Abstract
The most potent of the dioxins, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), is a persistent and ubiquitous environmental contaminant. And the health impact of exposure to TCDD is of great concern to the general public. Recent data indicate that l-glutamine (Gln) has antioxidant properties and may influence hepatotoxicity. The objective of the present study was undertaken to explore the effectiveness of Gln in alleviating the hepatotoxicity of TCDD on primary cultured rat hepatocytes. Gln (0.5, 1 and 2 mM) was added to cultures alone or simultaneously with TCDD (0.005 and 0.01 mM). The hepatocytes were treated with TCDD and Gln for 48 h. Then cell viability was detected by [3-(4,5-dimethyl-thiazol-2-yl) 2,5-diphenyltetrazolium bromide] (MTT) assay and lactate dehydrogenase (LDH) release, while total antioxidant capacity (TAC), total glutathione (TGSH) and total oxidative stress (TOS) levels were determined to evaluate the oxidative injury. The DNA damage was also analyzed by liver micronucleus assay (MN) and 8-oxo-2-deoxyguanosine (8-OH-dG). The results of MTT and LDH assays showed that TCDD decreased cell viability but not l-glutamine. TCDD also increased TOS level in rat hepatocytes and significantly decreased TAC and TGSH levels. On the basis of increasing doses, the dioxin in a dose-dependent manner caused significant increases of micronucleated hepatocytes (MNHEPs) and 8-OH-dG as compared to control culture. Whereas, in cultures exposured with Gln alone, TOS levels were not changed and TAC and TGSH together were significantly increased in dose-dependent fashion. The presence of Gln with TCDD modulated the hepatotoxic effects of TCDD on primary hepatocytes cultures. Noteworthy, Gln has a protective effect against TCDD-mediated DNA damages. As conclusion, we reported here an increased potential therapeutic significance of l-glutamine in TCDD-mediated hepatic injury for the first time.
Keywords: l-Glutamine, TCDD, Liver, Cell viability, Micronucleus assay, 8-OH-dG, Antioxidant capacity, Oxidative stress, Rat hepatocytes, DNA
Introduction
Polychlorinated dibenzo-p-dioxins are persistent environmental pollutants. The most potent congener, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), acting through aryl hydrocarbon receptor (AhR)-mediated signalling pathways, produces various toxic and biochemical effects, such as reproductive and developmental defects, immunotoxicity, liver damage, wasting syndrome, and cancer (Lu et al. 2011). There is consistency between humans and rodents in the target organs affected by dioxins (liver, oral cavity, cardiovascular system, immune system, thyroid, pancreas, and lung) (Yoshizawa et al. 2007). Induction of cytochrome P450 1a1 (Cyp1a1), via binding of the AhR to the dioxin response element (DRE) in the Cyp1a1 gene, is a major cause of oxidative stress and wasting syndrome after dioxin exposure (Parke et al. 1991; Nebert et al. 2000; Kopf et al. 2010). Treatment of C57BL/6 mice with TCDD (15 μg/kg, ip) increases mitochondrial ROS, which is dependent on the AhR; however, this low-toxic dose of TCDD also increases reduced glutathione (GSH) in liver mitochondria (Senft et al. 2002).
The generation of reactive oxygen species (ROS) can lead to oxidative stress, cell damage, and disease. A hallmark of oxidative stress is lipid peroxidation, which disrupts the structural integrity of cell membranes and can also lead to the formation of aldehydes, which in turn further damage lipids, protein, and DNA. Cells possess defense mechanisms to protect against free radical damage including antioxidant enzymes (superoxide dismutase; SOD, catalase; (CAT) and glutathione peroxidase; GSH-Px) and glutathione which scavenge free radicals to form non-radical products (Kern et al. 2002).
ROS are produced in response to exposure to environmental toxins, such as TCDD (Turkez et al. 2012a, b). In experimental animals, TCDD exposure damaged a number of target organs including liver, thymus, and adipose tissue (Kern et al. 2002; Turkez et al. 2012a, b). After the administration of TCDD to rodents changes were observed which associated with increased oxidative stress, including increased superoxide formation, lipid peroxidation (Kern et al. 2002), and DNA single strand breaks (Shertzer et al. 1998). In addition, another study (Slezak et al. 2000) demonstrated that indices of oxidative stress were present after both acute and subchronic administration of TCDD to mice, but the ROS production required only low tissue levels of TCDD in the subchronic exposure mice. Hence, there is an increasing interest to find the appropriate agents with antioxidative protection for hepatocyte cells. On the other hand, there are equivocal findings of chromosomal aberrations in humans exposed in vivo to TCDD (IARC 1997) and the increases in production 8-OH-dG in the liver of mice (Hung et al. 2006). Today, it is reported that TCDD-like chemicals alter expression of numerous genes in liver, but it is still unclear which pathways lead to major toxicities such as hepatotoxicity, wasting and lethality (Forgacs et al. 2010; Moffat et al. 2010).
In certain situations, people require the reasonable substitution of different dietary components. These include, amongst other things, the amino acid glutamine, which plays a central role in this study. Gln is the most occurring free amino acid found in the human body (Rohde et al. 1996). The nonessential amino acid Gln has recently been the focus of extensive scientific interest because of its importance in cell and tissue cultures and its physiologic role in animals and humans. Abundant evidence suggests that Gln may become a “conditionally essential” amino acid in the critically ill (Lacey and Wilmore 1990). Gln has been shown to restore muscle mass in cachexia due to cancer, rheumatoid arthritis, AIDS, and in critically ill trauma patients (Kuhls et al. 2007; Berk et al. 2008). Depletion of Gln stores can lead to severe complications, such as infection, poor wound healing, impaired immunity, increased intestinal permeability, and finally multiple organ failure (Müller et al. 2010; Abraham and Isaac 2011). Gln appears to be a unique amino acid, serving as a preferred respiratory fuel for rapidly proliferating cells; a regulator of acid–base balance through the production of urinary ammonia; a carrier of nitrogen between tissues; and an important precursor of nucleic acids, nucleotides, amino sugars, and proteins (Bort et al. 2010; Slivac et al. 2010). Gln is a key amino acid used to produce urea in liver (Darmaun 2000). The protective effect of Gln is already known and it was demonstrated a remarkable dependence of the hepatocyte function (Yang et al. 2010). It was also indicated that Gln via increasing activity of antioxidant enzymes and/or GSH level decreases oxidative stress (Matés et al. 2002; Kawada et al. 2009). However, no attention was paid to the effects of supplementation with Gln in hepatoprotection against TCDD; and also the information regarding Gln upon micronucleus (MN) formations in hepatocyte cells remains unknown. Since, in recent years, several natural formulations relating to reducing or eliminating TCDD toxicity have been in focus (Kwon et al. 2004), in our present study, we examined the effects of supplementation with Gln in TCDD-induced hepatotoxicity on the viability of cells (with LDH and MTT assays). We also evaluated for the first time the role of supplementation with Gln on antioxidant capacity (with TAC, TGSH and TOS levels) and DNA damage (with MN rates and 8-OH-dG levels) after TCDD-treatment.
Materials and methods
Test compounds and chemicals
TCDD (CAS No. 1746-01-6) and l-glutamine (Gln, CAS No. 56-85-9) was purchased from Sigma-Aldrich® (USA). All other chemicals that used in experiments were also purchased from Sigma-Aldrich® and Fluka® (Germany).
Animals
Male rats of Sprague–Dawley strain (from Medical Experimental Research Center, Ataturk University, Turkey), of 200–300 g body weight, were used throughout the present studies. They were allowed water and standard laboratory chow ad libitum and were maintained under standard light, temperature, and relative humidity conditions. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NRC 1996). The study protocol was approved by the local ethical committee.
