Abstract
In recent years, several automated scale-down bioreactor systems have been developed to increase efficiency in cell culture process development. ambr™ is an automated workstation that provides individual monitoring and control of culture dissolved oxygen and pH in single-use, stirred-tank bioreactors at a working volume of 10–15 mL. To evaluate the ambr™ system, we compared the performance of four recombinant Chinese hamster ovary cell lines in a fed-batch process in parallel ambr™, 2-L bench-top bioreactors, and shake flasks. Cultures in ambr™ matched 2-L bioreactors in controlling the environment (temperature, dissolved oxygen, and pH) and in culture performance (growth, viability, glucose, lactate, Na+, osmolality, titer, and product quality). However, cultures in shake flasks did not show comparable performance to the ambr™ and 2-L bioreactors.
Keywords: Scale-down system, Chinese hamster ovary, Cell culture automation, ambr™, Single-use bioreactor
Introduction
Several scale-down cell culture systems have been developed in recent years to reduce the timelines and costs associated with cell culture process development (Bareither and Pollard 2011; Betts and Baganz 2006; Kondragunta et al. 2010; Kumar et al. 2004; Lewis et al. 2010). Scale-down systems such as shake flasks and bench-top bioreactors are typically used to develop cell culture processes for clinical and commercial manufacturing. Although shake flasks provide higher throughput than bench-top bioreactors, they do not offer the environmental control capabilities of a bench-top bioreactor. The newly developed advanced microscale bioreactor (ambr™) system uses 24 or 48 single-use, pre-sterilized, stirred-tank bioreactors on an automated workstation that enables pH and dissolved oxygen (DO) control for each individual vessel (Bareither and Pollard 2011). In a recent trial run for the ambr™ system, Lewis et al. (2010) observed reproducible growth profiles for a Chinese hamster ovary (CHO) cell line cultured simultaneously in all 24 vessels under identical process conditions. When they cultured three CHO cell lines in fed-batch mode in the ambr™ system and compared the performance to historic data from 7-L bioreactors, they observed comparable viability and antibody production profiles (Lewis et al. 2010).
Here we conducted a more extensive evaluation of the ambr™ system to determine its ability to support cell culture process development. We simultaneously cultured four representative CHO cell lines in the ambr™ system, 2-L bioreactors, and shake flasks, and we assessed (1) online controls (temperature, DO, and pH) provided by the ambr™ and 2-L bioreactor systems; (2) off-line pO2, pH and pCO2 profiles; and (3) culture performance (growth, metabolism, productivity, and product quality) in the three culture systems. We also cultured two additional CHO cell lines in parallel ambr™ and 2-L bioreactors to compare different DO control strategies.
Materials and methods
ambr™ system
The ambr™ system (TAP Biosystems) used in this study consisted of 24 single-use bioreactors that are arranged in two sets of 12. Each set is supplied with a temperature sensor and a heating fan for temperature control, and a stirring plate for agitation control. Each bioreactor is equipped with an agitator with a pitched-blade impeller (11 mm diameter, impeller power number 0.6), a pH sensor spot, and a DO sensor spot. The ambr™ system was programmed to take pH and DO readings every 90 s, and to use these readings to control pH and DO via PID feedback loops. Gases for pH and DO control were mixed prior to entering each bioreactor via a sparge tube. A liquid handler was used to extract culture samples and to perform liquid additions using disposable pipette tips. To maintain aseptic operations, the system was placed inside a biological safety cabinet.
Cell lines
Cell lines 1 and 2 are different clones that secrete recombinant monoclonal antibody A, and were derived from a CHO dihydrofolate reductase-deficient (DHFR-) host as described previously (Guo et al. 2010). Cell lines 3 and 4 are different clones that secrete recombinant monoclonal antibody B, and were derived from a CHO-K1 host that utilizes the glutamine synthetase selection marker. Cell lines 5 and 6 are different clones that secrete recombinant monoclonal antibody C, and were also derived from a CHO-K1 host that utilizes the glutamine synthetase selection marker. To generate these cell lines, the host cells (CHO DHFR- or CHO-K1) were transfected with a DNA plasmid encoding genes for the appropriate selection marker, light chain, and heavy chain. The transfected cells were passaged every 3–4 days in proprietary chemically-defined media (derived from Ham’s F12/Dulbecco’s Modified Eagle’s Medium) containing selective pressure (methotrexate for cell lines 1 and 2, and methionine sulfoximine for cell lines 3, 4, 5, and 6).
2-L bioreactor production cultures
For each cell line tested, two glass stirred-tank bioreactors (Applikon, Foster City, CA) were seeded at ~1.7 × 106 cells/mL in a proprietary, chemically-defined medium. One culture was inoculated at 1.5 L. The other culture was inoculated at 2.0 L, from which 0.5 L was removed to seed the parallel ambr™ and shake flasks cultures after inoculating the 2-L bioreactor. Each identically-configured stirred-tank bioreactors had a maximum working volume of 2 L, a vessel diameter of 0.13 m, and vessel height of 0.25 m. The bioreactor process setpoints were controlled by DCUs (B. Braun Biotech, Allentown, PA). The cultures were maintained with setpoints for pH at 7.0, dissolved oxygen at 30 % of air saturation, and agitation (via single pitched-blade impeller with 45 mm diameter and impeller power number 1.5) at 350 rpm. Culture pH was maintained by addition of 1 M Na2CO3 to increase pH, or by sparging of CO2 gas to decrease pH. At the time of inoculation, each bioreactor culture started with a constant 10 mL/min air sparge, corresponding to 0.0067 vvm of air flow. Separate mass flow controllers were used for air and oxygen, and DO was controlled in cascade mode by sparging with air and then with the required amount of supplemental oxygen. Culture temperature was maintained at 37 °C initially for all cell lines from days 0–3. On day 3, the culture temperature was shifted to 35 °C for cell lines 1 and 2, and to 33 °C for cell lines 3 and 4. On day 3, a single nutrient feed consisting of a proprietary blend of chemically-defined ingredients was added to all cultures, increasing the culture volumes by 20 %. When glucose dropped below 4 g/L, the culture received a concentrated glucose feed consisting of a 500 g/L glucose stock solution to increase culture glucose by 12 g/L.
