Abstract
Rates of sulfate reduction (SR) and anaerobic oxidation of methane (AOM) in hydrothermal deep-sea sediments from Guaymas Basin were measured at temperatures of 5 to 200°C and pressures of 1 × 105, 2.2 × 107, and 4.5 × 107 Pa. A maximum SR of several micromoles per cubic centimeter per day was found at between 60 and 95°C and 2.2 × 107 and 4.5 × 107 Pa. Maximal AOM was observed at 35 to 90°C but generally accounted for less than 5% of SR.
Hydrothermal sediments of the Guaymas Basin contain highly diverse anaerobic thermophilic microorganisms, including methanogens, sulfate-reducing bacteria, and presumably also methanotrophs (2, 6, 15, 18, 19, 23). Thermogenic reactions in the subsurface sediments provide a complex mixture of methane, other hydrocarbons, and volatile fatty acids as substrates for these microorganisms (8). Acetate concentrations in Guaymas sediments are extremely variable, ranging from 10 to >1,000 μM, with maximum concentrations occurring in hot sediments (12). Methane concentrations are 12 to 16 mM (22), but various other hydrocarbons derived during thermal alteration of organic material have also been found in high concentrations (11, 16, 17). In previous investigations, high rates of sulfate reduction (SR) were found at sediment surfaces, where freshly deposited organic material as well as bottom-water sulfate is available. Subsurface maxima in SR are found where upward moving fluids advect high concentrations of volatile fatty acids and a variety of hydrocarbons (4, 7, 8, 12, 16, 21). It is not known whether methane is a relevant electron donor for SR in hydrothermal sediments because the activity of thermophilic methanotrophs in Guaymas sediments has not yet been investigated. So far, high rates of methane-dependent SR have been found only in cold environments, at in situ temperatures of −1.5 to +12°C (5). Accordingly, physiological experiments with anaerobic-methanotrophic (ANME) groups from cold seeps have shown temperature optima for the anaerobic oxidation of methane (AOM) of between 5 and 15°C (13, 14). However, typical biomarker and 16S rRNA gene signatures of AOM consortia suggest the presence of thermophilic methanotrophs in hydrothermal surface sediments of Guaymas Basin (15, 19). The primary aims of the present study were to reveal the effects of temperature and pressure on SR and AOM in such sediments and to test whether SR is fueled by methane.
Hydrothermal sediment was retrieved from a vented site, covered with a Beggiatoa mat, by the submersible ALVIN (dive 3780, cruise AT-07, 5 May 2002; 27°0′32"N, 111°24′26"W; 2,013 m water depth). The maximum temperature recorded at this site was 130°C at a sediment depth of 20 cm (D. Albert, personal communication), a setting similar to that of previous sampling of vent cores (3, 5, 19). The core was very gassy and expanded during retrieval. Immediately after recovery, the complete sediment sample was stored under anoxic conditions at 4°C until further measurements were performed in the home laboratory. For the experiments, 1 part of Guaymas sediment was mixed and diluted with 4 parts of a standard mineral salt solution (14). All preparations were carried out inside a glove box under an atmosphere of N2-CO2. Methane-saturated slurries were prepared by equilibrating the sediment slurry with a 100% methane headspace in a glass flask. Because AOM occurs in two steps (oxidation of methane to carbon dioxide and reduction of sulfate to sulfide), the radiotracers 14CH4 and 35SO4 were added in trace amounts to replicate subsamples for measurement of carbon dioxide and sulfide production. Measurements of SR and AOM were performed according to methods described elsewhere (10, 20). All data were calculated as activity per volume of undiluted sediment. For SR, two replicates were incubated for each temperature or pressure setting. For AOM, two (experiments 2 and 3) or four (experiment 4) replicates were used for each temperature setting. Abiotic controls, obtained by fixing the sample prior to addition of tracer, were also employed. The average control value plus the standard deviation was substracted from each data point value before calculation of activity. The effects of temperature and pressure on SR and AOM were studied by using a high-pressure thermal gradient block (10). Experiment 1 was carried out to measure the combined effects of pressure and temperature on SR (Table 1). In addition, temperature gradient experiments were carried out with different preincubations times (experiment 2, 1 day; experiments 3 and 4, 7 days) and methane concentrations (experiments 2 and 3, 0.1 mM; experiment 4, 1 mM).
