Abstract
Several lichen species have been used for medicinal purposes throughout the ages, and they are reported to be effective in the treatment of different disorders including ulcer and cancer. It is revealed that lichens may be easily accessible sources of natural drugs and possible food supplements after their safety evaluations. The main objective in this study was to evaluate the roles of aqueous extracts of Xanthoria elegans (at 25, 50 and 100 μg/ml) upon mitomycin C (MMC; at 10−7 M) induced genotoxic and oxidative damages in cultured human lymphocytes. X. elegans were collected from the Erzurum and Artvin provinces (in Turkey) during August 2010. After the application of MMC and X. elegans extract (XEE), separate and together, human whole blood cultures were assessed by four genotoxicity end-points including chromosomal aberration, micronucleus, sister chromatid exchange (SCE) and 8-oxo-2-deoxyguanosine (8-OH-dG) assays. In addition, biochemical parameters [total antioxidant capacity (TAC) and total oxidative stress (TOS)] were examined to determine oxidative effects. According to our results, the frequencies of cytogenetic endpoints and 8-OH-dG levels were significantly increased by MMC compared with controls in human peripheral lymphocytes. MMC caused oxidative stress by altering TAC and TOS levels. On the contrary, XEE led to increases of TAC level without changing TOS level. XEE had no genotoxic effect. Furthermore, our findings revealed that MMC induced increases in the mean frequencies of four genotoxic indices were diminished by XEE in dose dependent manner, indicating its protective role towards cells from MMC exerted injury. In conclusion, the results obtained in the present study indicate for the first time that XEE is a potential source of natural antigenotoxicants.
Keywords: Antigenotoxicity, DNA damage, Human blood cultures, Mitomycin C, Oxidative status, Xanthoria elegans
Introduction
Lichens, symbiotic associations between algae and fungi, are effective in the treatment of diseases such as hemorrhoids, bronchitis, dysentery, and tuberculosis (Kim and Choç 2007; Gülçin et al. 2002). Their biological activities and chemical compositions have long been investigated for anti-microbial (Candan et al. 2007), anti-viral (Fazio et al. 2007), anti-tumor (Rezanka and Dembitsky 2006), anti-proliferative (Ren et al. 2009), anti-inflammatory (Silva et al. 2010; Süleyman et al. 2003), anti-mutagenic (Geyikoglu et al. 2007; Turkez et al. 2010; Turkez and Dirican 2012) and anti-oxidant (Kohlhardt-Floehr et al. 2010; Aydin and Turkez 2011a, b; Turkez et al. 2012a) properties till today. On the other hand, lichens were used as raw materials in paint and cosmetic industry, as environmental indicators, as food and as medicine for various purposes (Cocchietto et al. 2002; Dilsizoğlu et al. 2004; Yazıcı and Aslan 2006; Svoboda 2007). The anti-inflammatory effect of methanol extract of the lichen species Peltigera rufescens was reported for its reducing effect on the neutrophil-derived free radicals and its ameliorating effect on the anti-oxidant defense systems (Tanas et al. 2010). Likewise, Halici et al. (2005) reported that, the water extract of the lichen, Usnea longissima, has protective effect in indomethacin-induced ulcers, which can be attributed to its anti-oxidant potential.
Nowadays, extensive efforts are made to investigate therapeutic substances capable of reducing the genotoxicity of various natural and man-made mutagens in human life (Turkez et al. 2005; Turkez and Geyikoglu 2010). These include vitamins, fatty acids and antibody (Edenharder et al. 1999; Rao et al. 2001; Yoshida et al. 2010; Turkez et al. 2012b). Concomitant treatment with the anti-oxidants provided protection against oxidative damage by mutagens in experimental animals (Abubakar et al. 2003; Esparza et al. 2003; Geyikoglu et al. 2005; Turkez and Geyikoglu 2010). To our best knowledge, no investigation on the anti-genotoxicity potential of X. elegans extracts (XEEs) on mitomycin C (MMC)-induced cytotoxicity in human blood cells was reported in the literature. Thus, the aim of the present study was to elucidate the potential beneficial role of XEE against MMC-induced genotoxic and oxidative damage. To do this, chromosomal aberrations (CA), micronucleus (MN) and sister chromatid exchange (SCE) rates and total antioxidant capacity (TAC), total oxidative stress (TOS) and 8-oxo-2-deoxyguanosine (8-OH-dG) levels were assessed after following treatments with XEE and MMC (a well known reference mutagen) in cultured human blood cells. CA, MN SCE and 8-OH-dG are rapid, reliable and sensitive tests for evaluating the presence and extent of DNA damage in human populations that are exposed to genotoxic substances in environments and their lifestyles (Turkez and Geyikoglu 2010; Turkez et al. 2012c). In addition, important oxidative parameters, TAC and TOS were used to monitor their anti-oxidant or pro-oxidant activities in vitro (Turkez and Togar 2010).
