Abstract
A short half-life and low levels of growth factors in an injured microenvironment necessitates the sustainable delivery of growth factors and stem cells to augment the regeneration of injured tissues. Our aim was to investigate the ability of VEGF165 expressing bone marrow mesenchymal stem cells (BMMSCs) to differentiate into hepatocytes when cultured with hepatocyte growth factor (HGF) and epidermal growth factor (EGF) in vitro. We isolated, cultured and identified rabbit BMMSCs, then electroporated the BMMSCs with VEGF165-pCMV6-AC-GFP plasmid. G418 was used to select transfected cells and the efficiency was up to 70%. The groups were then divided as follows: Group A was electroporated with pCMV6-AC-GFP plasmid + HGF + EGF and Group B was electroporated with VEGF165-pCMV6-AC-GFP plasmid +HGF + EGF. After 14 days, BMMSCs were induced into short spindle and polygonal cells. Alpha-fetoprotein (AFP) was positive and albumin (ALB) was negative in Group A, while both AFP and ALB were positive in group B on day 10. AFP and ALB in both groups were positive on day 20, but the quantity of AFP in group B decreased with prolonged time and was about 43.5% less than group A. The quantity of the ALB gene was increased with prolonged time in both groups. However, there was no significant difference between group A and B on day 10 and 20. Our results demonstrated that VEGF165-pCMV6-AC-GFP plasmid modified BMMSCs still had the ability to differentiate into hepatocytes. The VEGF165 gene promoted BMMSCs to differentiate into hepatocyte-like cells under the induction of HGF and EGF, and reduced the differentiation time. These results have implications for cell therapies.
Keywords: Stem cells, Differentiation, VEGF165, Hepatocyte growth factor, Epidermal growth factor
Introduction
Bone marrow mesenchymal stem cells (BMMSCs) have important clinical value in cell replacement therapy, gene therapy and tissue/organ reconstruction. BMMSCs induced under specific conditions can differentiate into various tissues and cells in different micro-environments. A number of studies have demonstrated that, when cultured with epidermal growth factor (EGF), hepatocyte growth factor (HGF) and basic fibroblast growth factor (bFGF) (Chivu et al. 2009; Forte et al. 2006; Snykers et al. 2011), liver cells (Gu et al. 2009; Lange et al. 2005) and liver injury serum (Li et al. 2010; Lysy et al. 2008) can induce BMMSCs to differentiate into hepatocytes in vitro and in vivo (Piryaei et al. 2011; Stock et al. 2010). Since growth factor protein is expensive and has a short half-life in vivo and large doses may result in hemodynamic abnormalities and serious side effects, gene-therapy has become a targeted approach for delivery (Supp and Boyce 2002). With the unique advantages of being easily obtained and amplified in vitro, allowing easy genetic manipulation and weak immunogenicity, BMMSCs have become an appropriate cell carrier for gene therapy (Park et al. 2003; Tsuda et al. 2005). The family of vascular endothelial growth factor (VEGF) molecules are potent initiators and regulators of angiogenesis with VEGF165 being the most powerful and widely distributed within the body for promoting angiogenesis (Neufeld et al. 1999; Zhao et al. 2007). BMMSCs transfected with the VEGF gene have been used in the treatment of cardiovascular disease (Liu et al. 2009; Pons et al. 2009; Zisa et al. 2009), bone regeneration (Wang et al. 2010), neurodegenerative (An et al. 2010), and Achilles tendon repair (Hou et al. 2009). However, there has not been research on VEGF gene modified BMMSCs and its role in the liver disease process.
HGF has been identified as a potent mitogen for hepatocytes (Forte et al. 2006) and plays an important role in liver development and regeneration (Michalopoulos and DeFrances 2005). EGF is another important factor found to play a role in the proliferation and differentiation of liver cells which promotes mitosis in the early stages of liver regeneration (Skarpen et al. 2005). In acute liver cell injury, HGF and EGF were significantly increased in the blood and also over-expressed in oval cell activation, proliferation and differentiation (Hatch et al. 2002; Hong et al. 2004).
Within the context of this work, we utilized HGF and EGF as the hepatocyte inducing protocol, to investigate the ability of BMMSCs transfected with VEGF to differentiate into hepatocytes in vitro.
Materials and methods
Isolation and culture of BMMSCs
BMMSCs were harvested from the bone marrow of 2 month old New Zealand rabbits (male or female, weight about 1 kg) that were obtained from the Laboratory Animal Unit of the Central South University (ChangSha, China). Bone marrow of humerus and femur were flushed with 40 ml D-Hanks solution (GIBCO, Carlsbad/CA, USA). The cell suspension was centrifuged over a Ficoll step gradient (density 1.077 g/ml; Ficoll-Histopaque 1077, Sigma-Aldrich, St. Louis, MO) at 200 g for 20 min. BMMSCs were collected and cultured in DMEM (Gibco, USA), supplemented with 20% fetal bovine serum (FBS, Gibco, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, USA) at 37 °C and 5% CO2 for 5 days. After removing the suspension cells, adherent BMMSCs were grown to 90% confluence and then passaged.
Flow cytometry
Approximately 1 × 106 cells of 3rd generation BMMSCs were harvested and re-suspended in 1 ml PBS. The cell suspension was incubated with primary monoclonal sheep anti-rabbit antibodies including: anti-CD45 (1:100); anti-CD44 (1:100); anti-CD34 (1:100); and anti-CD29 (1:100) for 1 h at 4 °C, and then washed with 0.1 M PBS three times. The cell suspension was incubated with FITC-conjugated secondary sheep antibody (1:200) for 40 min at 4 °C, and was then washed with 0.1 M PBS three times again. 0.01 M PBS was used as a negative control. All antibodies were purchased from eBioscience (USA).
Electroporation and G418 selection
The 3rd generation BMMSCs were harvested and resuspended in electroporation buffer [containing 750 μl Opti-DMEM (Gibco, USA) without serum and 50 μg VEGF165-pCMV6-AC-GFP plasmid (OriGene, Rockville/MD, USA) or pCMV6-AC-GFP plasmid (OriGene, USA)]. The cell concentration was 2 × 106 cell/ml. The electroporation parameters were: capacity at 1,000 μF, voltage at 280 V and internal resistance was infinite. After electroporation, the cells were transferred into a new 25 cm2 flask with complete medium, and analyzed for GFP expression under a fluorescence microscope 48 h post electroporation. The medium was changed every 3 days and contained G418 (200 μg/ml). We used cell flow cytometry to test the transfection efficiency.