ambr™ production cultures
The culture material taken from the newly inoculated 2-L bioreactors for each cell line was used to seed six ambr™ vessels at 14 mL working volume. The ambr™ system, with vessel dimensions of 28 mm × 16 mm × 58 mm, maintained the culture temperature at 37 °C, agitation at 1,400 rpm, and DO at 30 % of air saturation. The air sparge rate was set at a constant 0.15 mL/min. Oxygen was sparged as needed to maintain the DO at setpoint. When pH dropped below 6.97, 0.5 M Na2CO3 was added to increase pH to the target of 7.0. When pH rose above 7.05, sparging of CO2 was triggered to decrease pH. Over the course of the 14-day fed-batch process, ambr™ cultures received the same temperature shift, nutrient feed, and glucose feeds as the parallel 2-L bioreactors for each cell line. On days 0, 2, 3, 5, and 7, 60 μL of antifoam (Dow Corning) was added to each ambr™ culture. From each replicate, 200–350 μL was taken for each cell count, and 900 μL was taken for each off-line pH, pCO2, and metabolite analysis. For titer and product quality analyses, a 1.4-mL sample was taken from three of the six replicates for each cell line on day 10, and a 1.4-mL sample was taken from the other three replicates on day 12.
Shake flask production cultures
The culture material taken from the newly inoculated 2-L bioreactors for each cell line was also used to seed two 125-mL shake flasks at 40 mL each. The shake flasks were agitated on an orbital shaker (150 rpm with 25 mm throw), and maintained in a humidified incubator at 37 °C with 5 % CO2 overlay. Over the course of the 14-day process, shake flask cultures received the same temperature shift, nutrient feed, and glucose feed criteria as the parallel 2-L bioreactors and ambr™ vesssels for each cell line.
Off-line sample analyses
Samples were analyzed for viable cell concentration (VCC) and viability using the Vi-Cell XR (Beckman Coulter), and for pO2, pH, pCO2, Na+, glucose, and lactate using the Bioprofile 400 (Nova Biomedical). All samples from 2-L and ambr™ bioreactors were analyzed on BioProfile 400 within a few minutes after sampling to minimize off-gassing. The same Vi-Cell XR, BioProfile 400, and osmometer (Model 2020, Advanced Instruments) were used for all samples to eliminate instrument-to-instrument variability. Antibody titer was measured using high-pressure liquid chromatography (HPLC) with a protein A column. Antibody product quality assays were conducted using cell culture supernatant samples purified by PhyTip protein A column. Antibody glycan distribution was analyzed by capillary electrophoresis (CE) with fluorescence detection (Gennaro and Salas-Solano 2008) while molecular size distribution was analyzed by size-exclusion chromatography (SEC). Protein charge heterogeneity was measured using imaged capillary isoelectric focusing (icIEF); all charge heterogeneity samples were pre-treated with carboxypeptidase B. All protein product quality assays were developed in-house, and detailed protocols have been published (Hopp et al. 2009). Amino acid concentrations in the cultures at time of harvest were quantified by ultra-performance liquid chromatography (Waters AcQuity UPLC, Waters, Milford, MA) after the free amino acids in the cell culture supernatant samples were derivatized with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) to generate highly-fluorescent derivatives (Cohen and Michaud 1993).
Calculations
The growth rate between any two time points was calculated by taking the natural log of the ratio of VCC at the later time point to the VCC at the earlier time point, and dividing this number by the time interval. The average cell-specific productivity, qp, was calculated as the final antibody titer divided by the integral of VCC over the culture duration. The tip speed of impellers was calculated as πDiN, where Di represents the impeller diameter, and N represents the agitation rate. The impeller power was calculated as PNoN3D5iρ, where PNo represents the impeller power number, N represents the agitation rate, Di represents the impeller diameter, and ρ represents the liquid density (which is assumed to be 1 g/mL).
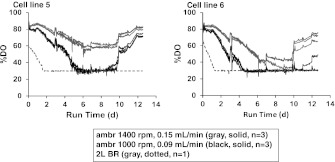
Experiment testing agitation rate and air flow rate using cell lines 5 and 6
In this experiment, the 2-L bioreactors were operated as described earlier in the “2-L bioreactor production cultures” section. The process setpoints were controlled by TruBio (Finesse Solutions, San Jose, CA). Culture temperature for both cell lines 5 and 6 was shifted on day 3 to 33 °C. The culture material taken from the newly inoculated 2-L bioreactors for each cell line was used to seed six ambr™ vessels at the 14 mL working volume. The ambr™ system maintained the agitation rate at 1,400 rpm and the air flow rate at a constant 0.15 mL/min for 3 replicates. For the other 3 replicates, the ambr™ system maintained the agitation rate at 1,000 rpm and the air flow rate at a constant 0.09 mL/min. All other conditions for the ambr™ cultures were as described earlier in the “ambr™ production cultures” section.