Materials and methods
Plants and extraction
Xanthoria elegans lichen species were collected from the Erzurum and Artvin provinces (Turkey) during August 2010. After drying at room temperature, a stereo microscope, a compound microscope, and the usual spot tests were used in the identification of the samples using reference books (Poelt 1974; Purvis et al. 1992; Wirth 1995; Aslan and Yazici 2006). The specimens are stored in the herbarium of Kazım Karabekir, Faculty of Education, Atatürk University, Erzurum. For water extraction of X. elegans, 20 g sample was mixed with 400 ml distillated and boiling water using magnetic stirrer for 15 min. Then the extracts were filtered over Whatmann No. 1 paper.
Experimental design
Whole heparinized human blood from four healthy non-smoking donors between the ages of 22 and 25 with no history of exposure to any genotoxic agent was used in our experiments. Questionnaires were obtained for each blood donor to evaluate exposure history, and in addition, informed consent forms were signed by each donor. In all volunteers involved in this study, hematological and biochemical parameters were analyzed and no pathology was detected. MMC (C15H18N4O5; Sigma®, St, Louis/MO, USA, at 10−7M) and three concentrations (25, 50 and 100 mg/l) of lichen aqueous extracts were tested in cultured human lymphocytes. These compounds were added to the cultures just before incubation for cytogenetic analysis. Experiments were conformed to the guidelines of the World Medical Assembly (Declaration of Helsinki). The concentrations were selected according to the previous studies (Scarpato et al. 1990; Aydin 2011). After supplementation of MMC and XEEs, the blood was incubated for 72 h at 37 °C to adjust body conditions for testing genotoxicity. Experiments conforming to the guidelines of the World Medical Assembly (Declaration of Helsinki). Each individual whole blood culture without MMC or XEE was studied as a control group.
CA assay
Human peripheral blood lymphocyte cultures were set up according to a slight modification of the protocol described by Evans and O’Riordan (1975). A 0.5 ml aliquot of heparinized blood was cultured in 6 ml of culture medium (Chromosome Medium B; Biochrom, Berlin) with 5 mg/ml of phytohemagglutinin (Biochrom). The cultures were incubated in complete darkness for 72 h at 37 °C. Two hours prior to harvesting, 0.1 ml of colchicine (0.2 mg/ml, Sigma) was added to the culture flask. Hypotonic treatment and fixation were performed. To prepare slides, 3–5 drops of the fixed cell suspension were dropped on a clean slide and air-dried. The slides were stained in 3 % Giemsa solution in phosphate buffer (pH 6.8) for 15 min. For each treatment, 30 well-spreaded metaphases were analyzed to detect the presence of CA. Criteria to classify the different types of aberrations (chromatid or chromosome gap and chromatid or chromosome break) were in accordance with the recommendation of EHC (Environmental Health Criteria) 46 for environmental monitoring of human populations (IPCS 1985).
MN assay
The MN test was performed by adding cytochalasin B (Sigma®) after 44 h of culture as previously described by Fenech and Morley (1985). At the end of the incubation period, the lymphocytes were fixed with ice-cold methanol: acetic acid (3:1). The fixed cells were put directly on slides using a cytospin, and stained with May Grünwald-Giemsa. All slides were coded before scoring. The slides were scored according to criteria reported by Fenech (1993). At least 2,000 binucleated lymphocytes were examined per concentration (two cultures per concentration) for the presence of one, two or more micronuclei.
SCE assay
In order to provide successive visualization of SCEs, 5-bromo-2′-deoxyuridine (Sigma®) was added after culture initiation. At exactly 70 h and 30 min after beginning incubations, colcemid (Sigma®) was added to the cultures. After hypotonic treatment (0.075 M KCl) followed by three repetitive cycles of fixation in methanol/acetic acid solution (3:1, v/v), centrifugation, and resuspension, the cell suspension was dropped onto chilled, grease free microscopic slides, air-dried, aged, and then differentially stained for the inspection of SCE rate according to fluorescence plus Giemsa (FPG) procedure (Perry and Wolff 1974). For each treatment condition, 25 well-spread second division metaphases were scored by a single observer (E. Aydin), and the values obtained were calculated as SCEs per cell.
Nucleic acid oxidation
DNA oxidation was determined by measuring the amount of 8-OH-dG adducts. DNA was digested by incubation with DNAase I, endonuclease, and alkaline phosphatase (Schneider et al. 1993). The amount of 8-OH-dG was measured by high performance liquid chromatography (HPLC) with electrochemical detection as described previously (Floyd et al. 1993).