Reverse transcription PCR analysis for VEGF165
Five groups were determined, including BMMSCs electroporated after 2 days (A), BMMSCs selected by G418 after 20 days (B) and BMMSCs post-selection after 7 days (C), 14 days (D), and 21 days (E). The total RNA was extracted using the Trizol reagent (Sigma-Aldrich, Shanghai, China) according to standard protocols. Reverse transcription was performed with 2 μg RNA and an Access Quick RT-PCR System (Promega, Beijing, China) according to the manufacturer’s protocol. Gene-specific primers for human VEGF165 and β-actin were designed using the Primer Premier software (Premier Biosoft International, Palo Alto/CA, USA) as listed in Table 1. The length of the amplicon was 280 bp. pCMV6-AC-GFP plasmid modified BMMSCs were used as negative controls. The reaction mixture was subjected to 30 cycles of a PCR including a 5 min denaturation step at 96 °C. Each cycle consisted of a 30 s denaturation step at 95 °C, a 30 s annealing at 60 °C, and a 30 s extension at 72 °C. The last cycle was followed by a 10 min longation step at 72 °C. PCR products were electrophoresed and analyzed on a 2% agarose gel.
Table 1.
Gene-specific primers
| Primer | Sequence |
|---|---|
| VEGF165 | F:5′-CCTTGCTGCTCACCTCCAC-3′ |
| R:5′-ATCTGCATGGTAGATGTTGGA-3′ | |
| ALB | F:5′-GAGCCAAAGATTTCCCAAGG-3′ |
| R:5′-ACATTCAAGCAGGTCACCGT-3′ | |
| β-actin | F:5′-TCACCATGGATGATGATATCGC-3′ |
| R:5′-CGTGCTCGATGGGGTACTTCA-3′ |
Hepatocyte differentiation
The 3rd generation BMMSCs were electroporated with the aforementioned plasmids. G418 (Gibco, USA) was utilized for selection with an efficiency up to 70% which was tested by flow cytometry, followed by HGF (Peprotech, Rocky Hill/NJ, USA) and EGF (Peprotech, USA) BMMSC inductation. The groups were designated as follows: Group A was transfected with pCMV6-AC-GFP plasmid + HGF (60 ng/ml) + EGF (45 ng/ml) and Group B was transfected with VEGF165-pCMV6-AC-GFP plasmid + HGF (60 ng/ml) + EGF (45 ng/ml).
Immunohistochemistry and immunofluorescence
In each group, BMMSCs cultured on coverslips on day 10 and 20 were fixed with 4% paraformaldehyde for 30 min, and treated with 0.1% Triton for 10 min. Endogenous peroxidase was quenched with 0.3% hydrogen peroxide solution. After blocking with 3% BSA in PBS at room temperature for 1 h, the slides were incubated with goat anti-rabbit albumin (ALB) antibody (1:100 dilution, Bethyl Laboratories, Montgomery/TX, USA) or sheep polyclonal anti-human alpha-fetoprotein (AFP) C-terminus antibody (1:200 dilution, Santa Cruz Biotechnology, Santa Cruz/CA, USA) at 4 °C overnight. After washing three times, the slides were then incubated with HRP-labeled anti-sheep antibody (1:200, Bethyl Labotatories) for detecting ALB and rhodamine-conjugated anti-goat antibody (1:200, Bethyl Labotatories) for detecting AFP at room temperature for 45 min. Then the slides were washed with 0.1 M PBS three times. Finally, the first slide was stained with DAB (Dako Envision + system, Glostrup, Denmark) counterstained with hematoxylin, and dehydrated with xylene. The second slide was observed directly under a fluorescence microscope. Undifferentiated BMMSCs were used as negative controls.
Western blots
BMMSCs in each group were collected on day 10 and 20. Total protein was extracted (Beyotime, Jiangsu, China) and the concentration was determined by the Bradford assay (Bio-Rad Laboratories, Hercules/CA, USA). The samples were fractionated on 10% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. After washing, the membrane was blocked with 10% milk at room temperature for 1 h and incubated with sheep polyclonal anti-human AFP C-terminus antibody (1:2,000 dilution, Santa Cruz), goat anti-rabbit ALB antibody (1:500 dilution, Bethyl Laboratories) at 4 °C overnight. After washing, the membrane was incubated with peroxidase-conjugated mouse anti-goat antibodies (1:1,000 dilution; Bethyl Labotatories) at room temperature for 1 h. After washing, the immunoreactive bands were detected by ECL chemiluminescence reagents (Beyotime, China). The density of the bands was quantified with a laser densitometer (Bio-Rad, USA). Undifferentiated BMMSCs were used as negative controls. β-actin was used as an internal control.
RNA isolation and real-time PCR
BMMSCs in each group were collected on day 10 and 20. The total RNA was extracted using Trizol reagent (Sigma-Aldrich) according to standard protocols. Reverse transcription was performed with 2 μg total RNA and real-time PCR was conducted with a QuantiTect SYBR Green RT-PCR Kit (Promega) according to the manufacturer’s protocol. Gene-specific primers for albumin and β-actin were designed using the Primer Premier software (Premier Biosoft International, Palo Alto) and listed in Table 1. PCR was performed as listed in Table 2. The PCR of β-actin was carried out at 94 °C for 2 min, followed by 40 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s and collecting fluorescence at 83 °C for 15 s. The PCR of ALB was carried out at 94 °C for 2 min, followed by 40 cycles at 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 60 s and collecting fluorescence at 84 °C for 15 s. Undifferentiated BMMSCs were used as negative controls.
Table 2.
Amplification system of real-time PCR
| QuantiTect SYBR green | 12.0 μl |
| Upstream primer (10 ng/μl) | 1.0 μl |
| Downstream primer (10 ng/μl) | 1.0 μl |
| cDNA | 4.0 μl |
| Nuclease-free H2O | 2.0 μl |
| Total volume | 20 μl |
Statistical analysis
Data are presented as mean ± SD. The results were analyzed by ANOVA using SPSS 16.0. Results were considered significant when p < 0.05.
