Abstract
Small-diameter synthetic vascular grafts have high failure rate and tissue-engineered blood vessels are limited by the scalability. Here we engineered bioactive materials for in situ vascular tissue engineering, which recruits two types of endogenous progenitor cells for the regeneration of blood vessels. Heparin was conjugated to microfibrous vascular grafts to suppress thrombogenic responses, and stromal cell-derived factor-1α (SDF-1α) was immobilized onto heparin to recruit endogenous progenitor cells. Heparin-bound SDF-1α was more stable than adsorbed SDF-1α under both static and flow conditions. Microfibrous grafts were implanted in rats by anastomosis to test the functional performance. Heparin coating improved the short-term patency, and immobilized SDF-1α further improved the long-term patency. SDF-1α effectively recruited endothelial progenitor cells (EPCs) to the luminal surface of the grafts, which differentiated into endothelial cells (ECs) and accelerated endothelialization. More interestingly, SDF-1α increased the recruitment of smooth muscle progenitor cells (SMPCs) to the grafts, and SMPCs differentiated into smooth muscle cells (SMCs) in vivo and in vitro. Consistently, SDF-1α-immobilized grafts had significantly higher elastic modulus. This work demonstrates the feasibility of simultaneously recruiting progenitor cells of ECs and SMCs for in situ blood vessel regeneration. This in situ tissue engineering approach will have broad applications in regenerative medicine.
Keywords: Vascular grafts, progenitor cells, in situ tissue engineering, SDF-1α
1. Introduction
Cardiovascular diseases account for 40% of deaths in the United States. Arterial replacement is a common treatment for vascular diseases, with over 500,000 vascular grafts being used in the bypass procedures for coronary artery and peripheral arteries each year. However, in many cases appropriate autologous venous and arterial grafts are damaged, have already been harvested, or are simply unusable [1]. Synthetic vascular grafts are limited to the grafts with inside-diameter (ID) larger than 5 mm due to the frequent thrombosis and occlusion in smaller grafts. In addition, the lack of endothelialization in synthetic grafts results in low patency rate in the long-term.
In the past decade, significant progress has been made to construct tissue-engineered blood vessels (TEBV) in vitro by using vascular cells with or without scaffolds [2–6]. In addition, adult stem cells such as bone marrow mesenchymal stem cells or mixed cell population have been used to make TEBV [7–11]. However, constructing cellular grafts involves extensive manipulation of cells in vitro and is time consuming, which limits the application to individualized and non-urgent therapies.
An interesting finding from the studies on stem cell-seeded grafts is that the stem cells in TEBVs are replaced by endogenous cells within days to weeks [7, 9], suggesting that the regeneration potential of endogenous cells could be harnessed for blood vessel regeneration. However, adult vascular cells, including endothelial cells (ECs) and smooth muscle cells (SMCs), have limited expansion capability. Therefore, it will be highly desirable to develop vascular grafts that can recruit endogenous stem cells or progenitor cells of both ECs and SMCs. CD34+ endothelial progenitor cells (EPCs) are present in circulating blood, and can differentiate into ECs [12–14]. Smooth muscle progenitor cells (SMPCs) in the local vascular tissues are less well understood, and various non-specific surface markers (e.g., Sca-1, c-kit, CD29, CD44, CD34 and CD146) have been reported for different types of SMPCs in vascular tissues [15–18]. Here we explored the approach to recruit both EPCs and SMPCs for in situ regeneration of blood vessels.
We and others have used electrospinning techniques to fabricate fibrous scaffolds for vascular graft construction [7, 19–22]. The fibrous structure simulates the microstructure in native arteries, and allows the integration of the grafts with surrounding cells and tissues. To prevent platelet adhesion and thrombus formation on the luminal surface of the grafts, poly(ethylene glycol) and anticoagulant (e.g., hirudin) have been used to modify the surface of micro/nano fibers [23]. Here we used heparin instead of hirudin as an anticoagulant because heparin could also be used as an adaptor molecule to immobilize bioactive factors [24]. Recently, heparin has been used to coat ePTFE grafts. However, heparin-treated grafts have shown a significantly worse primary patency at 48 months than autologous saphenous [25], suggesting the additional or alternative bioactive factors are needed to modify the surface of vascular grafts.