TAC and TOS analysis
The major advantage of TAC test is to measure the anti-oxidant capacity of all anti-oxidants in a biological sample and not just the anti-oxidant capacity of a single compound (Kusano and Ferrari 2008). In this test, anti-oxidants in the sample reduce dark blue-green colored ABTS radical to the colorless reduced ABTS form. The change of absorbance at 660 nm is related with total anti-oxidant level of the sample. The assay is calibrated with a stable antioxidant standard solution which is traditionally named as Trolox Equivalent that is a vitamin E analog. Since the measurement of different oxidant molecules separately is not practical and their oxidant effects are additive, the total oxidant status (TOS) of a sample is measured and this is named total peroxide (TP), serum oxidation activity (SOA), reactive oxygen metabolites (ROM) or some other synonyms. In the TOS assay performed here, oxidants present in the sample oxidize the ferrous ion–chelator complex to ferric ion. The oxidation reaction is prolonged by enhancer molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with chromogen in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (μmol H2O2 Equiv./L). The automated Trolox equivalent total anti-oxidant capacity (TAC) and total oxidant status (TOS) assays were carried out in plasma samples obtained from blood cultures for 2 h by commercially available kits (Rel Assay Diagnostics®, Gaziantep, Turkey) (Erel 2004, 2005).
Statistics
The results are expressed as mean ± standard deviation (SD). Comparison between groups was carried out by one-way analysis of variance followed by Duncan multiple range tests with the level of significance set at P < 0.05.
Results
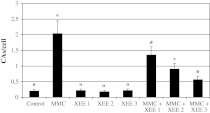
The results of the present study showed that, MMC (at 10−7M) caused increases of CAs frequencies. On the contrary, three XEE doses alone (at 25, 50 and 100 μg/ml) did not change the rate of CAs. Moreover, important statistical significances in the reduction of CA frequencies were found in the cultures concomitantly treated with XEE and MMC as compared to the group MMC treated alone (Fig. 1).
Fig. 1.
The frequencies of CAs in human lymphocytes treated with different concentrations of MMC and XEEs. (Values are expressed as mean ± SD for four cultures in each group. The bars are shown by different letter are significantly different from each other at a level of 5 %. MMC: 10−7 M mytomicin; XEE1: 25 µg/ml X. elegans water extract; XEE2: 50 µg/ml X. elegans water extract; XEE3: 100 µg/ml X. elegans water extract)
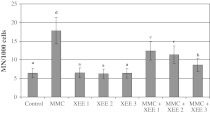
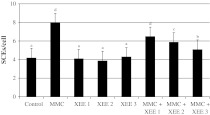
In the treatment for 72 h, a significant increase in induced MN rates was found at 10−7M MMC (P < 0.05). XEE at tested concentrations did not increase the rate of MNs. However, the positive effect of XEE in decreasing the incidence of MNs in comparison with an unprotected level was attained when cultures were treated simultaneously with MMC and XEE (Fig. 2). MMC also caused a statistically significant increase of SCE rates as compared to control group. On the contrary, three XEE applications alone also did not change the rate of SCEs. However, significant reductions of SCE rates were found in the cultures concomitantly treated with XEE and MMC as compared to the group MMC treated alone (Fig. 3).
Fig. 2.
The frequencies of MNs (%) in human lymphocytes treated with different concentrations of MMC and XEEs. Abbreviations are as in Fig. 1
Fig. 3.
Rate of SCEs in cultured human lymphocytes simultaneously exposed to MMC and XEEs. Abbreviations are as in Fig. 1
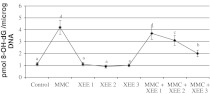
The status of 8-OH-dG in human lymphocytes of contol and all XEE concentrations was presented in Fig. 4. It was observed that MMC (at 10−7M) but not XEE applications significantly increased 8-OH-dG concentrations in the human blood cultures after 72 h. Moreover, the XEEs significantly decreased 8-OH-dG concentrations in MMC-treated human lymphocytes in a dose dependent manner.
Fig. 4.
8-OH-dG adducts in cultured human blood cells maintained 72 h in the presence of MMC, XEE and their combinations. Abbreviations are as in Fig. 1
Table 1 shows the effect of XEEs and MMC on oxidant status in human whole blood cultures were determined by TAC and TOS analysis. As shown in Table 1, the TAC value decreased with the addition of MMC while TOS value increased. In contrast to the XEE did not alter the TOS level but increased the TAC level in a dose dependent manner. Furthermore, XEEs had dose dependent inhibitory effects on oxidative damage in human blood cells by MMC.
Table 1.
