Summary
VEGF and mTOR-targeted therapies continue to play a critical role in the management of advanced and metastatic RCC. Ongoing research to identify novel agents continues to build upon the work done during the elucidation of the VHL/clear cell RCC pathway. It is hoped that ongoing and planned studies will enable development of therapeutic regimens that will incorporate agents with improved toxicity and better efficacy as well as defining a role for a multidisciplinary approach to the management of advanced RCC.
Keywords: Renal cell carcinoma (RCC), clear cell, von Hippel-Lindau (VHL), targeted therapy, tyrosine kinase inhibitor (TKI), vascular endothelial growth factor (VEGF), mammalian target of rapamycin (mTOR)
Introduction
Renal cell carcinoma (RCC) remains one of the most common adult malignancies in the United States (ranking seventh in men and eighth in women) with an estimated 58,240 new cases and 13,040 deaths in 2010 [1]. The overall incidence of kidney cancer is rising, at least in part due to increased use of cross-sectional imaging, such as CT scans and MRIs [2]. Despite increased detection and subsequent treatment, deaths from kidney cancer have not declined, largely because of the high mortality associated with metastatic disease [3].
The systemic management of advanced and metastatic RCC (mRCC) has changed rapidly over the past five years with FDA approval of six targeted agents directed against aberrant VEGF and mTOR pathways. VEGF-pathway antagonists have largely replaced cytokine-based therapies as the first-line treatment for many patients with clear cell RCC, although high dose IL-2 remains an appropriate option in selected patients [4–5]. Given the multiple treatment options currently available, an oncologist may feel like the proverbial “man with two watches”, never sure of the time or which systemic therapy to offer. The development of targeted therapies for clear cell RCC and recent clinical and pre-clinical reports will be the major focus of this article; targeted strategies for patients with non-clear cell RCC have recently been reviewed elsewhere [6]. Whenever possible, trials are identified by their NCT number so the reader may easily find the study of interest on the National Institutes of Health’s clinical trial registry website [7].
Study Endpoints and Quality of Life
In order to chart a course forward, it is useful to reflect on the developments in the field of kidney cancer therapeutics over the past decade. In 2002, Motzer and colleagues retrospectively analyzed data from six prospective trials that evaluated 463 subjects with advanced RCC who had been treated with interferon-α (IFN-α) as first-line therapy and found that progression free survival (PFS) and overall survival (OS) were 4.7 and 13 months, respectively [8]. Based on this, they proposed that IFN-α should be considered the standard therapy to which new treatments are compared. Subsequently, sunitinib [9], temsirolimus [10], and bevacizumab [11–12] were compared to IFN-α and found to be superior. In contrast, sorafenib [13], everolimus [14], and pazopanib [15] were found to be superior to placebo in their defining trials, although the intent of many of these protocols was to focus on subjects who had already failed cytokine or anti-VEGF therapy. (Table 1)
Table 1.
FDA-Approved Therapies for Advanced Clear Cell Renal Cell Carcinoma
| Therapy | Target | Treatment Line | Comparison Arm | Primary Endpoint |
|---|---|---|---|---|
| Bevacizumab + IFN-α (AVOREN) [11] |
VEGF | First-line | Placebo + IFN-α | OS |
| Bevacizumab + IFN-α (CALGB) [12] |
VEGF | First-line | IFN-α | OS |
| Pazopanib [15] | VEGFR | First-line or Cytokine Failure |
Placebo | PFS |
| Sorafenib [13] | VEGFR | Cytokine Failure | Placebo | OS |
| Sunitinib [9] | VEGFR | First-line | IFN-α | PFS |
| Everolimus [14] | mTOR | VEGFR Failure | Placebo | PFS |
| Temsirolimus [10] | mTOR | First-line | IFN-α | OS |
While IFN-α is no longer considered an appropriate comparator in randomized trials, no single agent has found universal favor as a suitable successor in first-line trials. In previously untreated patients, both sunitinib and temsirolimus (the latter in ‘poor risk’ RCC) have led to improved OS in randomized phase III trials, supporting the notion that new therapies should be compared to one of these agents depending on the patient population under study. However, other agents, including sorafenib and pazopanib are clearly active in RCC and constitute the comparator arm in several ongoing studies. It is hoped that head-to-head comparisons of these agents will eventually lead to the identification of a treatment standard that will then form the basis for the evaluation of new therapies.
