | 106.5 | 20b | 57.76 | 119 |
| 14b | 9.51 | 19.59 | 20c | 10.34 | 22.25 |
| 14c | 2.78 | 12.76 | 20d | 57.23 | 114 |
| 14d | 46.51 | 93.02 | 20e | 133.6 | >100 |
| 14e | 62.71 | 128.9 | 20f | 50.90 | >100 |
| 14f | 10.20 | 20.40 | 20h | 12.08 | 45.31 |
| 14h | 7.23 | 14.91 | 20i | 48.23 | 106 |
| 14i | 7.71 | 15.90 | 20l | 9.52 | 20.95 |
| 14l | 10.97 | 21.94 | |||
IC50 and IC90 = Sample concentrations that kill 50% and 90% cells compared to the solvent controls; Pentamidine: IC50 = 2.94 μM, IC90 = 5.88 μM; Amphotericin B: IC50 = 0.173 μM, IC90 = 0.358 μM.
Antimicrobial activity
Activity data is shown only for active compounds in Table 3. In Series 1 and 2, thirteen analogues have shown inhibitory activity against S. aureus and MRSA with IC50 in the range of 2.44-10 μM and MIC in the range of 5.99-20 μM. Of these, 12c having a nonyl substituent was the most potent having IC50 of 3.02 μM and 2.44 μM, and bactericidal activity at 23.98 μM and 12 μM against S. aureus and MRSA, respectively. The analogues 14c and 14h exhibited IC50 values of 3.01 μM and 3.18 μM, and were bactericidal at 5.8 μM and 5.48 μM respectively against S. aureus. The 14h inhibited the growth of E. coli with IC50 value of 5.96 μM and showed bactericidal activity at 10.96 μM. Only four compounds from Series 2 were active against P. aeruginosa (14c, 14h, 14i, and 14l) with 14h being most active with IC50 of 10.96 μM and MIC of 21.9 μM. The 14i was found to be the most effective against M. intracellulare with IC50 value of 5.95 μM and MBC of 12 μM.
Table 3.
In vitro antibacterial activity of tricyclic guanidine derivatives
| Compd No. |
S. aureus | MRSA | E.coli | P. aeruginosa | M. intracellulare | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| IC50 μM |
MIC μM |
MBC μM |
IC50 μM |
MIC μM |
MBC μM |
IC50 μM |
MIC μM |
MBC μM |
IC50 μM |
MIC μM |
MBC μM |
IC50 μM |
MIC μM |
MBC μM |
|
| 12b | 8.13 | 15.01 | 30 | 6.48 | 15 | - | 39.94 | - | - | - | - | - | 7.74 | 15 | 30 |
| 12c | 3.02 | 5.99 | 23.98 | 2.44 | 5.99 | 12 | 7.02 | - | - | - | - | - | 7.41 | 12 | 23.9 |
| 12f | 15.80 | 30.39 | 60.79 | 11.67 | 30.3 | - | - | - | - | - | - | - | 20.3 | 30 | 60 |
| 12h | 3.26 | 5.66 | 45.35 | 3.28 | 5.66 | 22.67 | - | - | - | - | - | - | 11.1 | 22.67 | 45.35 |
| 12i | 3.44 | 6.23 | 49.87 | 3.39 | 6.23 | 25 | - | - | - | - | - | - | 12 | 12.46 | 24.93 |