The TAC and TOS levels in cultured human blood cells maintained 2 h in the presence of MMC, XEE and their combinations Abbreviations are as in Fig. 1
| Treatments | TAC (mmol Trolox Equiv./l) | TOS (µmol H2O2 Equiv./l) |
|---|---|---|
| Control | 6.1 ± 0.9c | 11.8 ± 3.5a |
| MMC | 4.2 ± 0.6a | 16.7 ± 5.1e |
| XEE1 | 6.8 ± 0.9c | 11.1 ± 4.2a |
| XEE2 | 7.4 ± 1.1d | 11.4 ± 3.6a |
| XEE3 | 8.6 ± 1.4e | 11.9 ± 3.4a |
| MMC + XEE1 | 4.5 ± 0.5a | 15.7 ± 5.0d |
| MMC + XEE2 | 5.3 ± 0.8b | 14.0 ± 5.5c |
| MMC + XEE3 | 5.5 ± 1.1b | 13.4 ± 4.9b |
Discussion
In the present study we observed that MMC caused genotoxicity in human lymphocytes. In fact, CA test is regarded as a very important and useful indicator of exposure to biological and chemical agents (Padovani et al. 1997). MN assay provides a measure of both chromosome breakage and chromosome loss or non-disjunction in clastogenic and aneugenic events, respectively (Karaman et al. 2009). And damaged DNA can lead to aneuploidy and/or chromosomal instability, which is believed to be major contributor to tumor progression (Erol 2010). DNA damages and defective DNA repairs cause SCEs (Bozkurt et al. 2003). In accordance with our finding, cellular toxicity after MMC exposure may occur from MMC-induced insults such as the generation of free radicals, DNA monoadducts, and SCE formations; however, the most significant effects are due to the accumulation of covalent DNA interstrand cross-links (Tomasz 1995; Liao et al. 2012; Rencuzogullari et al. 2012).
In our investigation, it was determined that MMC led to development of oxidative stress (by altering TAC and TOS levels) and increases of 8-OH-dG levels. Similar to our findings, MMC was reported to be bioactivated by P450 reductase through one electron reduction and produces oxygen radicals (Seow et al. 2004). Ortega-Gutiérrez et al. (2009) revealed that MMC generated free radicals when metabolized. Activity of anti-oxidant enzymes like superoxide dismutase (SOD) and glutathione peroxidases (GSH-Px) content had declined after exposure to MMC in blood and liver samples of mice (Li et al. 2009). And, MMC induced oxidative DNA damage via increasing 8-OH-dG level in sea urchin embryos (Pagano et al. 2001).
In our present study, there is considerable evidence that XEE presented protective effects with increasing amounts without leading to any genetic damage in lymphocytes with. Several free radical scavenger agents, such as amifostine, proline-rich polypeptide and nitroxide, prevented the cytotoxic damage mediated by MMC in different organisms (Krishna et al. 1991; Hahn et al. 1997; Santini 2001; Aroutiounian et al. 2010). Likewise, it was suggested that apigenin and quercetin (bioflavonoids) exhibited protection against the MMC genotoxicity on mice bone marrow cells (Siddique and Afzal 2009; Mazumdar et al. 2011). In a previous study, it was determined that American ginseng extract was capable of suppressing the CA induced by MMC in mice (Pawar et al. 2007). Also, erythropoietin (a hormone produced by the kidney) decreased SCE formations induced by MMC (Digkas et al. 2010). According to our results, we could suggest that XEEs have important biological and pharmaceutical consequences, because the induction of CAs, MNs and SCEs here observed were also ameliorated by concurrent administration of XEE.
There are multiple forms of SOD, GSH-Px, glutathione reductase (GR) and catalase (CAT) in lichens (Weissman et al. 2006; Del Hoyo et al. 2011; Kotan et al. 2011). It was established that the water extracts of lichenes presented important effects against tissue damages, which could be attributed to their anti-oxidant potential (Rezanka and Dembitsky 2006; Kim and Choç 2007). In fact, Xanthoria species were shown to contain various anthraquinone pigments, and especially parietin (Brodo et al. 2001). Manojlovic et al. (2010) investigated the anthraquinones content of lichen Laurera benguelensis and reported that the lichen extracts showed high anti-oxidant activity due to its metabolites including parietin. In accordance with this finding, Yim et al. (1998) determined that the cellular protection afforded by the anthraquinone-containing fraction of herbal extracts might be related to its ability to sustain the glutathione anti-oxidant status under the oxidative stress conditions.
In the light of findings obtained in the present study, it is suggested that XEE supplements in foods could protect blood tissue against mutagens-induced oxidative DNA damage. So, the lichen X. elegans has the potential of being utilized as novel bioresources for naturally occurring anti-oxidant therapies.