TABLE 1.
Effect of pressure on SR rates at high temperatures (experiment 1)
| Pressure (Pa) | SR rate at temp (°C)a:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 73 | 80 | 85 | 95 | 100 | 105 | 115 | 120 | 130 | … | 195 | |
| 1 × 105 | 154 | 90 | 76 | ND | ND | ND | ND | ND | ND | ND | |
| 1 × 106 | ND | 128 | 75 | 100 | 13 | 0.2 | 0.3 | ND | ND | ND | |
| 2.2 × 107 | ND | 645 | ND | 2,786 | 5,564 | 0.0 | 1.8 | 0.1 | 0.4 | … | 0.1 |
| 4.5 × 107 | ND | 2,805 | 2,465 | 6,660 | 3,619 | 1.8 | 0.4 | 0.0 | 0.3 | … | 0.3 |
Values are in units of nanomoles per cubic centimeter per day. ND, not determined.
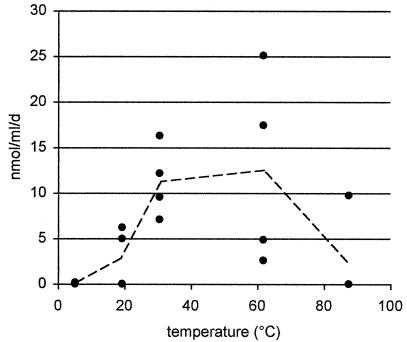
The temperature maximum of SR was between 60 and 90°C with a peak at 80°C, similar to results for intact sediment cores in previous studies (3, 21). At 80°C and 2.2 × 107 Pa, which represent the in situ conditions at the sampled site, SR was around 650 nmol cm−3 day−1 (Table 1). A maximum SR of almost 6,700 nmol cm−3 day−1 was reached at 4.5 × 107 Pa and 95°C. Under these conditions, the SR rate was 40 times higher than that in the 105- and 106-Pa incubations at the same temperatures. These rates are among the highest SR ever observed in a marine setting, comparable to the methane-driven SR values measured in Beggiatoa mats at cold seeps (1). SR ceased above 102°C even at high pressures (Table 1). Experiment 2 showed that a 1-day preincubation was insufficient for the microorganisms to adjust to the original temperature conditions, as SR and AOM rates were around 1 nmol cm−3 day−1 over the whole temperature range. In experiment 3, SR reached values similar to in situ (21) or ex situ (3) SR in samples from similar settings. The SR rate was significantly lower (P < 0.001) at low to intermediate temperatures (5 to 53°C; SR range, 0 to 15 nmol cm−3 day−1; average, 3.2 ± 2.5 nmol cm−3 day−1; n = 12) than at higher temperatures (62 to 85°C; SR range, 75 to 200 nmol cm−3 day−1; average, 95 ± 37 nmol cm−3 day−1; n = 8). Most interestingly, AOM rates were around 1% of SR rates at all temperatures. However, both processes exhibited a similar trend, as AOM rates in 62 to 85°C incubations (average, 1.6 ± 0.5 nmol cm−3 day−1; n = 8) were significantly higher (P < 0.001) than those for samples incubated at 5 to 53°C (average, 0.6 ± 0.2 nmol cm−3 day−1; n = 12). However, the identification of a clear temperature maximum was difficult because of the very low AOM rates. In experiment 4, samples were preincubated for a week at a methane concentration of ca. 1 mM. AOM rates increased to an average of 12 ± 5 nmol cm−3 day−1 at temperatures of 31 to 62°C (n = 8). At higher temperatures (>87°C), AOM rates declined again, with only one of four replicates showing measurable activity. At lower temperatures (<31°C), AOM rates were significantly lower (P < 0.005; average, 1.4 ± 1.7 nmol cm−3 day−1; n = 8), comparable to those for experiment 3 at similar temperatures (P = 0.17). Hence, our results suggest that a maximum for anaerobic oxidation of methane occurs at 30 to 60°C in the hydrothermal sediments of Guaymas Basin (Fig. 1). However, AOM contributed only 1 to 5% to SR at all temperatures, which is far from the 1:1 stoichiometry usually attributed to active AOM zones, where methane is the main substrate fueling SR (1, 5, 13, 14). The AOM rate was much higher in sediments from active cold seeps, reaching several hundred to 1,000 nmol cm−3 day−1 (13, 14, 20), compared to the turnover of a few nanomoles at Guaymas vents. However, at a cold-seep site, where other hydrocarbons were available in addition to methane, AOM was also reduced to 1 to 10% of SR (9). This may indicate that C2:C5 or higher hydrocarbons are a favorable substrate for sulfate reducers, outcompeting anaerobic methanotrophs in such environments.