| 12j | 6.89 | 12.22 | 48.90 | 11.63 | - | - | - | - | - | - | - | - | 16.8 | 24.45 | 24.45 |
| 12l | 8.19 | 16.39 | 65.57 | 8.59 | 16.3 | - | - | - | - | - | - | - | 13.5 | 32.78 | 65.57 |
| 14b | 6.25 | 14.40 | 57.63 | 4.26 | 14.4 | 28.8 | 14.26 | 28.8 | 28.8 | - | - | - | 8.12 | 14.40 | 14.40 |
| 14c | 3.01 | 5.80 | 5.80 | 3.10 | 5.8 | 11.6 | 6.33 | - | - | 45 | - | - | 9.81 | 11.6 | 23.20 |
| 14f | 4.60 | 7.28 | 14.57 | 5.07 | 14.5 | 14.5 | 29.82 | 58.3 | 58.3 | - | - | - | 7.05 | 14.5 | 29 |
| 14h | 3.18 | 5.48 | 21.93 | 2.89 | 5.48 | 10.96 | 5.96 | 10.96 | 10.96 | 10.96 | 21.9 | - | 8.26 | 10.96 | 43.86 |
| 14i | 3.20 | 6.02 | 24.09 | 5.54 | 12 | 12 | 12.38 | 24.09 | - | 37.88 | 48.2 | - | 5.95 | 12 | 12 |
| 14l | 12.32 | 31.34 | 31.34 | 8.27 | 15.6 | 15.6 | 31.91 | 62.69 | 62.69 | 30.31 | - | - | 11 | 15.6 | 31.2 |
| 17b | 8.21 | 38.02 | 38.02 | 7.90 | 19.0 | - | 69.12 | - | - | - | - | - | 13 | 19 | 38 |
| 17c | 17.28 | 32.68 | 65.35 | 27.38 | - | - | 57.22 | - | - | - | - | - | 35.3 | 65.35 | 65.35 |
| 17h | 16.40 | 31.54 | - | 8.51 | 15.7 | - | - | - | - | - | - | - | 34.1 | 63.09 | 31.54 |
| 17j | 19.0 | 33.11 | - | 56.85 | - | - | - | - | - | - | - | - | 34.9 | 66.22 | 66.22 |
| 17l | 64.09 | 80.32 | - | 56.06 | 80.3 | - | - | - | - | - | - | - | 79.5 | - | - |
| 20a | 24.33 | 40.16 | - | 26.78 | 40.1 | - | - | - | - | - | - | - | 34.4 | 80.32 | 80.32 |
| 20b | 36.49 | 72.20 | - | 29.09 | 36.1 | - | - | - | - | - | - | - | 44.3 | 72.20 | 72.20 |
| 20c | 8.74 | 15.67 | 31.34 | 11.28 | 31.3 | 31.3 | - | - | - | - | - | - | 25.9 | 62.6 | 62.6 |
| 20h | 8.52 | 15.10 | 30.21 | 8.30 | 15.1 | 30.2 | 47.88 | - | - | - | - | - | 15.8 | 30.2 | 30.2 |
| 20l | 4.44 | 7.93 | 15.87 | 7.07 | 15.8 | - | 23.27 | 63.49 | - | 48.19 | - | - | 6.73 | 15.87 | 31.74 |
IC50 = Concentration (μM) that affords 50% growth inhibition; MIC = Minimum Inhibitory Concentration (the lowest concentration in μM that allows no detectable growth); MBC = Minimum Bactericidal Concentration (the lowest concentration in μM that kills the organism); “-” = Not active at the highest test concentration of 20 μg/mL; Ciprofloxacin: IC50 = 0.3 μM, MIC = 1.5 μM, MBC = 1.5 μM (Sa); IC50 = 0.3 μM, MIC = 0.75 μM, MBC = 1.5 μM (MRSA); IC50 = 0.009 μM, MIC = 0.024 μM, MBC = 0.377 μM (Ec); IC50 = 0.311 μM, MIC = 3 μM, MBC = “-” (Pa); IC50 = 0.69 μM, MIC = 1.5 μM, MBC = 3 μM (Mi).