Results and discussion
ambr™ system: evaluation of environmental controls
Temperature
For the first three culture days, both 12-vessel blocks on the ambr™ system maintained temperature within ±0.1 °C of the 37.0 °C setpoint. Upon shifting to a lower temperature setpoint on day 3, the temperature stabilized after 2 h. Thereafter, the first block maintained temperature within ±0.1 °C of the 35.0 °C setpoint, while the second block maintained temperature between 33.18 and 33.22 °C, slightly higher than the 33.0 °C setpoint. The precision of temperature control and the time to stabilize at setpoint will be affected by environmental temperature because the ambr™ system controls temperature for each 12-vessel block by heating the air within the block, or by drawing in ambient air (for cooling).
DO
The ambr™ system generated comparable DO profiles amongst the replicates, and maintained DO at or above 30 % of air saturation throughout the run for all vessels (Fig. 1a). When DO dipped below the setpoint, supplemental oxygen kicked in to maintain DO within 5 % of the setpoint. The agitation rate for the 2-L bioreactors was chosen based on our experience with in-house CHO cell lines: it provides sufficient kLa to meet the oxygen requirements while avoiding cell damage. In selecting agitation rates for the ambr™ to scale from the 2-L bioreactors, we applied the same considerations towards oxygen transfer and shear sensitivity. Typical scale-up/down strategies include matching the power input per volume (P/V) or the maximum shear (as represented by impeller tip speed) across scales (Chisti 1993; Varley and Birch 1999). Based on the power numbers and the dimensions of the impellers, an agitation rate of 1,000 rpm in ambr™ would generate equivalent P/V as the 2-L bioreactors at the typical working volume, while an agitation rate of 1,400 rpm in ambr™ would generate equivalent tip speed as the 2-L bioreactors. For this first experiment, we chose 1,400 rpm to maximize oxygen transfer, to avoid vortex and bubble entrainment, and to ensure that the maximum amount of shear would be comparable to the 2-L bioreactor. At this selected condition for the ambr™ cultures, DO remained above 30 % of air saturation for the first 3–5 days of culture as well as towards the end of the culture. In the 2-L bioreactors, DO dropped to 30 % of air saturation within an hour of inoculation. However, we did not adjust the sparge strategy during this experiment when DO exceeded 30 % because CHO cell cultures are typically insensitive to DO at levels between 10 and 80 % of air saturation (Restelli et al. 2006; Trummer et al. 2006). In a subsequent experiment, by lowering the agitation and air flow rates for the ambr™ system, we observed an improved match in the DO profiles between the ambr™ and 2-L bioreactor cultures (Fig. 2). The culture performance was similar between the different agitation and aeration cases within the range studied (data not shown).
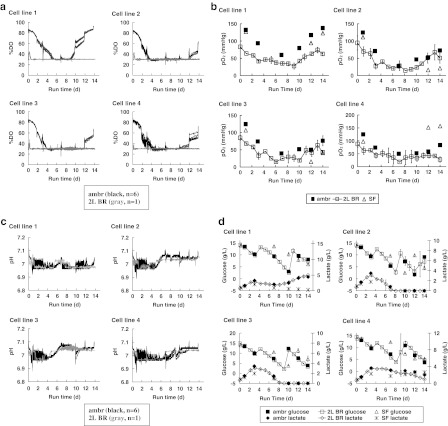
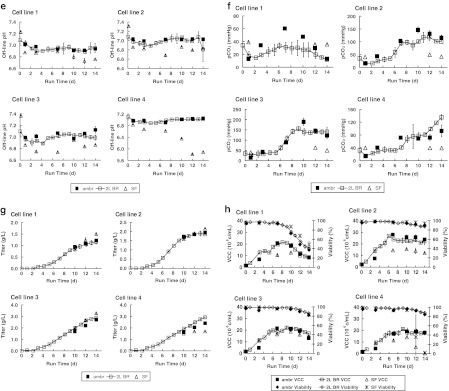
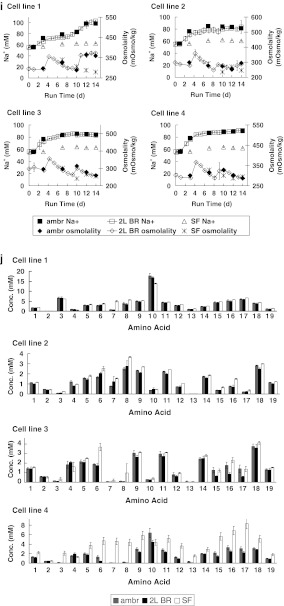
Fig. 1.
Profiles of fed-batch cultures in parallel ambr™ vessels (ambr; n = 6), 2-L bioreactors (2L BR; n = 2 except where denoted), and shake flasks (SF; n = 2 except where denoted) for four cell lines: a online dissolved oxygen, b off-line pO2 (SF n = 1), c online pH, d glucose and lactate, e off-line pH, f off-line pCO2 (SF n = 1), g antibody titer, h VCC and viability, i Na+ and osmolality, and j amino acids at harvest. Error bars represent ±1 SD
Fig. 2.