Acknowledgments
The authors are grateful to all volunteers for the blood samples.
References
- Abubakar MG, Taylor A, Ferns GAA. Aluminium administration is associated with enhanced hepatic oxidant stress that may be offset by dietary vitamin E in the rat. Int J Exp Pathol. 2003;84:49–54. doi: 10.1046/j.1365-2613.2003.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroutiounian RM, Hovhannisyan GG, Gasparyan GH, Margaryan KS, Aroutiounian DN, Sarkissyan NK, Galoyan AA. Proline-rich polypeptide-1 protects the cells in vitro from genotoxic effects of mitomycin C. Neurochem Res. 2010;35:598–602. doi: 10.1007/s11064-009-0104-8. [DOI] [PubMed] [Google Scholar]
- Aslan A, Yazici K. Contribution to the lichen flora of Giresun province of Turkey. Acta Bot Hung. 2006;48:231–245. doi: 10.1556/ABot.48.2006.3-4.1. [DOI] [Google Scholar]
- Aydin E (2011) The investigation of cytogenetic and oxidative effects of some lichen species located in Erzurum and Artvin cities. Master’s thesis, Atatürk University, Erzurum, Gradute School of Natural and Applied Sciences, Department of Biology
- Aydin E, Turkez H. Antioxidant and genotoxicity screening of aqueous extracts of four lichens collected from North East Anatolia. Fresen Environ Bull. 2011;20:2085–2091. [Google Scholar]
- Aydin E, Turkez H. Effects of lichenic extracts (Bryoria capillaris, Peltigera rufescens and Xanthoria elegans) on human blood cells: a cytogenetic and biochemical study. Fresen Environ Bull. 2011;20:2992–2998. [Google Scholar]
- Bozkurt G, Yuksel M, Karabogaz G, Sut N, Savran FO, Palanduz S, Yigitbasi ON, Algunes C. Sister chromatid exchanges in lymphocytes of nuclear medicine physicians. Mutat Res. 2003;535:205–213. doi: 10.1016/S1383-5718(02)00321-2. [DOI] [PubMed] [Google Scholar]
- Brodo IM, Sharnoff SD, Sharnoff S. Lichens of North America. Ch. 10 lichens and people. New Haven: Yale University Press; 2001. [Google Scholar]
- Candan M, Yılmaz M, Tay T, Erdem M, Türk A. Antimicrobial activity of extracts of the lichen Parmelia sulcata and its salazinic acid constituent. Z Naturforsch C. 2007;62:6196–6221. doi: 10.1515/znc-2007-7-827. [DOI] [PubMed] [Google Scholar]
- Cocchietto M, Skert N, Nimis PL, Sava G. A review on usnic acid, an interesting natural compound. Naturwissenschaften. 2002;89:137–146. doi: 10.1007/s00114-002-0305-3. [DOI] [PubMed] [Google Scholar]
- Hoyo A, Alvarez R, Campo EM, Gasulla F, Barreno E, Casano LM. Oxidative stress induces distinct physiological responses in the two Trebouxia phycobionts of the lichen Ramalina farinacea. Ann Bot. 2011;107:109–118. doi: 10.1093/aob/mcq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digkas EN, Chrisafi S, Passadaki T, Tsalkidis A, Hatzimichail A, Vargemezis V, Lialiaris TS. In vitro and in vivo cytogenetic effects of recombinant human erythropoietin on the frequency of sister chromatid exchanges alone or in combination with mitomycin C. Chemotherapy. 2010;56:239–247. doi: 10.1159/000316849. [DOI] [PubMed] [Google Scholar]
- Dilsizoğlu A, Kavuncuoğlu Z, Oba D. Old and new using areas, the lichens with unknown properties. Tubitak Sci Tech. 2004;439:86–89. [Google Scholar]
- Edenharder R, Worf-Wandelburg A, Decker M, Platt KL. Antimutagenic effects and possible mechanisms of action of vitamins and related compounds against genotoxic heterocyclic amines from cooked food. Mutat Res. 1999;444:235–248. doi: 10.1016/S1383-5718(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–2785. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Erol A. Systemic DNA damage response and metabolic syndrome as a premalignant state. Curr Mol Med. 2010;10:321–334. doi: 10.2174/156652410791065282. [DOI] [PubMed] [Google Scholar]
- Esparza L, Gomez M, Romeu M, Mulero M, Sanchez DJ, Mallol J, Domingo JL. Aluminum-induced pro-oxidant effects in rats: protective role of exogenous melatonin. J Pineal Res. 2003;35:32–39. doi: 10.1034/j.1600-079X.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- Evans HJ, O’Riordan ML. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat Res. 1975;31:135–148. doi: 10.1016/0165-1161(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Fazio AT, Adler MT, Bertoni MD, Sepúlveda CS, Damonte EB, Maier MS. Lichen secondary metabolites from the cultured lichen mycobionts of Teloschistes chrysophthalmus and Ramalina celastri and their antiviral activities. Z Naturforsch C. 2007;62:543–549. doi: 10.1515/znc-2007-7-813. [DOI] [PubMed] [Google Scholar]
- Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-L. [DOI] [PubMed] [Google Scholar]
- Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Watson JJ, Wong PK, Altmiller DH, Rickard RC. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1993;1:163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Geyikoglu F, Turkez H, Aslan A. The protective roles of some lichen species on colloidal bismuth subcitrate genotoxicity. Toxicol Ind Health. 2007;23:487–492. doi: 10.1177/0748233708089044. [DOI] [PubMed] [Google Scholar]
- Geyikoglu F, Turkez H, Keles MS (2005) The role of fruit juices in the prevention of aluminum sulphate toxicity in human blood in vitro. Fres Environ Bull 14:878–883
- Gülçin İ, Oktay M, Küfrevioğlu Öİ, Aslan A. Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol. 2002;79:325–329. doi: 10.1016/S0378-8741(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Hahn SM, Sullivan FJ, Deluca AM, Sprague M, Hampshire VA, Krishna MC, Russo A, Mitchell JB. Protection of mitomycin C induced skin extravation with the nitroxide, 3-carbamoyl-proxyl (3-CP) Int J Oncol. 1997;10:119–123. [PubMed] [Google Scholar]
- Halici M, Odabasoglu F, Suleyman H, Cakir A, Aslan A, Bayir Y. Effects of water extract of Usnea longissima on antioxidant enzyme activity and mucosal damage caused by indomethacin in rats. Phytomedicine. 2005;12:656–662. doi: 10.1016/j.phymed.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Environmental health criteria 46. Guidelines for the study of genetic effects in human populations. Geneva: WHO; 1985. pp. 45–54. [Google Scholar]
- Karaman A, Kadı M, Kara F. Sister chromatid exchange and micronucleus studies in patients with Behcet’s disease. J Cutan Pathol. 2009;36:831–837. doi: 10.1111/j.1600-0560.2008.01180.x. [DOI] [PubMed] [Google Scholar]
- Kim MS, Choç HB. Melanogenesis inhibitory effects of methanolic extracts of Umbilicaria esculenta and Usnea longissima. J Microbiol. 2007;45:578–582. [PubMed] [Google Scholar]
- Kohlhardt-Floehr C, Boehm F, Troppens S, Lademann J, Truscott TG. Prooxidant and antioxidant behaviour of usnic acid from lichens under UVB-light irradiation-studies on human cells. J Photochem Photobiol B Biol. 2010;101:97–102. doi: 10.1016/j.jphotobiol.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Kotan E, Alpsoy L, Anar M, Aslan A, Agar G. Protective role of methanol extract of Cetraria islandica (L.) against oxidative stress and genotoxic effects of AFB1 in human lymphocytes in vitro. Toxicol Ind Health. 2011;27:599–605. doi: 10.1177/0748233710394234. [DOI] [PubMed] [Google Scholar]
- Krishna MC, DeGraff W, Tamura S, Gonzalez FJ, Samuni A, Russo A, Mitchell JB. Mechanisms of hypoxic and aerobic cytotoxicity of mitomycin C in Chinese hamster V79 cells. Cancer Res. 1991;51:6622–6628. [PubMed] [Google Scholar]
- Kusano C, Ferrari B. Total antioxidant capacity: a biomarker in biomedical and nutritional studies. J Cell Mol Biol. 2008;7:1–15. [Google Scholar]
- Li F, Xu J, Zhou J, Zhao L, Sheng J, Sun G, Hu Q. Inhibition of mitomycin C-induced chromosomal aberrations by micrometer powder of selenium-enriched green tea in mice spermatocytes. Mutat Res. 2009;675:11–16. doi: 10.1016/j.mrgentox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Liao PH, Lin RH, Yang ML, Li YC, Kuan YH (2012) Evaluation of differential representative values between Chinese hamster cells and human lymphocytes in mitomycin C-induced cytogenetic assays and caspase-3 activity. Toxicol Ind Health 28:174–180 [DOI] [PubMed]
- Manojlovic NT, Vasiljevic PJ, Gritsanapan W, Supabphol R, Manojlovic I. Phytochemical and antioxidant studies of Laurera benguelensis growing in Thailand. Biol Res. 2010;43:169–176. doi: 10.4067/S0716-97602010000200004. [DOI] [PubMed] [Google Scholar]