The choice of an appropriate endpoint for evaluating the efficacy of targeted agents remains a matter of considerable debate. OS, PFS, and overall response rate (ORR) have each been used as the primary endpoint in pivotal trials of these agents. (Table 1) An improvement in OS provides the most convincing evidence that a new therapy is superior to the existing standard of care. However, OS analyses in randomized trials may be confounded by the availability and widespread use of active agents in patients who progress on their assigned therapy. Final results from two randomized phase III studies comparing IFN-α with or without bevacizumab were recently reported and demonstrated a significant improvement in PFS but not OS in the bevacizumab arms [11–12]. While these results may represent a true discordance between PFS and OS, it is also possible that a survival benefit was obscured by the use of subsequent lines of treatment. In view of these concerns, PFS is likely to be the primary endpoint of choice in many future trials and is widely accepted as the basis for regulatory approval in RCC [16].
In the absence of unequivocal survival benefits or direct head-to-head comparisons between the various drugs approved for mRCC, quality of life (QOL) assessments could help oncologists and their patients identify the therapies that offer a reasonable chance at prolonging life while favorably impacting on its quality. This is especially important considering that the side effects accompanying currently available targeted therapies are not likely to be offset by a durable complete response (CR), which, for example, may prompt some patients to accept the acute toxicity associated with high-dose interleukin-2 (IL-2) in exchange for the chance at cure. Since there is significant overlap in the mechanisms of action of individual drugs in a given class of targeted agents, their toxicity profiles are quite similar. However, newer agents currently in clinical trials such as tivozanib and axitinib, which are highly selective inhibitors of the VEGF receptors, appear to be associated with a better toxicity profile while retaining efficacy. Incorporation of QOL endpoints into trials comparing these agents with sunitinib or sorafenib should be considered and may guide treatment preferences even in the absence of differences in efficacy endpoints. Validated kidney cancer-specific QOL instruments are available, but they have not been universally adopted [17–18].
Molecular Mechanisms Underlying Renal Tumorigenesis
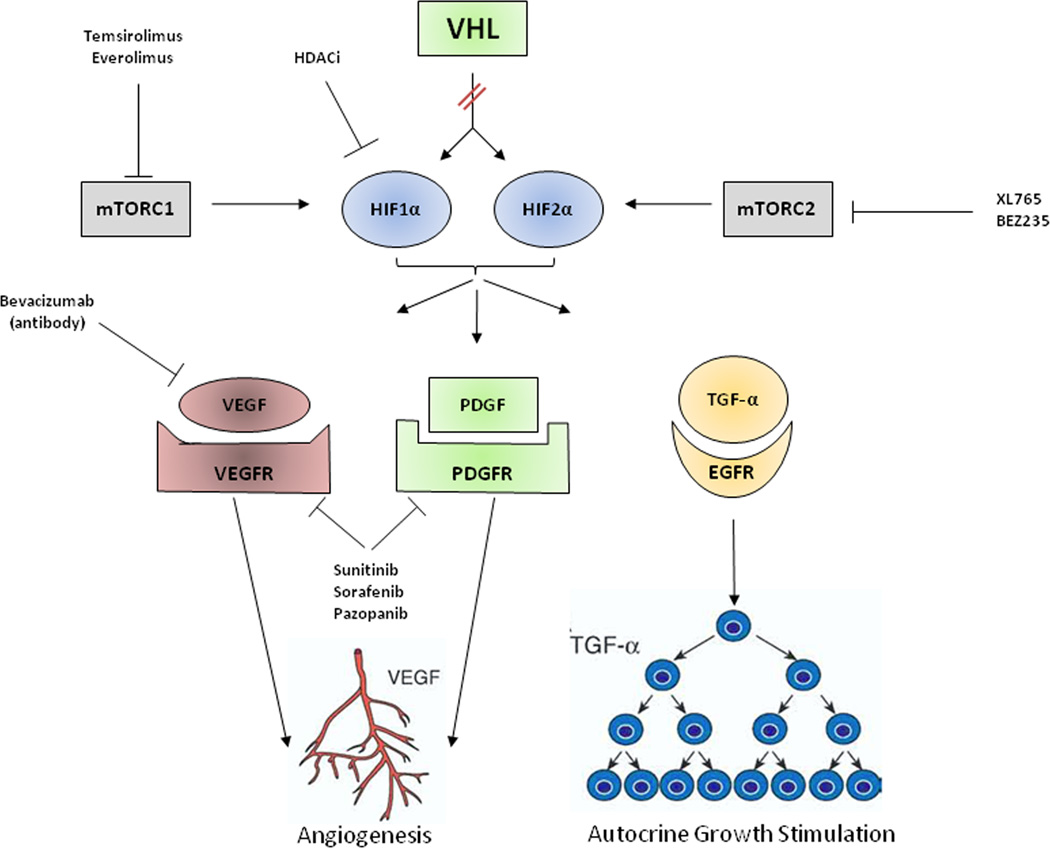
The majority of sporadic clear cell RCC tumors harbor mutations in the VHL tumor suppressor gene [19]. (Figure 1) Loss of functional VHL protein (pVHL) results in the activation of proangiogenic and growth factor pathways via constitutive stabilization of the alpha subunits of a group of transcriptionally active proteins called the hypoxia inducible factors (HIF) [20]. HIF plays a central role in renal tumorigenesis by acting as a transcription factor for genes that are involved in angiogenesis, tumor cell proliferation, cell survival and progression, metastatic spread, apoptosis and glucose metabolism [21]. The alpha subunits of HIF are also regulated at the translational level by growth factors through the phosphatidylinositol-3 kinase PI3K-AKT-mTOR signal transduction pathway [22]. Elucidation of the VHL/HIF pathway has led to the successful evaluation and regulatory approval of agents targeting the VEGF and mTOR axes. While these therapies are clearly active in clear cell RCC, the vast majority of tumors eventually become refractory to therapy through a variety of different, as yet poorly understood, mechanisms. Novel agents as well as rational combinations are in development for the treatment of mRCC in an attempt to address these resistance mechanisms, and reduce severe side effects.
