Abstract
BACKGROUND
Although numerous studies have demonstrated improved short-term outcomes after laparoscopic resection of colon cancer, the benefits of laparoscopic-assisted proctectomy (LAP) for rectal cancer are less clear. The current report addresses the need for a large multi-institutional study on early outcomes after proctectomy for cancer.
STUDY DESIGN
Patients who underwent elective LAP or open proctectomy for cancer during 2005 to 2009were identified from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. The frequency of postoperative complications and other early outcomes was determined. Multivariate logistic regression identified predictors of 30-day morbidity. Propensity scores, stratified by quintiles, were included in all multivariable models to partially adjust for nonrandom assignment of treatment.
RESULTS
Of 5,420 patients who underwent surgery for rectal cancer, 4,380 underwent open proctectomy and 1,040 (19.2%) LAP. The LAP group had a lower frequency of blood transfusion (12.3% versus 4.3%; p < 0.0001) and a longer mean operative time (242 versus 219 minutes; p < 0.0001). Median length of stay was 5 days after LAP and 7 days after open resection (p < 0.0001). Although no difference in 30-day mortality was detected, the frequency of complications was less after LAP (20.5% versus 28.8%; p < 0.0001). Specifically, the frequencies of superficial surgical site infection, sepsis, respiratory complications, renal failure, and venous thromboembolism were each lower in the LAP group. After adjusting for potential confounders, the likelihood of 30-day morbidity was significantly greater in open versus laparoscopic proctectomy (odds ratio = 1.41; 95% CI, 1.19–1.68).
CONCLUSIONS
Compared with open proctectomy, LAP is associated with decreased length of stay and 30-day morbidity. If ongoing randomized clinical trials confirm oncologic equivalency, LAP might eventually replace open resection as the standard of care for the treatment of patients with resectable rectal cancer.
More than 40,000 individuals are diagnosed with rectal cancer each year in the United States.1 For patients with localized disease, proctectomy is the standard of care and represents the only chance for cure. Proctectomy with total mesorectal excision for cancer is technically challenging, and postoperative complications are not uncommon. Although several studies have demonstrated improved short-term outcomes after laparoscopic-assisted resection of colon cancer,2–9 the benefits of laparoscopic-assisted proctectomy (LAP) for cancer of the rectum are less clear, and the American Society of Colon and Rectal Surgeons has not endorsed the technique outside of clinical trials.10 The objective of this study was to compare early outcomes after LAP and open proctectomy for cancer using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, which provides information on surgical outcomes from more than 230 community and academic hospitals nationwide. We hypothesized that the risk-adjusted likelihood of 30-day morbidity is lower in patients with rectal cancer treated with LAP versus open proctectomy.
METHODS
Data acquisition and patient selection
The ACS NSQIP provides risk-adjusted outcomes data to participating hospitals for the purpose of quality improvement. The program focuses on 30-day postoperative outcomes, including mortality and 21 categories of morbidity. Data collection at each participating institution is performed by a dedicated surgical clinical reviewer, with support and oversight from a nurse coordinator. The surgical clinical reviewer, using medical chart extraction and other methods, collects detailed data on patient demographics, comorbidities, laboratory values, operative variables, and 30-day postoperative outcomes, including complications, mortality, reoperation, and length of stay (LOS). Descriptions of the qualifications, training, and auditing of data collection personnel, case inclusion criteria, sampling and data collection strategy, and variable and outcomes definitions are available online from the ACS NSQIP Web site.11
Patients who underwent elective resection for rectal cancer were identified from the 2005–2009 ACS NSQIP Participant Use Files, which include data collected from 237 academic and community hospitals throughout the United States. Laparoscopic and open procedures were identified using Current Procedural Terminology codes. Only those patients with malignant neoplasms of the rectum, with an ICD-9 postoperative diagnosis code of 154.1, were included. Cases of rectosigmoid cancer and anal cancer were not included. We excluded high-risk patients with any of the following characteristics: emergency operation, disseminated cancer (defined as cancer that has spread to one or more sites in addition to the primary site, indicating that the cancer is widespread, fulminant, or near terminal), American Society of Anesthesiologists (ASA) class 5 (moribund), preoperative ventilator dependence, severe sepsis or septic shock (defined as documented organ and/or circulatory dysfunction in a patient with signs and symptoms of sepsis), acute renal failure (defined as increasing azotemia and a rise in creatinine >3 mg/dL in the 24 hours before surgery), and coma.
Outcomes
Thirty-day outcomes included sepsis (sepsis or septic shock), respiratory complications (pneumonia, ventilator dependence for >48 hours, or unplanned reintubation), superficial surgical site infection, abscess (deep surgical site infection or organ/space infection), dehiscence, venous thromboembolism (pulmonary embolism or deep vein thrombosis), cardiac complications (acute myocardial infarction or cardiac arrest requiring resuscitation), neurologic complications (stroke or coma), renal failure (postoperative progressive renal insufficiency with a rise in serum creatinine >2 mg/dL, or acute renal failure requiring dialysis), hemorrhage (bleeding requiring transfusion of at least 4 U packed red blood cells), urinary tract infection, and peripheral nerve injury. Additional 30-day outcomes included reoperation and mortality. Hospital LOS after the index operation was also recorded. We defined prolonged LOS as longer than 10 days.
Variables
Potential explanatory variables included demographics, preoperative health status and comorbidities, preoperative laboratory values, and operative variables including laparoscopic versus open resection. Demographics included age, sex, and race (white, black, or other). Variables related to preoperative health included functional status (independent versus totally or partially dependent), body mass index (classified according to World Health Organization definitions12), weight loss (≥10 lb in 6 months), current smoking, alcohol use (>2 drinks per day), chronic steroid use, and recent blood transfusion or operation. Comorbidities included diabetes mellitus, COPD, coronary artery disease, congestive heart failure (CHF), hypertension, peripheral vascular disease, neurological disease or event, dyspnea, pneumonia, and bleeding disorder. Preoperative laboratory values consisted of WBC count, hematocrit, platelet count, INR, sodium, BUN, creatinine, serum glutamic oxaloacetic transaminase (SGOT), alkaline phosphatase, and albumin. Standard NSQIP definitions of abnormal laboratory values were used. Variables related to neoadjuvant therapy included chemotherapy (within 30 days before surgery) and radiation therapy (within 90 days before surgery). Operative variables included wound class, ASA class, number of blood transfusions, and length of operation. The work relative value units score was used as a measure of procedure complexity.