Many biochemical factors are able to influence EPC mobilization and homing to ischemic tissues [26–28]. In particular, stromal cell–derived factor-1α (SDF-1α) is a potent factor for EPC homing as well as neovascularization [26, 29, 30]. In this study, we investigated the effects of immobilized SDF-1α on the recruitment of EPCs and SMPCs to vascular grafts. We hypothesized that heparin could suppress acute thrombogenic responses and that SDF-1α could recruit EPCs to facilitate endothelialization and improve long-term patency. In addition, SMPC recruitment could promote the remodeling of the vascular grafts, which has not been addressed previously.
2. Materials and methods
2.1. Fabrication of vascular grafts
Microfiber scaffolds were fabricated by using poly(L-lactic acid) (PLLA) (MW 67,400, Sigma-Aldrich) and polycaprolactone (PCL, MW 2,000, Polysciences). The polymer blends (e.g., 19% PLLA and 5% PCL; w/v) were completely dissolved in 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP, Aladdin). Microfibrous grafts were made by electrospinning polymer fibers onto a rotating stainless steel mandrel (1-mm diameter and 300 rpm) [23]. The negative voltage of 4.5 kV was applied to the mandrel, and a positive voltage of 4 kV was applied to the spinneret by using a high-voltage generator (Gamma High Voltage, Ormond Beach, FL). The electrospinning process was allowed to proceed until an approximately 200-µm wall thickness was achieved. The structure of the scaffolds was characterized by using a scanning electron microscope (Hitachi TM-1000). The half-life of the degradation of PLLA, the major component in the grafts, is about one year.
2.2. Heparin conjugation and SDF-1α immobilization
Heparin-functionalized microfibers were fabricated by using di-NH2-PEG as a linker molecule [24]. Briefly, the density of reactive carboxylic groups on the microfibers was increased by briefly treating the scaffolds with 0.01N NaOH (Sigma-Aldrich). Di-NH2-PEG molecules (MW 3400, Sigma) were then covalently attached to the carboxylic groups on the microfibers by using EDC and Sulfo-NHS (Pierce Biotechnology). Heparin was conjugated to the free amines on the di-NH2-PEG molecules via EDC and sulfo-NHS. Following heparin conjugation, SDF-1α (R&D Systems) in PBS (500ng/ml) were incubated with the scaffolds over night at 4°C to a llow for its binding to heparin and its immobilization on the scaffolds. For comparison, same concentration of SDF-1α was passively adsorbed onto microfibers of untreated grafts as control.
To visualize the uniformity of SDF-1α to heparin-functionalized scaffolds, immunofluorescent staining was performed by using an SDF-1α antibody (R&D System) and Alexa-Fluor 546 labeled secondary antibody.
The stability and in vitro release of immobilized SDF-1α was evaluated over a period of 7 days under both static and flow conditions. For static release experiments, 1ml of PBS was used as incubation medium for each graft. PBS was removed and replenished every 24 hours. For release experiments under flow, silicon tubing was used to connect grafts to a large reservoir (100 L) containing PBS. The reservoir was placed at height above the graft to supply PBS at a flow rate of 476 ± 6 ml/hr, which corresponded to a physiologically relevant shear stress of ~13 dynes/cm2. A peristaltic pump was used to pump PBS back into the reservoir to maintain the circulation of PBS. Grafts were collected on days 0, 1, 3 and 7 followed by complete digestion in 0.1 N NaOH for 36 hours. Following digestion, samples were neutralized with 0.1 N HCl. The amount of immobilized SDF-1α remaining was then directly quantified using an ELISA kit (R&D System).