Figure 1. Clear Cell Renal Cell Carcinoma Pathways and Targeted Therapies.
The VHL gene is mutated in the majority of sporadic clear cell kidney cancer. As a result of mutation, the VHL protein cannot target and degrade hypoxia-inducible factor (HIF) 1 α /2α. HIF overaccumulates and causes increased transcription of downstream genes, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) and transforming growth factor alpha (TGF-α).
Current therapeutic approaches include antibodies, such as bevacizumab, that targets VEGF, tyrosine kinase inhibitors such as sunitinib, sorafenib, and pazopanib that target the VEGF and PDGF receptors and rapalogues such as temsirolimus and everolimus that target the mammalian target of rapamycin complex 1 (mTORC1).
Future approaches could include agents that target HIF directly including histone deacetylase inhibitors (HDACi) and indirectly via inhibition of the mammalian target of rapamycin complex 2 (mTORC2).
Update on Clinical Trials
The most recent addition to the growing list of FDA-approved agents with activity in RCC is pazopanib, a second generation multi-targeted TKI that inhibits VEGF-R1/2/3, PDGF-Rα/β and c-kit. Pazopanib was approved for use in metastatic RCC based on results from a recently published randomized phase III study in 435 subjects with mRCC who had received no more than one prior cytokine therapy [15]. Subjects were randomly assigned to receive either pazopanib or placebo. Pazopanib was associated with a statistically significant improvement in PFS (median PFS 9.2 versus 4.2 month, HR 0.46, 95% CI 0.34–0.62, p< 0.0001), the primary endpoint of this trial, as well as a significantly higher overall response rate (30% vs. 3%, p<0.0001). Final overall survival data from this trial are awaited. Pazopanib is now being compared with sunitinib as first line therapy for advanced RCC in the phase III COMPARZ study (NCT00720941), and is being evaluated as second line therapy in mRCC patients previously treated with VEGF-targeted therapy in a single arm phase II study (NCT00731211).
In addition to the plethora of VEGF-pathway antagonists already on the market, a number of novel agents directed against this pathway are currently undergoing evaluation in phase II–III trials. Some of these agents, with more selective activity against the VEGF receptors (such as tivozanib) represent an attempt to improve on the toxicity profile of currently available agents, while others are designed to provide additional activity against pathways thought to complement the VEGF axis in promoting angiogenesis and tumor growth. Axitinib is a newer generation, selective VEGF-R inhibitor with activity against all three VEGF receptors. In a phase II trial of patients with cytokine refractory RCC, axitinib demonstrated an ORR of 44% which included two CRs [23]. The median time to progression was 15.7 months and median OS 29.9 months. A second phase II study demonstrated activity of this agent in subjects who had progressed on prior anti-VEGFR therapy; in 62 subjects who had received prior sorafenib, treatment with axitinib resulted in an ORR of 22.6% with a PFS of 7.4 months [24]. Axitinib is currently being evaluated in the phase III randomized AXIS trial (NCT00678392) versus sorafenib in subjects who have failed one prior cytokine or TKI therapy.
