Abstract
A common dietary contaminant, aflatoxin B1 (AFB1), has been shown to be a potent mutagen and carcinogen in humans and many animal species. Since the eradication of AFB1 contamination in agricultural products has been rare, the use of natural or synthetic free radical scavengers could be a potential chemopreventive strategy. Boron compounds like borax (BX) and boric acid are the major components of industry and their antioxidant role has recently been reported. In the present report, we evaluated the capability of BX to inhibit the rate of micronucleus (MN) and sister chromatid exchange (SCE) formations induced by AFB1. There were significant increases (P < 0.05) in both SCE and MN frequencies of cultures treated with AFB1 (3.12 ppm) as compared to controls. However, co-application of BX (1, 2 and 5 ppm) and AFB1 resulted in decreases of SCE and MN rates as compared to the group treated with AFB1 alone. Borax gave 30–50 % protection against AFB1 induced SCEs and MNs. In conclusion, the support of borax was especially useful in aflatoxin-toxicated blood tissue. Thus, the risk on target tissues of AFB1 could be reduced and ensured early recovery from its toxicity.
Keywords: Aflatoxin B1, Borax, Chemoprevention, Genotoxicity, Human blood cultures
Introduction
Aflatoxins are contaminants of improperly stored foods. They do not only contaminate our food stuffs but are also found in edible tissues, milk and eggs after consumption of contaminated feed by farm animals (Bennett and Klich 2003). Among various aflatoxins, aflatoxin B1 (AFB1) is the most potent carcinogen ever tested (Umarani et al. 2008). AFB1 is produced by some strains of Aspergillus and ingestion of aflatoxin-contaminated food and feed is known to cause hepatotoxicity, teratogenicity, immunotoxicity and even death in farm animals and humans (Guindon et al. 2007). The mechanism of cellular damage caused by AFB1 has not been fully elucidated but reactive oxygen species (ROS) and lipid peroxidation (LPO) have been considered to be main mechanisms in the toxicity of AFB1 (Rastogi et al. 2001; Shon et al. 2004). Finally, AFB1 causes micronucleus (MN), sister chromatid exchanges (SCEs), unscheduled DNA synthesis, and chromosomal strand breaks as well as forms adducts in rodent and human cells (Groopman and Kensler 1999). In 2005, Geyikoglu and Turkez reported similar findings in human lymphocytes in an in vitro model for the first time. They have suggested that the blood is a valuable tissue for monitoring the genotoxicity of AFB1. On the other hand, boron is a ubiquitous constituent of man’s external environment and it is essential for the growth of many plants. Boron deficiency and supplementation exert measurable biological effects in human and animal tissues (in osteoporosis, arthritis, plasma lipid profiles and brain function) (Devirian and Volpe 2003). Boric acid (BA) and Borax (BX) are able to strengthen the tissue antioxidant defenses (Hunt and Idso 1999; Pawa and Ali 2006). Boron compounds also show no potential for genotoxicity in cultured mammalian cells (Turkez et al. 2007, 2010a; Geyikoglu and Turkez 2008; Turkez 2008).
So far, antioxidants have attracted much interest with respect to their protective effect against free radical damage that may be the cause of many diseases including cancer (Shon et al. 2004). Since the complete avoidance of exposure to AFB1-producing mould is very difficult, chemoprevention is an attractive strategy for protecting humans and animals from the risk of cancer caused by exposure to this mycotoxin. Thus, this study investigated the efficacy of BX against AFB1-induced DNA damages. The SCE and MN tests covering a wide range of induced genetic damage as primary DNA damage were performed on peripheral lymphocytes.
Materials and methods
Experimental design
Blood samples were obtained by vein puncture from three healthy non-smoking donors. AFB1 (C17H12O6, CAS No. 1162-65-8, 98 % purity, Sigma Chemical Co., St Louis, MO. USA) (in concentration of 3.12 ppm) and BX (Na2B4O7:10H2O, CAS No. 1303-96-4, Eti Mine Works General Management, Turkey) (in concentrations of 1, 2 and 5 ppm) were dissolved in distilled water. These compounds were added to the cultures just before incubation for cytogenetic analysis. Experiments conformed to the guidelines of the World Medical Assembly (Declaration of Helsinki). These investigations are based on the previous works (Yamashoji and Isshiki 2001; Geyikoglu and Turkez 2005; Turkez et al. 2007). After supplementation of BX and AFB1, the blood was incubated for 72 h at 37 °C to adjust body conditions for testing SCE and MN. Each individual whole blood culture without BX or AFB1 was studied as a control group.