Results
Morphological observation and representative phenotypes of BMMSCs
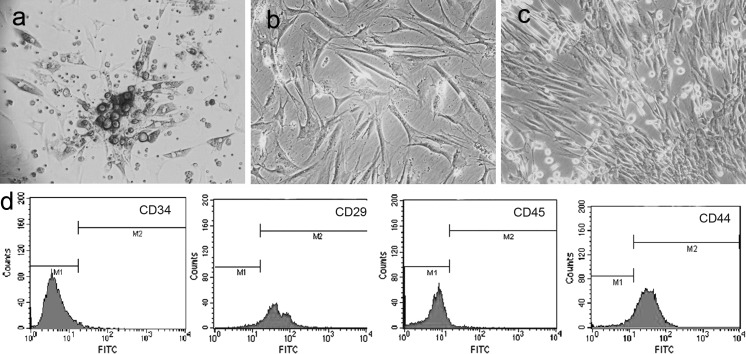
Based on observations after 24 h inoculation, adherent cells were attached to the culture dish. After 72 h, the adherent cells formed several cell clones (Fig. 1a). After 15–18 days, the cells were close to fusion (Fig. 1b). The BMMSCs were maintained in vitro through serial passages to obtain a uniform pattern (Fig. 1c) and phenotypic characterization was confirmed. CD29 (93.41 ± 3.2%, n = 3) and CD44 (91.55 ± 4.1%) were significantly over-expressed on BMMSCs while there was no obvious expression of CD45 (3.95 ± 2.9%) and CD34 (3.36 ± 3.6%; Fig. 1d).
Fig. 1.
The morphology and representative phenotype of BMMSCs. Primary BMMSCs 72 h (a, ×200), primary BMMSCs 18 days (b, ×200), and third-generation BMMSCs (c, ×200). Flow cytometry determination of MSC surface markers CD34, CD29, CD45 and CD44 (d)
Electroporation efficiency and G418 selection
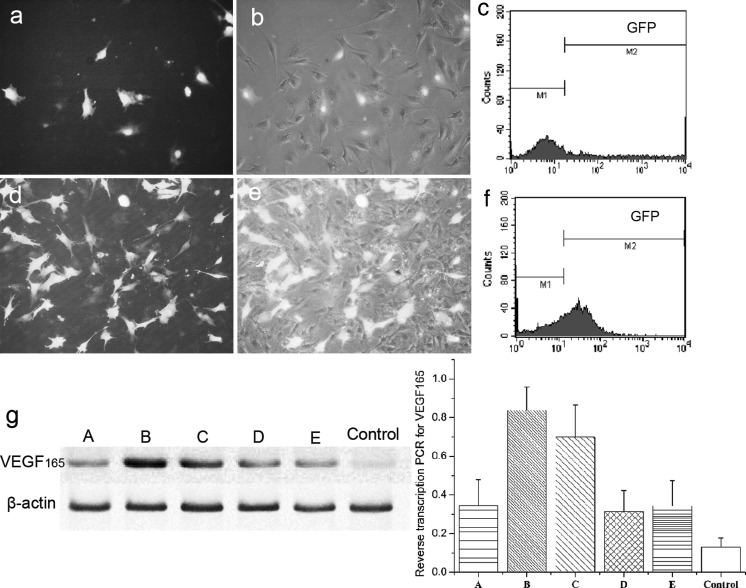
BMMSCs electroporated with VEGF165-pCMV6-AC-GFP plasmid were collected after 48 h (Fig. 2a, b), and the transfection efficiency tested by flow cytometry was 34.58 ± 5.08% (Fig. 2c). Transfection efficiency was up to 70.46 ± 4.17% (Fig. 2f) after using G418 (200 μg/ml) selection at approximately 20 days (Fig. 2d, e).
Fig. 2.
GFP and VEGF gene expression of electroporated BMMSCs. Green fluorescence represents the successful electroporation of plasmid into BMMSCs. Electroporated cells after 48 h (a and b). G418 selection after 20 days (d and e) (bright vision) b and e, ×200) dark vision (a and d, ×200). Transfection efficiency as determined by flow cytometry of BMMSCs electroporated after 48 h (c), and selected after 20 days (f). Reverse transcription PCR analysis for VEGF165 levels (the RT-PCR conditions A–E are described in the Materials and Methods section) (g). Values represent mean ± SD. Significance was set at p < 0.05
Reverse transcription PCR analysis for VEGF165
The mRNA levels of VEGF165 were examined by RT-PCR (Fig. 2g). The VEGF gene of the VEGF165-pCMV6-AC-GFP transfected group was tested in group A, and there was a significant increase in group B selected by G418 after 20 days (p < 0.05). After selection, there was a small decrease in expression with prolonged time, but there were no significant differences between groups D and E (p > 0.05).
Morphology of induced BMMSCs
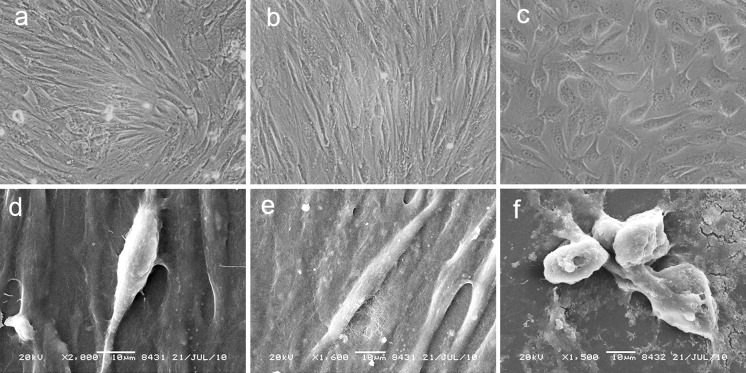
The BMMSCs presented a fibroblast-like morphology before differentiation (Fig. 3a, d) and became more slender after electroporation (Fig. 3b, e). However, after 20 days induction, cells developed shorter spindle and polygonal shapes. Also, the amount of polygonal cells increased and hepatocyte-like cells formed colonies with prolonged time (Fig. 3c). Electron microscopy showed that cells in both groups developed short spindle and polygonal shapes after 20 days induction (Fig. 3f). In contrast, BMMSCs cultured in basal medium did not exhibit significant changes in cell morphology on day 20.
Fig. 3.