Tivozanib is an oral pan-VEGF-R inhibitor that was recently studied in a phase II placebo-controlled randomized discontinuation trial in 272 RCC subjects of whom 83% had clear cell RCC [25]. The trial highlighted the favorable toxicity profile associated with this agent, with fewer than 10% of subjects developing CTCAE grade 3/4 hypertension as well as a low incidence of palmar plantar syndrome, diarrhea, fatigue, stomatitis, and proteinuria. PFS was reported to be 364 days in the clear cell RCC group versus 220 days in subjects with non-clear cell histology. The favorable toxicity profile of tivozanib might allow combination of this agent with mTOR inhibitors in clinically meaningful doses. Indeed, a phase I–II trial of this agent in combination with temsirolimus is currently ongoing. Tivozanib is also being evaluated in the phase III TIVO-1 trial (NCT01030783) versus sorafenib in previously untreated patients with advanced RCC.
Other agents with new and distinct anti-angiogenic profiles undergoing evaluation in patients with advanced RCC include regorafenib and dovitinib. Regorafenib inhibits both VEGFR-2 and TIE2 TK and may have advantages over inhibition of the VEGF axis alone. Data from a phase II trial of regorafenib in subjects with mRCC were presented at the joint 15th ECCO Congress in 2009 and demonstrated a 31% PR and 50% stable disease rate. Dovitinib, an orally available TKI, has activity against VEGF-R1/2/3, PDGF-R, c-kit, FLT3 and additionally fibroblast growth factor (FGF) receptor 1, 2 and 3. Activating mutations in FGF-Rs or their ligands have been detected in a number of tumor types including RCC and signaling via this pathway has been suggested as a resistance mechanism to VEGF-R inhibition [26]. Dovitinib is currently being investigated in phase II studies of mRCC with some preliminary reports of PRs and stable disease [27]. Further elucidation of mechanisms resulting in resistance to VEGF pathway antagonists and the development of strategies directed against these mechanisms are areas of active investigation.
In addition to the VEGF-R axis, a number of other molecules such as AKT are being pursued as possible therapeutic targets. Perifosene targets the pleckstrin homology domain of AKT, thereby preventing its translocation to the plasma membrane and subsequent activation by PDK1 and mTORC2 complexes. In two phase II studies in subjects with advanced RCC who had progressed on prior VEGF-R TKI and/or mTOR inhibitor, perifosene led to disease stabilization as well as some objective responses with manageable toxicities [28–29].
Combination strategies designed to provide maximal blockade of key components of the HIF pathway have typically involved attempts to exploit potential synergy between VEGF pathway and mTOR inhibitors. The majority of these combinations have been associated with significant toxicity, precluding their evaluation in phase II–III trials. An exception is the combination of bevacizumab and everolimus, a reasonably well tolerated regimen which was evaluated in a single arm phase II trial [30]. The combination was active in both treatment-naïve patients (ORR 30%, median PFS 9.1 months) and in those who had progressed on sunitinib or sorafenib (ORR 23%, median PFS 7.1 months). Everolimus, with or without bevacizumab, is currently being studied in a randomized placebo controlled trial in patients refractory to first-line VEGFR inhibitors under the aegis of CALGB (NCT01198158).
The prognostic criteria identified by Motzer and colleagues from Memorial-Sloan Kettering Cancer Center in patients with mRCC undergoing therapy with cytokines have formed the basis for risk-stratification in both clinical practice and experimental trials [8]. These criteria were partially validated and extended (with the addition of elevated neutrophil and platelet counts) by Heng and colleagues in a group of 645 subjects who had received a variety of VEGF pathway antagonists [31]. Efforts at external validation of these criteria using an independent data set are ongoing, and may help refine prognostication in patients receiving targeted agents.