Statistical analysis
All surgical outcomes and all potential control variables were compared between the elective LAP and open proctectomy groups and the complication and no complication groups, using chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables. To adjust for nonrandom assignment of open versus laparoscopic resection, a propensity score model was constructed based on multiple logistic regression using all of the preoperative patient characteristics, comorbidities, and laboratory values. Propensity scores were stratified into quintiles so that patients within each of the 5 groups had similar baseline risk factors. To confirm that the imbalances between the LAP and open groups were reduced after stratification, a 2-way analysis model was used with main effect as quintiles and LAP variable. The propensity score quintiles were included in the final multivariate model along with control variables that remained significant at p < 0.10 between the complication and no complication groups. To avoid multicollinearity problems, Pearson’s correlation between these selected variables were examined. Only one variable was included in the final multivariable model from each set of correlated variables. Adjusted odds ratios (AORs) and 95% confidence intervals for 30-day morbidity were obtained from the final multivariate logistic model including propensity score quintiles. Analyses were performed using SAS 9.1.3 for Windows (SAS Institute). All tests of significance were at the p < 0.05 level, and p values were 2-tailed.
RESULTS
Characteristics of the open and LAP groups
We identified 5,420 patients who underwent proctectomy for rectal cancer from 2005 to 2009 and otherwise met inclusion criteria for the study. LAP was used in 1,040 (19.2%), and 4,380 patients had open resection. Table 1 lists the characteristics of the 2 patient groups. The 2 groups were similar in terms of age and race/ethnicity distribution. The proportion of female patients was higher in the LAP group. The mean body mass index was higher in the open group. There were no significant differences between the 2 groups for recent weight loss or functional status.
Table 1.
Characteristics of Patients (n = 5,420) Who Underwent Open or Laparoscopic-Assisted Proctectomy for Cancer
| Characteristic | Open (n = 4,380) |
LAP (n = 1,040) |
Unadjusted p value |
Propensity score–adjusted p value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD) | 62.6 (13) | 61.9 (13) | 0.09 | 0.70 |
| Female sex (%) | 38.4 | 43.5 | 0.002* | 0.96 |
| Race/ethnicity (%) | 0.09 | 1.00 | ||
| White | 77.5 | 76.1 | ||
| Black | 6.6 | 5.6 | ||
| Other | 15.8 | 18.4 | ||
| Preoperative health and comorbidities | ||||
| BMI, mean (SD) | 28.1 (6.6) | 27.1 (5.8) | <0.001* | 0.75 |
| Weight loss (>10% in 6 mo) (%) | 6.5 | 5.1 | 0.10 | 0.90 |
| Nonindependent functional status (%) | 2.8 | 1.9 | 0.13 | 0.79 |
| Current smoker (%) | 18.8 | 18.0 | 0.55 | 0.98 |
| Alcohol use (>2 drinks per day) (%) | 4.8 | 6.6 | 0.013* | 0.94 |
| Diabetes mellitus (%) | 15.2 | 12.7 | 0.042* | 0.91 |
| COPD (%) | 4.4 | 2.5 | 0.005* | 0.63 |
| Dyspnea (%) | 8.9 | 6.9 | 0.044* | 0.81 |
| Coronary artery disease (%) | 10.2 | 8.6 | 0.12 | 0.94 |
| CHF (%) | 0.3 | 0.3 | 0.79 | 0.95 |
| Hypertension (%) | 48.3 | 44.0 | 0.013* | 0.86 |
| Peripheral vascular disease (%) | 0.9 | 0.7 | 0.42 | 0.95 |
| Neurologic disease (%) | 6.3 | 4.8 | 0.07 | 0.91 |
| Steroid use (%) | 1.4 | 1.0 | 0.29 | 0.97 |
| Bleeding disorder (%) | 2.7 | 2.5 | 0.76 | 0.95 |
| Neoadjuvant chemotherapy (%) | 7.0 | 4.6 | 0.005* | 0.74 |
| Neoadjuvant radiation therapy (%) | 38.7 | 31.6 | <0.001* | 0.99 |
| Preoperative laboratory values, mean (SD) | ||||
| WBC (×103 cells/µL) | 6.2 (2.4) | 6.2 (2.2) | 0.24 | 0.42 |
| Hematocrit (%) | 38.6 (4.6) | 39.2 (4.7) | <0.001* | 0.43 |
| Platelets (×103 cells/µL) | 255 (81) | 251 (73) | 0.56 | 0.55 |
| INR | 1.04 (0.16) | 1.02 (0.11) | 0.42 | 0.77 |
| Sodium (mmol/L) | 140 (2.8) | 140 (2.7) | 0.21 | 0.90 |
| BUN (mg/dL) | 14.7 (6.5) | 14.3 (5.7) | 0.50 | 0.79 |
| Creatinine (mg/dL) | 0.95 (0.44) | 0.92 (0.37) | 0.003* | 0.41 |
| SGOT (U/L) | 23.7 (16) | 23.8 (11) | 0.032* | 0.07 |
| Alkaline phosphatase (IU/L) | 81.0 (32) | 79.0 (36) | 0.011* | 0.09 |
| Serum albumin (g/dL) | 3.93 (0.57) | 4.00 (0.49) | <0.001* | 0.25 |
| Operative variables | ||||
| Wound class (%) | 0.034* | |||
| Contaminated | 7.6 | 5.6 | ||
| Dirty or infected | 1.6 | 1.2 | ||
| ASA class (%) | <0.001* | |||
| 3 (Severe disturbance) | 45.9 | 38.8 | ||
| 4 (Life-threatening disturbance) | 3.1 | 2.7 | ||
| Blood transfusions (%) | <0.001* | |||
| 1–2 U | 8.9 | 3.0 | ||
| >2 U | 3.4 | 1.3 | ||
| Length of operation, min, mean (SD) | 219 (99) | 242 (96) | <0.001* | |
| Ostomy (%) | 58.2 | 46.1 | <0.001* | |
| Abdominoperineal resection (%) | 40.6 | 31.0 | <0.001* | |
| Work RVUs, mean (SD) | 28.8 (4.5) | 31.0 (6.1) | <0.001* | |
| NSQIP risk score, mean (SD) | ||||
| Probability of morbidity | 0.207 (0.080) | 0.182 (0.076) | <0.001* | |
| Probability of mortality | 0.010 (0.015) | 0.008 (0.012) | <0.001* |
Statistical significance at the p < 0.05 level.
ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as kg/m2); CHF, congestive heart failure; LAP, laparoscopic-assisted proctectomy; RVUs, relative value units; SGOT, serum glutamic oxaloacetic transaminase.
Several preoperative comorbid conditions were more prevalent in the open group, including diabetes mellitus, COPD, dyspnea, and hypertension. The prevalence of alcohol use was higher in patients who underwent LAP and there was no difference in smoking. There were no significant differences in the frequencies of other comorbid conditions such as coronary artery disease, CHF, peripheral vascular disease, neurologic disease, chronic steroid use, and bleeding disorders. Use of both neoadjuvant chemotherapy and radiation therapy was higher in the open resection group. An analysis of preoperative laboratory values demonstrated significant differences in mean values for WBC count, hematocrit, creatinine, SGOT, alkaline phosphatase, and serum albumin in the patients who underwent LAP. After adjusting for nonrandom assignment of treatment with propensity score quintiles, there were no significant differences between the LAP and open resection groups for any of the preoperative factors (Table 1, last column).
There were significant differences between the open and LAP groups for all of the operative variables that we analyzed, including wound class, ASA class, blood transfusions, length of operation, ostomy creation, abdominoperineal resection (APR), and work relative value units (Table 1). The percentage of contaminated, dirty, or infected wounds was higher in the open group. Similarly, the open group had a greater proportion of patients with an ASA class corresponding to severe or life-threatening systemic disturbance. Use of intraoperative blood transfusions was also higher in the open resection group. The mean operative time was considerably longer in the LAP group compared with the open. Mean work relative value units, a proxy for procedure complexity, was higher in the LAP group. About 58% of the patients who underwent open resection were given a colostomy or ileostomy, compared with 46% in the LAP group (p < 0.0001). The frequency of APR was also higher in the open resection group. There were small but statistically significant differences between the 2 groups for the NSQIP Probability of Morbidity and Mortality scores. The NSQIP Probability of Morbidity score for open patients was 0.207, compared with 0.182 in LAP patients (p < 0.0001). NSQIP Probability of Mortality scores were 0.010 and 0.008, respectively (p < 0.0001).
Adverse outcomes after proctectomy
The rate of any 30-day complication in the open resection group was 28.8%, compared with 20.5% in patients who underwent LAP (p < 0.0001).Table 2 displays the rates of each type of complication, as well as other adverse outcomes. The open proctectomy group had significantly higher rates of superficial surgical site infection (11.8% versus 6.0%; p < 0.0001), sepsis (7.2% versus 4.7%; p = 0.0041), respiratory complications such as pneumonia (4.5% versus 2.8%; p = 0.0113), renal failure (2.0% versus 0.8%; p = 0.0072), and venous thromboembolism (1.7% versus 0.7%; p = 0.0122). There were no significant differences between the 2 groups in the incidence of abscess, urinary tract infection, dehiscence, cardiac complications, hemorrhage, neurologic complications, or peripheral nerve injury.
Table 2.
Frequency of 30-Day Adverse Outcomes in 5,420 Patients Who Underwent Open Resection or Laparoscopic-Assisted Proctectomy for Cancer
| Adverse outcomes | Open (%) (n = 4,380) |
LAP (%) (n = 1,040) |
p Value |
|---|---|---|---|
| Any complication* | 28.8 | 20.5 | <0.001† |
| Superficial SSI | 11.8 | 6.0 | <0.001† |
| Abscess | 7.4 | 7.6 | 0.81 |
| Sepsis and septic shock | 7.2 | 4.7 | 0.004† |
| Urinary tract infection | 5.5 | 4.4 | 0.16 |
| Respiratory complication | 4.5 | 2.8 | 0.011† |
| Renal failure | 2.0 | 0.8 | 0.007† |
| Dehiscence | 1.9 | 1.4 | 0.37 |
| VTE | 1.7 | 0.7 | 0.012† |
| Cardiac complication | 0.8 | 0.7 | 0.58 |
| Hemorrhage | 0.7 | 0.5 | 0.38 |
| Neurologic complication | 0.4 | 0.3 | 0.63 |
| Peripheral nerve injury | 0.3 | 0.1 | 0.34 |
| Prolonged LOS (>10 d) | 20.1 | 11.8 | <0.001† |
| Reoperation | 6.9 | 6.5 | 0.70 |
| Mortality | 1.1 | 0.6 | 0.14 |
Includes all listed outcomes other than prolonged length of stay, reoperation, and mortality.
Statistical significance at the p < 0.05 level.
LAP, laparoscopic-assisted proctectomy; LOS, length of stay; SSI, surgical site infection; VTE, venous thromboembolism.