Morphology of the induced BMMSCs. Light microscopy (a, ×200), scanning electron microscopy (d) of unelectroporated BMMSCs, electroporated BMSCs after 48 h (b × 200, and e), BMMSCs induced after 20 days (c, ×200 and f). Scale bars in d, e, f, 10 μm
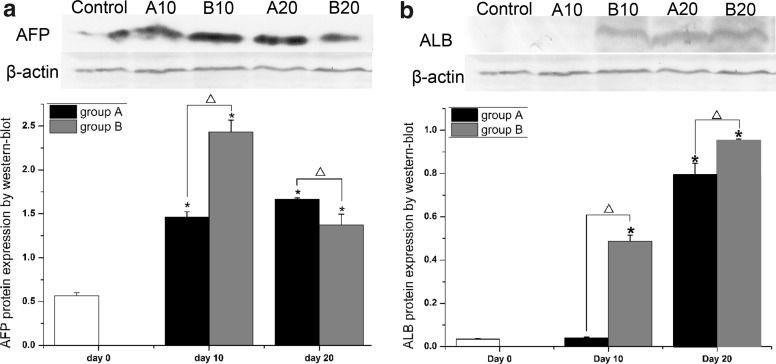
Immunocytochemistry and immunofluorescence
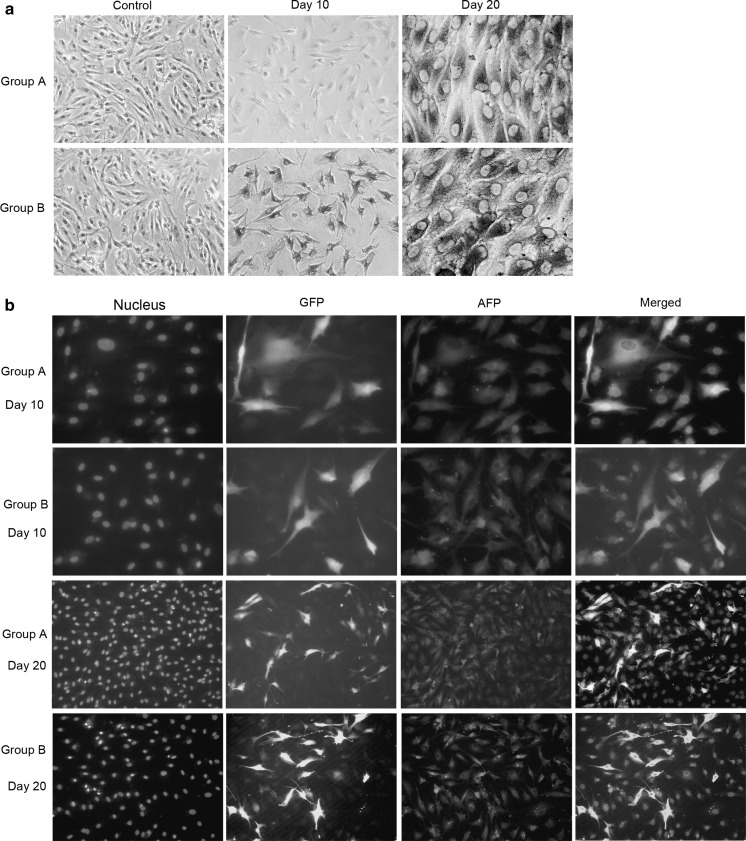
Immunocytochemistry and immunofluorescence revealed the location of ALB and AFP. There were uniform distributions of the brown-yellow granules (representing ALB positive) and red fluorescence (representing AFP positive) in the cytoplasm and nuclei of the positive cells. Green fluorescence represented the expression of GFP. Undifferentiated BMMSCs were used as negative controls for ALB. ALB was negative in group A and positive in group B on day 10 (Fig. 4a). ALB was positive in both groups on day 20 (Fig. 4a). AFP was positive in both groups on days 10 and 20 (Fig. 4b). The ALB expression was earlier in group B than in group A, but AFP expression occurred at the same time in both groups. In contrast, ALB and AFP expressions were negative in BMMSCs cultured in basal medium.
Fig. 4.
The ALB and AFP expression of induced gene-modified BMMSCs on days 10 and 20. Immunohistochemistry for detecting ALB (a). Immunofluorescence for detecting AFP (b)
Western blot
The relative expression of ALB and AFP in the induced BMMSCs was analyzed with Western blotting. The expression level of hepatocyte-specific proteins was normalized with β-actin as an internal protein loading control. There was no expression of ALB in group A on day 10 (p > 0.05), and apparent expression in group B (p < 0.05). The expression of AFP decreased with prolonged time in group B and was less than in group A on day 20 (Fig. 5a). There was higher ALB expression in group B compared to group A (Fig. 5b) on day 20. Therefore, the expression of ALB was earlier and at a higher level in group B than in group A on day 10 and 20. This was consistent with immunocytochemistry results.
Fig. 5.
Western-blot results for detecting ALB and AFP of the induced gene-modified BMMSCs on the 10th day and 20th day. The results shown are representative of two independent experiments, each of them performed in triplicate. Values represent mean ± SD. Significance was set at p < 0.05 and assessed compared with day 0 (asterisk) and compared between groups (triangle)
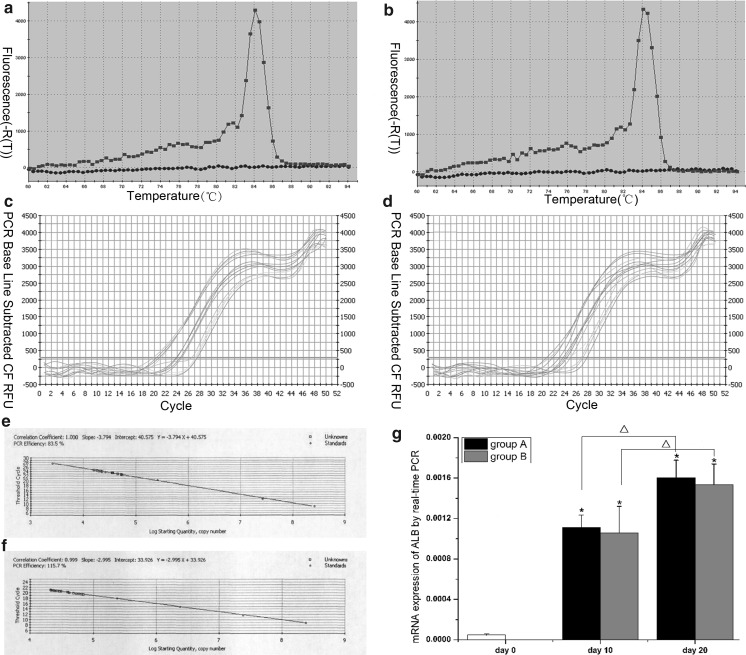
Real-time PCR
To determine whether morphologic changes and the expression of ALB were sustained and associated with the induction of hepatocyte-specific genes, the total RNA of both groups was isolated on day 10 and 20. Real-time PCR was used to analyze the expression of the ALB gene (Fig. 6). Undifferentiated BMMSCs were used as negative controls. The ALB gene was detected in both groups on day 10 and 20, and the quantity of the ALB gene was significantly increased with prolonged time . However, there was no significant difference between group A and B on day 10 and 20 (Fig. 6 g).