FIG. 1.
Effects of temperature on AOM in hydrothermal sediments of Guaymas Basin (experiment 4). Each point represents one replicate subsample (n = 4 per setting). •, AOM; ---, average AOM.
There remains the question of whether the ANME-1 and ANME-2 groups detected in the surface sediments of Beggiatoa cores from Guaymas Basin (15, 19) are also present in hydrothermal subsurface sediments and are responsible for AOM at temperatures above 30°C. The previously observed substantial decrease in archaeal methanotroph biomarker lipids with increasing sediment depth (i.e., increasing temperature) (15, 19) indicates that the methane-rich, hot subsurface sediments are not a preferred environment for ANME populations. In conclusion, although AOM proceeds at higher temperatures in subsurface sediments of the Guaymas basin, it is clearly not the dominant carbon cycling process.
Acknowledgments
We thank Dan Albert for providing samples, Imke Müller for carrying out AOM analyses, and Beth Orcutt, Samantha Joye, and Andreas Teske for fruitful discussions and comments on the manuscript.
This study was supported by the program MUMM (Mikrobielle Umsatzraten von Methan in gashydrathaltigen Sedimenten; FN 03G0554A) of the Bundesministerium für Bildung und Forschung (BMBF; Germany). Further support came from the Max Planck Society (Germany).
Footnotes
This is publication GEOTECH-42 of the program GEOTECHNOLOGIEN of the Bundesministerium für Bildung und Forschung and the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Boetius, A., K. Ravenschlag, C. J. Schubert, D. Rickert, F. Widdel, A. Giesecke, R. Amann, B. B. Jørgensen, U. Witte, and O. Pfannkuche. 2000. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407:623-626. [DOI] [PubMed] [Google Scholar]
- 2.Burggraf, S., H. W. Jannasch, B. Nicolaus, and K. O. Stetter. 1990. Archaeoglobus profundus sp. nov. represents a new species within the sulfate-reducing archaebacteria. Syst. Appl. Microbiol. 13:24-28. [Google Scholar]
- 3.Elsgaard, L., M. F. Isaksen, B. B. Jørgensen, A.-M. Alayse, and H. W. Jannasch. 1994. Microbial sulfate reduction in deep-sea sediments at the Guaymas Basin hydrothermal vent area: influence of temperature and substrates. Geochim. Cosmochim. Acta 58:3335-3343. [Google Scholar]
- 4.Gundersen, J. K., B. B. Jørgensen, E. Larsen, and H. W. Jannasch. 1992. Mats of giant sulfur bacteria on deep-sea sediments due to fluctuating hydrothermal flow. Nature 360:454-456. [Google Scholar]
- 5.Hinrichs, K.-U., and A. Boetius. 2003. The anaerobic oxidation of methane: new insights in microbial ecology and biogeochemistry, p. 457-477. In G. Wefer, D. Billett, D. Hebbeln, B. B. Jørgensen, M. Schlüter, and T. van Weering (ed.), Ocean margin systems. Springer-Verlag, Heidelberg, Germany.
- 6.Jannasch, H. W., C. O. Wirsen, S. J. Molyneaux, and T. A. Langworthy 1988. Extremely thermophilic fermentative archaebacteria of the genus Desulfurococcus from deep-sea hydrothermal vents. Appl. Environ. Microbiol. 54:1203-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jørgensen, B. B., M. F. Isaksen, and H. W. Jannasch. 1992. Bacterial sulfate reduction above 100°C in deep-sea hydrothermal vent sediments. Science 258:1756-1757. [DOI] [PubMed] [Google Scholar]
- 8.Jørgensen, B. B., L. X. Zawacki, and H. W. Jannasch. 1990. Thermophilic bacterial sulfate reduction in deep-sea sediments at the Guaymas Basin hydrothermal vent site (Gulf of California). Geochim. Cosmochim. Acta 37:695-710. [Google Scholar]
- 9.Joye, S. B., A. Boetius, B. N. Orcutt, J. P. Montoya, H. N. Schulz, M. J. Erickson, and S. Lugo. The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps. Chem. Geol., in press.
