Abstract
Herceptin/Trastuzumab is a humanized IgG1κ light chain antibody used to treat some forms of breast cancer. A phage-displayed recombinant antibody library was used to obtain an scFv (designated 2B4) to a linear synthetic peptide representing Herceptin’s heavy chain CDR3. ELISAs and piezoimmunosensor/quartz crystal microbalance (QCM) assays were used to characterize 2B4-binding activity to both native and heat denatured Herceptin. The 2B4 scFv specifically bound to heat denatured Herceptin in a concentration dependent manner over a wide (35–220.5 nM) dynamic range. Herceptin denatures and forms significant amount of aggregates when heated. UV-Vis characterization confirms that Herceptin forms aggregates as the temperature used to heat Herceptin increases. QCM affinity assay shows that binding stoichiometry between 2B4 scFv and Herceptin follows a 1:2 relationship proving that 2B4 scFv binds strongly to the dimers of heat denatured Herceptin aggregates and exhibits an affinity constant of 7.17 × 1013 M−2. The 2B4-based QCM assay was more sensitive than the corresponding ELISA. Combining QCM with ELISA can be used to more fully characterize non-specific binding events in assays. The potential theoretical and clinical implications of these results and the advantages of using QCM to characterize human therapeutic antibodies in samples are also discussed.
Introduction
Chimeric, humanized and fully human therapeutic antibodies and antibody fragments are gaining widespread use for the treatment of various human diseases such as arthritis, autoimmune diseases, allergy, cardiovascular disease, transplant rejection, cancer and viral infections [1, 2]. These therapeutic protein drugs can be extraordinarily expensive, with some treatments costing $100,000 or more per year. Therapeutic proteins that aggregate or denature upon storage may lose biological activity and cannot be used in humans. Proteins such as platelet factor 4, phosphorylase b, stem cell factor, hexokinase PI, HIV protease subunits, and growth hormone can self-associate significantly to form dimer and larger protein aggregates [3]. Proteins that self-associate and aggregate can contribute to the pathology associated with several human disorders including idiopathic cryoglobulinemia and rheumatoid arthritis. Therefore, aggregated proteins cannot be used as therapeutics in humans. Herceptin, Avastin, Cetuximab and Rituximab are human therapeutic IgG1κ light chain antibodies. Human recombinant antibodies can also form multimers upon storage. It was reported that a human recombinant IgG1κ light chain antibody to VEGF existed predominantly as a monomer when stored at a pH below 5.5; but formed non-covalent aggregates when stored under higher pH, temperature and ionic conditions. The non-covalent aggregates reverted back to monomers when the antibody was diluted [3]. Assuming that the monomeric antibody retains biological activity, it could be successfully used as a therapeutic in humans.
It can be costly and time consuming to determine if therapeutic antibodies in solution have aggregated or denatured when produced or stored. We used phage display and QCM to develop a rapid, highly sensitive scFv-based piezoimmunosensor assay to detect aggregated and degraded Herceptin in solution. This and similar assays can potentially be used to monitor therapeutic antibodies to quickly identify optimal conditions under which antibodies can be produced, formulated, stored and used to retain biological activity.
Phage-display has been used since the mid to late 1980s to select for peptides and proteins (e.g. scFv and Fab recombinant antibodies) specific for a wide range of target molecules for use in assays [4, 5]. Recombinant scFv antibodies are small heterodimers that are composed of antibody variable heavy (VH) and light (VL) chains joined together and stabilized by a peptide linker.[6, 7] Recombinant scFvs represent the smallest functional VH-VL domains of antibodies necessary for antigen-binding activity. The key advantages in using scFv antibody fragments for immunoassays are that antigen-specific scFvs can be rapidly selected using phage display, genetically engineered to assume unique characteristics, and inexpensively produced in bacteria. Recombinant scFv are small (~27,000 daltons) in comparison to traditional IgG antibodies (~150,000), can penetrate tumors more readily, are rapidly cleared from the body and can exhibit great antigen-binding specificity and low immunogenicity.[8] We have successfully used phage-display to select for scFv specific for P450 enzymes (e.g. CYP2B19 and CYP1B1),[9, 10] a bacterial toxin (e.g. H. pylori BabA),[11] phospho-Akt,[12] protein adducts (e.g. teucrin A and the isoketal known as levuglandin), [13, 14] a radiation-induced antigen (P-selectin),[15] metal nanoclusters,[16] the angiotensin II receptor[17] and a putative early breast cancer biomarker.[18] These scFvs have been used either for mass spectrometry-based proteomic analysis,[14] or have been used to image tumors[15] or inhibit tumor cell growth.[12]