Fig. 6.
Real-time PCR analysis of the mRNA levels for ALB. Melting curve for β-actin (a) and for ALB (b). Amplification curve for β-actin (c) and for ALB (d). Standard curve for β-actin (e) and ALB (f). The relative quantity of the ALB gene (g). Values represent mean ± SD. Significance was set at p < 0.05 and assessed compared with day 0 (asterisk) and compared between groups (triangle)
Discussion
Stem cells have generated a great deal of interest in recent years because of their potential therapeutic use. Under the influence of environmental factors, they can proliferate, differentiate and replace damaged cells from adult tissues. A number of studies have demonstrated that stem cells can differentiate into hepatocytes in vitro. Numerous cytokines and growth factors have been shown to have potent effects on hepatic growth and differentiation in vitro (Lee et al. 2004; Talens-Visconti et al. 2007). They include HGF, EGF, transforming growth factor (TGF), bFGF, insulin, insulin-like growth factor, and oncostatin M (OSM). HGF and EGF are also considered important factors in the hepatocyte differentiation pathway (Forte et al. 2006; Sato et al. 1999; Tamama et al. 2006).
Hepatocyte growth factor is identified as a potent mitogen for hepatocytes. The secretion of HGF after liver injury is increased (Shi et al. 2011). It can accelerate tissue regeneration following an acute insult, as well as amelioration of tissue fibrosis and dysfunction in chronic conditions (Ishiki et al. 1992; Pulavendran et al. 2011; Shiota et al. 2000). EGF is another important factor in the proliferation and differentiation of liver cells. HGF and EGF induce the expression of all members of the TGF-beta family, and they also produce a marked increase in CK19 expression that correlates with the appearance of cells that resemble oval cells. In hepatic failure and acute liver injury, the increased blood HGF and EGF levels play an important functional role in liver regeneration (Flohr et al. 2009; Mizuguchi et al. 2001). There are many studies using HGF and EGF in the hepatocyte inducing protocol, and the concentrations of HGF and EGF showed wide ranges (Snykers et al. 2009). The concentration of HGF ranges from 20 ng/ml to 150 ng/ml (Banas et al. 2007; Kazemnejad et al. 2008; Talens-Visconti et al. 2006; Yamamoto et al. 2008), and the concentration of EGF ranges from 10 ng/ml to 50 ng/ml (Kazemnejad et al. 2008; Lange et al. 2005; Shi et al. 2005). Wang et al. (2004) induced isolated BMMSCs with HGF in vitro at a concentration of 50 ng/ml, which mimicked the injured liver microenvironment. In our previous study, we showed the combination of HGF (60 ng/ml) and EGF (45 ng/ml) was the best hepatocyte inducing protocol.
Vascular endothelial growth factor is not only an angiogenic factor but also plays a role in the survival of the liver (Akiyoshi et al. 1998). In liver cirrhosis or acute-on-chronic liver failure patients, serum VEGF levels were significantly lower than those of the control group, suggesting that serum VEGF levels may be associated with hepatocyte regeneration grades (Akiyoshi et al. 1998; Shi et al. 2011). The VEGF165-pCMV6-AC-GFP plasmid has been successfully transfected into BMMSCs by electroporation in our experiment, and had a stable expression after G418 selection. However, the expression decreased with a prolonged time after selection. Other studies also established a method for transfecting the human VEGF165 gene into MSCs in which the target gene and protein could be expressed effectively (Guo-ping et al. 2010; Tarnowski et al. 2010).
There are many studies on combining MSCs and VEGF gene therapy for protecting neurons against hypoxic ischemic injury (An et al. 2010), promoting MSC proliferation (Crespo-Diaz et al. 2011), enhancing the efficacy of MSCs in the swine model of myocardial infarction (Liu et al. 2009) and promoting cardiac function and MSC survival (Pons et al. 2009). Some studies have shown that MSCs could also achieve the biological effects by secreting VEGF. VEGF secreted by MSCs has been used in gastric ulcer and oral ulcer healing (El-Menoufy et al. 2010; Hayashi et al. 2008), in murine ischemic limbs leading to an increase in vessel density (Hoffmann et al. 2010), in inhibiting the apoptosis of lung cells (Zhen et al. 2010), in facilitating neurological function (Deng et al. 2010), enhancing the repair and regeneration of critical sized bone defects (Kanczler et al. 2010) and improving cardiac function in failing rat hearts (Tao et al. 2009). Therefore, the VEGF165 gene is the best target gene in gene therapy for promoting angiogenesis. Liver cells get nutrition and oxygen from a complex network of blood vessels. Ajioka et al. (1999) showed that VEGF-transfected liver cells formed a large number of clones and the meaningful vascular network promoted the growth of liver cells. However, there has been no research on the influence of the VEGF gene on BMMSCs differentiating into hepatocytes. In our experiment, we used HGF and EGF as the hepatocyte inducing protocol, and studied the ability of BMMSCs transfected with VEGF165-pCMV6-AC-GFP plasmid to differentiate into hepatocytes in vitro, and we provided a reliable basis for gene and cell combination therapy in liver failure. The results confirmed that gene modified BMMSCs still had the ability to differentiate into hepatic cells under the induction of HGF and EGF.