Patients who underwent open resection had a higher rate of intraoperative blood transfusion (12.3% versus 4.3%; p < 0.0001). Median LOS was 7 days (interquartile range 5 to 10) in the open group versus 5 days (interquartile range 4 to 8) in the LAP group (p < 0.0001). The frequency of prolonged LOS, defined as >10 days, was higher in the open group (20.1% versus 11.8%; p < 0.0001). There were no differences in the rates of 30-day reoperation or mortality between the 2 groups (Table 2).
Predictors of morbidity after proctectomy
The following preoperative variables were significantly associated with 30-day complication after proctectomy for cancer in univariate analysis: age, sex, race/ethnicity, body mass index, recent weight loss, functional status, smoking, diabetes mellitus, COPD, dyspnea, coronary artery disease, CHF, hypertension, peripheral vascular disease, neurologic disease, and chronic steroid use (Table 3). There was no association between alcohol use, bleeding disorder, or use of neoadjuvant therapy and 30-day morbidity.
Table 3.
Characteristics of Patients (n = 5,420) Who Underwent Proctectomy for Cancer and Did or Did Not Have a Postoperative Complication within 30 Days
| Characteristic | No complication (n = 3,944) | Complication (n = 1,476) | p Value |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 62.0 (13) | 63.7 (13) | <0.001* |
| Female sex (%) | 40.7 | 35.7 | <0.001* |
| Race/ethnicity (%) | <0.001* | ||
| White | 78.0 | 75.3 | |
| Black | 5.6 | 8.5 | |
| Other | 16.4 | 16.2 | |
| Preoperative health and comorbidities | |||
| BMI, mean (SD) | 27.6 (6.2) | 28.8 (6.9) | <0.001* |
| Weight loss >10% in 6 mo (%) | 5.4 | 8.3 | <0.001* |
| Nonindependent functional status (%) | 2.0 | 4.3 | <0.001* |
| Current smoker (%) | 17.9 | 20.7 | 0.019* |
| Alcohol use (>2 drinks per day) (%) | 4.8 | 5.8 | 0.14 |
| Diabetes mellitus (%) | 13.0 | 19.3 | <0.001* |
| COPD (%) | 2.9 | 7.0 | <0.001* |
| Dyspnea (%) | 7.7 | 10.7 | <0.001* |
| Coronary artery disease (%) | 8.5 | 13.4 | <0.001* |
| CHF (%) | 0.2 | 0.8 | 0.001* |
| Hypertension (%) | 45.4 | 53.2 | <0.001* |
| Peripheral vascular disease (%) | 0.6 | 1.7 | <0.001* |
| Neurologic disease (%) | 5.3 | 8.1 | <0.001* |
| Steroid use (%) | 1.0 | 2.2 | 0.001* |
| Bleeding disorder (%) | 2.5 | 3.1 | 0.18 |
| Neoadjuvant chemotherapy (%) | 6.6 | 6.5 | 0.93 |
| Neoadjuvant radiation therapy (%) | 37.5 | 37.1 | 0.79 |
| Preoperative laboratory values | |||
| WBC (×103 cells/µL), mean (SD) | 6.1 (2.3) | 6.5 (2.4) | <0.001* |
| Hematocrit (%), mean (SD) | 38.9 (4.6) | 38.3 (4.6) | <0.001* |
| Platelets (×103 cells/µL), mean (SD) | 253 (77) | 260 (87) | 0.039* |
| INR, mean (SD) | 1.02 (0.14) | 1.05 (0.17) | <0.001* |
| Sodium (mmol/L), mean (SD) | 140 (2.7) | 140 (2.9) | 0.11 |
| BUN (mg/dL), mean (SD) | 14.5 (6.2) | 14.9 (6.8) | 0.30 |
| Creatinine (mg/dL), mean (SD) | 0.93 (0.31) | 0.99 (0.64) | 0.001* |
| SGOT (U/L), mean (SD) | 23.9 (15) | 23.2 (14) | <0.001* |
| Alkaline phosphatase (IU/L), mean (SD) | 79 (32) | 83 (35) | 0.017* |
| Serum albumin (g/dL), mean (SD) | 3.97 (0.55) | 3.87 (0.56) | <0.001* |
| Operative variables | |||
| Wound class (%) | <0.001* | ||
| Contaminated | 6.6 | 9.0 | |
| Dirty or infected | 1.2 | 2.4 | |
| ASA class (%) | <0.001* | ||
| 3 (Severe disturbance) | 41.8 | 51.8 | |
| 4 (Life-threatening disturbance) | 2.3 | 5.0 | |
| Blood transfusions (%) | <0.001* | ||
| 1–2 U | 6.7 | 10.4 | |
| >2 U | 2.2 | 5.2 | |
| Length of operation (min), mean (SD) | 217 (97) | 240 (103) | <0.001* |
| Ostomy (%) | 54.5 | 59.4 | 0.001* |
| Abdominoperineal resection (%) | 37.0 | 43.6 | <0.001* |
| Work RVUs, mean (SD) | 29.2 (5.0) | 30.6 (4.8) | 0.47 |
| Type of resection (%) | <0.001* | ||
| LAP | 21.0 | 14.4 | |
| Open resection | 79.0 | 85.6 |
Statistical significance at the p < 0.05 level.
ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as kg/m2); CHF, congestive heart failure; LAP, laparoscopic-assisted proctectomy; RVUs, relative value units; SGOT, serum glutamic oxaloacetic transaminase.
Of the preoperative laboratory values, WBC count, hematocrit, platelet count, international normalized ratio, creatinine, SGOT, alkaline phosphatase, and albumin were significantly associated with morbidity in univariate analysis. Operative variables that had a significant association with postoperative complication included wound class, ASA class, blood transfusion, length of operation, ostomy creation, abdominoperineal resection, and open versus laparoscopic-assisted resection.