The heavy chain CDR3 region of an antibody generally confers antigen-binding specificity and typically displays the most unique amino acid sequence among different antigen-specific antibodies present in a sample. As such, antibodies (i.e. anti-Ids) that specifically bind to the heavy chain CDR3 region of another antibody (e.g. Herceptin) will generally bind to that antibody (e.g. Herceptin) and not to other antibodies such as those present in normal serum. Since the linear heavy chain CDR3 peptide can mimic an epitope on an antibody, it is likely the antibody selected using a linear chain CDR peptide of an antigen will bind to the antigen either in native state or when it was denatured as long as the linear peptide region is intact. We carried out phage antibody selections on a Herceptin heavy chain CDR3 linear synthetic peptide to obtain an scFv, designated 2B4. We were interested in determining if we could use the 2B4 scFv, ELISAs and QCM to detect native and heat denatured Herceptin in QCM assays. We selected Herceptin as our therapeutic monoclonal antibody to monitor the effects elevated temperatures have on human therapeutic antibody aggregation and denaturation. Herceptin is used to treat some forms of breast cancer. Herceptin selectively binds to the human epidermal growth factor receptor known as Her2 [19] on breast cancer cells and it successfully inhibits cancer cell proliferation [20]. Herceptin can induce cardiac dysfunction in some individuals [21, 22]. As shown in Figure 1, the Herceptin antibody heavy chain CDR3 amino acid sequence is WGGDGFYAMDY. This sequence is preceded by the 3 amino acids CSR. A peptide (CSRWGGDGFYAMDY) was synthesized to contain, among other things, the Herceptin heavy chain CDR3 amino acid sequence (WGGDGFYAMDY) and the naturally occurring cysteine (C). The cysteine free sulfhydryl (-SH) group was used to couple the peptide to maleimide-activated carrier proteins (e.g. maleimide-activated BSA) for phage selections to obtain the 2B4 scFv specific for the Herceptin heavy chain CDR3 region and Herceptin IgG. We were interested in determining if we could use the 2B4 scFv, ELISAs and QCM to detect native and heat denatured Herceptin. Tornetta et. al has used an anti-idiotypic antibodies as recognition elements to detect Herceptin[23]. Our past work shows that scFv is smaller than anti-idiotypic antibody and could significantly increase the analytical performance of a biosensor. Here we focus on the investigation of the binding potential of a scFv antibody with native and heat denatured therapeutical humanized monoclonal antibody Herceptin by ELISAs and QCM to monitor the effects of elevated temperatures have on a human therapeutic antibody properties. The 2B4 scFv was subsequently biotinylated, immobilized onto NeutrAvidin coated Au QCM sensor surfaces and used to capture and detect heat-treated Herceptin.
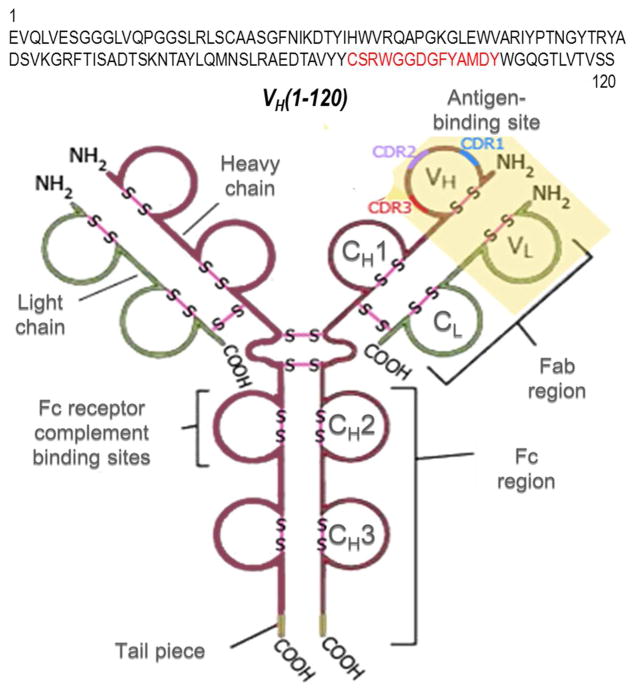
Figure 1. Structure of a Herceptin Molecule.
There are two pairs of heavy (H, Dark Red) and light (L, Light Green) chains in one Herceptin IgG. Each pair of H and L chain contains constant domains (CL, CH1, CH2, and CH3) and variable regions (VL, and VH) with their CDRs (CDR1, CDR2, and CDR3). The heavy chain CDR3 region of Herceptin is highlighted and displayed in red both in the linear amino acid sequence VH(1–120) and the Y-shaped immunoglobulin structure. The antigen binding site, Fab and Fc region, Fc receptor complement binding sites, N-terminal (-NH2) and C-terminal (-COOH) ends of each polypeptide, and the positions of S-S disulfide bonds are delineated and indicated.
Experimental Section
Materials
NeutrAvidin™ biotin-binding protein (Thermo Scientific, Cat. No. 31000) was received in lyophilized condition. Human therapeutic antibodies such as Herceptin® (Trastuzumab), Avastin® (Bevacizumab), Rituxan® (Rituximab) and Yervoy were provided as reconstituted solutions (21 μg/μL Herceptin, 25 μg/μL Avastin, 10 μg/μL Rituxan and 5μg/μl Yervoy respectively) in Sterile Water for Injection (SWFI) by Beaumont Hospital, Royal Oak, Michigan or the Vanderbilt University Medical Center pharmacy. UltraPure™ distilled water (Invitrogen Cat. No. 10977-015) and Hepes buffered saline (HBS) and HBS containing Tween 20 (HBS-P) (G. E. Healthcare Cat. No. BR-1003-69 and BR-1003-68, respectively) were used as provided. All other related chemicals were purchased from Sigma-Aldrich Corp. (St. Louis, MO), were of Bio-reagent grade quality and used without further purification.