Cytogenetic tests
Cultures were set up according to a slight modification of the protocol described by Evans and O’Riordan (1975). A 0.5 mL aliquot of heparinized blood was cultured in 6 mL of culture medium (Chromosome Medium B, Biochrom, Berlin, Germany) with 5 μg/mL of phytohemagglutinin (Biochrom). Above-mentioned doses of tested compounds were added into the culture tubes. With the aim of providing successive visualization of SCEs, 5-bromo-2′-deoxyuridine (Sigma) was added at culture initiation. The cultures were incubated in complete darkness for 72 h at 37 °C. Exactly 70 h and 30 min after beginning the incubations, demecolcine (N-Diacetyl-N-methylcolchicine, Sigma) was added to the cultures. After hypotonic treatment (0.075 M KCl), followed by three repetitive cycles of fixation in methanol/acetic acid solution (3:1, v/v), centrifugation, and resuspension, the cell suspension was dropped onto chilled, grease-free microscopic slides, air-dried, aged for 3 days, and then differentially stained for the inspection of the SCE rate according to fluorescence plus Giemsa procedure (Perry and Wolff 1974). For each treatment condition, well-spread twenty five second division metaphases containing 42–46 chromosomes in each cell were scored by one observer (F. Geyikoglu), and the values obtained were calculated as SCEs per cell.
The MN test was performed by adding cytochalasin B (Sigma) after 44 h of culture. At the end of the 72 h incubation period, the lymphocytes were fixed with ice-cold methanol/acetic acid (1:1, v/v). The fixed cells were put directly on slides using a cytospin, and stained with Giemsa solution. All slides were coded before scoring. The criteria for scoring MN were as described by Fenech (1993). At least 1,000 binucleated lymphocytes were examined per concentration for the presence of one, two or more MN.
Statistical analysis
The experimental data were analyzed using one-way analysis of variance (ANOVA) and Duncan’s tests to determine whether any treatment significantly differed from the controls or each others. Results are presented as mean ± SD values and the P-levels of 0.05 was regarded as statistically significant.
Results
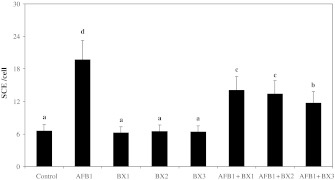
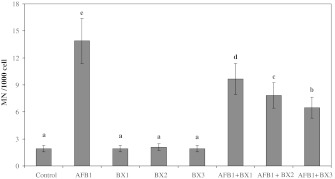
Figures 1 and 2 show the results of SCE and MN analyzed in human peripheral lymphocytes treated for 72 h with different concentrations of AFB1 in the presence or absence of BX. The SCE and MN frequencies observed in cultures treated with AFB1 (3.12 ppm) were significantly higher than control values. However, BX did not lead to genetic damages at all tested concentrations. Moreover, it was successful against AFB1-induced genotoxicity in blood cells. When 3.12 ppm AFB1 was treated with BX, SCEs/cell and MN/1,000 cell values got significantly decreased as compared to AFB1 treated alone. The results also showed that borax did not completely inhibit induction of SCE and MN formation by AFB1.
Fig. 1.
Sister chromatid exchange frequencies after the treatment of human lymphocytes with aflatoxin B1 and borax. Values are expressed as mean for three cultures in each group; means in the figure followed by different letters present significant differences at the P < 0.05 level; AFB1: 3.12 ppm aflatoxin B1; BX1: 1 ppm borax; BX2: 2 ppm borax, BX3: 5 ppm borax
Fig. 2.