Variables that differed between the open and LAP groups at the p < 0.05 level in univariate analysis were used to construct a multivariable model of morbidity. This model also included the LAP propensity score variable, with indicators for quintiles, to adjust for nonrandom assignment of treatment. Logistic regression was used to calculate AOR and 95% confidence intervals for 30-day postoperative complications after proctectomy. Results of this analysis are displayed in Table 4. After adjusting for LAP propensity score and the other variables, age and sex were not significantly associated with one of the primary outcomes, 30-day morbidity. AOR of morbidity was higher in blacks compared with whites (AOR = 1.50; 95% CI, 1.18–1.92).
Table 4.
Adjusted Odds Ratios and 95% Confidence Intervals for 30-Day Complication after Proctectomy for Cancer
| Characteristic | Adjusted odds ratio (95% CI) for complication |
|---|---|
| Demographics | |
| Age (y) | |
| Younger than | 50 Reference |
| 50–59 | 0.96 (0.78–1.17) |
| 60–69 | 0.95 (0.77–1.17) |
| 70–79 | 1.02 (0.81–1.28) |
| 80 and older | 1.25 (0.95–1.63) |
| Sex | |
| Female | Reference |
| Male | 1.12 (0.97–1.29) |
| Race | |
| White | Reference |
| Black | 1.50 (1.18–1.92)* |
| Other | 1.14 (0.96–1.35) |
| Preoperative health and comorbidities | |
| BMI | |
| <18.5 (underweight) | 0.68 (0.45–1.03) |
| 18.5–24.9 (normal weight) | Reference |
| 25–29.9 (overweight) | 1.13 (0.97–1.33) |
| 30–34.9 (obese I) | 1.31 (1.08–1.58)* |
| 35–39.9 (obese II) | 1.47 (1.13–1.91)* |
| >40 (obese III) | 1.53 (1.11–2.11)* |
| Weight loss | |
| No | Reference |
| Yes | 1.45 (1.13–1.86)* |
| Diabetes mellitus | |
| No | Reference |
| Yes | 1.20 (1.00–1.43) |
| Smoking | |
| No | Reference |
| Yes | 1.18 (1.00–1.40) |
| Functional status | |
| Independent | Reference |
| Partially or totally dependent | 1.43 (0.99–2.07) |
| COPD | |
| No | Reference |
| Yes | 1.72 (1.26–2.34)* |
| Coronary artery disease | |
| No | Reference |
| Yes | 1.21 (0.99–1.50) |
| Hypertension | |
| No | Reference |
| Yes | 1.03 (0.89–1.19) |
| Peripheral vascular disease | |
| No | Reference |
| Yes | 2.23 (1.23–4.07)* |
| Neurologic disease | |
| No | Reference |
| Yes | 1.14 (0.88–1.47) |
| Dyspnea | |
| No | Reference |
| Yes | 0.93 (0.74–1.16) |
| Steroids | |
| No | Reference |
| Yes | 1.68 (1.02–2.77)* |
| Bleeding disorder | |
| No | Reference |
| Yes | 0.99 (0.67–1.44) |
| Preoperative laboratory values | |
| WBC | |
| Low (<4.5 × 103 cells/µL) | 0.80 (0.68–0.95)* |
| Normal (4.5–11 × 103 cells/µL) | Reference |
| High (>11 × 103 cells/µL) | 1.37 (0.97–1.93) |
| Creatinine | |
| Normal (≤1.2 mg/dL) | Reference |
| High (>1.2 mg/dL) | 1.03 (0.83–1.28) |
| Alkaline phosphatase | |
| Normal (≤125 IU/L) | Reference |
| High (>125 IU/L) | 1.22 (0.90–1.64) |
| Serum albumin | |
| Normal (≥3.4 g/dL) | Reference |
| 2.5–3.3 g/dL | 1.19 (0.93–1.52) |
| <2.5 g/dL | 0.99 (0.53–1.86) |
| Operative variables | |
| Wound class | |
| Clean or clean-contaminated | Reference |
| Contaminated | 1.18 (0.94–1.49) |
| Dirty or infected | 1.48 (0.94–2.35) |
| ASA class | |
| 1 or 2 (No or mild disturbance) | Reference |
| 3 (Severe disturbance) | 1.18 (1.02–1.36)* |
| 4 (Life-threatening disturbance) | 1.44 (1.00–2.07) |
| Abdominoperineal resection | |
| No | Reference |
| Yes | 1.20 (1.05–1.37)* |
| Blood transfusions | |
| 0 U | Reference |
| 1 – 2 U | 1.13 (0.90–1.42) |
| >2 U | 1.68 (1.20–2.34)* |
| Length of operation (h) | |
| <4 | Reference |
| ≥4 | 1.38 (1.20–1.58)* |
| Type of operation | |
| LAP | Reference |
| Open resection | 1.41 (1.19–1.68)* |
Statistical significance at the p < 0.05 level.
ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as kg/m2); LAP, laparoscopic-assisted proctectomy.
Compared with normal weight, obesity class I (AOR = 1.31; 95% CI, 1.08–1.58), class II (AOR = 1.47; 95% CI, 1.13–1.91), and class III (AOR = 1.53; 95% CI, 1.11–2.11) were all associated with morbidity. Weight loss of >10% of total body weight in the 6 months before surgery was also a risk factor for having a complication (AOR = 1.45; 95% CI, 1.13–1.86). Other comorbid conditions that were associated with morbidity included COPD (AOR = 1.72; 95% CI, 1.26–2.34), peripheral vascular disease (AOR = 2.23; 95% CI, 1.23–4.07), and chronic steroid use (AOR = 1.68; 95% CI, 1.02–2.77). The only preoperative laboratory variable that was significantly associated with morbidity was WBC count, where a low value (<4.5 × 103 cells/µL) was protective.
Operative variables that were associated with morbidity included ASA class 3 (AOR = 1.18, 95% CI, 1.02–1.36), APR (AOR = 1.20; 95% CI, 1.05–1.37), transfusion of >2 U blood (AOR = 1.68; 95% CI, 1.20–2.34), and duration of operation longer than 4 hours (AOR = 1.38; 95% CI, 1.20–1.58). Importantly, after adjusting for possible confounders and treatment propensity score, the type of resection was significantly associated with 30-day morbidity. Compared with LAP, the AOR of complication after open resection was 1.41 (95% CI, 1.19–1.68).