Phage selection, characterization, purification and biotinylation of the 2B4 scFv
The scFv recombinant antibodies specific for Herceptin were selected by utilizing a large (~2.9 billion member) phage-displayed recombinant antibody library according to modifications of previously described methods[24]. Briefly, to obtain scFv specific for Herceptin, the phage-displayed scFv library was panned against a Herceptin heavy chain CDR3 synthetic peptide (CSRWGGDGFYAMDY) conjugated to bovine serum albumin (BSA). Phage scFv bound to the peptide were eluted using Herceptin, and used to infect E. coli TG1 cells. E. coli TG1 cells were plated onto agar medium. After two rounds of phage scFv selections, individual bacterial colonies were picked and induced to express soluble E-tagged scFv. Individual E-tagged scFv bound to Herceptin in ELISA were detected using an anti-E tag monoclonal antibody conjugated to peroxidase (G.E. Healthcare, Cat. No. 27-9413-01), a color developer (ABTS) and hydrogen peroxide. E. coli producing scFv reactive with Herceptin but not a negative control human IgG1κ light chain antibody (e.g. Synagis, MedImmune) were purified using the RPAS Purification Module (G.E. Healthcare cat# 17-1362-01) according to the manufacturer’s instructions, then biotinylated (see supporting materials S1–3).
2B4 scFv Preparation and Characterization by QCM
The 2B4 scFv-based QCM assay format is depicted in Scheme 1. The gold quartz crystal electrode used for 2B4 characterization was an AT cut quartz wafer coated with 1000-Å of gold onto ~0.23 cm2 geometric area (International Crystal Co.). The non-polished gold quartz surface was cleaned as described earlier.[25–28] The freshly prepared gold surface was immersed in a 1 mg/ml NeutrAvidin in HBS solution for 2 h at room temperature, washed thoroughly with HBS buffer to remove unbound NeutrAvidin, dried gently with pure N2, incubated with biotinylated 2B4 scFv solution (60 ng/μL) for more than 8 h at 4°C, washed and dried as described above, then used for the QCM assay. During each measurement, the scFv modified gold surface was mounted at the side of a Kel-F cell filled with 1 mL of HBS buffer. To reduce the potential electromagnetic noise, the cell was placed in a Faraday cage and stirred continuously [29]. A Network/Spectrum/Impedance Analyzer (Agilent 4395A) was used to monitor frequency (ΔF) and damping resistance change (ΔR) on the modified gold surface electrode in real time. A schematic of the QCM experimental set-up is shown in Figure S1. Electrochemical characterization of the 2B4 scFv-modified gold quartz crystal electrode is illustrated in Figure S2 and described in S4 with data presented in Table S1.
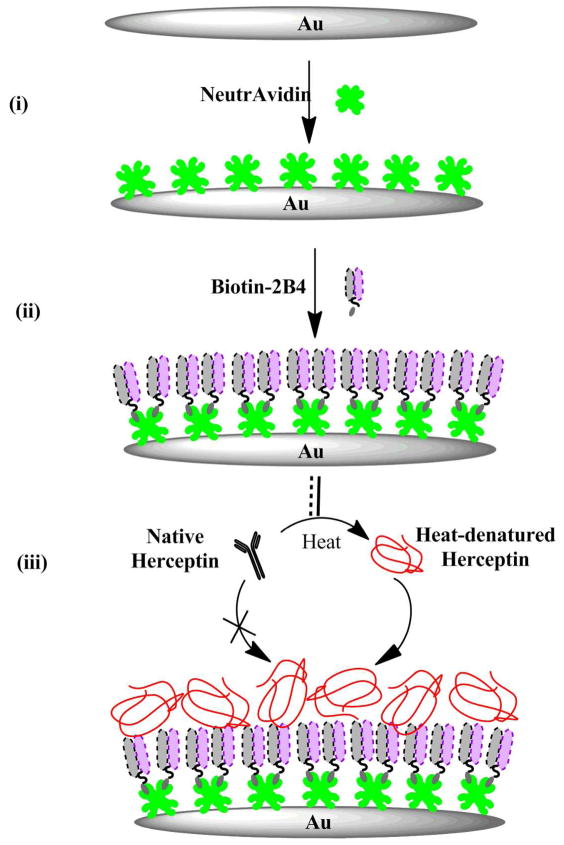
Scheme 1. * Schematic depicting QCM assay used to detect heat-denatured Herceptin.
(i) NeutrAvidin was drop-coated onto the freshly prepared bare Au quartz crystal electrode; (ii) Biotinylated 2B4 scFv were tethered to the Au surface electrode via biotin-NeutrAvidin interaction; (iii) Heat-denatured Herceptin was captured by the 2B4 scFv on the Au quartz crystal electrode and detected by the QCM assay (No response to the intact Herceptin was observed). * Note: Figure not drawn to scale.
Therapeutic antibodies thermal pretreatment
An Eppendorf® Thermomixer Shaker was used to heat Herceptin and negative control (e.g., Avastin, Rituxan) therapeutic antibodies and serum samples. Prior to QCM analysis, 15–25μl of therapeutic antibodies were heated at temperature 37 °C, 61 °C and 71 °C for 30min with shaking at 300 rpm; and at temperature 83 °C, 85 °C, 90 °C, 95 °C and 99 °C for 5min with shaking at 300 rpm, respectively. Samples were then cooled to room temperature prior to assay. Measurements of Herceptin in biograde water denatured upon heating were performed using Cary 100 UV-vis spectrometer and results are shown in Figure S3.