Online DO profiles of fed-batch cultures in parallel 2-L bioreactors (2L BR) and ambr™ vessels (ambr) with different agitation rates and air flow rates
The spikes in the DO profiles before DO dropped to setpoint corresponded to antifoam and batch feed additions. The spikes in the DO profiles after triggering oxygen supplementation corresponded to bioreactor sampling. Liquid additions and sampling are expected to cause DO spikes because they disturb the headspace and alter the working volume. The time for the DO traces to equilibrate to setpoint after such manipulations would depend on the controller setup, the cell metabolism and its associated sparge requirement. The slight difference in DO amongst the replicates of cell line 1 and cell line 4 from day 10 to day 12 (Fig. 1a) is attributed to volume differences: three out of six replicates were sampled on day 10 for titer and product quality. These three replicates had lower volume and therefore higher DO levels under constant air flow compared to the other replicates. After sampling the second set of three replicates on day 12 for titer and product quality, all replicates achieved equal working volumes, and the resulting DO profiles between vessels were comparable. Cell lines 2 and 3 also had the same volume difference amongst replicates on days 10 and 12. However, the volume difference did not result in different DO levels because these cell lines consumed more oxygen and required oxygen supplementation to maintain DO at setpoint.
Off-line pO2 measurements indicate that there was no oxygen limitation in all three vessel types (Fig. 1b). The off-line pO2 profile for the ambr™ cultures trended with the online DO profile (Fig. 1a). If DO in all the cultures was maintained at 30 % of air saturation throughout the off-line sampling process, we would expect the off-line pO2 readings to approximate 50 mmHg. We attribute the off-line pO2 readings that were lower than 50 mmHg to the drop in DO between the time of culture sampling and the time of assay by the Bioprofile 400 for high cell density cultures. Using oxygen uptake rates of 0.6–1.5 × 10−13 mol O2 cell−1 h−1 measured for CHO cells (Keane et al. 2003; Lin et al. 1993), pO2 levels would decrease at 15–40 mmHg per minute for non-agitated culture samples at high cell densities (~20 × 106 cells/mL). In this experiment, the sampling-to-assay time ranged from one minute for ambr™ cultures to several minutes for 2-L bioreactor and shake flask cultures. Therefore, the off-line pO2 readings may provide DO trends over time in the cultures, but they would not accurately reflect actual DO levels in the cultures.
pH
We configured the pH control strategy in the ambr™ system to maintain pH around 7.0 and to match that of the 2-L bioreactor. The ambr™ replicates showed similar online pH profiles, with pH readings between 6.96 and 7.07 (Fig. 1c). All four cell lines required base addition approximately every 1–2 h during the first 4 days of culture. By testing the pH increase per unit volume of base added to our culture media, we empirically determined the volume of base to add upon each base trigger. Taking into consideration the narrow pH range between the base trigger pH (6.97) and target pH (7.0), as well as the minimum volume for liquid addition (10 μL), we selected a diluted base (0.5 M Na2CO3) for pH adjustment. This approach enabled us to limit any pH overshoot during base addition by the ambr™ to ~0.03–0.04 units. Although we could further lower the base concentration to reduce the pH overshoot, this would increase the frequency of base addition. To avoid triggering CO2 sparge from the ~0.03–0.04 overshoot in pH, we set the CO2 trigger at pH 7.05.
All cell lines required CO2 to maintain pH when lactate was consumed (Fig. 1d). The timing of switches between base and CO2 addition was similar in ambr™ and 2-L bioreactors for all four cell lines. For example, in both ambr™ and 2-L bioreactors, cell line 1 required base addition from the initial part of the culture until day 5. Between day 5 and day 7, cultures in both types of bioreactor systems required CO2 addition to maintain pH because of lactate consumption. After day 7, cultures in both bioreactor systems required base because of lactate production. In line with the spikes in the online DO profiles, the spikes in the online pH profiles corresponded to headspace and volume disturbances during additions and sampling.
Based on off-line pH measurements, both the ambr™ and 2-L bioreactor cultures successfully maintained pH around 7.0 (Fig. 1e). The average offset between online and off-line pH values over all measurements was 0.05 units for both bioreactor systems. Off-line pH measurements also indicated that culture pH in shake flasks drifted downward for all cell lines, to as low as 5.8 for cell line 4. The pCO2 profiles in the ambr™ cultures were more similar to those of the corresponding 2-L bioreactor cultures than to the parallel shake flask cultures (Fig. 1f). Because we used shake flasks with vented caps and maintained the agitated shake flask cultures in incubators with 5 % CO2 overlay, we expected the pCO2 levels measured in these bicarbonate-buffered cultures to be in equilibrium with the CO2 levels of their environment. Our off-line pCO2 measurements for the shake flask cultures—showing that pCO2 levels remained relatively constant at approximately 40 mmHg throughout the duration of the shake flask culture—are consistent with this expectation.
Cell culture performance comparison
The ambr™ and 2-L bioreactor cultures trended similarly in titer, growth, and metabolites, whereas the shake flask cultures showed greater deviation from the bioreactor cultures (Fig. 1d, g–j).