- Mazumdar M, Giri S, Giri A. Role of quercetin on mitomycin C induced genotoxicity: analysis of micronucleus and chromosome aberrations in vivo. Mutat Res. 2011;721:147–152. doi: 10.1016/j.mrgentox.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Ortega-Gutiérrez S, López-Vicente M, Lostalé F, Fuentes-Broto L, Martínez-Ballarín E, García JJ. Protective effect of melatonin against mitomycin C-induced genotoxic damage in peripheral blood of rats. J Biomed Biotechnol. 2009;2009:791432. doi: 10.1155/2009/791432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovani L, Tronati L, Mauro F, Testa A, Appolloni M, Azzidei P, Caporossi D, Tedeschi B, Vernole P. Cytogenetic effects in lymphocytes from children exposed to radiation fall-out after the chernobyl accident. Mutat Res. 1997;395:249–254. doi: 10.1016/S1383-5718(97)00137-X. [DOI] [PubMed] [Google Scholar]
- Pagano G, Degan P, Biase A, Iaccarino M, Warnau M. Diepoxybutane and mitomycin C toxicity is associated with the induction of oxidative DNA damage in sea urchin embryos. Hum Exp Toxicol. 2001;20:651–655. doi: 10.1191/096032701718890577. [DOI] [PubMed] [Google Scholar]
- Pawar AA, Tripathi DN, Ramarao P, Jena G. Protective effects of American ginseng (Panax quinquefolium) against mitomycin C induced micronuclei in mice. Phytother Res. 2007;21:1221–1227. doi: 10.1002/ptr.2245. [DOI] [PubMed] [Google Scholar]
- Perry P, Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Poelt J. Bestimmungsschlüssel Europäischer Flechten. Vaduz: J. Cramer; 1974. p. 757. [Google Scholar]
- Purvis OW, Coppins BJ, Hawksworth DL, James PW, Moore DM. The lichen flora of Great Britain and Ireland. London: Natural History Museum Publications in association with The British Lichen Society; 1992. p. 710. [Google Scholar]
- Rao MV, Chinoy NJ, Suthar MB, Rajvanshi MI. Role of ascorbic acid on mercuric chloride-induced genotoxicity in human blood cultures. Toxicol In Vitro. 2001;15:649–654. doi: 10.1016/S0887-2333(01)00081-9. [DOI] [PubMed] [Google Scholar]
- Ren MR, Hur JS, Kim JY, Park KW, Park SC, Seong CN, Jeong IY, Byun MW, Lee MK, Seo KI. Anti-proliferative effects of Lethariella zahlbruckneri extracts in human HT-29 human colon cancer cells. Food Chem Toxicol. 2009;47:2157–2162. doi: 10.1016/j.fct.2009.05.042. [DOI] [PubMed] [Google Scholar]
- Rencuzogullari E, Yildiz AM, Buyukleyla M. The genotoxic and anti-genotoxic effects of Stachys petrokosmos leaf extract in human lymphocytes using microsomal fractions. Cytotechnology. 2012;64:83–94. doi: 10.1007/s10616-011-9396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezanka T, Dembitsky VM. The colleflaccinosides, two chiral bianthraquinone glycosides with antitumor activity from the lichen Collema flaccidum collected in Israel and Russia. Nat Prod Res. 2006;20:969–980. doi: 10.1080/14786410500218674. [DOI] [PubMed] [Google Scholar]
- Santini V. Amifostine: chemotherapeutic and radiotherapeutic protective effects. Expert Opin Pharmacother. 2001;2:479–489. doi: 10.1517/14656566.2.3.479. [DOI] [PubMed] [Google Scholar]
- Scarpato R, Migliore L, Barale R. The micronucleus assay in Anodonta cygnea for the detection of drinking water mutagenicity. Mutat Res. 1990;245:231–237. doi: 10.1016/0165-7992(90)90151-9. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Jr, Phillips JR, Pye Q, Maidt ML, Price S, Floyd RA. Methylene blue and rose bengala photoinactivation of RNA bacteriophages: comparative studies of 8-oxoguanine formation in isolated RNA. Arch Biochem Biophys. 1993;301:91–97. doi: 10.1006/abbi.1993.1119. [DOI] [PubMed] [Google Scholar]
- Seow HA, Penketh PG, Baumann RP, Sartorelli AC. Bioactivation and resistance to mitomycin C. Methods Enzymol. 2004;382:221–233. doi: 10.1016/S0076-6879(04)82012-3. [DOI] [PubMed] [Google Scholar]
- Siddique YH, Afzal M. Antigenotoxic effect of apigenin against mitomycin C induced genotoxic damage in mice bone marrow cells. Food Chem Toxicol. 2009;47:536–539. doi: 10.1016/j.fct.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Silva JA, Bomfim RR, Estevam Cdos S, Antoniolli AR, Araújo AA, Thomazzi SM. Pharmacological properties of lichen Cladonia clathrata. Pharm Biol. 2010;48:745–752. doi: 10.3109/13880200903273914. [DOI] [PubMed] [Google Scholar]