Results and discussion
ELISA Characterization of 2B4 scFv and biotinylated 2B4-scFv
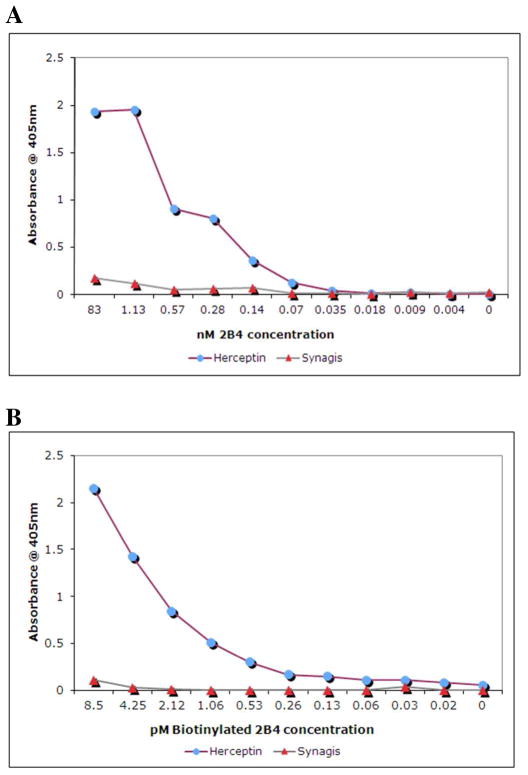
Affinity purified 2B4 scFv was assayed against Herceptin and Synagis by ELISA. E-tagged 2B4 scFv was detected using the anti-E tag/HRP conjugate and ABTS/H2O2. The 2B4 scFv ELISA results are depicted in Figure 2A. The purified scFv was also biotinylated and used to detect Herceptin in ELISA. Biotinylated 2B4 bound to Herceptin was detected using streptavidin/HRP and ABTS/H2O2. The biotinylated 2B4 ELISA results are depicted in Figure 2B. Assay results suggest that both unlabeled (Figure 2A) and biotinylated 2B4-scFv (Figure 2B) bound to Herceptin, and to a much lesser extent, to Synagis. The 2B4 scFv apparently exhibits high antigen-binding strength as only nM or pM concentration is needed to obtain an ELISA assay signal.
Figure 2. ELISA results of 2B4 scFv characterization.
(A) Affinity purified 2B4; and (B) The biotinylated 2B4 was assayed against Herceptin by ELISA. Synagis was used as a negative control.
ELISA characterization of biotinylated 2B4-scFv binding specificity
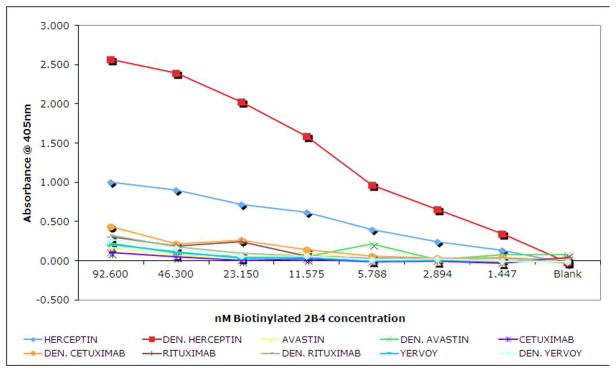
ELISAs were used to determine 2B4-scFv binding specificity. Native and heat-denatured therapeutic antibodies were coated onto microtiter plates at 25ng/well and probed with biotinylated 2B4-scFv diluted serially from 92.6 nM to 1.447 nM. Biotinylated 2B4–scFv bound more strongly to denatured Herceptin, ~2.5 times less so to native (non-heat denatured) Herceptin and only slightly to native and heat-deantured negative control therapeutic antibodies (e.g. Avastin, Rituxan, etc.) (Figure 3). These results suggest that biotinylated 2B4-scFv has greater binding specificity for heat denatured Herceptin than for native Herceptin or other native of denatured therapeutic antibodies.
Figure 3. 2B4-scFv binding specificity.
Figure depicts ELISA results for biotinylated 2B4-scFv on native and heat denatured Herceptin and the negative control therapeutic antibodies Avastin, Cetuximab, Rituximab and Yervoy.