Hepatocyte isolation and cultivation
Rats were sacrificed by CO2 overdose, and the livers were removed immediately. Isolated hepatocytes from rats were prepared by the collagenase perfusion technique (Wang et al. 2002). The liver was perfused through the hepatic portal vein with calcium-free Hanks balanced salt solution to remove blood for about 10 min at a flow rate of 2.5 mL/min. As soon as the liver became grayish brown in color, a second buffer solution containing collagenase (Hank’s balanced salt supplemented with 4 mM calcium chloride and 0.5 mg collagenase/mL) was perfused at the same rate until the liver appeared to have broken up. After treatment the liver was minced into 3- to 4-mm pieces with a sterile scalpel. Following mechanical dissociation, the cells were filtered through a gauze and centrifuged at 1,350 rpm for 5 min. Then, the hepatocytes were collected in Dulbecco's modified eagle medium (DMEM) containing bovine serum albumin (0.1%) (Sigma Chemical Co., St. Louis/MO, USA) and bovine insulin (5μg/mL). The cell suspension was filtered through a gauze again and allowed to sediment for 20 min to eliminate cell debris, blood, and sinusoidal cells. The cells were then washed three times by centrifugation at 50 g, tested by Trypan blue dye exclusion for viability (always in the range of 82–93 %). The hepatocytes were then suspended in a mixture of 75 % (v/v) Eagle’s minimum essential medium and 25 % (v/v) medium 199, supplemented with 10 % (v/v) fetal calf serum containing streptomycin (100 μg/mL), penicilin (100 IU/mL), bovine insulin (5 µg/mL), bovine serum albumin (1 mg/mL) and NaHCO3 (2.2 mg/L). For the experimental procedure, hepatocytes were plated in multiwell tissue culture plates (3 × 105 cells in a well area of 3.8 cm2; 8 × 105 cells in a well area of 9.6 cm2). The medium was changed 3–4 h later. The effect of TCDD and Gln was studied after 48 h of exposure in cultures maintained with a medium deprived of fetal calf serum but supplemented with hydrocortisone hemisuccinate (for inducing differentiation of the hepatocyte-like cells into more granular cells) (7 × 10−7M) (Rakba et al. 1999). Hepatocytes were cultured for an additional 8 h before treatment.
Treatments
After 8 h of plating, when primary hepatocytes got adhered and attained their epithelial morphology, culture medium was aspirated and replaced with an equal volume of medium supplemented with different concentrations of TCDD (0.005 and 0.01 mM) and Gln (0.5, 1 and 2 mM; physiological concentration and its two and four times concentrations) kept in 5 % CO2 incubator for 48 h (n = 6). This protocol is based on the works of Bechoua et al. (1999) and Katic et al. (2010).
MTT assay
Viability of cells was assessed by measuring the formation of formazan from 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) in a spectrophotometrically test. Hepatocytes were incubated with 0.7 mg/mL MTT for 30 min at 37 °C at the end of the experiment. After washing with PBS the blue formazan was extracted from cells with isopropanol/formic acid (95:5) and spectrophotometric signal was determined at 560 nm (Lewerenz et al. 2003). Cell viability rate was calculated as the percentage of MTT absorption as follows:
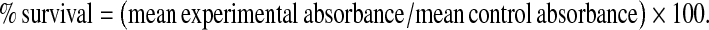 |
Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) activity was measured in the culture medium as an index of cytotoxicity, employing an LDH kit (Bayer Diagnostics®, France) adapted to the auto analyzer (ADVIA 1650, USA). Enzyme activity was expressed as the extracellular LDH activity percentage of the total activity on the plates.
Total antioxidant capacity and total oxidant status assays
The major advantage of TAC test is to measure the antioxidant capacity of all antioxidants in a biological sample and not just the antioxidant capacity of a single compound. In this test, antioxidants in the sample reduce dark blue-green colored ABTS radical to colorless reduced ABTS form. The change of absorbance at 660 nm is related with the total antioxidant level of the sample. The assay is calibrated with a stable antioxidant standard solution which is traditionally named as Trolox Equivalent that is a vitamin E analog. In TOS assay performed here, oxidants present in the sample oxidize the ferrous ion-chelator complex to ferric ion. The oxidation reaction is prolonged by enhancer molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with chromogen in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (μmol H2O2 Equiv./L). The automated Trolox equivalent total antioxidant capacity (TAC) and total oxidant status (TOS) assays were carried out in samples obtained from hepatocyte cultures after 48 h of incubation by commercially available kits (Rel Assay Diagnostics®, Gaziantep, Turkey).
Total glutathione
The glutathione content (GSH) of cell suspensions was determined by the 5, 5′-dithio-bis (2-nitrobenzoic acid)-glutathione disulfide (DTNB-GSSG) reductase recycling assay. Briefly, 200 μL of cell suspension was added to 200 μL of 10% (w/v) 5-sulfosalicylic acid for protein precipitation and centrifuged 2 min at 12,000 rpm. Supernatant aliquots were taken out for measurement of total glutathione (TGSH) following the DTNB oxidation at 415 nm and compared with a standard curve (Lima et al. 2004).
Liver micronucleus assay
Liver micronucleus (MN) assay was done by using the method of Suzuki et al. (2009). Immediately prior to evaluation, 10–20 μL of hepatocyte suspension was mixed with an equal volume of acridine orange (AO)—4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) stain solution (AO, 0.5 mg/mL; DAPI, 10 μg/mL) for fluorescent staining. Approximately 10–20 μL of the mixture was dropped onto a glass slide and covered with a cover glass. Samples of well-isolated hepatocytes were evaluated with the aid of a fluorescence microscope counting the number of MNHEPs in 2000 hepatocytes for each animal. MNHEPs were defined as hepatocytes with round or distinct MNs that stained like the nucleus, with a diameter 1/4 or less than that of the nucleus, and confirmed by focusing up and down, taking into account hepatocyte thickness. The test was performed by one observed.
Nucleic acid oxidation
After treating hepatocytes with TCDD and Gln, samples were lysed with lysis buffer. They were then incubated with RNase A (200 μg/mL) for 1 h at 37 °C and proteinase K for 18–24 h at 50 °C. The DNA was extracted with phenol–chloroform–isoamyl alcohol and precipitated with absolute alcohol; then the DNA was dissolved in sterile water, and the concentration was determined with a UV spectrophotometer at 260 nm. After adjusting the DNA concentration to 100 μg DNA/50 μl sterile water and heating to 100 °C for 30 min to denature the DNA, 2 μL sodium acetate and 1 μL nuclease P1 (5 mg/mL) were added to the DNA and heated to 37 °C for 1 h. Then 16 μL Tris–HCl (1 M, pH 7.2) and 1.3 units of alkaline phosphatase (type III from Escherichia coli) were added and reacted at 37 °C for 1 h. Finally, the amount of 8-OH-dG was measured by high-performance liquid chromatography (HPLC) with electrochemical detection as described previously (Floyd et al. 1986; Schneider et al. 1993; Hwang et al. 2005).
Statistical analysis
The experimental data were analyzed using one-way analysis of variance (ANOVA) and Fischer’s least significant difference (LSD) tests to determine whether any treatment significantly differed from the controls or each others. Results presented as mean ± SD values and the level of 0.05 was regarded as statistically significant.
Results
In vitro cellular toxicity
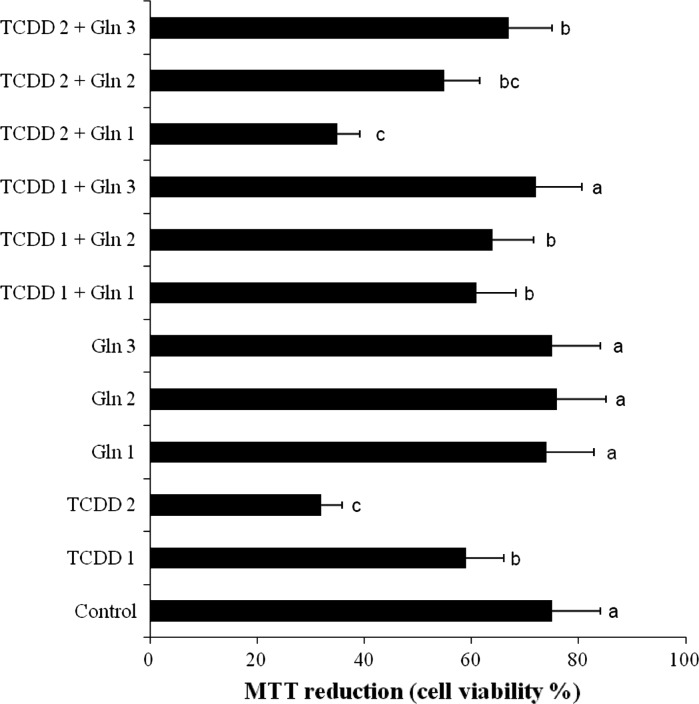
The results of cell viability measured by MTT assay is shown in Fig. 1. When assayed in vitro on the hepatocyte cells using the MTT assay, the values for the 0.005 and 0.01 mM TCDD-treated cells ranged from 1.3 to 2.4 fold lower than that for the control cell line, respectively. However, the 0.5, 1 and 2 mM doses of Gln provided in vitro activities on cell viability against the tested TCDD compound and no cytotoxicity was detected for normal cells (non TCDD treated).