The analogues from Series 3 and 4 were also active against all strains; ten compounds have shown moderate to potent antibacterial activity against both the strains of S. aureus with IC50 values in the range of 4.44-64 μM and all were bactericidal at <80 μM. The most potent activity was shown by 20l having IC50 of 4.44 μM and 7.07 μM, MIC of 7.93 μM and 15.8 μM and bactericidal at 15.87 μM against S. aureus and MRSA respectively. Four analogues showed moderate activity against E. coli; 20l having IC50 value of 23.27 μM and MIC of 63.49 μM. None of analogues in Series 3 and 4 were active against P. aeruginosa. Ten analogues exhibited the promising activity against M. intracellulare; 20l being the most active with IC50 of 6.73 μM, MIC of 15.87 μM and bactericidal at 31.74 μM. In comparison, batzelladine K (17l) showed a weak activity against S. aureus, MRSA and M. intracellulare (IC50 64.09, 56 and 79.5 μM respectively).
Antifungal activity
Activity data is shown only for active compounds Table 4. The tricyclic guanidine analogues showed activities to a variable extent against various strains. In Series 1, 12c having nonyl substitution produced the most potent activity with IC50 of 3.02, 2.18, 2.11 and 5.46 μM, MIC and MFC of 5.99, 2.99, 2.99 and 11.99 μM against C. albicans, C. glabrata, C. neoformans and A. fumigatus respectively; while in Series 2, analogue 14c showed IC50 of 3.83, 3.75 and 2.39 μM, and fungicidal at 5.80, 5.80 and 2.90 μM against C. albicans, C. glabrata and C. neoformans respectively. 14c was the most potent in Series 2 against C. krusei (IC50 = 1.87 μM, MIC and MFC = 2.90 μM). The most potent activity against C. neoformans was observed with analogues 14h and 14i (IC50 = 0.46 and 1.03 μM, MIC and MFC = 0.68 and 1.51 μM, respectively).
Table 4.
In vitro antifungal activity of tricyclic guanidine derivatives
| Compd No. |
Candida albicans | Candida glabrata | Candida krusei | Cryptococcus neoformans | Aspergillus fumigatus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| IC50 μM |
MIC μM |
MFC μM |
IC50 μM |
MIC μM |
MFC μM |
IC50 μM |
MIC μM |
MFC μM |
IC50 μM |
MIC μM |
MFC μM |
IC50 μM |
MIC μM |
MFC μM |
|
| 12b | 18 | 60 | 60 | - | - | - | 19.76 | 60 | 60 | 8.73 | 15 | 30 | 33.54 | - | - |
| 12c | 3.02 | 5.99 | 5.99 | 2.18 | 2.99 | 2.99 | 2.47 | 2.99 | 2.99 | 2.11 | 2.99 | 2.99 | 5.46 | 11.99 | 11.99 |
| 12f | - | - | - | - | - | - | 33.22 | - | - | 29.54 | 60.79 | 30.39 | - | - | - |
| 12h | - | - | - | - | - | - | 35.89 | 45.35 | 45.35 | 6.71 | 11.33 | 11.33 | - | - | - |
| 12j | - | - | - | - | - | - | - | - | - | 23.71 | 48.9 | 48.9 | - | - | - |
| 12l | - | - | - | - | - | - | 39.21 | - | - | 28.68 | 32.78 | 32.78 | - | - | - |
| 14b | 7.03 | 28.81 | 57.63 | 35.96 | - | - | 13.91 | 28.81 | 28.81 | 4.20 | 3.60 | 3.60 | 35.64 | - | - |
| 14c | 3.89 | 5.80 | 5.80 | 3.75 | 5.80 | 5.80 | 1.87 | 2.90 | 2.90 | 2.39 | 5.80 | 2.90 | 10.11 | 23.20 | 23.20 |
| 14f | 16.44 | 29.15 | 58.30 | 34.66 | 58.30 | - | 11.39 | 29.15 | 29.15 | 12.47 | 14.57 | 14.57 | 35.27 | - | - |
| 14h | 34.93 | - | - | - | - | - | 12.34 | 21.93 | 43.86 | 0.46 | 0.68 | 0.68 | - | - | - |