Characterization of biotinylated 2B4 scFv by QCM
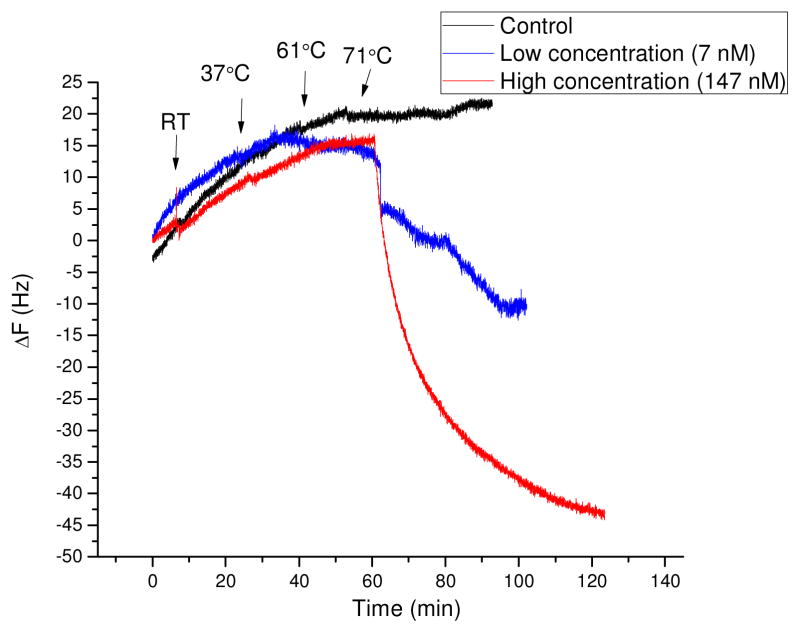
ELISA results (Figure 2 and 3) indicate that 2B4 scFv and biotinylated 2B4 scFv recognized both native and heat denatured Herceptin coated onto wells of a microtiter plate surface. The 2B4 scFv to Herceptin was further characterized by QCM to determine if 2B4 scFv could capture and detect Herceptin present in a solution. If the 2B4 scFv captured Herceptin when analyzed by QCM, then a gold sensor frequency change (ΔF) would occur. The biotinylated 2B4 scFv was immobilized onto a NeutrAvidin-coated QCM surface and used to capture Herceptin out of solution. Our initial results indicated that biotinylated 2B4 scFv immobilized onto a NeutrAvidin-coated QCM sensor surface did not capture native or non-heat denatured Herceptin out of solution (Figure 4). However, the 2B4 scFv was shown to bind to native and heat-denatured Herceptin in ELISA (Figure 3). Proteins (e.g. Herceptin) can become denatured when immobilized onto a solid surface (e.g. the surface of a microtiter plate). The 2B4 scFv was selected by phage display against a linear heavy chain CDR3 peptide of Herceptin. Since linear peptides do not always assume the correct structural conformation of an epitope on a native protein, then it was possible that the linear CDR3 peptide used for phage selections to obtain the 2B4 scFv may have assumed the conformation of an epitope on denatured Herceptin. Therefore, we hypothesized that the 2B4 scFv would capture Herceptin out of solution, if Herceptin was denatured. Since heat can denature proteins, Herceptin was pre-heated to room temperature (RT), 37, 61 or 71°C then assayed by QCM to determine if 2B4 scFv was specific for denatured Herceptin. As shown in Figure 4, QCM analysis indicated that 2B4 scFv did capture heat denatured Herceptin, when Herceptin was heated to 71°C. The 2B4 scFv did not capture Herceptin, when Herceptin was exposed to temperatures lower than 71°C (Figure 4). These results are supported in part by the findings published by Vermeer et. al. who demonstrated that IgG antibodies (similar to Herceptin) unfold at 71°C [30]. The 2B4 scFv QCM assay signal decreased from −25 to −65Hz ΔF, respectively, when 71°C treated Herceptin was spiked into the 1 ml QCM cell at low (~7 nM) and high (~147 nM) concentrations, and thus demonstrated that the 2B4 scFv captured heat denatured Herceptin (Figure 4). Electrochemical methods (Cyclic Voltammetry (CV) and Electrochemical Impedance Spectroscopy (EIS) were used to further characterize the 2B4 scFv QCM sensor surface and the interactions between the 2B4 scFv and native and heat denatured Herceptin. Redox [Fe(CN)3−/Fe(CN)4−] probe peaks, as an indication of sensor surface binding events, sequentially decreased upon NeutrAvidin, biotinylated 2B4 scFv and heat-denatured Herceptin surface deposition (Figure S2 and Table S1). Taken together, these results confirmed that the 2B4 scFv specifically bound to heat denatured Herceptin.
Figure 4. QCM frequency change (ΔF) vs time.
A NeutrAvidin/biotin 2B4 modified Au QCM electrode was exposed sequentially to Herceptin incubated at RT or pre-heated to 37°C, 61°C or 71 °C, at low (Blue, ~7 nM) and high (Red, ~147 nM) Herceptin concentration. NeutrAvidin modified Au without the 2B4 scFv was used for the negative control.
Characterization of Herceptin at heat denaturation conditions
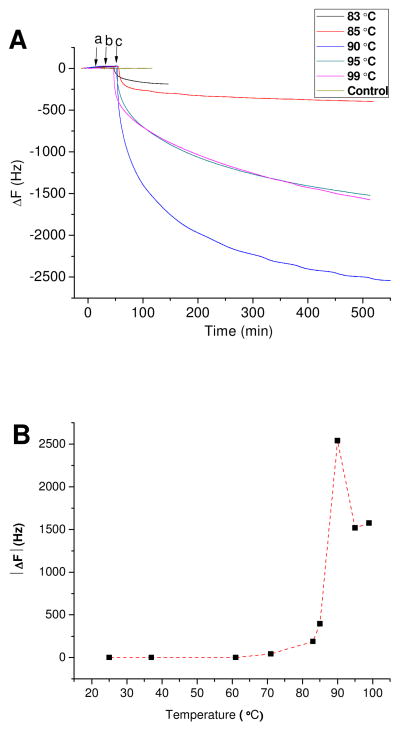
In general, the melting point (Tm) of a protein is indicative of its thermal stability. Kelley, R.F., et al. reported that the Tm of Herceptin Fab was around 83 °C [31]. Antibody IgG may exhibit greater thermal stability than antibody Fab fragments. Based on the evidence obtained thus far, 2B4 scFv binds to and captures Herceptin heated to 71°C. The QCM assay was used to determine if 71°C was optimal for Herceptin heat-denaturation and 2B4 scFv binding and capture activity. Herceptin was heated from 83–99°C and assayed by the 2B4 scFv QCM assay. Avastin and Rituxan were heated similarly and used as negative controls (Figure 5A).