The differentiation of hepatoblasts into hepatocytes is a steady process. It is known that embryonic, fetal, and adult hepatocytes differ in molecular phenotypes (Shafritz et al. 2006; Theise 2006), and involve different cytokines (Duncan 2003; Kamiya et al. 1999). EGF promotes mitosis in the early stage of liver regeneration. HGF, OSM, glucocorticoids and endothelial cells play an important role in maturation and terminal differentiation stages. In vivo hepatogenesis implies serial expression of early (AFP, HNF3b and TTR), mid-late (ALB, HNF1a, HNF4a, and CK18), and late (TO, TAT, C/EBPa, and CYPs) markers (Clotman and Lemaigre 2006; Kinoshita and Miyajima 2002; Kyrmizi et al. 2006; Snykers et al. 2009). The first expression of ALB is in early to mid-late embryos and maintains expression in fetal and adult hepatocytes (Shiojiri 1981). AFP, on the other hand, is expressed very early in embryonic development and during the fetal stages. Its expression gradually levels off with increasing development and disappears entirely in adult life (Cascio and Zaret 1991). Therefore, AFP is a reliable marker for discriminating between distinct developmental stages.
Our experiment showed that VEGF165 over-expressing BMMSCs reduced the secretion of AFP with prolonged time compared with group A, while the secretion of ALB protein increased. It demonstrated that the VEGF165 gene could play an important role in the maturation and terminal differentiation stage of liver cells. It could also promote BMMSCs to differentiate into hepatocyte-like cells under the induction of HGF and EGF. Although the expression of AFP was significantly reduced in group B on day 20 compared with group A, it did not disappear. We therefore speculate that our hepatocyte-like cells were in the mid-late stage. Our next research interests may involve prolonging the induction time and using the late (TO, TAT, C/EBPa, and CYPs) markers.
Data also demonstrated that the expression level of the ALB gene was not totally consistent with the expression of ALB protein. That is, real-time PCR showed that the quantity of the ALB gene was increased with prolonged time. However, there was no significant difference between group A and B on day 10 and 20. While Western blots showed that there was no expression of ALB protein in group A (p > 0.05) and significant expression in group B (p < 0.05) on day 10, and there was significant difference between group A and B on day 10 and 20. These results suggested that the VEGF165 gene may influence protein synthesis of ALB, and promote BMMSCs to differentiate into hepatocyte-like cells.
In conclusion, our study provided evidence that electroporated BMMSCs still have the ability to differentiate into hepatocyte-like cells. VEGF165-pCMV6-AC-GFP plasmid promotes BMMSCs to differentiate into hepatocyte-like cells under the induction of HGF and EGF, and reduces the differentiation time compared with control group.
Acknowledgments
This study was supported by The National Natural Science Foundation of China, No. 30070235, 30470508 and 30870695; The Natural Science Foundation of Hunan Province, No. 06JJ2008, 07JJ6040.
References
- Ajioka I, Akaike T, Watanabe Y. Expression of vascular endothelial growth factor promotes colonization, vascularization, and growth of transplanted hepatic tissues in the mouse. Hepatology. 1999;29:396–402. doi: 10.1002/hep.510290233. [DOI] [PubMed] [Google Scholar]
- Akiyoshi F, Sata M, Suzuki H, Uchimura Y, Mitsuyama K, Matsuo K, Tanikawa K. Serum vascular endothelial growth factor levels in various liver diseases. Dig Dis Sci. 1998;43:41–45. doi: 10.1023/A:1018863718430. [DOI] [PubMed] [Google Scholar]
- An SS, Jin HL, Kim KN, Kim DS, Cho J, Liu ML, Oh JS, Yoon DH, Lee MH, Ha Y (2010) Neuroprotective effect of combined hypoxia-induced VEGF and bone marrow-derived mesenchymal stem cell treatment. Childs Nerv Syst 26:323–331. doi:10.1007/s00381-009-1040-2 [DOI] [PubMed]
- Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- Cascio S, Zaret KS. Hepatocyte differentiation initiates during endodermal-mesenchymal interactions prior to liver formation. Development. 1991;113:217–225. doi: 10.1242/dev.113.1.217. [DOI] [PubMed] [Google Scholar]
- Chivu M, Dima SO, Stancu CI, Dobrea C, Uscatescu V, Necula LG, Bleotu C, Tanase C, Albulescu R, Ardeleanu C, Popescu I (2009) In vitro hepatic differentiation of human bone marrow mesenchymal stem cells under differential exposure to liver-specific factors. Transl Res 154:122–132. doi:10.1016/j.trsl.2009.05.007 [DOI] [PubMed]
- Clotman F, Lemaigre FP. Control of hepatic differentiation by activin/TGFbeta signaling. Cell Cycle. 2006;5:168–171. doi: 10.4161/cc.5.2.2341. [DOI] [PubMed] [Google Scholar]
- Crespo-Diaz R, Behfar A, Butler GW, Padley DJ, Sarr MG, Bartunek J, Dietz AB, Terzic A (2011) Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant 20:797–811. doi:http://dx.doi.org/10.3727/096368910X543376 [DOI] [PubMed]
- Deng YB, Ye WB, Hu ZZ, Yan Y, Wang Y, Takon BF, Zhou GQ, Zhou YF (2010) Intravenously administered BMSCs reduce neuronal apoptosis and promote neuronal proliferation through the release of VEGF after stroke in rats. Neurol Res 32:148–56. doi:http://dx.doi.org/10.1179/174313209X414434 [DOI] [PubMed]
- Duncan SA (2003) Mechanisms controlling early development of the liver. Mech Dev 120:19–33. doi:http://dx.doi.org/10.1016/S0925-4773(02)00328-3 [DOI] [PubMed]
- El-Menoufy H, Aly LA, Aziz MT, Atta HM, Roshdy NK, Rashed LA, Sabry D. The role of bone marrow-derived mesenchymal stem cells in treating formocresol induced oral ulcers in dogs. J Oral Pathol Med. 2010;39:281–289. doi: 10.1111/j.1600-0714.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- Flohr TR, Bonatti HJ, Brayman KL, Pruett TL. The use of stem cells in liver disease. Curr Opin Organ Transplant. 2009;14:64–71. doi: 10.1097/MOT.0b013e328320fd7b. [DOI] [PubMed] [Google Scholar]
- Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, Prat M, Di Nardo P (2006) Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells 24:23–33. doi:10.1634/stemcells.2004-0176 [DOI] [PubMed]
- Gu JY, Shi XL, Zhang Y, Ding YT. Study on the effects and mechanisms of bone marrow mesenchymal stem cells on porcine primary hepatocyte culture in vitro. Zhonghua Gan Zang Bing Za Zhi. 2009;17:867–871. [PubMed] [Google Scholar]
- Guo-ping W, Xiao-chuan H, Zhi-hui Y, Li G. Influence on the osteogenic activity of the human bone marrow mesenchymal stem cells transfected by liposome-mediated recombinant plasmid pIRES-hBMP2-hVEGF165 in vitro. Ann Plast Surg. 2010;65:80–84. doi: 10.1097/SAP.0b013e3181b4bc5d. [DOI] [PubMed] [Google Scholar]
- Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N, Kawano S (2008) Topical transplantation of mesenchymal stem cells accelerates gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol 294:G778–G786. doi:10.1152/ajpgi.00468.2007 [DOI] [PubMed]
- Hoffmann J, Glassford AJ, Doyle TC, Robbins RC, Schrepfer S, Pelletier MP. Angiogenic effects despite limited cell survival of bone marrow-derived mesenchymal stem cells under ischemia. Thorac Cardiovasc Surg. 2010;58:136–142. doi: 10.1055/s-0029-1240758. [DOI] [PubMed] [Google Scholar]
- Hong H, Chen JZ, Zhou F, Xue L, Zhao GQ. Influence of serum from liver-damaged rats on differentiation tendency of bone marrow-derived stem cells. World J Gastroenterol. 2004;10:2250–2253. doi: 10.3748/wjg.v10.i15.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Mao Z, Wei X, Lin L, Chen L, Wang H, Fu X, Zhang J, Yu C (2009) Effects of transforming growth factor-beta1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix Biol 28:324–35. doi:http://dx.doi.org/10.1016/j.matbio.2009.04.007 [DOI] [PubMed]
- Ishiki Y, Ohnishi H, Muto Y, Matsumoto K, Nakamura T. Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo. Hepatology. 1992;16:1227–1235. [PubMed] [Google Scholar]
- Kamiya A, Kinoshita T, Ito Y, Matsui T, Morikawa Y, Senba E, Nakashima K, Taga T, Yoshida K, Kishimoto T, Miyajima A (1999) Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J 18:2127–2136. doi:10.1093/emboj/18.8.2127 [DOI] [PMC free article] [PubMed]
- Kanczler JM, Ginty PJ, White L, Clarke NM, Howdle SM, Shakesheff KM, Oreffo RO (2010) The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials 31:1242–1250. doi:http://dx.doi.org/10.1016/j.biomaterials.2009.10.059 [DOI] [PubMed]
- Kazemnejad S, Allameh A, Gharehbaghian A, Soleimani M, Amirizadeh N, Jazayeri M. Efficient replacing of fetal bovine serum with human platelet releasate during propagation and differentiation of human bone-marrow-derived mesenchymal stem cells to functional hepatocyte-like cells. Vox Sang. 2008;95:149–158. doi: 10.1111/j.1423-0410.2008.01075.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Miyajima A (2002) Cytokine regulation of liver development. Biochim Biophys Acta 1592:303–312. doi:http://dx.doi.org/10.1016/S0167-4889(02)00323-3 [DOI] [PubMed]
- Kyrmizi I, Hatzis P, Katrakili N, Tronche F, Gonzalez FJ, Talianidis I. Plasticity and expanding complexity of the hepatic transcription factor network during liver development. Genes Dev. 2006;20:2293–2305. doi: 10.1101/gad.390906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, Fiegel HC (2005) Hepatocytic gene expression in cultured rat mesenchymal stem cells. Transplant Proc 37:276–279. doi:http://dx.doi.org/10.1016/j.transproceed.2004.11.087 [DOI] [PubMed]
- Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- Li TZ, Kim JH, Cho HH, Lee HS, Kim KS, Lee SW, Suh H. Therapeutic potential of bone-marrow-derived mesenchymal stem cells differentiated with growth-factor-free coculture method in liver-injured rats. Tissue Eng Part A. 2010;16:2649–2659. doi: 10.1089/ten.tea.2009.0814. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhao SH, Lu MJ, Jiang SL, Yan CW, Zhang Y, Meng L, Tang Y, Meng XM, Wei YJ, Wang LL, Dai HJ, Xu J (2009) Gelatin microspheres containing vascular endothelial growth factor enhances the efficacy of bone marrow mesenchymal stem cells transplantation in a swine model of myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi 37:233–239 [PubMed]
- Lysy PA, Campard D, Smets F, Najimi M, Sokal EM. Stem cells for liver tissue repair: current knowledge and perspectives. World J Gastroenterol. 2008;14:864–875. doi: 10.3748/wjg.14.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances M. Liver regeneration. Adv Biochem Eng Biotechnol. 2005;93:101–134. doi: 10.1007/b99968. [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Kamohara Y, Hui T, Neuman T, Mitaka T, Demetriou AA, Rozga J (2001) Regulation of c-met expression in rats with acute hepatic failure. J Surg Res 99:385–396. doi:http://dx.doi.org/10.1006/jsre.2001.6216 [DOI] [PubMed]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Park J, Ries J, Gelse K, Kloss F, Mark K, Wiltfang J, Neukam FW, Schneider H. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10:1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- Piryaei A, Valojerdi MR, Shahsavani M, Baharvand H. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev. 2011;7:103–118. doi: 10.1007/s12015-010-9126-5. [DOI] [PubMed] [Google Scholar]
- Pons J, Huang Y, Takagawa J, Arakawa-Hoyt J, Ye J, Grossman W, Kan YW, Su H. Combining angiogenic gene and stem cell therapies for myocardial infarction. J Gene Med. 2009;11:743–753. doi: 10.1002/jgm.1362. [DOI] [PubMed] [Google Scholar]
- Pulavendran S, Rose C, Mandal AB. Hepatocyte growth factor incorporated chitosan nanoparticles augment the differentiation of stem cell into hepatocytes for the recovery of liver cirrhosis in mice. J Nanobiotechnol. 2011;9:15. doi: 10.1186/1477-3155-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Mitaka T, Mizuguchi T, Mochizuki Y, Hirata K. Effects of nicotinamide-related agents on the growth of primary rat hepatocytes and formation of small hepatocyte colonies. Liver. 1999;19:481–488. doi: 10.1111/j.1478-3231.1999.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Shafritz DA, Oertel M, Menthena A, Nierhoff D, Dabeva MD. Liver stem cells and prospects for liver reconstitution by transplanted cells. Hepatology. 2006;43:S89–S98. doi: 10.1002/hep.21047. [DOI] [PubMed] [Google Scholar]