| 14i | 25.90 | 48.19 | - | - | - | - | 16.84 | 24.09 | 24.09 | 1.03 | 1.51 | 1.51 | 47.78 | - | - |
| 14l | 9.09 | 31.34 | 62.69 | 34.16 | 62.69 | 62.69 | 12.94 | 31.34 | 31.34 | 4.29 | 7.83 | 7.83 | 59.34 | - | - |
| 17b | 22.09 | 38.0 | 76 | - | - | - | 24.37 | 76 | 38 | 17.60 | 38 | 38 | 47.37 | - | - |
| 17c | - | - | - | 17.32 | 32.68 | 65.36 | 9.57 | 16.34 | 16.34 | 2.61 | 8.17 | 8.17 | - | - | - |
| 17h | - | - | - | - | - | - | - | - | - | 16.49 | 31.54 | 31.54 | - | - | - |
| 17j | - | - | - | - | - | - | - | - | - | 35.49 | 66.22 | 66.22 | - | - | - |
| 17l | - | - | - | - | - | - | - | - | - | 76.18 | - | - | - | - | - |
| 20a | - | - | - | - | - | - | - | - | - | 19.96 | 40.16 | 40.16 | - | - | - |
| 20b | - | - | - | - | - | - | - | - | - | 15.34 | 18.0 | 18.0 | - | - | - |
| 20c | - | - | - | 19.0 | 31.34 | 62.69 | 6.99 | 15.67 | 15.67 | 3.66 | 7.83 | 15.67 | - | - | - |
| 20h | - | - | - | - | - | - | - | - | - | 3.56 | 7.55 | 7.55 | - | - | - |
| 20l | 16.79 | 31.74 | 31.74 | 19.30 | 31.74 | 63.49 | 8.98 | 15.87 | 15.87 | 2.12 | 3.96 | 3.96 | 34.25 | - | - |
IC50 = Concentration (μM) that affords 50% growth inhibition; MIC= minimum inhibitory concentration (the lowest concentration in μM that allows no detectable growth); MFC = minimum fungicidal concentration (the lowest concentration in μM that kills the organism); “-” = No activity at the highest test concentration of 20 μg/mL. Amphotericin B: IC50 = 0.30 μM, MIC = 1.35 μM, MFC = 1.35 μM (Ca); IC50 = 0.39 μM, MIC = 0.68 μM, MFC = 1.35 μM (Cg); IC50 = 0.56 μM, MIC = 1.35 μM, MFC = 1.35 μM (Ck); IC50 = 0.86 μM, MIC = 1.35 μM, MFC = 2.57 μM (Afu); IC50 = 0.26 μM, MIC = 0.68 μM, MFC = 0.68 μML (Cn).
In Series 3 and 4, 17b was moderately active against C. albicans and A. fumigatus (IC50 = 22 and 46.73 μM) and 17c, 20c and 20l were active against C. glabrata (IC50 of 17.32-19.30 μM) whereas, they showed promising activity against C. krusei (IC50 values of 9.57, 6.99 and 8.98 μM respectively, and MIC and MFC value of 15 μM), and C. neoformans (IC50 of 2.61, 3.66 and 2.12 μM, MIC of 8.17, 7.83 and 3.96 μM, and MFC of 8.17, 15.67 and 3.96 μM respectively). Batzelladine K (17l) was inactive against all pathogens except C. neoformans (IC50 76.18 μM).
Anti-HIV activity
None of tested analogues were found to inhibit the growth of HIV-1 (supporting information-Table 5). Batzelladine K also demonstrated no anti-HIV activity.
Conclusion
We have synthesized four series of tricyclic guanidine analogues based on batzelladine K structural framework. The analogues were evaluated for antimalarial, antileishmanial, antibacterial, antifungal and anti-HIV activities. The 14h and 20l produced the most potent antimalarial activity against chloroquine-sensitive D6 (IC50 of 1.25 and 0.88 μM respectively) and chloroquine-resistant W2 (IC50 of 1.64 and 1.07 μM respectively) strains of P. falciparum. The most potent analogues having nonyl substituent, 12c and 14c, not only exhibited antileishmanial activities but also showed potent antibacterial and antifungal activities.