Figure 5. (A) The frequency change (ΔF) vs Time, and (B) The absolute frequency change (|ΔF|) vs Temperature curve for Heat-denatured Herceptin.
The 2B4 scFv quartz transducer was exposed to 1 μL of heat-treated Avastin (25 μg/μl), Rituxan (10 μg/μl) and Herceptin (21 μg/μl) stock concentrations. NeutrAvidin modified Au – only- was used as the QCM electrode negative control.
The 2B4 modified gold QCM electrode demonstrated negligible binding activity to the negative controls but bound heat-treated Herceptin. The increase in absolute values for the 2B4 scFv QCM frequency-shift (|ΔF|), as an indication of enhanced 2B4 scFv binding activity, was 189 Hz at 83 °C, 395 Hz at 85 °C, 2540 Hz at 90 °C, 1519 Hz at 95 °C, and 1575 Hz at 99 °C (Figure 5A). The data were used to generate a |ΔF| versus temperature curve to determine that 90°C heat denaturation temperature was optimal for 2B4/Herceptin binding activity (Figure 5B).
UV-vis absorption Spectroscopy is commonly used to characterize the relative levels of protein aggregations in solution[32]. As shown in Figure S3, when Herceptin was heated, an increase in optical density due to an increase in light scattering is observed between 350 nm to 650nm even at the concentration of Herceptin of ~5 μM. The wavelength between 350 nm-650 nm provides the best sensitivity for measuring aggregations generated scattering while avoiding absorption due to the protein itself. This result confirmed that at elevated temperature Herceptin self-association occurs and forms aggregates.
Characterization of 2B4-scFv QCM assay analytical figure of merits
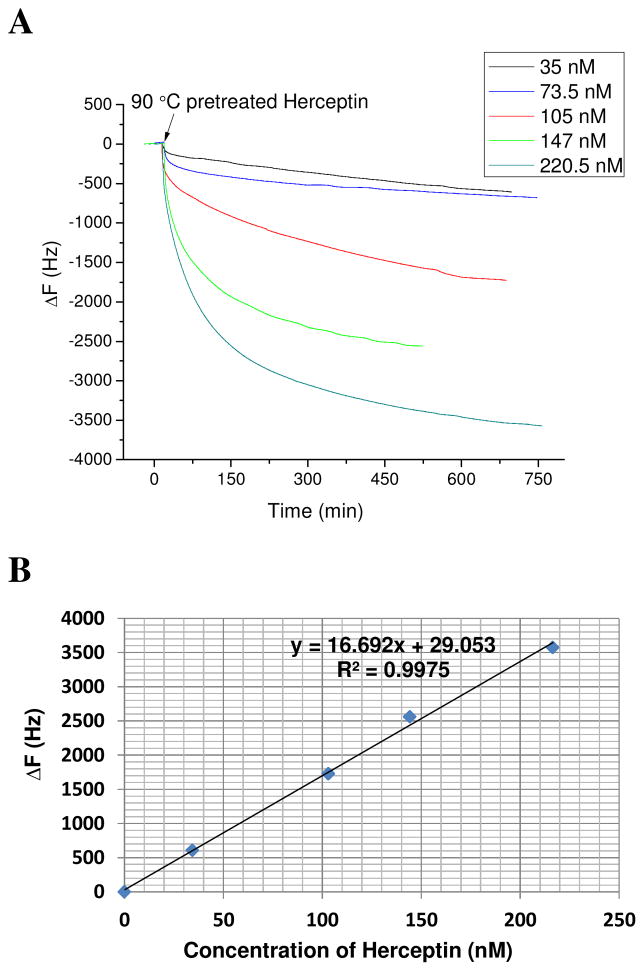
The dynamic range for the 2B4 scFv QCM assay was determined using low to high concentrations of heat-denatured Herceptin. As described previously, the 2B4 scFv in QCM exhibited highest binding activity to 90°C heat-treated Herceptin (Figure 5). The 2B4 scFv QCM assay was used to determine 90°C treated Herceptin (ranging in concentrations from 35–220.5 nM) assay sensitivity. An increase in concentration of heat-denatured Herceptin added to the 2B4 scFv QCM cell resulted in an increase in QCM frequency change (ΔF). The ΔF did not reach saturation even when the concentration of heat-denatured Herceptin reached 220.5 nM (Figure 6A). A nearly direct correlation (R2 = 0.9975) between frequency shift (ΔF) and Herceptin concentration was obtained using the 2B4 scFv QCM assay. The 2B4 scFv-modified Au quartz transducer exhibited a sensitivity of 16.7 Hz/nM (Figure 6B) and a detection limit of 0.21 nM when a Signal/Noise ratio of 3:1 was used.
Figure 6. 2B4 scFv QCM assay dynamic range.