- Süleyman H, Odabasoglu F, Aslan A, Cakir A, Karagoz Y, Gocer F, Halici M, Bayir Y. Anti-inflammatory and antiulcerogenic effects of the aqueous extract of Lobaria pulmonaria (L.) Hoffm. Phytomedicine. 2003;10:552–557. doi: 10.1078/094471103322331539. [DOI] [PubMed] [Google Scholar]
- Svoboda D. Evaluation of the European method for mapping lichen diversity (LDV) as an indicator of environmental stress in the Czech Republic. Biologia. 2007;62:424–431. doi: 10.2478/s11756-007-0085-5. [DOI] [Google Scholar]
- Tanas S, Odabasoglu F, Halici Z, Cakir A, Aygun H, Aslan A, Suleyman H. Evaluation of anti-inflammatory and antioxidant activities of Peltigera rufescens lichen species in acute and chronic inflammation models. J Nat Med. 2010;64:42–49. doi: 10.1007/s11418-009-0367-z. [DOI] [PubMed] [Google Scholar]
- Tomasz M. Mitomycin C: small, fast and deadly (but very selective) Chem Biol. 1995;2:575–579. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Turkez H, Dirican E. A modulator against mercury chloride-induced genotoxic damage: Dermatocarpon intestiniforme (L.) Toxicol Ind Health. 2012;28:58–63. doi: 10.1177/0748233711404036. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F. The anti-genotoxic effect of taurine on aluminum sulphate induced DNA damage in human peripheral lymphocytes. IUFS J Biol. 2010;69:25–32. [Google Scholar]
- Turkez H, Togar B. The genotoxic and oxidative damage potential of olanzapine in vitro. Toxicol Ind Health. 2010;26:583–588. doi: 10.1177/0748233710373090. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoğlu F, Keleş MS. Biochemical response to colloidal bismuth subcitrate: dose-time effect. Biol Trace Elem Res. 2005;105:151–158. doi: 10.1385/BTER:105:1-3:151. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Aslan A, Karagöz Y, Türkez O, Anar M. Antimutagenic effects of lichen Pseudovernia furfuracea (L.) Zoph. extracts against the mutagenicity of aflatoxin B1 in vitro. Toxicol Ind Health. 2010;26:625–631. doi: 10.1177/0748233710377779. [DOI] [PubMed] [Google Scholar]
- Turkez H, Aydin E, Sisman T, Aslan A (2012a) Role of Peltigera rufescens (Weis) Humb. (a lichen) on imazalil-induced genotoxicity: analysis of micronucleus and chromosome aberrations in vitro. Toxicol Ind Health (doi:10.1177/0748233711414615) (in press) [DOI] [PubMed]
- Turkez H, Geyikoglu F, Mokhtar YI, Togar B. Eicosapentaenoic acid protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced hepatic toxicity in cultured rat hepatocytes. Cytotechnology. 2012;64:15–25. doi: 10.1007/s10616-011-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Yousef MI, Geyikoglu F (2012c) Propolis protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity in rat hepatocytes. Food Chem Toxicol (doi:10.1016/j.fct.2011.09.018) (in press) [DOI] [PubMed]
- Weissman L, Fraiberg M, Shine L, Garty J, Hochman A. Responses of antioxidant in the lichen Ramalina lacera may serve as an early-warning bioindicator system for the detection of air pollution stres. FEMS Microbiol Ecol. 2006;58:41–53. doi: 10.1111/j.1574-6941.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- Wirth V (1995) Die Flechten Baden-Würrttembergs, Teil 1-2. Ulmer, Stuttgart, p 1006
- Yazıcı K, Aslan A. Distribution of epiphytic lichens and air pollution in the city of Trabzon, Turkiye. Bull Environ Contam Toxicol. 2006;77:838–845. doi: 10.1007/s00128-006-1220-7. [DOI] [PubMed] [Google Scholar]
- Yim TK, Wu WK, Mak DH, Ko KM. Myocardial protective effect of an anthraquinone-containing extract of Polygonum multiflorum ex vivo. Planta Med. 1998;64:607–611. doi: 10.1055/s-2006-957531. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Watanabe K, Takahashi S, Ichikawa K. Protective effects of HFE7A, mouse anti-human/mouse Fas monoclonal antibody against acute and lethal hepatic injury induced by Jo2. Cytotechnology. 2010;62:313–323. doi: 10.1007/s10616-009-9244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]