- 10.Kallmeyer, J., T. G. Ferdelman, K.-H. Jansen, and B. B. Jørgensen. 2003. A high-pressure thermal gradient block for investigating microbial activity in multiple deep-sea samples. J. Microbiol. Methods 1846:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Kawka, O. E., and B. R. T. Simoneit. 1994. Hydrothermal pyrolysis of organic matter in Guaymas Basin. I. Comparison of hydrocarbon distributions in subsurface sediments and seabed petroleums. Org. Geochem. 22:947-978. [Google Scholar]
- 12.Martens, C. S. 1990. Generation of short chain organic acid anions in hydrothermally altered sediments of the Guaymas Basin, Gulf of California. Appl. Geochem. 5:71-76. [Google Scholar]
- 13.Michaelis, W., R. Seifert, K. Nauhaus, T. Treude, V. Thiel, M. Blumenberg, K. Knittel, A. Gieseke, K. Peterknecht, T. Pape, A. Boetius, R. Amann, B. B. Jørgensen, F. Widdel, J. Peckmann, N. V. Pimenov, and M. B. Gulin. 2002. Microbial reefs in the Black Sea fueled by anaerobic oxidation of methane. Science 297:1012-1015. [DOI] [PubMed] [Google Scholar]
- 14.Nauhaus, K., A. Boetius, M. Krüger, and F. Widdel. 2002. In vitro demonstration of anaerobic oxidation of methane coupled to sulphate reduction in sediment from a marine gas hydrate area. Environ. Microbiol. 4:296-305. [DOI] [PubMed] [Google Scholar]
- 15.Schouten, S., S. G. Wakeham, E. C. Hopmans, and J. S. Sinninghe Damste. 2003. Biogeochemical evidence that thermophilic archaea mediate the anaerobic oxidation of methane. Appl. Environ. Microbiol. 69:1680-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simoneit, B. R. T., and P. F. Lonsdale. 1982. Hydrothermal petroleum in mineralized mounds at the seabed of Guaymas Basin. Nature 295:198-202. [Google Scholar]
- 17.Simoneit, B. R. T., and M. Schoell. 1995. Carbon isotope systematics in individual hydrocarbons in hydrothermal petroleums from the Guaymas Basin, Gulf of California. Org. Geochem. 23:857-863. [Google Scholar]
- 18.Stetter, K. O. 1988. Archaeoglobus fulgidus gen. nov. sp. nov.: a new taxon of extremely thermophilic archaebacteria. Syst. Appl. Microbiol. 10:172-173. [Google Scholar]
- 19.Teske, A., K.-U. Hinrichs, V. Edgcomb, A. de Vera Gomez, D. Kysela, S. P. Sylva, M. L. Sogin, and H. W. Jannasch. 2002. Microbial diversity of hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Appl. Environ. Microbiol. 68:1994-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treude, T., A. Boetius, K. Knittel, K. Wallmann, and B. B. Jørgensen. 2003. Anaerobic oxidation of methane above gas hydrates (Hydrate Ridge, OR). Mar. Ecol. Prog. Ser. 264:1-14.
- 21.Weber, A., and B. B. Jørgensen. 2002. Bacterial sulfate reduction in hydrothermal sediments of the Guaymas Basin, Gulf of California, Mexico. Deep-Sea Res. Part I 49:827-841. [Google Scholar]
- 22.Whelan, J. A. 1988. Origins of methane in hydrothermal systems. Chem. Geol. 71:183-198. [Google Scholar]
- 23.Zhao, H., A. G. Wood, F. Widdel, and M. P. Bryant. 1988. An extremely thermophilic Methanococcus from a deep sea hydrothermal vent and its plasmids. Arch. Microbiol. 150:178-183. [Google Scholar]