- Shi XL, Qiu YD, Wu XY, Xie T, Zhu ZH, Chen LL, Li L, Ding YT (2005) In vitro differentiation of mouse bone marrow mononuclear cells into hepatocyte-like cells. Hepatol Res 31:223–231. doi:http://dx.doi.org/10.1016/j.hepres.2005.01.011 [DOI] [PubMed]
- Shi XL, Gu JY, Zhang Y, Han B, Xiao JQ, Yuan XW, Zhang N, Ding YT. Protective effects of ACLF sera on metabolic functions and proliferation of hepatocytes co-cultured with bone marrow MSCs in vitro. World J Gastroenterol. 2011;17:2397–2406. doi: 10.3748/wjg.v17.i19.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojiri N. Enzymo- and immunocytochemical analyses of the differentiation of liver cells in the prenatal mouse. J Embryol Exp Morphol. 1981;62:139–152. [PubMed] [Google Scholar]
- Shiota G, Kunisada T, Oyama K, Udagawa A, Nomi T, Tanaka K, Tsutsumi A, Isono M, Nakamura T, Hamada H, Sakatani T, Sell S, Sato K, Ito H, Kawasaki H (2000) In vivo transfer of hepatocyte growth factor gene accelerates proliferation of hepatic oval cells in a 2-acetylaminofluorene/partial hepatectomy model in rats. FEBS Lett 470:325–330. doi:http://dx.doi.org/10.1016/S0014-5793(00)01337-5 [DOI] [PubMed]
- Skarpen E, Oksvold MP, Grosvik H, Widnes C, Huitfeldt HS. Altered regulation of EGF receptor signaling following a partial hepatectomy. J Cell Physiol. 2005;202:707–716. doi: 10.1002/jcp.20171. [DOI] [PubMed] [Google Scholar]
- Snykers S, Kock J, Rogiers V, Vanhaecke T. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells. 2009;27:577–605. doi: 10.1634/stemcells.2008-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snykers S, Kock J, Tamara V, Rogiers V. Hepatic differentiation of mesenchymal stem cells: in vitro strategies. Methods Mol Biol. 2011;698:305–314. doi: 10.1007/978-1-60761-999-4_23. [DOI] [PubMed] [Google Scholar]
- Stock P, Bruckner S, Ebensing S, Hempel M, Dollinger MM, Christ B. The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nat Protoc. 2010;5:617–627. doi: 10.1038/nprot.2010.7. [DOI] [PubMed] [Google Scholar]
- Supp DM, Boyce ST. Overexpression of vascular endothelial growth factor accelerates early vascularization and improves healing of genetically modified cultured skin substitutes. J Burn Care Rehabil. 2002;23:10–20. doi: 10.1097/00004630-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Talens-Visconti R, Bonora A, Jover R, Mirabet V, Carbonell F, Castell JV, Gomez-Lechon MJ. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006;12:5834–5845. doi: 10.3748/wjg.v12.i36.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talens-Visconti R, Bonora A, Jover R, Mirabet V, Carbonell F, Castell JV, Gomez-Lechon MJ (2007) Human mesenchymal stem cells from adipose tissue: differentiation into hepatic lineage. Toxicol In Vitro 21:324–329. doi:http://dx.doi.org/10.1016/j.tiv.2006.08.009 [DOI] [PubMed]
- Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:686–695. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- Tao ZW, Li LG, Geng ZH, Song MB, Zheng JR, Yu SY, Dang T, Kang HL, Zhu SJ. Autologous mesenchymal stem cell implantation promotes myocardial expressions of growth factors and improves cardiac function in failing rat hearts. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37:495–500. [PubMed] [Google Scholar]
- Tarnowski M, Szydlo A, Aniol J, Koryciak-Komarska H, Lesiak M, Gutmajster E, Sieron AL, Kusz D. Optimization of genetic engineering and homologous recombination of collagen type I genes in rat bone marrow mesenchymal stem cells (MSC) Cell Reprogram. 2010;12:275–282. doi: 10.1089/cell.2009.0084. [DOI] [PubMed] [Google Scholar]
- Theise ND. Gastrointestinal stem cells. III. Emergent themes of liver stem cell biology: niche, quiescence, self-renewal, and plasticity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G189–G193. doi: 10.1152/ajpgi.00041.2005. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Wada T, Yamashita T, Hamada H. Enhanced osteoinduction by mesenchymal stem cells transfected with a fiber-mutant adenoviral BMP2 gene. J Gene Med. 2005;7:1322–1334. doi: 10.1002/jgm.777. [DOI] [PubMed] [Google Scholar]
- Wang PP, Wang JH, Yan ZP, Hu MY, Lau GK, Fan ST, Luk JM (2004) Expression of hepatocyte-like phenotypes in bone marrow stromal cells after HGF induction. Biochem Biophys Res Commun 320:712–716. doi:http://dx.doi.org/10.1016/j.bbrc.2004.05.213 [DOI] [PubMed]
- Wang L, Pei GX, Gao LB, Jiang S, Mu TW, Chen SY, Qin JJ, Jin D, Lou AJ, Zhao PR (2010) Tissue engineering vascularized bone repairing segmental femoral bone defects in rabbits. Zhonghua Yi Xue Za Zhi 90:1637–1641 [PubMed]
- Yamamoto Y, Banas A, Murata S, Ishikawa M, Lim CR, Teratani T, Hatada I, Matsubara K, Kato T, Ochiya T (2008) A comparative analysis of the transcriptome and signal pathways in hepatic differentiation of human adipose mesenchymal stem cells. FEBS J 275:1260–1273. doi:10.1111/j.1742-4658.2008.06287.x [DOI] [PubMed]
- Zhao DM, Wang HB, Yang JF, Wu SQ, Liu JL, Xu FY, Qiu LP, Cai JL. Effect of vascular endothelial growth factor 165 gene transfection on bone defects and its mRNA expression in rabbits. Chin Med J (Engl) 2007;120:1187–1191. [PubMed] [Google Scholar]
- Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu Y, Zhang Z. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy. 2010;12:605–614. doi: 10.3109/14653241003745888. [DOI] [PubMed] [Google Scholar]
- Zisa D, Shabbir A, Suzuki G, Lee T (2009) Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun 390:834–838. doi:http://dx.doi.org/10.1016/j.bbrc.2009.10.058 [DOI] [PMC free article] [PubMed]