Fig. 1.
Hepatoprotective effect of Gln on TCDD induced toxicity in cultured rat hepatocytes by the MTT assay. TCDD 1: 0.005 mM TCDD; TCDD 2: 0.01 mM TCDD; Gln 1: 0.5 mM l-glutamine; Gln 2: 1 mM l-glutamine; Gln 3: 2 mM l-glutamine; means (n = 6) in the figure followed by the different letters present significant differences from each other at the p < 0.05 level
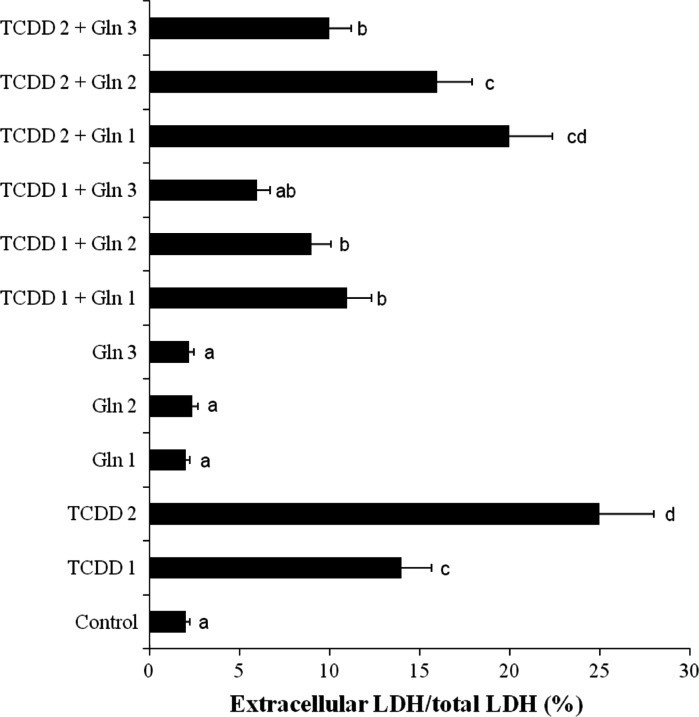
TCDD-induced hepatocellular damage was clearly evidenced by increases in LDH levels compared with the observations of controls (Fig. 2). Although LDH was not affected by Gln doses alone, decrease of the levels of enzyme reached statistical significance at all doses of l-glutamine against TCDD toxicity.
Fig. 2.
Hepatoprotective effect of Gln on TCDD induced toxicity in cultured rat hepatocytes by the LDH assay. TCDD 1: 0.005 mM TCDD; TCDD 2: 0.01 mM TCDD; Gln 1: 0.5 mM l-glutamine; Gln 2: 1 mM l-glutamine; Gln 3: 2 mM l-glutamine; means (n = 6) in the figure followed by the different letters present significant differences from each other at the p < 0.05 level
Biochemical tests
Table 1 shows the effects of Gln on biochemical parameters in the tissue cells for all experimental groups. The hepatic TAC and TGSH levels decreased (p < 0.05) in the 0.005 and 0.01 mM TCDD treated groups. On the contrary, TOS increased in cells cultured under the effect of TCDD. The hepatocytes of control groups maintained optimal value of the antioxidant status. On the other hand, the cells treated with 0.5, 1 and 2 mM Gln alone showed increases in the levels of antioxidant capacity. However, TOS levels were unchanged in both control and Gln groups. Moreover, application of Gln at all doses significantly (p < 0.05) increased the reduced ratios of TAC and GSH by TCDD.
Table 1.
The oxidative assessments in hepatocyte cultures exposed simultaneously to TCDD and Gln
| Treatments | TAC (mmol Trolox Equiv./L) | TOS (μmol H2O2 Equiv./L) | TGSH content (nmol/million cells) |
|---|---|---|---|
| Control | 5.04 ± 0.47c | 8.26 ± 2.14a | 41.54 ± 6.22c |
| TCDD 1 | 4.11 ± 0.38b | 11.82 ± 2.61c | 35.65 ± 5.17b |
| TCDD 2 | 3.40 ± 0.43a | 16.41 ± 2.93d | 29.53 ± 5.33a |
| Gln 1 | 5.21 ± 0.45c | 8.32 ± 2.07a | 43.29 ± 7.11c |
| Gln 2 | 5.64 ± 0.28d | 8.27 ± 2.22a | 48.61 ± 5.54d |
| Gln 3 | 5.96 ± 0.52d | 8.36 ± 2.14a | 56.65 ± 5.83e |
| TCDD 1 + Gln 1 | 4.30 ± 0.44b | 10.81 ± 2.68bc | 37.93 ± 5.77b |
| TCDD 1 + Gln 2 | 4.59 ± 0.38bc | 10.24 ± 2.27b | 40.86 ± 6.55c |
| TCDD 1 + Gln 3 | 4.97 ± 0.51c | 9.78 ± 2.44b | 43,25 ± 5.73c |
| TCDD 2 + Gln 1 | 3.87 ± 0.42ab | 15.20 ± 3.27 cd | 33.42 ± 4.60b |
| TCDD 2 + Gln 2 | 4.22 ± 0.43b | 13.41 ± 3.08c | 34.91 ± 6.13b |
| TCDD 2 + Gln 3 | 4.35 ± 0.47b | 10.96 ± 2.78bc | 37.24 ± 5.68b |
TCDD 1: 0.005 mM TCDD; TCDD 2: 0.01 mM TCDD; Gln 1: 0.5 mM l-glutamine; Gln 2: 1 mM l-glutamine; Gln 3: 2 mM l-glutamine; means (n = 6) in the table followed by the different letters present significant differences from each other at the p < 0.05 level
Genotoxicity assessment
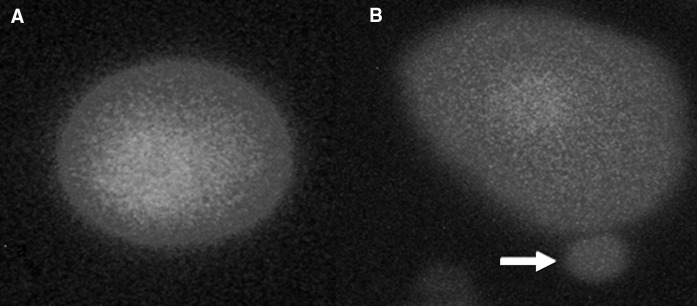
Table 2 shows the results of the liver MN assay in cultured rat hepatocytes. The tested doses of TCDD induced statistically significant increases in formations of MNHEPs although Gln did not change the MNHEP numbers (at all doses) as compared to control group. Also, Gln minimized the increased MNHEPs rates by TCDD in a clear dose dependent manner (Fig. 3).
Table 2.