Titer and growth
The four recombinant CHO cell lines used in this study were chosen to span a range of titers in 2-L bioreactors (Fig. 1g). Day 14 titers (Table 1) in ambr™ cultures deviated from the 2-L bioreactor cultures by an average of 8 % (5, 5, 5, and 17 %, respectively, for cell lines 1, 2, 3, and 4), which is close to the 5 % coefficient of variation typically expected for the titer assay. Day 14 titers (Table 1) in shake flask cultures deviated from 2-L bioreactor cultures by an average of 26 %. For cell lines 1, 2, and 3, day 14 titers were 30, 17, and 14 % higher in shake flasks than in the 2-L bioreactors. In their studies using two recombinant CHO cell lines, Tissot et al. (2011) also observed titers that were 20–25 % higher in agitated cultures without pH and DO controls than in bioreactors with pH and DO controls. For cell line 4, day 14 titers were ~40 % lower in shake flasks than in the 2-L bioreactors. The early titer plateau for this cell line in shake flasks is attributed to an early viability decline (Fig. 1h) that coincided with the low culture pH (Fig. 1e).
Table 1.
Culture performance in parallel ambr™, 2-L bioreactors, and shake flasks for four cell lines
| Cell line | Vessel | Maximumd growth ratee (1/d) | Day 14 titer (g/L)e | Cell-specific productivity (pg/cell/d)e |
|---|---|---|---|---|
| 1 | ambr™a | 0.38 ± 0.01 | 1.22 ± 0.02 | 7.2 ± 0.3 |
| 2-L Bioreactorb | 0.38 ± 0.01 | 1.16 ± 0.09 | 6.4 ± 0.5 | |
| Shake flaskc | 0.28 ± 0.01 | 1.50 ± 0.05 | 12.6 ± 0.5 | |
| 2 | ambr™a | 0.35 ± 0.00 | 1.94 ± 0.04 | 7.6 ± 0.2 |
| 2-L Bioreactorb | 0.33 ± 0.00 | 1.92 ± 0.11 | 7.7 ± 0.5 | |
| Shake flaskc | 0.26 ± 0.00 | 2.16 ± 0.00 | 12.8 ± 0.6 | |
| 3 | ambr™a | 0.39 ± 0.01 | 2.71 ± 0.01 | 14.1 ± 0.3 |
| 2-L Bioreactorb | 0.35 ± 0.02 | 2.85 ± 0.05 | 13.4 ± 0.4 | |
| Shake flaskc | 0.35 ± 0.01 | 3.27 ± 0.11 | 17.2 ± 0.0 | |
| 4 | ambr™a | 0.34 ± 0.01 | 2.41 ± 0.07 | 13.5 ± 0.6 |
| 2-L Bioreactorb | 0.35 ± 0.01 | 2.92 ± 0.13 | 15.2 ± 0.2 | |
| Shake flaskc | 0.28 ± 0.01 | 1.70 ± 0.00 | 15.0 ± 0.2 |
an = 6
bn = 2
cn = 2
dMeasured from days 0–7
eAverage ±1 standard deviation
The four cell lines were also chosen to test the ability of the ambr™ system to support typical CHO cell growth. In all cases, ambr™ cultures showed similar maximum growth rates, VCCs, and viabilities as 2-L bioreactor cultures (Table 1, Fig. 1h). The maximum growth rate was calculated based on cell growth between days 0–7 because all the cultures reached a growth plateau after day 7. Cultures in shake flask generally showed lower growth than in 2-L bioreactors: peak VCC values were, on average, 33 % lower. For all cell lines tested, the average cell-specific productivities calculated from titer and VCC values differed by less than 13 % between ambr™ and 2-L bioreactors (Table 1). The calculated cell-specific productivities were 96, 65, and 28 % higher in shake flasks than in bioreactors for cell lines 1, 2, and 3, respectively. Although the off-line pH for these three cell lines were lower in shake flasks than in bioreactors, the pH in shake flasks exceeded 6.6 throughout, and should not cause viability issues (Cook and Fox 1988). For cell line 4, off-line pH in shake flasks dipped below 6.4 by day 10 and below 6.0 by day 12; the corresponding viability decline (Fig. 1h) and titer plateau (Fig. 1g) are attributed to cell death at the low culture pH (<6.4). The average cell-specific productivity for cell line 4 was similar for cultures in shake flasks and in 2-L bioreactors due to the combination of lower growth and lower titer compared to 2-L bioreactors.
Lower pH can result in lower growth and higher specific productivity for CHO cell lines (Sauer et al. 2000; Trummer et al. 2006; Tsao et al. 2005; Yoon et al. 2005). The extent of the effect of pH on cell growth, cell-specific productivity, and titer is cell line dependent, as seen with the four cell lines tested in the first experiment. In shake flask cultures, while some cell lines can achieve similar growth—as observed here for cell line 3 and as reported for other cell lines cultured without tight pH and DO control (Tissot et al. 2011; Yuk et al. 2011)—or similar cell-specific productivity (as observed here for cell line 4), other cell lines may show lower growth (as observed here for cell lines 1, 2, and 4) or higher cell-specific productivity (as observed here for cell lines 1 and 2). The resulting effect on titer would reflect the net effect on both growth and cell-specific productivity.
Glucose and lactate
The ambr™ cultures showed similar glucose profiles and required glucose feeds at the same time as 2-L bioreactor cultures (Fig. 1d). By contrast, all the corresponding shake flask cultures consumed less glucose, similar to observations by Tissot et al. (2011) with CHO cells cultured in orbitally-shaken vessels without pH and DO control. The four cell lines also exhibited a range of lactate production and consumption behaviors. For all these cell lines, ambr™ cultures exhibited similar lactate profiles as 2-L bioreactor cultures (Fig. 1d). By contrast, shake flask cultures exhibited peak lactate levels that were 20–25 % lower than 2-L bioreactor cultures, also similar to observations by Tissot et al. (2011). The higher glucose levels and lower lactate profiles observed in the shake flasks (as compared to the ambr™ and 2-L bioreactor cultures) are consistent with the expected decrease in glucose consumption and lactate production when CHO cells are cultured at lower pH (Trummer et al. 2006; Tsao et al. 2005; Yoon et al. 2005).