The micronucleus rates after the treatment of human lymphocytes with aflatoxin B1 and borax. Abbreviations are as in Fig. 1
Discussion
The results obtained by us indicate a significant increase in the ratios of the SCEs and MNs by AFB1 in lymphocytes, which is in accordance with the previous reports (Groopman and Kensler 1999; Geyikoglu and Turkez 2005; Turkez and Geyikoglu 2010). The SCEs are formed by toxic oxygen metabolites in cultured human leukocytes and other mammalian cells (Weitberg et al. 1983). AFB1 toxicity is also related to LPO and oxidation of DNA in vivo and in vitro (Shen et al. 1996). So this xenobiotic could contribute to the modification of the genome leading to carcinogenesis (Amici et al. 2007). Lipid peroxides enter the nucleus where they react with Fe+2 to generate the alkoxyl radical which attacks DNA (Fraga and Tappel 1988). Also, intracellular calcium levels increase as a result of oxidative damage of cell membranes, calcium then enters the nucleus where it can activate nucleases which cause DNA strand breaks (McConkey et al. 1989). DNA damages and defective DNA repairs were reported to cause SCEs (Bozkurt et al. 2003). On the other hand, MNs are the results of acentric fragments or lagging chromosomes that fail to incorporate into either of the daughter nuclei during telophase of mitotic cells (Albertini et al. 2000). It was established that BX alone was non-genotoxic. In accordance to this finding, previous reports indicated that boron compounds had no mutagenic potential (Turkez et al. 2007, 2010a, b, 2011, 2012; Turkez 2008; Geyikoglu and Turkez 2008; Turkez and Geyikoglu 2010).
In the current study, red blood cells (RBCs) were present in the incubation medium. It is known that glutathione (GSH) is a major component of RBCs that plays a central role in the antioxidant defenses of cells (Meister 1983). Moreover, GSH is a cofactor of the enzyme glutathione peroxidase (GSH-Px) (Leopold 1976). Antioxidant enzymes, such as GSH-Px, have important roles in scavenging free radicals, and prevent their interactions with cellular DNA (Ferguson et al. 2004). However, depletion of GSH leads to oxidative stress. The decrease in cellular glutathione content can affect signaling pathways that participate in various physiological responses from cell proliferation to gene expression (Rahman et al. 2002). Thus, it is possible that AFB1-induced oxidative stress acted as an intermediate for the observed genetic damage. On the contrary, it has been shown that pretreatment with boron (as borax) could replenish the depleted level of GSH, with a concomitant decrease in LPO (Pawa and Ali 2006). This finding corroborates the recently reported evidence that boron itself is not an antioxidant, but is thought to strengthen the antioxidant defense system of the tissue (Hunt and Idso 1999).
Our study also revealed that the dramatic reduction of AFB1-induced SCEs and MN formations were due to the protective roles of borax. It is known that AFB1 has pro-oxidant effects (Geyikoglu and Turkez 2005; Adam and Malgorzata 2008; Turkez and Sisman 2007; Turkez et al. 2010b). Treatment with BX could cause reductions in the activity levels of some pro-oxidant enzymes consequently ameliorating the tissue damage (Pawa and Ali 2006). There is no evidence on induction of activities of enzymes present in the blood by boron compounds in this investigation. However, this compound could strengthen the antioxidant defense mechanism against AFB1. All pro-oxidant factors lead to antioxidant depletion (Hedenborg 1988), thus, chemical-induced genotoxicity occurs, including oxidation of purine nucleotides of nucleic acids and that of SH groups of proteins (Hooiveld et al. 1998; Xi et al. 2004). In conclusion, defective DNA repair and DNA damage form SCEs (Bozkurt et al. 2003). As a matter of fact, Pawa and Ali (2006) reported that the formation of malondialdehyde (MDA) was ameliorated by borax in thioacetamide (a toxic chemical substance) treated rats. They suggested that the protective effect of boron (as borax) might also be due to the inhibition of free radical formation.
The protective effects could be important cytogenetic characteristics of borax, yet how these activities relate to the anti-mutagenic properties of this agent is difficult to understand. In conclusion, this study firstly reveals that borax provides an important protection against AFB1-induced genetic damages in blood tissue. In the light of the findings obtained in the present study, it is suggested that foods with boron supplements could protect blood tissue against mycotoxin-induced DNA damage. The present results also suggest potential new directions for further study on the biological effects of boron compounds.
Acknowledgments
We are grateful to volunteers for the blood samples. The author is grateful to Dr. Savaş Yeşilyurt for his proofreading the article.