Hepatoprotective effect of Gln on TCDD induced toxicity in cultured rat hepatocytes by the liver MN assay
| Treatments | MNHEP (%)/2000 HEP |
|---|---|
| Control | 0.25 ± 0.08a |
| TCDD 1 | 0.86 ± 0.27c |
| TCDD 2 | 1.74 ± 0.35d |
| Gln 1 | 0.26 ± 0.14a |
| Gln 2 | 0.29 ± 0.09a |
| Gln 3 | 0.22 ± 0.11a |
| TCDD 1 + Gln 1 | 0.79 ± 0.27c |
| TCDD 1 + Gln 2 | 0.61 ± 0.22bc |
| TCDD 1 + Gln 3 | 0.43 ± 0.19b |
| TCDD 2 + Gln 1 | 1.39 ± 0.45cd |
| TCDD 2 + Gln 2 | 1.01 ± 0.47cd |
| TCDD 2 + Gln 3 | 0.88 ± 0.43c |
HEP hepatocyte, MNHEPs number of micronucleated hepatocytes. TCDD 1: 0.005 mM TCDD; TCDD 2: 0.01 mM TCDD; Gln 1: 0.5 mM l-glutamine; Gln 2: 1 mM l-glutamine; Gln 3: 2 mM l-glutamine; means (n = 6) in the table followed by the different letters present significant differences from each other at the p < 0.05 level
Fig. 3.
a Sample hepatocyte from 2 mM Gln-treated culture, b Sample hepatocyte from 0.01 mM TCDD-treated culture (arrow shows MN formation)
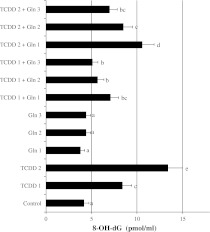
The status of 8-OH-dG in liver cells of control and experimental groups was presented in Fig. 4. Firstly, the hepatocyte levels of 8-OH-dG, a sensitive marker of oxidative DNA damage, were quantified with regard to TCDD administration. It was observed that TCDD significantly increased 8-OH-dG concentrations in the liver cell. Whereas, Gln at 0.5, 1 and 2 mM did not have any effect on the level of 8-OH-dG. Moreover, the Gln doses significantly decreased 8-OH-dG concentrations in TCDD-treated hepatocytes.
Fig. 4.
8-OH-dG adducts in rat hepatocyte cultures in the presence of TCDD, Gln and their combinations. TCDD 1: 0.005 mM TCDD; TCDD 2: 0.01 mM TCDD; Gln 1: 0.5 mM l-glutamine; Gln 2: 1 mM L-glutamine; Gln 3: 2 mM L-glutamine; means (n = 6) in the figure followed by the different letters present significant differences from each other at the p < 0.05 level
Discussion
This study examined the effects of TCDD on markers of oxidative stress, DNA damage, cell viability and hepatotoxicity in rat hepatocyte cultures. Because liver is the major organ involved in detoxification, and an important organ in the generation of ROS, hepatocytes were examined after the addition of TCDD in vitro. Also, we examined the effect of Gln treatment on hepatic injury resulting from TCDD. Our results indicated that although Gln prevented hepatic damage, it especially could play a beneficial role by preventing MNHEPs and changes in antioxidant status.
In present study, the TCDD elicited severe instances of hepatocyte damage (increasing LDH). Whereas, Gln at high dose has a pronounced effect on the relative potency of TCDD. The LDH in serum as a biological marker for liver damage increases (Park et al. 2010). Cell necrosis leads to a rise in concentration of the LDH enzyme in serum and tissue. The LDH released into the medium provides an index of cell death and membrane permeability to LDH, and an increase in LDH activity in the medium occurs as a result of cell membrane disintegration and enzyme leakage (Yokogawa et al. 2004). In addition, cytotoxicity, the degree to which a chemical could cause cell damage, was assessed in this study by the means of MTT assay. As shown in Fig. 1, the MTT assay results revealed that TCDD was cytotoxic to hepatocyte cells. Overall, TCDD significantly decreased (p < 0.05) the viability of hepatocytes. Consistent with our finding, MTT assay demonstrated that the viability of human adrenocortical, pancreatic and mammary cells is significantly decreased in after TCDD treatment (Bradshaw et al. 2002).
The physiological plasma levels of Gln were reported to be between 0.5 and 0.7 mM in humans (Oehler et al. 2002). Dose dependent protective effects of 0.5, 1 and 2 mM Gln supplementations were observed in human cultured lymphocytes by Greig et al. (2001). Again the results of animal experiments indicated that 1.25 mM Gln supplementation could exhibit cellular protectivity (Khogali et al. 2002). In the present study, Gln via increasing levels of TAC and GSH decreased oxidative stress in relation to the applied dose. In fact, the highest dose of Gln (2 mM) was determined more effective than 0.5 and 1 mM Gln. This novel finding may explain in part Gln protective effects. As a matter of fact, Gln serves as substrate for glutathione synthesis and was found diminished with Gln withdrawal that is associated with an increase in ROS levels (Gao et al. 2009). The relative potency of TCDD is also estimated by comparing the different dose levels for a particular response.
Previous studies have suggested that oxidative stress following production of ROS plays a role in TCDD-induced toxicity (Slezak et al. 2000), while others have observed superoxide production, lipid peroxidation, and oxidative damage in fetal and placental tissues from mammals upon treatment with TCDD (Hassoun et al. 2000). The results of Sul et al. (2009) revealed that treatment with 100, 300, 500 and 1,000 nM TCDD decreased the viability of neuroblastoma (N2a) cells and increased DNA damage in a dose-dependent manner. Additionally, a malondialdehyde (MDA) assay was performed to determine if TCDD induces lipid peroxidation. The results of this assay revealed that 100, 300 and 500 nM TCDD induced lipid peroxidation in a dose-dependent manner. These results indicate that treatment of N2a cells with TCDD increases lipid peroxidation and induce oxidative stress. Long-term exposure of mice to TCDD resulted in the induction of biomarkers of oxidative stress, including production of reactive oxygen species and lipid peroxidation in the hepatic tissues of mice (Hassoun et al. 2000).
It has been suggested that free radicals and ROS are quenched by amino acids, such as Gln (Roth et al. 2002). Therefore, under conditions of increased oxidative stress, dietary antioxidants become critical in maintaining a desirable oxidant: antioxidant balance. Our results revealed that Gln alone in hepatic tissue exhibited significant antioxidant activity. Increased TAC observed herein, was in agreement with previous rat models for liver (Szijártó 2008). The present study explored the ability of 0.01 mM dose as a protective agent that induces improvement of antioxidant capacity against TCDD. Gln supplementation resulted in particular protection of antioxidant status in hepatocytes after TCDD treatments, since: (1) the ratios of TAC and TGSH in Gln/TCDD hepatocytes were re-established to control/Gln levels; (2) significant decreases were observed in the level of TOS in the hepatocytes of Gln and TCDD applied cultures. Gln in increasing dose showed induction of TAC and GSH in TCDD models, with values of 3.4–18.3 % and 4.2–36.4 %, respectively. This means that, Gln had significantly high antioxidant activity in hepatocyte cultures. This is not surprising since Gln has been reported as an efficient scavenger and quencher of ROS in lipid bilayers (Kumar and Anandan 2007). Therefore, Gln provides enhancement of the immune response (Müller et al. 2010) and protection against diseases such as cancer through scavenging of oxygen radicals (Thébault et al. 2010). However, the exact mechanisms of the protective effect of Gln against injury of organs and tissues are still incompletely understood (Jia et al. 2006).
Our present findings suggested that the total antioxidant capacity from Gln was due to GSH and/or enhance enzymatic antioxidant defense against TCDD. Because, total antioxidant capacity comes from non-enzymatic antioxidants like glutathione (GSH), as well as enzymatic ones such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) (Murri et al. 2010). GSH, is one of the most abundant intracellular antioxidants in animal cells (Kidd 1997), detoxifies endogenous ROS and provides protection against oxidative damage (Schauer et al. 2004). In female B6C3F1 mice, exposure to TCDD results in a significant depletion of GSH by oxidative stress in liver (Slezak et al. 2000). The observed decreases in GSH-related enzyme activities indicated that TCDD may induce oxidative stress in rat hepatocytes at relevant low doses of TCDD by altering GSH metabolic mechanisms at the cellular level. GSH-Px enzyme metabolizes peroxides such as H2O2 and protects cell membranes from lipid peroxidation (Twaroski et al. 2001). It is suggested that amino acids may protect against different aspects of toxicity by serving as precursors for glutathione, or by converting to tricarboxylic acid cycle intermediates for energy production (Ralph et al. 2010). The energy potential of Gln is almost equivalent to that of glucose (Nelson and Cox 2008). The liver is the major organ of Gln consumption. Gln provides carbons for energy and gluconeogenesis, nitrogen for ureagenesis, and substrates for nucleotide and glutathione biosynthesis in order to support cell repair and detoxification reactions (Austgen et al. 1991). Gln was reported to be responsible for NADPH and ATP production. Since Gln was a precursor of GSH, its supplementation in the clinical diet could be used to maintain high levels of GSH and to avoid oxidative stress damage (Amores-Sánchez and Medina 1999). Again, increased utilization of cellular Gln delayed and prevented neutrophil apoptosis (Tihan et al. 2011). As a matter of fact, the antiapoptotic effects of Gln on T cells were investigated and it was found that Gln not only regulated intracellular oxidative balance but also precluded especially T cell apoptosis (Chang et al. 2002). In accordance to this finding, a statistically significant increase in bcl-2 gene expression was thought to be the most important step of apoptosis preventing the effect of Gln in the period following its entry into the cell (Pithon-Curi et al. 2003; Ran et al. 2004; Tihan et al. 2011). Again, it was determined that Gln administration to damaged tissues regulated release of free radicals (Kudsk 2002; Peng et al. 2004). Gln has been shown to protect gut mucosa against oxidative damage through the induction of heat-shock protein 70 (HSP70) while HSP induction has a significant protective effect against liver injury (Saad et al. 1995). Gln has also been found to reduce the expression of pro-inflammatory cytokines in intestinal immune and peripheral blood mononuclear cells in patients with acute pancreatitis (de Beaux et al. 1998; Coeffier et al. 2001; Schuster et al. 2006). On the other hand, Gln supplementation rapidly improved the expression of proliferating cell nuclear antigen (PCNA) after cisplatin-induced intestinal mucosal injury (Tazuke et al. 2011). Tsai et al. (2011) suggested that dietary Gln supplementation decreased oxidative stress-related gene expression, increased the antioxidant potential and may consequently attenuate renal oxidative damage in rats with streptozotocin (STZ)—induced diabetes. On the contrary, enteral glutamine supplementation offered no advantage in patients with peritonitis or abdominal trauma (Kumar et al. 2007).