Na+, osmolality, and amino acids
Since the four cell lines showed different lactate profiles (Fig. 1d), they are also expected to differ in their Na+ and osmolality profiles (Fig. 1i) when cultured using Na2CO3 addition for pH control. In the shake flask cultures, Na+ profiles are identical for all four cell lines, consistent with the lack of pH adjustment. For each cell line, both the ambr™ and 2-L bioreactors maintained pH at ~7.0 (Fig. 1c), and showed similar Na+ profiles. Because the Na+ contribution from the production media and nutrient feeds should be identical, and evaporation rates are comparably low in both systems (<2 %), similar Na+ profiles indicate similar base usage throughout the culture duration. The increase in Na+ concentration can be used to estimate the base requirement, and thus a predictor for basic titrant needed during process scale-up. Consistent with the similar Na+ (Fig. 1i), growth (Fig. 1h), glucose and lactate (Fig. 1d) profiles, the osmolality profiles were also similar between the ambr™ and 2-L bioreactor cultures (Fig. 1i).
Analysis of the spent media at the end of the culture showed similar amino acid concentrations between the ambr™ and the 2-L bioreactor cultures for all four cell lines (Fig. 1j). The shake flask cultures showed higher amino acid concentrations in the spent media than the ambr™ and 2-L bioreactor cultures for cell line 4; the decline in viability during the last few days of the shake flask cultures for cell line 4 should lower nutrient consumption. Although the amino acid assay can measure 21 amino acids, two amino acids were below the level of detection in all the cultures; therefore the data here show the concentrations for only 19 amino acids.
Product quality
Samples were taken from the three culture systems on days 10, 12 and 14 and analyzed for key product quality attributes: glycan distribution, molecular size distribution, and charge heterogeneity. For each cell line tested, all product quality attributes analyzed were comparable in the three culture systems in general (Table 2). The glycan distribution quantifies the three main species of N-linked glycans in the Fc domain of IgG with zero (G0), or one (G1) terminal galactose residue, or with terminal mannose residues (Man 5), as detailed by Gennaro and Salas-Solano (2008). The molecular size distribution quantifies the high molecular weight species (HMWS), monomer (main), and low molecular weight species (LMWS). The charge heterogeneity distribution quantifies the acidic, main, and basic species captured by icIEF (Zhang et al. 2011).
Table 2.
Day 14 product quality (glycan distribution, molecular size distribution, and charge heterogeneity) for samples from ambr™, 2-L bioreactor, and shake flask cultures
| Cell Line | Vessel | % G0d | % G1d | % Man5d |
|---|---|---|---|---|
| Glycan distribution | ||||
| 1 | ambr™a | 81.3 ± 0.4 | 14.8 ± 0.3 | 0.6 ± 0.0 |
| 2-L Bioreactorb | 82.7 ± 0.1 | 12.2 ± 1.9 | 0.5 ± 0.0 | |
| Shake flaskc | 84.4 ± 0.1 | 10.5 ± 0.1 | 0.5 ± 0.1 | |
| 2 | ambr™a | 89.5 ± 0.2 | 6.0 ± 0.2 | 0.5 ± 0.0 |
| 2-L Bioreactorb | 89.9 ± 0.3 | 5.2 ± 0.1 | 0.5 ± 0.0 | |
| Shake flaskc | 89.4 ± 0.3 | 5.1 ± 0.1 | 0.7 ± 0.0 | |
| 3 | ambr™a | 88.0 ± 0.3 | 5.9 ± 0.2 | 0.6 ± 0.1 |
| 2-L Bioreactorb | 84.3 ± 3.2 | 9.0 ± 2.2 | 0.6 ± 0.2 | |
| Shake flaskc | 88.4 ± 0.3 | 3.9 ± 0.0 | 1.0 ± 0.0 | |
| 4 | ambr™a | 88.6 ± 0.2 | 5.9 ± 0.1 | 0.5 ± 0.1 |
| 2-L Bioreactorb | 87.6 ± 0.3 | 6.4 ± 0.3 | 0.5 ± 0.0 | |
| Shake flaskc | 87.3 ± 0.1 | 4.9 ± 0.1 | 1.2 ± 0.1 | |
| Cell Line | Vessel | %HMWSd | %Maind | %LMWSd |
|---|---|---|---|---|
| Molecular size distribution | ||||
| 1 | ambr™a | 2.0 ± 0.1 | 97.8 ± 0.1 | 0.2 ± 0.1 |