(A) Comparison of frequency change vs time. Biotin-2B4 NeutrAvidin modified Au QCM electrode was exposed to 1 μl volumes of Herceptin spiked into 1 ml of HBS P at 5 different concentrations ranging from 35–220.5 nM; (B) Frequency change vs concentration of 90 °C pretreated Herceptin.
Binding affinity of 2B4 scFv with heat-denatured Herceptin by QCM assay
Herceptin, when heated, apparently self-associates to form oligomers (see Figures 5 and S3). However, we did not know how many heavy chain CDR3 regions would be exposed and available for 2B4 scFv binding when Herceptin aggregated and formed oligomers. QCM data used to determine the affinity constant (Ka) for the 2B4 scFv/heat-denatured Herceptin interaction suggested that Herceptin formed dimers when heated to 90°C and that only one heavy chain CDR3 region was exposed and available for 2B4 scFv binding. The QCM data supported the following assumptions and formulas that were subsequently used to determine the Ka for the 2B4 scFv/heat-treated Herceptin interactions. The Affinity constant Ka can be determined based on the following reactions.
| (1) |
| (2) |
Based on a Langmuir adsorption isotherm and above reaction stoichiometry, the association constant (Ka) for the scFv-2B4 and heat denatured Herceptin interaction can be obtained by the following equation.
| (3) |
Where, ΔMmax is the maximum concentration of heat-denatured Herceptin that can bind to the 2B 4 scFv sensor surface, when all the 2B4 scFv are converted to scFv-denatured Herceptin dimer complexes. ΔM is the concentration of heat-denatured Herceptin at equilibrium, and [Herceptin] is the concentration of monomer Herceptin. The scFv-2B4/heat denatured Herceptin binding amount at the nanogram level (ΔM) can be obtained from the time relationship of frequency shift (ΔF) at various heat-denatured Herceptin concentrations (see Figure 6). Then [Herceptin]2/ΔM as the function of [Herceptin]2 was plotted (Figure 7). The linearity of data depicted in Figure 7 suggests that 2B4 scFv binds with the heat denatured Herceptin dimmers and not other oligomer of Herceptin. The ratio of the slope to the intercept of Figure 7 was used to obtain the association constant (Ka), 7.17 × 1013 M−2, by applying to equation 3. This affinity constant is close to the biotin –avidin binding affinity constant, the highest in nature. The self- association of some proteins has been shown to largely depend on pH and ionic strength [3]. However, under our assay conditions, the results suggest that the dimerization of heat treated Herceptin in biograde water, in which ions are lacking, is probably due to of hydrophobic interaction. However, pH and ionic strength may also contribute to Herceptin aggregation in which Herceptin is stored under other conditions. At 90°C, both the Fc and Fab region of an IgG antibody can be denatured and involved in Herceptin aggregation. The involvement of the Fc region in Herceptin dimerization could be further studied using other antibodies that contain identical Fc regions such as Avastin. Investigations are currently under way to determine the regions on Herceptin that are responsible for Herceptin aggregation and 2B4 scFv binding activity when Herceptin is heated.
Figure 7.
Plot of [Herceptin]2/ΔM vs [Herceptin]2 of NeutrAvidin/Biotin-2B4 modified piezoimmunosensor to obtain the scFv-2B4/Herceptin binding association constant (Ka).
Conclusions
We have demonstrated in this report that a synthetic Herceptin heavy chain CDR3 peptide can be used as an antigen for phage recombinant antibody selections to obtain an scFv (designated 2B4) specific for denatured Herceptin dimer. The 2B4 scFv bound optimally to 90°C heat-denatured Herceptin and much less so to Herceptin heated at higher or lower temperatures or when left untreated. When formatted for use in a QCM assay, the Ka for the interaction between the 2B4-scFv and 90°C heat-denatured Herceptin was high at 7 × 10−13M−2. Conceivably, this and other scFv-based QCM assays can be used to analyze Herceptin and other therapeutic antibodies to determine antibody thermal stability, aggregation and denaturation conditions. Similar scFv-based QCM assays can also be used to follow and optimize large-scale antibody production, purification and storage conditions in industry to determine if antibodies are properly folded, remain stable and suitable for use in humans.
Supplementary Material
Acknowledgments
X Zeng and R. Mernaugh like to thank support from NIH R21 (EB006495) for this work. R. Mernaugh was supported in part by the Vanderbilt Institute of Chemical Biology. We like to thank Ms. Peiling Lin’s help in UV-vis experiment.