It is reported that the GSH content of cells becomes depleted if they are deprived of energy substrates. Thus, by providing an energy source, and by acting as a precursor to GSH, Gln may increase the GSH content of hepatocytes and protect them from toxic substances (Ralph et al. 2010). An in vitro study by Babu et al. (2001) found that Gln prevented damage to the liver, and this was possibly mediated by GSH synthesis. Gln-supplemented nutrition significantly preserved hepatic glutathione in an animal model of pre-infusion with Gln and hepatic injury (Hong et al. 1992). Roth et al. (2002) reported that there is a significant correlation between the Gln supply and intracellular GSH content. The administration of Gln reduced liver injury after bile duct ligation in a model of obstructive jaundice (Margaritis et al. 2005). In addition, preceding ingestion of a Gln suppressed liver injury in D-galactosamine-induced acute hepatitis (Komano et al. 2008). Glutamine has a significant effect on suppression of apoptosis due to the increase of antioxidant activity. The studies showed that Gln preserved total GSH levels after oxidative damage, making it a component of the cellular antioxidant defense (Jia et al. 2006).
Oxidative stress has been postulated to be one of the deleterious factors for liver, and it was also reported that antioxidant levels were significantly reduced in hepatic diseases (Gonzales et al. 2005). Oxidative DNA damage is closely associated with TCDD-induced liver cancer (Knerr and Schrenk 2006). Forgacs et al. (2010) suggested that TCDD altered the expression of genes associated with mitochondrial function including complexes I (NADH dehydrogenase), III (cytochrome c reductase), IV (cytochrome c oxidase), and V (ATP synthase) which might contribute to TCDD-elicited mitochondrial toxicity. ROS produced by activated macrophages and a consequent rise of lipid peroxidation caused direct activation of hepatic cells, leading to liver lesions (Svegliati et al. 1998). In our study, the level of TOS also significantly increased after treatment by TCDD compared to control cells. Consistent with our finding, TCDD increased oxidative stress in hepatic tissue of rats in vivo (Hassoun et al. 2000; Turkez et al. 2012a, b). The observations suggested that anti-oxidant therapy has an important role in preventing hepatic damages (Nakamura et al. 2010). Treatment with Gln seems to preserve the antioxidant capacity, to protect the structure and mitochondrial function and to decrease the production of free radicals. The effects of Gln may also be due to the hepatic synthesis of antioxidant enzymes, protecting the tissue from the action of free radicals in a hepatic ischemia–reperfusion and TCDD-induced injury models (Yang 1993; Turkez et al. 2012a, b). The antioxidant function of Gln has also been confirmed by decreasing the production of cytokines against liver injury (Urbina et al. 2004). Glutamine increased the activity of SOD in liver tissue after chemotherapy stress in TCDD model of rat (Xia and Wu 1997).
The dose dependency of TAC suppression in hepatocyte cultures is also driven by the range of dose–response data available in our study. The studies provide some evidence that TCDD toxicity generally is related to increasing dose in tissues (Slezak et al. 2000). The relative potency of TCDD is also estimated by comparing the different dose levels for a particular response. The dose dependency of TAC suppression in hepatocyte cultures is driven by the range of dose–response data available in our study. The detrimental effects in hepatic tissue of TCDD might also lead to disruption in the functional integrity of hepatocytes (Czepiel et al. 2010).
On the other hand, Gln catabolism was associated with decreased glutathione levels and antioxidant capacity, which correlated with increased apoptosis (Lora et al. 2004). Our results not only supported that Gln was potent hepatoprotective agent after MTT and LDH assays, but also explained the action on MNHEPs and 8-OH-dG of Gln. TCDD-induced oxidative stress caused 8-OH-dG and single strand breaks in DNA from liver and brain tissues of rats (Hassoun et al. 2000). In our investigation, 8-OH-dG significantly increased by the effect of TCDD. In addition, MN test was performed for the first time and revealed that TCDD exposure increased the rate of MNHEPs in hepatocytes. The Micronucleus assay is a reliable test to indicate chromosomal damage (Yilmaz et al. 2008), MNHEPs production as 8-OH-dG is one of the key pieces of evidence for the possible involvement of antioxidant activity in oxidative DNA damage (Tung-Kwang et al. 2010). ROS can alter vital cell components like polyunsaturated fatty acids, proteins and nucleic acids. The increased production of reactive oxygen species, lipid peroxidation, and DNA and membrane damage are always associated with TCDD exposure (Shertzer et al. 1998). Gln acted as a potent oxygen radical scavenger shown to be beneficial to carcinoma prognosis (Todorova et al. 2010). Recently, Gln has been reported to possess anticancer properties (Halder et al. 2010). Glutamine decreased DNA damage by protecting breast cancer cells from free radicals and reactive oxygen species generated by radiation in young healthy females (Cano et al. 2010). Because, it is an important precursor of nucleic acids. And the amide nitrogen of Gln is the major component for the biosynthesis of nucleotides (e.g. purines and pyrimidines for DNA and RNA) (Neu et al. 2002). Again, Gln is considered as an important precursor of amino sugars and proteins (Gao et al. 2009).
Collectively, Gln treatment offered beneficial effects against TCDD injury in hepatic tissue. The observed effect of Gln on antioxidant status, and DNA was reflected by a major protection against liver damage and hepatocyte lost. The data were essential to properly address the usage of Gln as a nutritional therapy for prevention of TCDD toxicity.
Contributor Information
Hasan Turkez, Email: hasanturkez@yahoo.com.
Fatime Geyikoglu, Phone: +90-442-2314333, FAX: +90-442-2360948, Email: geyikogluff@yahoo.com.