| 2-L Bioreactorb | 2.0 ± 0.0 | 97.8 ± 0.0 | 0.3 ± 0.0 | |
| Shake flaskc | 1.8 ± 0.1 | 97.9 ± 0.0 | 0.3 ± 0.0 | |
| 2 | ambr™a | 2.9 ± 0.2 | 96.4 ± 0.1 | 0.8 ± 0.1 |
| 2-L Bioreactorb | 1.6 ± 0.6 | 97.1 ± 0.6 | 1.3 ± 0.0 | |
| Shake flaskc | 2.3 ± 0.0 | 96.7 ± 0.0 | 1.0 ± 0.1 | |
| 3 | ambr™a | 1.6 ± 0.1 | 98.2 ± 0.0 | 0.2 ± 0.0 |
| 2-L Bioreactorb | 1.5 ± 0.2 | 98.3 ± 0.1 | 0.3 ± 0.1 | |
| Shake flaskc | 1.5 ± 0.0 | 98.0 ± 0.4 | 0.5 ± 0.4 | |
| 4 | ambr™a | 1.8 ± 0.1 | 98.0 ± 0.0 | 0.2 ± 0.1 |
| 2-L Bioreactorb | 1.2 ± 0.1 | 98.5 ± 0.1 | 0.3 ± 0.0 | |
| Shake flaskc | 2.3 ± 0.1 | 97.5 ± 0.0 | 0.3 ± 0.0 | |
| Cell Line | Vessel | %Acidicd | %Maind | %Basicd |
|---|---|---|---|---|
| Charge heterogeneity | ||||
| 1 | ambr™a | 22.9 ± 0.1 | 69.9 ± 0.2 | 7.2 ± 0.1 |
| 2-L Bioreactorb | 25.7 ± 1.1 | 68.3 ± 1.5 | 6.1 ± 0.4 | |
| Shake flaskc | 22.7 ± 0.2 | 71.9 ± 0.2 | 5.4 ± 0.4 | |
| 2 | ambr™a | 25.9 ± 0.3 | 69.5 ± 0.6 | 4.6 ± 0.4 |
| 2-L Bioreactorb | 26.6 ± 1.4 | 69.2 ± 2.5 | 4.3 ± 1.0 | |
| Shake flaskc | 25.4 ± 1.1 | 71.0 ± 1.2 | 3.6 ± 0.1 | |
| 3 | ambr™a | 24.0 ± 0.3 | 60.3 ± 0.7 | 15.7 ± 0.5 |
| 2-L Bioreactorb | 26.9 ± 1.6 | 60.1 ± 0.4 | 13.0 ± 1.1 | |
| Shake flaskc | 25.7 ± 0.3 | 64.3 ± 1.6 | 10.0 ± 1.2 | |
| 4 | ambr™a | 24.9 ± 1.2 | 56.1 ± 1.2 | 19.0 ± 0.2 |
| 2-L Bioreactorb | 27.7 ± 0.4 | 56.3 ± 1.8 | 16.0 ± 2.2 | |
| Shake flaskc | 33.4 ± 0.2 | 56.0 ± 0.3 | 10.6 ± 0.5 | |
an = 4
bn = 2
cn = 2
dAverage ±1 standard deviation
Automation and other capabilities
In this evaluation, the ambr™ system successfully maintained sterility and executed all automated steps. The ambr™ workstation also reduced manual operations by automating liquid additions such as bolus nutrient feeds and glucose feeds, and sampling for off-line analyses. For example, the workstation transferred samples from the culture vessels into Vi-Cell cups, and diluted the samples prior to cell counting by the Vi-Cell XR system. By automating routine operations, the ambr™ system increased throughput and operational ease over the 2-L bioreactors. The single-use feature of the ambr™ vessels also provided additional time savings over the 2-L bioreactors.
Conclusions
For the four CHO cell lines chosen to represent the range of growth, metabolism, and productivities typically observed in our fed-batch cultures, we observed consistent performance among replicates in ambr™ bioreactors, and similar performance between the ambr™ and 2-L bioreactor cultures. However, the performance in shake flask cultures—in terms of growth, viability, metabolism, titer, and cell-specific productivity—was generally not representative of the performance in the bioreactor cultures. By providing pH and DO control and an automated liquid handling system, the ambr™ system overcomes some of the limitations of conventional small-scale cultures vessels such as shake flasks. By supplying single-use, pre-calibrated, and instrumented vessels, the ambr™ system provides a higher throughput platform for cell culture process development in a stirred-tank bioreactor environment.
Acknowledgments
The authors thank cell line development groups for generating the CHO cell lines evaluated; B6 media preparation group for supplying all the media and solutions used; cell banking group for supplying ampoules; Peter Harms and Louis Cheung for discussions on methods of higher throughput for off-line analysis; Analytical Operations group, especially Yun Tang, Kevin Lin, and Renee Yang, for providing analytical support for titer, product quality and amino acid assays; Andy Lin and John Joly for guidance and support.
Contributor Information
Wei-Ting Hsu, Phone: +1-650-4672591, FAX: +1-650-2252006, Email: wendyh@gene.com.