Pre-clinical Developments
Significant progress has been made in drug development for clear cell RCC during the past decade as a result of our ability to better characterize the biochemical consequences of VHL inactivation. Further insights into mTOR biology and HIF regulation have led to the development of strategies designed to optimize inhibition of this pathway. It is now known that mTOR exists in two biochemically and functionally distinct complexes, mTORC1 and mTORC2. Recent evidence suggests that HIF-2α, a HIF isoform believed to play a significant role in mediating clear cell renal tumorigenesis, is regulated by mTORC2 rather than mTORC1 [32]. Conventional mTOR inhibitors, such as temsirolimus and everolimus, inhibit mTORC1 but not mTORC2. In addition, these agents initiate a negative feedback loop leading to the paradoxical increase in mTORC2 activity and AKT activation. Several new PI3K/mTORC1/2 complex inhibitors are currently in development to overcome the limitations of selective mTORC1 inhibition. In contrast to rapalogues, which primarily downregulate HIF-1a, these dual mTOR inhibitors induce profound reduction of both HIF-1a and HIF-2α levels and are associated with more potent antitumor activity in animal xenograft models [33]. XL765 (NCT00485719), AZD8055 (NCT00973076) and BEZ235 (NCT01195376) are a few of the dual mTOR inhibitors that are currently in phase I trials in advanced solid malignancies.
Recent pre-clinical studies with histone deacetylase (HDAC) inhibitors have shown anti-neoplastic effects on RCC cells. These agents are well known to effect gene expression through the hyperacetylation of histones and have been reported to reduce HIF-1α and VEGF transcription [34]. In addition, HDAC inhibitors have been shown to promote proteosomal degradation of HIF-1α in an oxygen/pVHL-independent manner [35]. Clinically, a number of these agents are in human trials. Vorinostat has been tested as monotherapy and demonstrated prolonged disease stabilization in RCC [36]. Additional phase II trials are underway as monotherapy (NCT00278395) and as combination therapy with bevacizumab (NCT00324870) or ridaforolimus (NCT01169532).
Although much of the excitement surrounding drug development for RCC has focused on signal transduction inhibitors, continued work with immunomodulation to reproduce the elusive CRs and long-term remissions obtained with IL-2, albeit in a small proportion of patients, continues. Therapeutic interest has arisen in interleukin-21 (IL-21), a member of the IL-2 family. This cytokine has demonstrated evidence of antitumor activity superior to IL-2 in an animal model of RCC [37]. Recombinant IL-21 was well tolerated in early studies and demonstrated a stable disease rate of 89% in a phase I trial of IL-21 in subjects with mRCC or melanoma. Four subjects with RCC had a PR [38]. Combination therapy with sorafenib is underway in a phase I-II study in subjects with metastatic clear cell RCC (NCT00389285).
Neoadjuvant Therapy and the Role of Cytoreductive Nephrectomy in the Era of Targeted Therapy
While agents targeting the VEGF and mTOR pathways have an established role in the management of mRCC, we are yet to understand how these agents can be integrated with surgical approaches to maximize clinical benefit. Several studies have explored the feasibility and utility of VEGF pathway inhibitors in the neoadjuvant setting. The theoretical advantages of administering systemic therapy before surgery are many and include assessment of primary tumor response, tumor downstaging, and decreasing circulating tumor cells [39–40]. Early reports suggest that these therapies lead to modest regression of primary tumors, with sufficient downstaging in some patients to permit surgical extirpation of previously unresectable tumors [41–42]. Although studies have demonstrated the general tolerability of targeted agents, there is still limited data on the safety of surgical resection following treatment with these agents, and several reports have shown increased perioperative complications after treatment with sunitinib, sorafenib, or bevacizumab [43–44].
The concept of neoadjuvant therapy is attractive on many fronts; however, the approach must be validated in randomized clinical trials before being widely adopted. The role of cytoreductive nephrectomy in patients with metastatic disease receiving VEGF targeted agents is another area that deserves further study. The current paradigm of debulking nephrectomy is based on data generated in the era of cytokine therapy, but is commonly used as a prelude to targeted therapy.
Several ongoing studies are designed to clarify these critical issues. The SURTIME study (NCT01099423) from the EORTC is a phase III trial of 458 subjects with their primary tumor in situ and synchronous mRCC. Subjects are randomized to sunitinib followed by nephrectomy or nephrectomy followed by sunitinib with PFS as the primary endpoint. A second study, CARMENA (NCT00930033), is a randomized phase III trial comparing sunitinib therapy alone versus cytoreductive nephrectomy followed by sunitinib therapy.