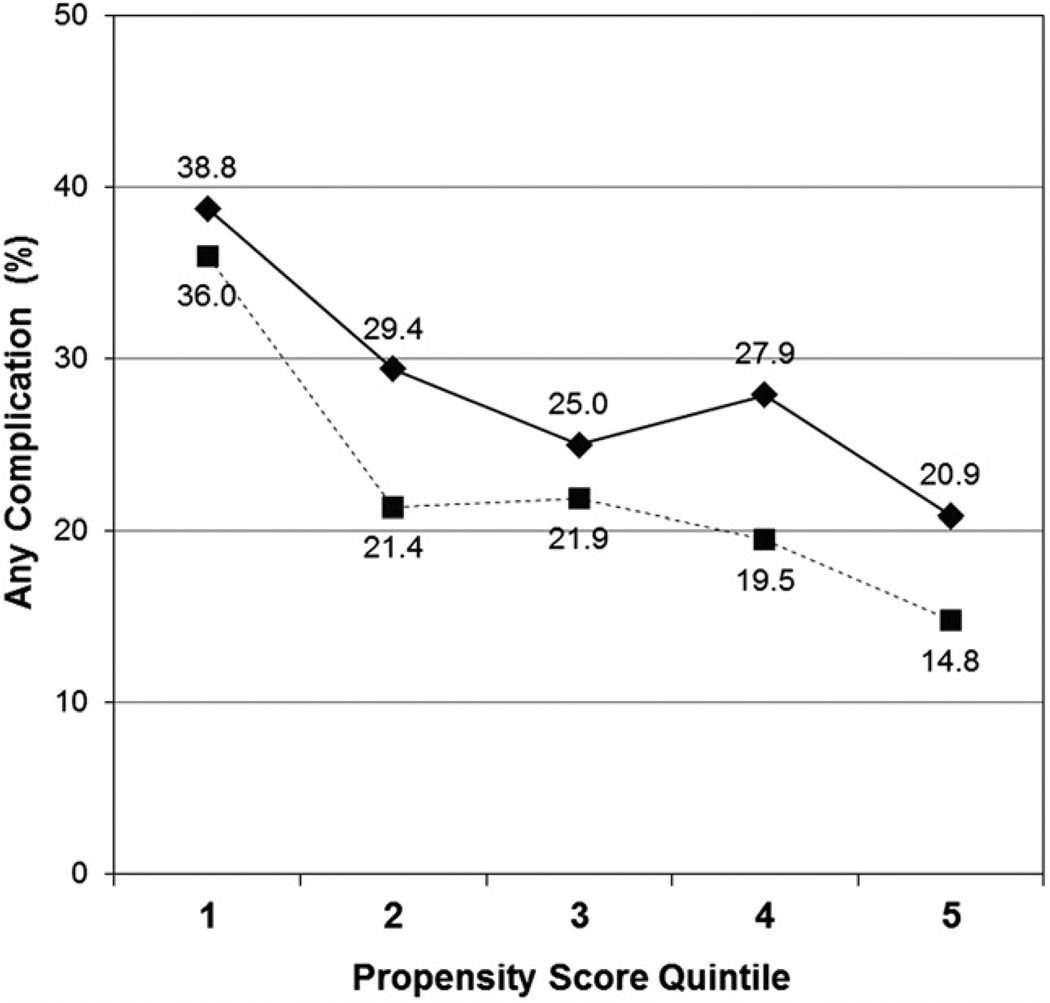
To confirm that there was a difference in risk-adjusted morbidity between the open resection and LAP groups, we determined 30-day complication rates after stratifying by LAP propensity score quintiles. Results of this analysis are displayed in Table 5 and Figure 1. In each propensity score quintile, the frequency of morbidity was lower in the LAP group compared with the open resection group. Differences were statistically significant for quintiles 2, 4, and 5.
Table 5.
Unadjusted 30-Day Complication Rates after Open Proctectomy or Laparoscopic-Assisted Proctectomy for Cancer, Stratified by Propensity Score Quintiles
| Propensity score quintile |
Complications in open group (%) |
Complications in LAP group (%) |
p Value |
|---|---|---|---|
| 1 | 38.8 | 36.0 | 0.61 |
| 2 | 29.4 | 21.4 | 0.034* |
| 3 | 25.0 | 21.9 | 0.40 |
| 4 | 27.9 | 19.5 | 0.011* |
| 5 | 20.9 | 14.8 | 0.019* |
These results are graphically displayed in Figure 1.
Statistical significance at the p < 0.05 level.
LAP, laparoscopic-assisted proctectomy.
Figure 1.
Unadjusted 30-day complication rates after open proctectomy or laparoscopic-assisted proctectomy for cancer, stratified by propensity score quintiles. Solid line, open proctectomy; dotted line, laparoscopic-assisted proctectomy.
DISCUSSION
The objective of this study was to determine the frequency of perioperative complications and other early outcomes after open and laparoscopic-assisted proctectomy for cancer. We found that LAP was associated with decreased 30-day morbidity. Specifically, the unadjusted rates of superficial surgical site infection, sepsis, respiratory complications, renal failure, and venous thromboembolism were considerably lower in patients who underwent laparoscopic-assisted resection. Although mean operative time was longer in the LAP group, the requirement for blood transfusion was less. In addition, hospital LOS was 2 days less in patients who underwent LAP. There was no significant difference in the unadjusted rate of 30-day mortality between the 2 groups. After adjusting for nonrandom assignment of treatment and other potential confounding factors, the difference morbidity rate persisted.