Mokhtar I. Yousef, Email: yousefmokhtar@yahoo.com
References
- Abraham P, Isaac B. The effects of oral glutamine on cyclophosphamide-induced nephrotoxicity in rats. Hum Exp Toxicol. 2011;30:616–623. doi: 10.1177/0960327110376552. [DOI] [PubMed] [Google Scholar]
- Amores-Sánchez MI, Medina MA. Glutamine, as a precursor of glutathione, and oxidative stress. Mol Genet Metab. 1999;67:100–105. doi: 10.1006/mgme.1999.2857. [DOI] [PubMed] [Google Scholar]
- Austgen TR, Chen MK, Flynn TC, Souba WW, Chen MK, Flynn TC, Souba WW. The effects of endotoxin on the splanchnic metabolism of glutamine and related substrates. J Trauma. 1991;31:742–751. doi: 10.1097/00005373-199106000-00003. [DOI] [PubMed] [Google Scholar]
- Babu R, Eaton S, Drake DP, Spitz L, Pierro A. Glutamine and glutathione counteract the inhibitory effects of mediators of sepsis in neonatal hepatocytes. J Pediatr Surg. 2001;36:282–286. doi: 10.1053/jpsu.2001.20690. [DOI] [PubMed] [Google Scholar]
- Bechoua S, Dubois M, Dominguez Z, Goncalves A, Nemoz G, Lagarde M, Prigent AF. Protective effect of docosahexaenoic acid against hydrogen peroxide-induced oxidative stress in human lymphocytes. Bioch Pharmacol. 1999;57:1021–1030. doi: 10.1016/S0006-2952(99)00012-X. [DOI] [PubMed] [Google Scholar]
- Berk L, James J, Schwartz A, Hug E, Mahadevan A, Samuels M, Kachnic L. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG0122) Support Care Cancer. 2008;16:1179–1188. doi: 10.1007/s00520-008-0403-7. [DOI] [PubMed] [Google Scholar]
- Bort JA, Stern B, Borth N. CHO-K1 host cells adapted to growth in glutamine-free medium by FACS-assisted evolution. Biotechnol J. 2010;5:1090–1097. doi: 10.1002/biot.201000095. [DOI] [PubMed] [Google Scholar]
- Bradshaw TD, Trapani V, Vasselin DA, Westwell AD. The aryl hydrocarbon receptor in anticancer drug discovery: friend or foe? Curr Pharm Des. 2002;8:2475–2490. doi: 10.2174/1381612023392784. [DOI] [PubMed] [Google Scholar]
- Cano KE, Li YJ, Chen Y. NMR metabolomic profiling reveals new roles of SUMOylation in DNA damage response. J Proteome Res. 2010;9:5382–5388. doi: 10.1021/pr100614a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WK, Yang KD, Chuang H, Jan JT, Shaio MF. Glutamine protects activated human T cells from apoptosis by up-regulating glutathione and Bcl-2 levels. Clin Immunol. 2002;104:151–160. doi: 10.1006/clim.2002.5257. [DOI] [PubMed] [Google Scholar]
- Coeffier M, Miralles-Barrachina O, Pessot F, Lalaude O, Daveau M, Lavoinne A. Influence of glutamine on cytokine production by human gut in vitro. Cytokine. 2001;13:148–154. doi: 10.1006/cyto.2000.0813. [DOI] [PubMed] [Google Scholar]
- Czepiel J, Biesiada G, Gajda M, Szczepański W, Szypuła K, Dabrowski Z, Mach T. The effect of TCDD dioxin on the rat liver in biochemical and histological assessment. Folia Biol. 2010;58:85–90. doi: 10.3409/fb58_1-2.85-90. [DOI] [PubMed] [Google Scholar]
- Darmaun D. Role of glutamine depletion in severe illness. Diab Nutr Metab. 2000;13:25–30. [PubMed] [Google Scholar]
- Beaux AC, O’Riordain MG, Ross JA, Jodozi L, Carter DC, Fearon KC. Glutamine-supplemented total parenteral nutrition reduces blood mononuclear cell interleukin-8 release in severe acute pancreatitis. Nutrition. 1998;14:261–265. doi: 10.1016/S0899-9007(97)00477-2. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Watson JJ, Wong PK, Altmiller DH, Rickard RC. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1:163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Forgacs AL, Burgoon LD, Lynn SG, LaPres JJ, Zacharewski T. Effects of TCDD on the expression of nuclear encoded mitochondrial genes. Toxicol Appl Pharmacol. 2010;246:58–65. doi: 10.1016/j.taap.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang T, Lee Y, Kita K, Ochi T, Zeller K, Marzo AM, Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23 enhances mitochondrial glutaminase and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales S, Polizio AH, Erario MA, Tomaro ML. Glutamine is highly effective in preventing in vivo cobalt-induced oxidative stress in rat liver. World J Gastroenterol. 2005;11:3533–3541. doi: 10.3748/wjg.v11.i23.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig JE, Keast D, Palmer TN. Effects of glutamine and ethanol in vitro on lymphocytes from human alcohol abusers and non-abusers. Addiction Biol. 2001;6:73–82. doi: 10.1080/13556210020020139. [DOI] [Google Scholar]
- Halder AK, Adhikary N, Maity MK, Jha T. Synthesis, pharmacological activity and comparative QSAR modeling of 1,5-N, N’-substituted-2-(substituted naphthalenesulphonyl) glutamamides as possible anticancer agents. Eur J Med Chem. 2010;45:1760–1771. doi: 10.1016/j.ejmech.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Li F, Abushaban A, Stohs SJ. The relative abilities of TCDD and its congeners to induce oxidative stress in the hepatic and brain tissues of rats after subchronic exposure. Toxicol. 2000;145:103–113. doi: 10.1016/S0300-483X(99)00221-8. [DOI] [PubMed] [Google Scholar]
- Hong RW, Rounds JD, Helton WS, Robinson MK, Wilmore DW. Glutamine preserves liver glutathione after lethal hepatic injury. Ann Surg. 1992;215:114–119. doi: 10.1097/00000658-199202000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YC, Huang GS, Sava VM, Blagodarsky VA, Hong MY. Protective effects of tea melanin against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity: antioxidant activity and aryl hydrocarbon receptor suppressive effect. Biol Pharm Bull. 2006;29:2284–2291. doi: 10.1248/bpb.29.2284. [DOI] [PubMed] [Google Scholar]
- Hwang JM, Tseng TH, Tsai YY, Lee HJ, Chou FP, Wang CJ, Chu CY. Protective effects of baicalein on tert-butyl hydroperoxide-induced hepatic toxicity in rat hepatocytes. J Biomed Sci. 2005;12:389–397. doi: 10.1007/s11373-005-1572-8. [DOI] [PubMed] [Google Scholar]
- IARC (1997) Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans, vol 69. International Agency for Research on Cancer, Lyon [PMC free article] [PubMed]
- Jia CJ, Dai CL, Zhang X, Cu K, Xu F, Xu YQ. Alanyl-glutamine dipeptide inhibits hepatic ischemia-reperfusion injury in rats. World J Gastroenterol. 2006;12:1373–1378. doi: 10.3748/wjg.v12.i9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katic J, Cemeli E, Baumgartner A, Laubenthal J, Bassano I, Stolevik SB, Granum B, Namork E, Nygaard UC, Lovik M, Leeuwen D, Loock KV, Anderson D, Fuci A, Decordier I. Evaluation of the genotoxicity of 10 selected dietary/environmental compounds with the in vitro micronucleus cytokinesis-block assay in an interlaboratory comparison. Food Chem Toxicol. 2010;48:2612–2623. doi: 10.1016/j.fct.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Kawada H, Kojima M, Kimura T, Natori S, Sasaki K, Sasaki H. Effect of 5-S-GAD on UV-B-induced cataracts in rats. Jpn J Ophthalmo. 2009;53:531–535. doi: 10.1007/s10384-009-0695-2. [DOI] [PubMed] [Google Scholar]
- Kern PA, Fishman RB, Song W, Brown AD, Fonseca V. The effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on oxidative enzymes in adipocytes and liver. Toxicol. 2002;171:117–125. doi: 10.1016/S0300-483X(01)00564-9. [DOI] [PubMed] [Google Scholar]
- Khogali SE, Pringle SD, Weryk BV, Rennie MJ. Is glutamine beneficial in ischemic heart disease? Nutrition. 2002;18:123–126. doi: 10.1016/S0899-9007(01)00768-7. [DOI] [PubMed] [Google Scholar]
- Kidd PM. Glutathione: systemic protectant against oxidative and free radical damage. Altern Med Rev. 1997;1:155–176. [Google Scholar]