References
- Adam G, Malgorzata KL. Protective effects of melatonin and N-acetylserotonin on aflatoxin B1-induced lipid peroxidation in rats. Cell Biochem Funct. 2008;26:314–319. doi: 10.1002/cbf.1438. [DOI] [PubMed] [Google Scholar]
- Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DE, Tice R, Waters MD, Aitio A. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res. 2000;463:111–172. doi: 10.1016/S1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Amici M, Cecarini V, Pettinari A, Bonfili L, Angeletti M, Barocci S, Biagetti M, Fioretti E, Maria EA. Binding of aflatoxins to the 20S proteasome: effects on enzyme functionality and implications for oxidative stress and apoptosis. Biol Chem. 2007;388:107–117. doi: 10.1515/BC.2007.012. [DOI] [PubMed] [Google Scholar]
- Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt G, Yuksel M, Karabogaz G, Sut N, Savran FO, Palanduz S, Yigitbasi ON, Algunes C. Sister chromatid exchanges in lymphocytes of nuclear medicine physicians. Mutat Res. 2003;535:205–213. doi: 10.1016/S1383-5718(02)00321-2. [DOI] [PubMed] [Google Scholar]
- Devirian TA, Volpe SL. The physiological effects of dietary boron. Crit Rev Food Sci Nutr. 2003;43:219–231. doi: 10.1080/10408690390826491. [DOI] [PubMed] [Google Scholar]
- Evans HJ, O’Riordan ML. Human lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat Res. 1975;31:135–148. doi: 10.1016/0165-1161(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-L. [DOI] [PubMed] [Google Scholar]
- Ferguson LR, Philpott M, Karunasinghe N. Dietary cancer and prevention using antimutagens. Toxicology. 2004;198:147–159. doi: 10.1016/j.tox.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Fraga CG, Tappel AL. Damage to DNA concurrent with lipid peroxidation in rat liver slices. Biochem J. 1988;252:893–896. doi: 10.1042/bj2520893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyikoglu F, Turkez H. Protective effect sodium selenite on genotoxicity to human whole blood cultures induced by aflatoxin B1. Braz Arch Biol Technol. 2005;48:905–910. doi: 10.1590/S1516-89132005000800006. [DOI] [Google Scholar]
- Geyikoglu F, Turkez H. Boron compounds reduce vanadium tetraoxide genotoxicity in human lymphocytes. Environ Toxicol Pharmacol. 2008;26:342–347. doi: 10.1016/j.etap.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Groopman JD, Kensler TW. The light at the end of the tunnel for chemical- specific biomarkers: daylight or headlight? Carcinogenesis. 1999;20:1–11. doi: 10.1093/carcin/20.1.1. [DOI] [PubMed] [Google Scholar]
- Guindon KA, Bedard LL, Massey TE. Elevation of 8-hydroxydeoxyguanosine in DNA from isolated mouse lung cells following in vivo treatment with aflatoxin b1. Toxicol Sci. 2007;98:57–62. doi: 10.1093/toxsci/kfm073. [DOI] [PubMed] [Google Scholar]
- Hedenborg M. Titanium dioxide induced chemiluminescence of human polymorphonuclear leukocytes. Int Arch Occup Environ Health. 1988;61:1–6. doi: 10.1007/BF00381600. [DOI] [PubMed] [Google Scholar]
- Hooiveld M, Heederick DJJ, Kogevinas M, Boffetta P, Needham LL, Patterson DG, Bueno-de-Mesquita HB., Jr Second follow-up of a Dutch cohort occupationally exposed to phenoxy herbicides, chlorophenols, and contaminants. Am J Epidemiol. 1998;147:891–901. doi: 10.1093/oxfordjournals.aje.a009543. [DOI] [PubMed] [Google Scholar]
- Hunt CD, Idso JP. Dietary boron as a physiological regulator of the normal inflammatory response: a review and current research progress. J Trace Elem Exp Med. 1999;12:221–233. doi: 10.1002/(SICI)1520-670X(1999)12:3<221::AID-JTRA6>3.0.CO;2-X. [DOI] [Google Scholar]
- Leopold F. Glutathione peroxidase brought into focus. In: Pryor WA, editor. Free radicals in biology. New York: Academic Press; 1976. pp. 223–254. [Google Scholar]