The ACS NSQIP is an excellent data source for the study of early outcomes after surgery for colorectal cancer. Personnel use medical record review and other techniques to collect data on >200 variables. Many of these variables, eg, preoperative laboratory values, operative characteristics such ASA class, and patient-related factors such as body mass index and smoking, are not recorded in large administrative databases such as the Surveillance, Epidemiology and End Results–Medicare linked database. The ability of ACS NSQIP to capture 30-day outcomes regardless of hospital admission status is a major strength compared with other databases, such as the Healthcare Cost and Utilization Project Nationwide Inpatient Sample, which do not monitor events that occur after discharge. In 2009, the ACS NSQIP Participant Use File contained information on surgical outcomes from 237 hospitals nationwide. Findings based on multi-institutional ACS NSQIP data might be more generalizable than those based on the experience of a single high-volume academic colorectal surgery center.
After adjusting for potential confounders, we found a statistically and clinically significant association between resection technique and 30-day morbidity. Compared with open resection, the AOR for any complication with LAP was 1.41 (95% CI, 1.19–1.68).Median LOS was lower by 2 days in patients who underwent LAP. Other studies have also reported improved early outcomes with LAP for cancer. Aziz and colleagues conducted a meta-analysis of 20 studies (n = 2,071) comparing laparoscopic and open surgery for rectal cancer. LAP was associated with decreased times to stoma function, first bowel movement, and discharge from the hospital. Subgroup analysis of APRs showed decreased requirement for parenteral analgesia and reduced frequency of wound infections after LAP.13 Ding and colleagues conducted a case-control study comparing LAP and open proctectomy for rectal cancer at 3 centers in China, and found that LAP was associated with a substantially lower use of parenteral narcotics, time to first flatus and bowel movement, time to diet, time to ambulation, and LOS. They observed no difference in morbidity or mortality.14
Several randomized controlled trials (RCTs) have also provided information on early outcomes after laparoscopic versus open proctectomy for rectal cancer. Although these have demonstrated some benefits of a laparoscopic approach, none have shown substantial improvements in postoperative complications. In 2004, Leung and colleagues15 at the Chinese University of Hong Kong published the results of an RCT comparing laparoscopic and open resection of rectosigmoid carcinoma (n = 403). Times to first flatus, first bowel movement, resumption of normal diet, and independent walking were all considerably lower in the LAP group, as was LOS. There were no substantial differences between the 2 groups for postoperative morbidity or mortality.15 Subgroup analysis of the 381 patients with rectal cancer in the United Kingdom Medical Research Council Laparoscopic-Assisted Surgery in Colorectal Cancer trial revealed a 1-day shorter median time to first bowel movement and a 2-day shorter LOS in the laparoscopic group. The incidence of 30-day complications in the laparoscopic and open groups was 40% and 37%, respectively.16 Ng and colleagues17 reported the results of an RCT comparing laparoscopic versus open APR for rectal cancer (n = 99). The laparoscopic approach was associated with a lower analgesic requirement and shorter times to first bowel movement and mobilization. There were no differences in early morbidity or mortality, or in 5-year disease-free or overall survival.17 In 2009, Lujan and colleagues18 published the results of another RCT comparing LAP with open proctectomy for cancer in Spain (n = 204). As in the other RCTs, LAP was associated with faster recovery and decreased LOS. They found no differences in complication rates or in disease-free or overall survival.18
Although most RCTs have not shown a difference between LAP and open proctectomy for postoperative complications, in our multi-institutional observational study the difference was statistically and clinically significant (20.5% versus 28.8%; p < 0.0001). After partially adjusting for nonrandom assignment of treatment with propensity score analysis and controlling for other potential confounders, laparoscopic versus open resection was still an important predictor of 30-day morbidity. It is possible that selection bias exists in our study, despite out attempts to minimize it. The discrepancy in morbidity findings between our study and earlier RCTs might also be due in part to differences in sample size and statistical power. Our sample consisted of 5,420 patients, although the largest published RCT had 403 study subjects. Several large multicenter RCTs comparing laparoscopic and open proctectomy for cancer are underway. These include the multinational COLOR II, Japan Clinical Oncology Group 0404, and American College of Surgeons Oncology Group Z6051 trials.19 These studies will shed more light on the short-term advantages and long-term oncologic outcomes of LAP for rectal cancer.
The main limitations of our study are related to the source of the data. The ACS NSQIP is a voluntary program and the participating sites do not represent a statistically valid national sample of hospitals in the United States. The database contains many clinical variables, but essentially no information on patient socioeconomic status. The operative approach was dichotomized into open proctectomy and LAP based on Current Procedural Terminology codes, and we were unable to determine the rate of conversion from LAP to open. ACS NSQIP does not report cancer stage (other than “disseminated” or not), distance of the tumor from the dentate line, or tumor bulk. Hospital identifiers and geographic information are not included in the Participant Use File. Therefore, it is not possible to determine the association of hospital procedure volume on surgical outcomes using this database. There is a similar paucity of surgeon-level variables, and the database does not include information on surgeon specialty training, experience, or procedure volume. We were therefore unable to control for clustering among hospitals and surgeons. The ACS NSQIP does not track measures of physiologic recovery after colorectal surgery, including time to first bowel movement or stoma output, time to oral diet, need for parenteral narcotics, and time to independent ambulation. Similarly, the list of 30-day outcomes recorded in the ACS NSQIP omits readmission. We have previously shown that early readmission after colectomy for cancer is common, and that readmission is associated with 1-year mortality.20 The ACS NSQIP does not include information on the pathologic status of resection margins, the gross adequacy of the total mesorectal excision specimen, or the number of lymph nodes retrieved. Because the ACS NSQIP only records events up to 30 days after the operation, it cannot be used for the study of oncologic end points such as local recurrence, distant recurrence, cancer-specific mortality, and overall mortality; or important quality-of-life outcomes such as continence and sexual function.
Additionally, although we have attempted to control for bias introduced by nonrandom assignment of treatment using propensity score matching and stratification by probability of morbidity scores, we recognize that bias might exist due to unobserved or unknown confounders that the ACS NSQIP database fails to capture. Surgeons might deem patients to be poor candidates for laparoscopic surgery on the basis of nonquantifiable factors or a “gestalt” impression. We recognize that many patients in our study might have been evaluated in this subjective manner.