Analogue 20l having pentyl and methyl substitutions showed promising activities against all pathogens. However, none was active against HIV. The combination of broad spectrum of activities makes these tricyclic guanidines as a promising new structural class of compounds. Further exploration of these tricyclic guanidines can provide lead compounds for further development as antimicrobial agents.
Supplementary Material
Acknowledgements
Dr. Nafees Ahmed and Dr. Keyur Brambhatt thank the Council of Scientific and Industrial Research (CSIR) and Department of Biotechnology (DBT), New Delhi for the award of Senior Research Fellowships. Antimicrobial assays was supported by NIH grant No. AI 27094. Antimalarial and antileishmanial screening assays are partly supported by USDA-ARS cooperative scientific agreement number 58-6408-2-0009 (NCNPR) and US Department of Defense CDMRP grant number W81XWH-09-20093 (BLT). We thank John Trott, Marsha Wright and Surendra Jain for technical assistance in antimalarial, antibacterial/antifungal and antileishmanial assays, respectively. Anti-HIV screening was supported by Department of Biotechnology project (Grant No. BT/PR7020/Med/14/930/2005 Dt. 23/05/2006).
Footnotes
Supporting information
Supporting Information may be found in the online version of this article:
Experimental procedures for synthesis
Spectral data of intermediates and final compounds
Table 5: Anti HIV activity of compounds
References
- 1.Kaur K, Jain M, Khan SI, Jacob MR, Tekwani BL, Singh S, Singh PP, Jain R. Synthesis, antiprotozoal, antimicrobial, beta-hematin inhibition, cytotoxicity and methemoglobin (MetHb) formation activities of bis(8-aminoquinolines) Bioorg Med Chem. 2011;19:197–210. doi: 10.1016/j.bmc.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. http://www.who.int/leishmaniasis/resources/en/index.html.
- 3.Bharate SB, Khan SI, Yunus NAM, Chauthe SK, Jacob MR, Tekwani BL, Khan IA, Singh IP. Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg Med Chem. 2007;15:87–96. doi: 10.1016/j.bmc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Harms G, Feldmeier H. HIV infection and tropical parasitic diseases - deleterious interactions in both directions? Trop Med Int Health. 2002;7:479–488. doi: 10.1046/j.1365-3156.2002.00893.x. [DOI] [PubMed] [Google Scholar]
- 5.Mazu TK, Etukala JR, Zhu XY, Jacob MR, Khan SI, Walker LA, Ablordeppey SY. Identification of 3-phenylaminoquinolinium and 3-phenylaminopyridinium salts as new agents against opportunistic fungal pathogens. Bioorg Med Chem. 2011;19:524–533. doi: 10.1016/j.bmc.2010.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groll AH, Tragiannidis A. Recent Advances in Antifungal Prevention and Treatment. Semin Hematol. 2009;46:212–229. doi: 10.1053/j.seminhematol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, Brosse CD, Mai S, Truneh A, Carte B. Novel Alkaloids from the Sponge Batzella Sp - Inhibitors of HIV GP120-Human CD4 Binding. J Org Chem. 1995;60:1182–1188. [Google Scholar]
- 8.Patil AD, Freyer AJ, Offen P, Bean MF, Johnson RK. Three new tricyclic guanidine alkaloids from the sponge Batzella sp. J Nat Prod 1997. 1997;60:704–709. [Google Scholar]
- 9.Patil AD, Freyer AJ, Taylor PB, Carte B, Zuber G, Johnson RK, Faulkner DJ. Batzelladines F-I, novel alkaloids from the sponge Batzella sp.: Inducers of p56(lck)-CD4 dissociation. J Org Chem. 1997;6:1814–1819. [Google Scholar]
- 10.Hua HM, Peng J, Dunbar DC, Schinazi RF, Andrews AGDC, Cuevas C, Garcia-Fernandez LF, Kelly M, Hamann MT. Batzelladine alkaloids from the caribbean sponge Monanchora unguifera and the significant activities against HIV-1 and AIDS opportunistic infectious pathogens. Tetrahedron. 2007;63:11179–11188. [Google Scholar]