Rigzen P. S. Aulakh, Email: rigzena@gene.com
Donald L. Traul, Email: Don.Traul@tapbiosystems.com
Inn H. Yuk, Email: iyuk@gene.com
References
- Bareither R, Pollard D. A review of advanced small-scale parallel bioreactor technology for accelerated process development: current state and future need. Biotechnol Prog. 2011;27:2–14. doi: 10.1002/btpr.522. [DOI] [PubMed] [Google Scholar]
- Betts JI, Baganz F. Miniature bioreactors: current practices and future opportunities. Microb Cell Fact. 2006;5:21. doi: 10.1186/1475-2859-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisti Y. Animal cell culture in stirred bioreactors: observations on scale-up. Process Biochem. 1993;28:511–517. doi: 10.1016/0032-9592(93)85012-5. [DOI] [Google Scholar]
- Cohen SA, Michaud DP. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem. 1993;211:279–287. doi: 10.1006/abio.1993.1270. [DOI] [PubMed] [Google Scholar]
- Cook JA, Fox MH. Effects of chronic pH 6.6 on growth, intracellular pH, and response to 42.0 °C hyperthermia of Chinese Hamster Ovary cells. Cancer Res. 1988;48:2417–2420. [PubMed] [Google Scholar]
- Gennaro LA, Salas-Solano O. On-line CE-LIF-MS technology for the direct characterization of N-linked glycans from therapeutic antibodies. Anal Chem. 2008;80:3838–3845. doi: 10.1021/ac800152h. [DOI] [PubMed] [Google Scholar]
- Guo D, Gao A, Michels DA, Feeney L, Eng M, Chan B, Laird MW, Zhang B, Yu XC, Joly J, Snedecor B, Shen A. Mechanisms of unintended amino acid sequence changes in recombinant monoclonal antibodies expressed in Chinese hamster ovary (CHO) cells. Biotechnol Bioeng. 2010;107:163–171. doi: 10.1002/bit.22780. [DOI] [PubMed] [Google Scholar]
- Hopp J, Pritchett R, Darlucio M, Ma J, Chou JH. Development of a high throughput protein a well-plate purification method for monoclonal antibodies. Biotechnol Prog. 2009;25:1427–1432. doi: 10.1002/btpr.247. [DOI] [PubMed] [Google Scholar]
- Keane JT, Ryan D, Gray PP. Effect of shear stress on expression of a recombinant protein by Chinese Hamster Ovary cells. Biotechnol Bioeng. 2003;81:211–220. doi: 10.1002/bit.10472. [DOI] [PubMed] [Google Scholar]
- Kondragunta B, Drew JL, Brorson KA, Moreira AR, Rao G. Advances in clone selection using high-throughput bioreactors. Biotechnol Prog. 2010;26:1095–1103. doi: 10.1002/btpr.392. [DOI] [PubMed] [Google Scholar]
- Kumar S, Wittmann C, Heinzle E. Minibioreactors. Biotechnol Lett. 2004;26:1–10. doi: 10.1023/B:BILE.0000009469.69116.03. [DOI] [PubMed] [Google Scholar]
- Lewis G, Lugg R, Lee K, Wales R. Novel automated micro-scale bioreactor technology: a qualitative and quantitative mimic for early process development. Bioprocess J. 2010;9:22–25. [Google Scholar]
- Lin AA, Kimura R, Miller WM. Production of tPA in recombinant CHO cells under oxygen-limited conditions. Biotechnol Bioeng. 1993;42:339–350. doi: 10.1002/bit.260420311. [DOI] [PubMed] [Google Scholar]
- Restelli V, Wang MD, Huzel N, Ethier M, Perreault H, Butler M. The effect of dissolved oxygen on the production and the glycosylation profile of recombinant human erythropoietin produced from CHO cells. Biotechnol Bioeng. 2006;94:481–494. doi: 10.1002/bit.20875. [DOI] [PubMed] [Google Scholar]
- Sauer PW, Burky JE, Wesson MC, Sternard HD, Qu L. A high-yielding, generic fed-batch cell culture process for production of recombinant antibodies. Biotechnol Bioeng. 2000;67:585–597. doi: 10.1002/(SICI)1097-0290(20000305)67:5<585::AID-BIT9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tissot S, Oberbek A, Reclari M, Dreyer M, Hacker DL, Baldi L, Farhat M, Wurm FM. Efficient and reproducible mammalian cell culture bioprocesses without probes and controllers? N Biotechnol. 2011;28:382–390. doi: 10.1016/j.nbt.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Trummer E, Fauland K, Seidinger S, Schriebl K, Lattenmayer C, Kunert R, Vorauer-Uhl K, Weik R, Borth N, Katinger H, Muller D. Process parameter shifting: part I. Effect of DOT, pH, and temperature on the performance of Epo-Fc expressing CHO cells cultivated in controlled batch bioreactors. Biotechnol Bioeng. 2006;94:1033–1044. doi: 10.1002/bit.21013. [DOI] [PubMed] [Google Scholar]
- Tsao Y-S, Cardoso AG, Condon RGG, Voloch M, Lio P, Lagos JC, Kearns BG, Liu Z. Monitoring Chinese hamster ovary cell culture by the analysis of glucose and lactate metabolism. J Biotechnol. 2005;118:316–327. doi: 10.1016/j.jbiotec.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Varley J, Birch J. Reactor design for large scale suspension animal cell culture. Cytotechnology. 1999;29:177–205. doi: 10.1023/A:1008008021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SK, Choi SL, Song JY, Lee GM. Effect of culture pH on Erythropoietin production by Chinese Hamster Ovary cells grown in suspension at 32.5 and 37.0 °C. Biotechnol Bioeng. 2005;89:345–356. doi: 10.1002/bit.20353. [DOI] [PubMed] [Google Scholar]
- Yuk IH, Baskar D, Duffy PH, Hsiung J, Leung S, Lin AA. Overcoming challenges in WAVE bioreactors without feedback controls for pH and dissolved oxygen. Biotechnol Prog. 2011;27:1397–1406. doi: 10.1002/btpr.659. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lilyestrom W, Li C, Scherer T, Reis R, Zhang B. Revealing a positive charge patch on a recombinant monoclonal antibody by chemical labeling and mass spectrometry. Anal Chem. 2011;83:8501–8508. doi: 10.1021/ac2016129. [DOI] [PubMed] [Google Scholar]