CONCLUSIONS
Despite these limitations, the current study, based on a large sample drawn from 237 academic and community hospitals throughout the United States, has important implications. It confirms the results of previous randomized and nonrandomized studies that showed that laparoscopic resection is associated with decreased blood transfusions, longer operative time, and shorter LOS, and is not inferior to open proctectomy for 30-day mortality. In contrast to the highly selected patients in RCTs from high-volume colorectal surgery centers, patients in the ACS NSQIP population had a decreased risk-adjusted frequency of 30-day complications after LAP compared with open proctectomy. Although our findings suggest that LAP is associated with improved short-term outcomes, the incidence of early morbidity was still 1 in 5. Additional research is needed for development of interventions that will decrease complications and improve outcomes in patients with rectal cancer undergoing surgical resection.
Abbreviations and Acronyms
- ACS NSQIP
American College of Surgeons National Surgical Quality Improvement Program
- AOR
adjusted odds ratio
- APR
abdominoperineal resection
- ASA
American Society of Anesthesiologists
- CHF
congestive heart failure
- LAP
laparoscopic-assisted proctectomy
- LOS
length of stay
- RCT
randomized controlled trial
- SGOT
serum glutamic oxaloacetic transaminase
Footnotes
Disclosure Information: Nothing to disclose.
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in it represent the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or for the conclusions derived by the authors.
Presented at the American College of Surgeons 96th Annual Clinical Congress, Washington, DC, October 2010.
Author Contributions
Study conception and design: Greenblatt, Pugely, Kennedy
Acquisition of data: Greenblatt, Rajamanickam, Pugely, Kennedy
Analysis and interpretation of data: Greenblatt, Rajamanickam, Pugely, Heise, Foley, Kennedy
Drafting of manuscript: Greenblatt, Rajamanickam, Pugely, Heise, Foley, Kennedy
Critical revision: Greenblatt, Rajamanickam, Pugely, Heise, Foley, Kennedy
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy GD, Heise C, Rajamanickam V, et al. Laparoscopy decreases postoperative complication rates after abdominal colectomy: results from the national surgical quality improvement program. Ann Surg. 2009;249:596–601. doi: 10.1097/SLA.0b013e31819ec903. [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Bentrem DJ, Merkow RP, et al. Laparoscopic-assisted vs. open colectomy for cancer: comparison of short-term outcomes from 121 hospitals. J Gastrointest Surg. 2008;12:2001–2009. doi: 10.1007/s11605-008-0568-x. [DOI] [PubMed] [Google Scholar]
- 4.Abraham NS, Byrne CM, Young JM, Solomon MJ. Meta-analysis of non-randomized comparative studies of the short-term outcomes of laparoscopic resection for colorectal cancer. ANZ J Surg. 2007;77:508–516. doi: 10.1111/j.1445-2197.2007.04141.x. [DOI] [PubMed] [Google Scholar]
- 5.Tjandra JJ, Chan MK. Systematic review on the short-term outcome of laparoscopic resection for colon and rectosigmoid cancer. Colorectal Dis. 2006;8:375–388. doi: 10.1111/j.1463-1318.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 6.Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa H, Kabeshima Y, Watanabe M, et al. Randomized controlled trial of laparoscopic versus open colectomy for advanced colorectal cancer. Surg Endosc. 2003;17:636–640. doi: 10.1007/s00464-002-8516-4. [DOI] [PubMed] [Google Scholar]
- 8.Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of nonmetastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 9.Milsom JW, Bohm B, Hammerhofer KA, et al. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg. 1998;187:46–54. doi: 10.1016/s1072-7515(98)00132-x. discussion 54–55. [DOI] [PubMed] [Google Scholar]
- 10.Row D, Weiser MR. An update on laparoscopic resection for rectal cancer. Cancer Control. 2010;17:16–24. doi: 10.1177/107327481001700103. [DOI] [PubMed] [Google Scholar]
- 11.American College of Surgeons National Surgical Quality Improvement Program. [Accessed January 3, 2010]; Available at: https://acsnsqip.org. [Google Scholar]
- 12.The World Health Organization. International classification of adult underweight, overweight and obesity according to body mass index. [Accessed January 3, 2010]; Available at: http://apps.who.int/bmi/index.jsp?introPage_intro_3.html. [Google Scholar]
- 13.Aziz O, Constantinides V, Tekkis PP, et al. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol. 2006;13:413–424. doi: 10.1245/ASO.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 14.Ding KF, Chen R, Zhang JL, et al. Laparoscopic surgery for the curative treatment of rectal cancer: results of a Chinese three-center case-control study. Surg Endosc. 2009;23:854–861. doi: 10.1007/s00464-008-9990-0. [DOI] [PubMed] [Google Scholar]
- 15.Leung KL, Kwok SP, Lam SC, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187–1192. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 16.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 17.Ng SS, Leung KL, Lee JF, et al. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol. 2008;15:2418–2425. doi: 10.1245/s10434-008-9895-0. [DOI] [PubMed] [Google Scholar]
- 18.Lujan J, Valero G, Hernandez Q, et al. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. 2009;96:982–989. doi: 10.1002/bjs.6662. [DOI] [PubMed] [Google Scholar]
- 19.Soop M, Nelson H. Laparoscopic-assisted proctectomy for rectal cancer: on trial. Ann Surg Oncol. 2008;15:2357–2359. doi: 10.1245/s10434-008-0020-1. [DOI] [PubMed] [Google Scholar]
- 20.Greenblatt DY, Weber SM, O’Connor ES, et al. Readmission after colectomy for cancer predicts one-year mortality. Ann Surg. 2010;251:659–669. doi: 10.1097/SLA.0b013e3181d3d27c. [DOI] [PMC free article] [PubMed] [Google Scholar]