- McConkey DJ, Hartzell P, Jondal M, Orrenius S. Inhibition of DNA fragmentation in thymocytes and isolated thymocyte nuclei by agents that stimulate protein kinase C. J Biol Chem. 1989;264:13399–13402. [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Pawa S, Ali S. Boron ameliorates fulminant hepatic failure by counteracting the changes associated with the oxidative stress. Chem Biol Interact. 2006;160:89–98. doi: 10.1016/j.cbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Perry P, Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Lohani M, Dopp E, Pemsel H, Jonas L, Weiss DG, Schiffmann D. Evidence that titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ Health Perspect. 2002;110:797–800. doi: 10.1289/ehp.02110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi R, Srivastava AK, Rastogi AK. Long term effect of aflatoxin B(1) on lipid peroxidation in rat liver and kidney: effect of picroliv and silymarin. Phytother Res. 2001;15:307–310. doi: 10.1002/ptr.722. [DOI] [PubMed] [Google Scholar]
- Shen HM, Shi CY, Shen Y, Ong CN. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free Radic Biol Med. 1996;21:139–146. doi: 10.1016/0891-5849(96)00019-6. [DOI] [PubMed] [Google Scholar]
- Shon MY, Choi SD, Kahng GG. Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem Toxicol. 2004;42:659–666. doi: 10.1016/j.fct.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Turkez H. Effects of boric acid and borax on titanium dioxide genotoxicity. J Appl Toxicol. 2008;28:658–664. doi: 10.1002/jat.1318. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F. Boric acid: a potential chemoprotective agent against aflatoxin b1 toxicity in human blood. Cytotechnology. 2010;62:157–165. doi: 10.1007/s10616-010-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Sisman T. Anti-genotoxic effect of hydrated sodium calcium aluminosilicate on genotoxicity to human lymphocytes induced by aflatoxin B-1. Toxicol Ind Health. 2007;23:83–89. doi: 10.1177/0748233707076738. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Tatar A, Keleş S, Özkan A. Effects of some boron compounds on peripheral human blood. Z Naturforsch C. 2007;62:889–896. doi: 10.1515/znc-2007-11-1218. [DOI] [PubMed] [Google Scholar]
- Turkez H, Tatar A, Hacimuftuoglu A, Ozdemir E. Boric acid as a protector against paclitaxel genotoxicity. Acta Biochim Pol. 2010;57:95–97. [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Aslan A, Karagöz Y, Türkez O, Anar M. Antimutagenic effects of lichen Pseudovernia furfuracea (L.) Zoph. extracts against the mutagenicity of aflatoxin B(1) in vitro. Toxicol Ind Health. 2010;26:625–631. doi: 10.1177/0748233710377779. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Colak S. The protective effect of boric acid on aluminum-induced hepatotoxicity and genotoxicity in rats. Turk J Biol. 2011;35:293–301. [Google Scholar]
- Turkez H, Geyikoglu F, Tatar A, Keles MS, Kaplan İ. The effects of some boron compounds against heavy metal toxicity in human blood. Exp Toxicol Pathol. 2012;64:93–101. doi: 10.1016/j.etp.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Umarani M, Shanthi P, Sachdanandam P. Protective effect of Kalpaamruthaa in combating the oxidative stress posed by aflatoxin B1-induced hepatocellular carcinoma with special reference to flavonoid structure–activity relationship. Liver Int. 2008;28:200–213. doi: 10.1111/j.1478-3231.2007.01615.x. [DOI] [PubMed] [Google Scholar]
- Weitberg AB, Weitzman SA, Destrempes M, Latt SA, Stossel TP. Stimulated human phagocytes produce cytogenetic changes in cultured mammalian cells. N Engl J Med. 1983;308:26–30. doi: 10.1056/NEJM198301063080107. [DOI] [PubMed] [Google Scholar]
- Xi ZG, Chao FH, Yang DF, Sun YM, Li GX, Zhang HS, Zhang W, Yang YH, Liu HL. The effects of DNA damage induced by acetaldehyde. Huan Jing Ke Xue. 2004;25:102–105. [PubMed] [Google Scholar]
- Yamashoji S, Isshiki K. Rapid detection of cytotoxicity of food additives and contaminants by a novel cytotoxicity test, menadione-catalyzed H2O2 production assay. Cytotechnology. 2001;37:171–178. doi: 10.1023/A:1020580818979. [DOI] [PMC free article] [PubMed] [Google Scholar]