- Knerr S, Schrenk D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol Nutr Food Res. 2006;50:897–907. doi: 10.1002/mnfr.200600006. [DOI] [PubMed] [Google Scholar]
- Komano T, Egashira Y, Sanada H. L-Gln and L-Ser suppress the D-galactosamine-induced IL-18 expression and hepatitis. Biochem Biophys Res Commun. 2008;372:688–690. doi: 10.1016/j.bbrc.2008.05.114. [DOI] [PubMed] [Google Scholar]
- Kopf PG, Scott JA, Agbor LN, Boberg JR, Elased KM, Huwe JK, Walker MK. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117:537–546. doi: 10.1093/toxsci/kfq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183:390–398. doi: 10.1016/S0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- Kuhls DA, Rathmacher JA, Musngi MD, Frisch DA, Nielson J, Barbe A, MacIntyre AD, Coates JE, Fildes JJ. Betahydroxy-beta-methylbutyrate supplementation in critically ill trauma patients. J Traum. 2007;62:125–132. doi: 10.1097/TA.0b013e31802dca93. [DOI] [PubMed] [Google Scholar]
- Kumar HS, Anandan S. Biochemical studies on the cardioprotective effect of glutamine on tissue antioxidant defense system in isoprenaline-induced myocardial infarction in rats. J Clin Biochem Nutr. 2007;40:49–55. doi: 10.3164/jcbn.40.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kumar R, Sharma SB, Jain BK. Effect of oral glutamine administration on oxidative stress, morbidity and mortality in critically ill surgical patients. Indian J Gastroenterol. 2007;26:70–73. [PubMed] [Google Scholar]
- Kwon YI, Yeon JD, Oh SM, Chung KH. Protective effects of ursodeoxycholic acid against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced testicular damage in mice. Toxicol Appl Pharmacol. 2004;194:239–247. doi: 10.1016/j.taap.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nut Rev. 1990;48:297–309. doi: 10.1111/j.1753-4887.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Lewerenz V, Hanelt S, Nastevska C, El-Bahay C, Rouhrdanz E, Kahl R. Antioxidants protect primary rat hepatocyte cultures against acetaminophen-induced DNA strand breaks but not against acetaminophen-induced cytotoxicity. Toxicol. 2003;191:179–187. doi: 10.1016/S0300-483X(03)00256-7. [DOI] [PubMed] [Google Scholar]
- Lima CF, Carvalho F, Fernandes E, Bastos ML, Santos-Gomes PC, Fernandes-Ferreira M, Pereira-Wilson C. Evaluation of toxic/protective effects of the essential oil of Salvia officinalis on freshly isolated rat hepatocytes. Toxicol In Vitro. 2004;18:457–465. doi: 10.1016/j.tiv.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Lora J, Alonso FJ, Segura JA, Lobo C, Márquez J, Matés JM. Antisense glutaminase inhibition decreases glutathione antioxidant capacity and increases apoptosis in Ehrlich ascitic tumour cells. Eur J Biochem. 2004;271:4298–4306. doi: 10.1111/j.1432-1033.2004.04370.x. [DOI] [PubMed] [Google Scholar]
- Lu H, Cui W, Klaassen CD. Nrf2 protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced oxidative injury and steatohepatitis. Toxicol App Pharmacol. 2011;256:122–135. doi: 10.1016/j.taap.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaritis VG, Filos KS, Michalak MA, Scop CD, Spiliopoulou I, Nikolopoulou VN, Vagianos CE. Effect of oral glutamine administration on bacterial tanslocation, endotoxemia, liver and ileal morphology, and apoptosis in rats with obstructive jaundice. World J Surg. 2005;29:1329–1334. doi: 10.1007/s00268-005-7721-4. [DOI] [PubMed] [Google Scholar]
- Matés JM, Pérez-Gómez C, Núñez de Castro I, Asenjo M, Márquez J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol. 2002;34:439–458. doi: 10.1016/S1357-2725(01)00143-1. [DOI] [PubMed] [Google Scholar]
- Moffat ID, Boutros PC, Chen H, Okey AB, Pohjanvirta R. Aryl hydrocarbon receptor (AHR)-regulated transcriptomic changes in rats sensitive or resistant to major dioxin toxicities. BMC Genomics. 2010;11:263–269. doi: 10.1186/1471-2164-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Topp T, Weismüller K, Kwapisz M, Engel J. The influence of upstream IL-2 -330 (T/G) and TNF-α -308 (A/G) polymorphisms on glutamine-supplemented cytokine release. J Transl Med. 2010;8:113–121. doi: 10.1186/1479-5876-8-113. [DOI] [PubMed] [Google Scholar]
- Murri M, Garcia-Delgado R, Alcázar-Ramirez J, Linde F, Fernández-Ramos A, Cardona F, Tinahones FJ. Assessment of cellular and plasma oxidative stress in SAHS patients before and after continuous positive airway pressure treatment. Clin Lab. 2010;56:397–406. [PubMed] [Google Scholar]
- Nakamura MHS, Ikeda M, Hokari R, Kato N, Hibi T, Miura S. An antioxidant resveratrol significantly enhanced replication of hepatitis C virus. World J Gastroenterol. 2010;16:184–192. doi: 10.3748/wjg.v16.i2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59:65–85. doi: 10.1016/S0006-2952(99)00310-X. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM. Lehninger principles of biochemistry. New York: Freeman; 2008. [Google Scholar]
- Neu JJ, DeMarco V, Li N. Glutamine: clinical applications and mechanisms of action. Curr Opin Clin Nutr Metab Care. 2002;5:69–75. doi: 10.1097/00075197-200201000-00013. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington: National Academy Press; 1996. [Google Scholar]
- Oehler R, Pusch E, Dungel P, Zellner M, Eliasen MM, Brabec M, Roth E. Glutamine depletion impairs cellular stress response in human leucocytes. Br J Nutr. 2002;87:17–21. doi: 10.1079/BJN2001453. [DOI] [PubMed] [Google Scholar]
- Park CM, Cha YS, Youn HJ, Cho CW, Song YS. Amelioration of oxidative stress by dandelion extract through CYP2E1 suppression against acute liver injury induced by carbon tetrachloride in Sprague-Dawley rats. Phytother Res. 2010;24:1347–1353. doi: 10.1002/ptr.3121. [DOI] [PubMed] [Google Scholar]
- Parke DV, Ioannides C, Lewis DF. The role of the cytochromes P450 in the detoxication and activation of drugs and other chemicals. Can J Physiol Pharmacol. 1991;69:537–549. doi: 10.1139/y91-081. [DOI] [PubMed] [Google Scholar]
- Peng X, Yan H, You Z, Wang P, Wang S. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns. 2004;30:135–139. doi: 10.1016/j.burns.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Pithon-Curi TC, Schumacher RI, Freitas JJ, Lagranha C, Newsholme P, Palanch AC. Glutamine delays spontaneous apoptosis in neutrophils. Am J Physiol Cell Physiol. 2003;284:1355–1361. doi: 10.1152/ajpcell.00224.2002. [DOI] [PubMed] [Google Scholar]
- Rakba N, Melhaoui M, Loye P, Delcros JG, Morel I, Lescoat G. Bgugaine, a pyrrolidine alkaloid from Arisarum vulgare, is a strong hepatotoxin in rat and human liver cell cultures. Toxicol Lett. 1999;104:239–248. doi: 10.1016/S0378-4274(98)00375-0. [DOI] [PubMed] [Google Scholar]
- Ralph DM, Robinsona SR, Campbella MS, Bishop GM. Histidine, cystine, glutamine, and threonine collectively protect astrocytes from the toxicity of zinc. Free Radical Biol Med. 2010;49:649–657. doi: 10.1016/j.freeradbiomed.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Ran Q, Liang H, Gu M, Qi W, Walter CA, Roberts LJ. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J Biol Chem. 2004;279:55137–55146. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- Rohde T, MacLean DA, Klarlund Pedersen B. Glutamine, lymphocyte proliferation and cytokine production. Scand J Immunol. 1996;44:648–650. doi: 10.1046/j.1365-3083.1996.d01-352.x. [DOI] [PubMed] [Google Scholar]
- Roth E, Oehler R, Manhart N, Exner R, Wessner B, Strasser E. Regulative potential of glutamine—relation to glutathione metabolism. Nutrition. 2002;18:217–221. doi: 10.1016/S0899-9007(01)00797-3. [DOI] [PubMed] [Google Scholar]