- 11.Gallimore WA, Kelly M, Scheuer PJ. Alkaloids from the sponge Monanchora unguifera. J Nat Prod. 2005;68:1420–1423. doi: 10.1021/np050149u. [DOI] [PubMed] [Google Scholar]
- 12.Bewley CA, Ray S, Cohen F, Collins SK, Overman LE. Inhibition of HIV-1 envelope-mediated fusion by synthetic batzelladine analogues. J Nat Prod. 2004;67:1319–1324. doi: 10.1021/np049958o. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed N, Brahmbhatt KG, Singh IP, Bhutani KK. Total Synthesis of (+/−)-Batzelladine K: A Biomimetic Approach. Synthesis. 2010;15:2567–2570. [Google Scholar]
- 14.Makler MT, Hinrichs DJ. Measurement of the Lactate-Dehydrogenase Activity of Plasmodium-Falciparum as an Assessment of Parasitemia. Am J Trop Med Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 15.Borenfreund E, Babich H, Martin-Alguacil N. Rapid chemosensitivity assay with human normal and tumor cells in vitro. In Vitro Cell Dev Biol. 1990;26:1030–1034. doi: 10.1007/BF02624436. [DOI] [PubMed] [Google Scholar]
- 16.Mustafa J, Khan SI, Ma G, Walker LA, Khan IA. Synthesis and anticancer activities of fatty acid analogs of podophyllotoxin. Lipids. 2004;39:167–172. doi: 10.1007/s11745-004-1215-5. [DOI] [PubMed] [Google Scholar]
- 17.Harrison JH, Jollow DJ. Contribution of aniline metabolites to aniline-induced methemoglobinemia. Mol Pharmacol. 1987;32:423–431. [PubMed] [Google Scholar]
- 18.Mikus J, Steverding D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol Int. 2000;48:265–269. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 19.Ma G, Khan SI, Jacob MR, Tekwani BL, Li Z, Pasco DS, Walker LA, Khan IA. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob Agents Chemother. 2004;48:4450–4452. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NCCLS Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically M7-A5. National Committee on Clinical Laboratory Standards. 2000;20(2) [Google Scholar]
- 21.NCCLS Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard M27-A2. National Committee on Clinical Laboratory Standards. 2002;22(15) [Google Scholar]
- 22.NCCLS Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, M38-A. National Committee on Clinical Laboratory Standards. 2002;22(16) [Google Scholar]
- 23.NCCLS Susceptibility Testing of Mycobacteria, Nocardia, and Other Aerobic Actinomycetes; Tentative Standard-second edition, M24-T2. National Committee on Clinical Laboratory Standards. 2000;20(26) [PubMed] [Google Scholar]
- 24.Franzblau SG, Witzig RS, McLaughlin JC, Torres PM, Hernandez GA, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 1998;36:362–366. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denizot F, Lang RJ. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Goila-Gaur R, Salzweddel K, Kilgore NR, Reddick M, Matallana C, Castillo A, Zoumplis D, Martin DE, Orenstein JM, Allaway GP, Freed EO, Wild CT. A potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc Natl Acad Sci USA. 2003;100:13555–13560. doi: 10.1073/pnas.2234683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladha JS, Tripathy MK, Mitra D. Mitochondrial complex I activity is impaired during HIV-1-induced T-cell apoptosis. Cell Death Differ. 2005;12:1417–1428. doi: 10.1038/sj.cdd.4401668. [DOI] [PubMed] [Google Scholar]
- 28.Gervaix AWD, Leoni LM, Richman DD, Wong-Staal F, Corbeil J. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proc Natl Acad Sci USA. 1997;94:4653–4658. doi: 10.1073/pnas.94.9.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.House HO, Cronin TH. A Study of Intramolecular Diels-Alder Reaction. J Org Chem. 1965;30:1061–1070. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.