Adjuvant Therapy for Clear Cell RCC
A proportion of patients with seemingly organ confined and locally advanced RCC are at risk for progression after surgical resection. Adjuvant therapy has been used in the treatment of several malignancies with favorable results; however, cytokine treatment regimens, radiation therapy, and thalidomide have failed to improve outcomes in RCC when used in the adjuvant setting [45–47]. Several randomized, phase III trials are currently evaluating the impact of VEGF pathway antagonists on PFS, OS and safety/tolerability in the adjuvant setting. The S-TRAC trial (NCT00375674) is a randomized, two arm, double-blind trial started in 2007. Subjects at high risk of recurrence based on the University of California Los Angeles Integrated Staging System (UISS) are randomized to receive sunitinib versus placebo for 12 months; accrual is ongoing. The ASSURE trial (NCT00326898) is a large double blind trial with an accrual goal of close to 2000 patients. Subjects with pT1b, G3-4, pT2-T4, or any T with node positive RCC are randomized after nephrectomy to receive 1 year of sunitinib, sorafenib, or placebo. The primary endpoint is disease free survival and the anticipated completion date for data analysis is 2016. The last ongoing trial is the SORCE trial (NCT00492258) which is a three arm phase III study that randomizes subjects at high or intermediate risk of relapse using the Leibovich score to placebo for 3 years versus placebo for 2 years combined with sorafenib for 1 year versus sorafenib for 3 years. The trial’s estimated primary completion date is in late 2012. The results of these trials are eagerly awaited to determine the role of targeted therapy in the adjuvant setting.
Conclusions
Targeted therapies continue to be an integral component in the treatment of advanced and metastatic RCC. Novel agents and new combinations of existing treatments are being tested in trials throughout the world. Despite the successes of targeted therapies, much work remains to be done [48].
The knowledge that has been gained treating patients with clear cell RCC must also be translated into the non-clear histologies, which have traditionally been excluded from large kidney cancer trials [6]. Additional work to identify predictors of tumor sensitivity or resistance to therapy, and biomarkers for monitoring response to treatment, are urgently needed [49]. The role of neoadjuvant and adjuvant systemic therapy must also be defined. Urologists and oncologists must continue to support kidney cancer clinical trials at the local, regional, and national levels in order to enroll subjects to the trials that will attempt to answer these and other pressing questions relating to the management of advanced kidney cancer.
Purpose of review.
This article reviews the evolution of targeted therapies for clear cell renal cell carcinoma (RCC) and recent developments in the field. The vast majority of work in kidney cancer deals with clear cell RCC, which is the most common variant of this malignancy. The identification of loss of function of the von Hippel-Lindau protein as the basis for clear cell RCC, in addition to the well designed clinical trials that have ensued, provide an outstanding model for the development of mechanism-based targeted therapy in cancer.
Recent findings.
The treatment of advanced and metastatic RCC continues to be a major challenge for uro-oncologists despite the approval of six targeted therapies over the past five years. This rapid growth in therapeutic options has brought much needed improvements in overall and progression-free survival, although durable complete responses are rare. However, the plurality of treatments also poses challenges in terms of selecting the best therapy for a given patient, designing trials with appropriate comparison arms and endpoints, identifying safe and effective drug combinations or sequences, and determining the role of targeted therapies in the neoadjuvant and adjuvant settings.
Acknowledgments
This research was funded by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, et al. Rising incidence of renal cell cancer in the united states. JAMA. 1999;281(17):1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 3.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: A need to reassess treatment effect. J Natl Cancer Inst. 2006;98(18):1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI. Metastatic renal cell carcinoma: Many treatment options, one patient. J Clin Oncol. 2009;27(19):3225–3234. doi: 10.1200/JCO.2008.19.9836. [DOI] [PubMed] [Google Scholar]
- 5.Di Lorenzo G, Autorino R, Sternberg CN. Metastatic renal cell carcinoma: Recent advances in the targeted therapy era. Eur Urol. 2009 doi: 10.1016/j.eururo.2009.09.002. [DOI] [PubMed] [Google Scholar]
- **6. Singer EA, Bratslavsky G, Linehan WM, et al. Targeted therapies for non-clear renal cell carcinoma. Target Oncol. 2010;5(2):119–129. doi: 10.1007/s11523-010-0148-3. The most recent review of targeted therapies for non-clear RCC
- 7.NIH. Clinical trials registry. 2010 [cited 2010 November 24]; Available from: http://clinicaltrials.gov/ct2/home.