- Saad S, Kanai M, Awane M, Yamamoto Y, Morimoto T, Isselhard W. Protective effect of heat shock pretreatment with heat shock protein induction before hepatic warm ischemic injury caused by Pringle’s maneuver. Surgery. 1995;118:510–516. doi: 10.1016/S0039-6060(05)80367-8. [DOI] [PubMed] [Google Scholar]
- Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW, Messmer K, Bilzer M. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol. 2004;10:864–870. doi: 10.3748/wjg.v10.i6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JE, Jr, Phillips JR, Pye Q, Maidt ML, Price S, Floyd RA. Methylene blue and rose bengala photoinactivation of RNA bacteriophages: comparative studies of 8-oxoguanine formation in isolated RNA. Arch Biochem Biophys. 1993;30:91–97. doi: 10.1006/abbi.1993.1119. [DOI] [PubMed] [Google Scholar]
- Schuster H, Blanc MC, Neveux N, Bonnefont-Rousselot D, Tourneau A, Bandt JP, Cynober L. Protective effects of regulatory amino acids on ischemia-reperfusion injury in the isolated perfused rat liver. Scand J Gastroenterol. 2006;41:1342–1349. doi: 10.1080/00365520600682039. [DOI] [PubMed] [Google Scholar]
- Senft AP, Dalton TP, Nebert DW, Genter MB, Puga A, Hutchinson RJ, Kerzee JK, Uno S, Shertzer HG. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic Biol Med. 2002;33:1268–1278. doi: 10.1016/S0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- Shertzer HG, Nebert DW, Puga A, Ary M, Sonntag D, Dixon K, Robinson LJ, Cianciolo E, Dalton TP. Dioxin causes a sustained oxidative stress response in the mouse. Biochem Biophys Res Commun. 1998;253:44–48. doi: 10.1006/bbrc.1998.9753. [DOI] [PubMed] [Google Scholar]
- Slezak BP, Hatch GE, DeVito MJ, Diliberto JJ, Slade R, Crissman K, Hassoun E, Birnbaum LS. Oxidative stress in female B6C3F1 mice following acute and subchronic exposure to 2,3,7,8-tetrachlorodibenzo-pdioxin (TCDD) Toxicol Sci. 2000;54:390–398. doi: 10.1093/toxsci/54.2.390. [DOI] [PubMed] [Google Scholar]
- Slivac I, Blajić V, Radošević K, Kniewald Z, Gaurina Srček V. Influence of different ammonium, lactate and glutamine concentrations on CCO cell growth. Cytotechnology. 2010;62:585–594. doi: 10.1007/s10616-010-9312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul D, Kim HS, Choa EK, Lee M, Kim HS, Jung WW, Hwang KW, Parkb SY. 2(3), pp. 7,8-TCDD neurotoxicity in neuroblastoma cells is caused by increased oxidative stress, intracellular calcium levels, and tau phosphorylation. Toxicol. 2009;255:65–71. doi: 10.1016/j.tox.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Takasawa H, Kobayashi K. Evaluation of a liver micronucleus assay with 12 chemicals using young rats (II): a study by the collaborative study group for the micronucleus test/Japanese Environmental mutagen society–mammalian mutagenicity study group. Mutagenesis. 2009;24:9–16. doi: 10.1093/mutage/gen047. [DOI] [PubMed] [Google Scholar]
- Svegliati BG, D’Ambrosio L, Ferretti G, Casini A, Sario A, Salzano R, Ridolfi F, Saccomanno S, Jezequel AM, Benedetti A. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720–726. doi: 10.1002/hep.510270313. [DOI] [PubMed] [Google Scholar]
- Szijártó A. Methods of increasing ischemic tolerance in liver surgery. Magy Seb. 2008;61:128–135. doi: 10.1556/MaSeb.61.2008.3.5. [DOI] [PubMed] [Google Scholar]
- Tazuke Y, Maeda K, Wasa M, Satoko N, Fukuzawa M. Protective mechanism of glutamine on the expression of proliferating cell nuclear antigen after cisplatin-induced intestinal mucosal injury. Pediatr Surg Int. 2011;27:151–158. doi: 10.1007/s00383-010-2798-8. [DOI] [PubMed] [Google Scholar]
- Thébault S, Deniel N, Galland A, Lecleire S, Charlionet R, Coëffier M, Tron F, Vaudry D, Déchelotte P. Proteomics human duodenal proteome modulations by glutamine and antioxidants. Clin Appl. 2010;4:325–336. doi: 10.1002/prca.200800175. [DOI] [PubMed] [Google Scholar]
- Tihan DN, Erbil Y, Seven R, Arkaya S, Türkoğlu U, Hepgül G, Borucu I. The effect of glutamine on oxidative damage in an experimental abdominal compartment syndrome model in rats. Ulus Travma Acil Cerrahi Derg. 2011;17:1–8. doi: 10.5505/tjtes.2011.73555. [DOI] [PubMed] [Google Scholar]
- Todorova VK, Kaufmann Y, Hennings L, Klimberg VS. Oral glutamine protects against acute doxorubicin-induced cardiotoxicity of tumor-bearing rats. J Nutr. 2010;140:44–48. doi: 10.3945/jn.109.113415. [DOI] [PubMed] [Google Scholar]
- Tsai PH, Liu JJ, Yeh CL, Chiu WC, Yeh SL. Effects of glutamine supplementation on oxidative stress-related gene expression and antioxidant properties in rats with streptozotocin-induced type 2 diabetes. Br J Nutr. 2011;1:1–7. doi: 10.1017/S0007114511004168. [DOI] [PubMed] [Google Scholar]
- Tung-Kwang L, O’Brien KF, Wang W, Johnke RM, Sheng C, Benhabib SM, Wang T, Allison RR. Radioprotective effect of American ginseng on human lymphocytes at 90 minutes post-irradiation: a study of 40 cases. J Altern Complement Med. 2010;16:561–567. doi: 10.1089/acm.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Mokhtar YI, Togar B. Eicosapentaenoic acid protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced hepatic toxicity in cultured rat hepatocytes. Cytotechnology. 2012;64:15–25. doi: 10.1007/s10616-011-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Yousef MI (2012) Modulatory effect of l-glutamine on 2,3,7,8 tetrachlorodibenzo-p-dioxin induced liver injury in rats. Toxicol Ind Health. doi:10.1177/0748233711420474 (in press) [DOI] [PubMed]
- Twaroski LW, O’Brien ML, Robertson LW. Effects of selected polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes and selenium status: implications for oxidative stress. Biochem Pharmacol. 2001;62:273–278. doi: 10.1016/S0006-2952(01)00668-2. [DOI] [PubMed] [Google Scholar]
- Urbina JJO, Jorquera F, Culebras J, Villares C, González-Gallego J, Tuñón MJ. Influencia de la formulación de la glutamina en sus efectos sobre los sistemas antioxidantes y de destoxificación hepática en la rata. Nutr Hosp. 2004;2:73–82. [PubMed] [Google Scholar]
- Wang HX, Ma XC, Deng QL. Cytotoxicity of flutamide and 2-hydroxyflutamide and their effects on CYP1A2 mRNA in primary rat hepatocytes. Acta Pharmacol Sin. 2002;23:562–566. [PubMed] [Google Scholar]
- Xia JZ, Wu ZH. Metabolism of glutamine-dipeptide supplemented TPN decreasing injuries of liver and intestine in intraperitoneal chemotherapy rats. Parenteral Enteral Nut. 1997;4:78–82. [Google Scholar]
- Yang RR. The effect of glutamine on cholestasis caused by total parenteral nutrition. Zhonghua Wai Ke Za Zhi. 1993;31:94–96. [PubMed] [Google Scholar]
- Yang H, Ierapetritou MG, Roth CM. Effects of amino acid transport limitations on cultured hepatocytes. Biophys Chem. 2010;152:89–98. doi: 10.1016/j.bpc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Yilmaz S, Aksoy H, Unal F, Celik M, Yüzbaşloğlu D. Genotoxic action of fungicide Conan 5FL (hexaconazole) on mammalian cells in vivo and in vitro. Genetika. 2008;44:323–328. [PubMed] [Google Scholar]
- Yokogawa K, Watanabe M, Takeshita H, Nomura M, Mano Y, Miyamoto K. Serum aminotransferase activity as a predictor of clearance of drugs metabolized by CYP isoforms in rats with acute hepatic failure induced by carbon tetrachloride. Int J Pharm. 2004;269:479–489. doi: 10.1016/j.ijpharm.2003.09.045. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Heatherly A, Malarkey DE, Walker NJ, Nyska A. A critical comparison of murine pathology and epidemiological data of TCDD, PCB126, and PeCDF. Toxicol Pathol. 2007;35:865–879. doi: 10.1080/01926230701618516. [DOI] [PMC free article] [PubMed] [Google Scholar]