References
- 1.Brekke OH, Loset GA. New technologies in therapeutic antibody development. Curr Opin Pharmacol. 2003;3(5):544–50. doi: 10.1016/j.coph.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Majidi J, et al. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Human antibodies. 2009;18(3):81–100. doi: 10.3233/HAB-2009-0204. [DOI] [PubMed] [Google Scholar]
- 3.Moore JM, Patapoff TW, Cromwell ME. Kinetics and thermodynamics of dimer formation and dissociation for a recombinant humanized monoclonal antibody to vascular endothelial growth factor. Biochemistry. 1999;38(42):13960–7. doi: 10.1021/bi9905516. [DOI] [PubMed] [Google Scholar]
- 4.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228(4705):1315–7. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 5.Azzazy HM, Highsmith WE., Jr Phage display technology: clinical applications and recent innovations. Clinical biochemistry. 2002;35(6):425–45. doi: 10.1016/s0009-9120(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 6.Bird RE, et al. Single-chain antigen-binding proteins. Science. 1988;242(4877):423–6. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- 7.Huston JS, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(16):5879–83. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng X, Shen Z, Mernaugh R. Recombinant antibodies and their use in biosensors. Analytical and bioanalytical chemistry. 2011 doi: 10.1007/s00216-011-5569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du L, et al. Evidence that cytochrome P450 CYP2B19 is the major source of epoxyeicosatrienoic acids in mouse skin. Arch Biochem Biophys. 2005;435(1):125–33. doi: 10.1016/j.abb.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Shen Z, et al. Recombinant antibody piezoimmunosensors for the detection of cytochrome P450 1B1. Anal Chem. 2007;79(4):1283–9. doi: 10.1021/ac061211a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennig EE, et al. Heterogeneity among Helicobacter pylori strains in expression of the outer membrane protein BabA. Infect Immun. 2004;72(6):3429–35. doi: 10.1128/IAI.72.6.3429-3435.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin I, et al. Proapoptotic activity of cell-permeable anti-Akt single-chain antibodies. Cancer research. 2005;65(7):2815–24. doi: 10.1158/0008-5472.CAN-04-2898. [DOI] [PubMed] [Google Scholar]
- 13.Davies SS, et al. Localization of isoketal adducts in vivo using a single-chain antibody. Free Radic Biol Med. 2004;36(9):1163–74. doi: 10.1016/j.freeradbiomed.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Druckova A, et al. Identification of the protein targets of the reactive metabolite of teucrin a in vivo in the rat. Chem Res Toxicol. 2007;20(10):1393–408. doi: 10.1021/tx7001405. [DOI] [PubMed] [Google Scholar]
- 15.Hariri G, et al. Radiation-guided P-selectin antibody targeted to lung cancer. Ann Biomed Eng. 2008;36(5):821–30. doi: 10.1007/s10439-008-9444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edl J, Mernaugh R, Wright DW. Assays for selection of single-chain fragment variable recombinant antibodies to metal nanoclusters. Methods Mol Biol. 2005;303:113–20. doi: 10.1385/1-59259-901-X:113. [DOI] [PubMed] [Google Scholar]
- 17.Tamura M, et al. Specific single chain variable fragment (ScFv) antibodies to angiotensin II AT(2) receptor: evaluation of the angiotensin II receptor expression in normal and tumor-bearing mouse lung. J Mol Histol. 2008 doi: 10.1007/s10735-008-9172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley C, et al. Carcinogen-induced histone alteration in normal human mammary epithelial cells. Carcinogenesis. 2007;28(10):2184–92. doi: 10.1093/carcin/bgm100. [DOI] [PubMed] [Google Scholar]
- 19.Cho HS, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421(6924):756–60. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 20.Park JW, TD, Campbell MJ, et al. Biological therapy of breast cancer. Biodrugs. 2000;14(Oct):221–46. [Google Scholar]
- 21.Seidman A, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(5):1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 22.Ewer MS, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(31):7820–6. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 23.Tornetta M, et al. Isolation of human anti-idiotypic antibodies by phage display for clinical immune response assays. Journal of immunological methods. 2007;328(1–2):34–44. doi: 10.1016/j.jim.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, et al. Selection of active ScFv to G-protein-coupled receptor CCR5 using surface antigen-mimicking peptides. Biochemistry. 2004;43(39):12575–84. doi: 10.1021/bi0492152. [DOI] [PubMed] [Google Scholar]
- 25.Shen Z, et al. Engineered recombinant single-chain fragment variable antibody for immunosensors. Anal Chem. 2005;77(21):6834–42. doi: 10.1021/ac0507690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Z, et al. Single-chain fragment variable antibody piezoimmunosensors. Anal Chem. 2005;77(3):797–805. doi: 10.1021/ac048655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Z, et al. Engineering peptide linkers for scFv immunosensors. Anal Chem. 2008;80(6):1910–7. doi: 10.1021/ac7018624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang Y, et al. Immobilization of a human epidermal growth factor receptor 2 mimotopederived synthetic peptide on Au and its potential application for detection of herceptin in human serum by quartz crystal microbalance. Analytical chemistry. 2011;83(23):8928–36. doi: 10.1021/ac201430p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Z, et al. Nonlabeled quartz crystal microbalance biosensor for bacterial detection using carbohydrate and lectin recognitions. Anal Chem. 2007;79(6):2312–9. doi: 10.1021/ac061986j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeer AW, Norde W. The thermal stability of immunoglobulin: unfolding and aggregation of a multi-domain protein. Biophys J. 2000;78(1):394–404. doi: 10.1016/S0006-3495(00)76602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley RF, et al. Antigen binding thermodynamics and antiproliferative effects of chimeric and humanized anti-p185HER2 antibody Fab fragments. Biochemistry. 1992;31(24):5434–41. doi: 10.1021/bi00139a003. [DOI] [PubMed] [Google Scholar]
- 32.Sarciaux JM, et al. Effects of buffer composition and processing conditions on aggregation of bovine IgG during freeze-drying. Journal of pharmaceutical sciences. 1999;88(12):1354–61. doi: 10.1021/js980383n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.