- 8.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 10.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Bellmunt J, Negrier S, et al. Phase iii trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (avoren): Final analysis of overall survival. J Clin Oncol. 2010;28(13):2144–2150. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 12.Rini BI, Halabi S, Rosenberg JE, et al. Phase iii trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of calgb 90206. J Clin Oncol. 2010;28(13):2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : Final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 15.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase iii trial. J Clin Oncol. 2010;28(6):1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 16.Pal SK, Figlin RA. Targeted therapies for renal cell carcinoma: Understanding their impact on survival. Target Oncol. 2010;5(2):131–138. doi: 10.1007/s11523-010-0145-6. [DOI] [PubMed] [Google Scholar]
- 17.Cella D, Yount S, Du H, et al. Development and validation of the functional assessment of cancer therapy-kidney symptom index (fksi) J Support Oncol. 2006;4(4):191–199. [PubMed] [Google Scholar]
- 18.Cella D, Yount S, Brucker PS, et al. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value Health. 2007;10(4):285–293. doi: 10.1111/j.1524-4733.2007.00183.x. [DOI] [PubMed] [Google Scholar]
- 19.Beroukhim R, Brunet JP, Di Napoli A, et al. Patterns of gene expression and copy-number alterations in von-hippel lindau disease-associated and sporadic clear cell carcinoma of the kidney. Cancer Res. 2009;69(11):4674–4681. doi: 10.1158/0008-5472.CAN-09-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170(6 Pt 1):2163–2172. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 21.Semenza GL. Targeting hif-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 22.Hay N. The akt-mtor tango and its relevance to cancer. Cancer Cell. 2005;8(3):179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: A phase ii study. Lancet Oncol. 2007;8(11):975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- *24. Rini BI, Wilding G, Hudes G, et al. Phase ii study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27(27):4462–4468. doi: 10.1200/JCO.2008.21.7034. Axitinib, a newer TKI with a better side effect profile, produced 2 CRs in patients refractory to cytokines
- 25.Bhargava P, Esteves B, Nosov D, et al. Updated activity and safety results of a phase ii randomized discontinuation trial (rdt) of av-951, a potent and selective vegfr 1, 2 and 3 kinase inhibitor, in patients with renal cell carcinoma (rcc) J Clin Oncol. 2009:27. doi: 10.1200/JCO.2011.35.3524. [DOI] [PubMed] [Google Scholar]
- 26.Emoto N, Isozaki O, Ohmura E, et al. Basic fibroblast growth factor (fgf-2) in renal cell carcinoma, which is indistinguishable from that in normal kidney, is involved in renal cell carcinoma growth. J Urol. 1994;152(5 Pt 1):1626–1631. doi: 10.1016/s0022-5347(17)32492-8. [DOI] [PubMed] [Google Scholar]
- 27.Angevin E, Lopez J, Pande A, et al. Tk1258 (dovitinib lactate) in metastatic renal cell carcinoma (mrcc) patients refractory to approved targeted therapies: A phase i/ii dose finding biomarker study. J Clin Oncol. 2009:27. [Google Scholar]
- 28.Cho D, Figlin RA, Flaherty K, et al. A phase ii trial of perforsine in patients with advanced renal cell carcinoma (rcc) who have failed tyrosine kinase inhibitors. J Clin Oncol. 2009 [Google Scholar]
- 29.Vogelzang N, Hutson TE, Samlowski W, et al. Phase ii study of perifosene in metastatic renal cell carcinoma (rcc) progressing after prior therapy (rx) with a vegf receptor inhibitor. J Clin Oncol. 2009:27. [Google Scholar]
- **30. Hainsworth JD, Spigel DR, Burris HA, 3rd, et al. Phase ii trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol. 2010;28(13):2131–2136. doi: 10.1200/JCO.2009.26.3152. This study demonstrated the safety and efficacy of combining VEGF and mTOR inhibitors
- *31. Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. A potential prognostic model for patients with mRCC receiving targeted therapies
- **32. Toschi A, Lee E, Gadir N, et al. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mtorc1 and mtorc2. J Biol Chem. 2008;283(50):34495–34499. doi: 10.1074/jbc.C800170200. The first study to demonstrate the dependence of HIF-2 on mTORC2, identifying the rationale for dual mTORC1/2 inhibition
- **33. Cho DC, Cohen MB, Panka DJ, et al. The efficacy of the novel dual pi3-kinase/mtor inhibitor nvp-bez235 compared with rapamycin in renal cell carcinoma. Clin Cancer Res. 2010;16(14):3628–3638. doi: 10.1158/1078-0432.CCR-09-3022. A preclinical study demonstrating excellent responses in vivo in RCC cell lines to dual mTORC1/2 and PIK3CA inhibition
- 34.Ellis L, Hammers H, Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett. 2009;280(2):145–153. doi: 10.1016/j.canlet.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian DZ, Kachhap SK, Collis SJ, et al. Class ii histone deacetylases are associated with vhl-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res. 2006;66(17):8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 36.Kelly WK, O'Connor OA, Krug LM, et al. Phase i study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23(17):3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frederiksen KS, Lundsgaard D, Freeman JA, et al. Il-21 induces in vivo immune activation of nk cells and cd8(+) t cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008;57(10):1439–1449. doi: 10.1007/s00262-008-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson JA, Curti BD, Redman BG, et al. Phase i study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26(12):2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 39.Kroeger N, Gajda M, Zanow J, et al. Downsizing a tumor thrombus of advanced renal cell carcinoma with neoadjuvant systemic therapy and resulting histopathological effects. Urol Int. 2010;84(4):479–484. doi: 10.1159/000296301. [DOI] [PubMed] [Google Scholar]
- 40.Tan KV, Namdarian B, Costello AJ, et al. Potential use of circulating endothelial cells as a biomarker of renal cell carcinoma. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- **41. Shuch B, Riggs SB, LaRochelle JC, et al. Neoadjuvant targeted therapy and advanced kidney cancer: Observations and implications for a new treatment paradigm. BJU Int. 2008;102(6):692–696. doi: 10.1111/j.1464-410X.2008.07660.x. The first report of neoadjuvant treatment-induced shrinkage of the primary kidney prior to cytoredective nephrectomy
- 42.Thomas AA, Rini BI, Lane BR, et al. Response of the primary tumor to neoadjuvant sunitinib in patients with advanced renal cell carcinoma. J Urol. 2009;181(2):518–523. doi: 10.1016/j.juro.2008.10.001. discussion 523. [DOI] [PubMed] [Google Scholar]
- 43.Jonasch E, Wood CG, Matin SF, et al. Phase ii presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(25):4076–4081. doi: 10.1200/JCO.2008.21.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas AA, Rini BI, Stephenson AJ, et al. Surgical resection of renal cell carcinoma after targeted therapy. J Urol. 2009;182(3):881–886. doi: 10.1016/j.juro.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Messing EM, Manola J, Wilding G, et al. Phase iii study of interferon alfa-nl as adjuvant treatment for resectable renal cell carcinoma: An eastern cooperative oncology group/intergroup trial. J Clin Oncol. 2003;21(7):1214–1222. doi: 10.1200/JCO.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Margulis V, Matin SF, Tannir N, et al. Randomized trial of adjuvant thalidomide versus observation in patients with completely resected high-risk renal cell carcinoma. Urology. 2009;73(2):337–341. doi: 10.1016/j.urology.2008.08.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kjaer M, Iversen P, Hvidt V, et al. A randomized trial of postoperative radiotherapy versus observation in stage ii and iii renal adenocarcinoma. A study by the copenhagen renal cancer study group. Scand J Urol Nephrol. 1987;21(4):285–289. doi: 10.3109/00365598709180784. [DOI] [PubMed] [Google Scholar]
- 48.Vogelzang NJ. Another step toward the cure of metastatic renal cell carcinoma? J Clin Oncol. 2010 doi: 10.1200/JCO.2010.31.5044. [DOI] [PubMed] [Google Scholar]
- 49.Garcia JA, Cowey CL, Godley PA. Renal cell carcinoma. Curr Opin Oncol. 2009;21(3):266–271. doi: 10.1097/CCO.0b013e32832a05c8. [DOI] [PubMed] [Google Scholar]