2.3. Implantation and explantation of vascular grafts
All procedures were approved by the Institutional Review Board Service and the Institutional Animal Care and Use Committee at the University of California, Berkeley. Male Sprague-Dawley rats (weight, 260 to 280 g) were purchased from the Charles River animal facility. The rats were anesthetized with 2.0% isoflurane in 70% nitrous oxide and 30% oxygen. The left common carotid artery was dissected, clamped, and transected; and the graft was sutured end to end with 8 uninterrupted stitches by using a 10-0 needle. No heparin or any other anticoagulant was used at any point before, during, or after the implantation procedure. The patency of the graft was determined by examining the blood flow in the blood vessel at the distal end of the graft in the live animal under anesthesia. The graft was defined as being patent only if there was a restoration of blood flow in the distal vessel after squeezing and releasing the vessel with forceps. In detail, forceps were used to hold the distal blood vessel near the anastomotic site. The other forceps were used to squeeze the vessel gently toward the distal end for about 3–5 mm, and the blood vessel became flat. Then the forceps near the anastomotic site were released to determine whether blood flow could be restored to inflate the vessel. The animals were then euthanized and the vascular grafts were explanted. Histological analysis of the cross sections of the grafts was used to confirm the patency.
2.4. Isolation and culture of cells from implanted vascular grafts
Vascular grafts for in vitro cell isolation were harvested at 1 week post-surgery, and washed three times with sterile phosphate buffered saline (PBS) supplemented with 1% penicillin/streptomycin (P/S). The grafts were then cut open longitudinally. Sterile cotton tips were used to scrape off any blood or tissue attached to the luminal side. The graft was then cut into mm-size pieces and placed onto the surface coated with 1% CellStart (Invitrogen Corp.) in a 35 mm tissue culture dish. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen Corp.) with 10% fetal bovine serum (FBS, Thermo Fisher Scientific Inc.). Cell culture was maintained at 37°C in an incubator with 5% CO2. The medium was changed every other day. Cells started migrating out of the tissue explants within 2 days. After one week, the tissue explants were dislodged and removed from the culture and the cells were passaged to newly coated dishes and cultured as a monolayer. Some cultures were fixed with 4% paraformaldehyde for characterization at this time point. Others were maintained in 10% FBS medium and passaged once a week for one month routinely as needed to study the spontaneous differentiation of these cells.
2.5. Histological analysis
Samples for histological examination were snap-frozen in optimal cutting temperature (OCT) Compound (Tissue Tek,), and sectioned into 10-µm thickness using cryostat. Immunohistochemical staining was used to analyze the tissue sections with the following primary antibodies: SM-MHC (sc-79079, goat, Santa Cruz Biotechnology Inc.), CNN1 (1806-1, rabbit, Epitomics, Inc), SMA (1184-1, rabbit, Epitomics, Inc), elastin (ab21610, rabbit, Abcam Inc.), Collagen type I (ab34710, rabbit, Abcam Inc.), CD31 (550300, mouse, BD Biosciences), CD34 (AF4117, rat, R&D), CD133 (ab19898, rabbit, Abcam Inc.), CD45 (05–1410, mouse, Millipore), CXCR7 (51024-1-AP, rabbit, Proteintech Group), CXCR4 (ab2074, rabbit, Abcam Inc.), CD68 (MCA341R, mouse, AbD Serotec) and CD3 (ab8879, mouse, Abcam Inc.). Verhoeff’s Staining was performed by using Verhoeff’s Elastic Stain kits (American MasterTech Scientific. Inc). Immunohistochemistry images were captured with a Zeiss confocal microscope (LSM710).
2.6. En Face immunofluorescence staining
The grafts were explanted and fixed with 4% paraformaldehyde for 30 minutes. Each graft was cut into 3 slices longitudinally by using microscissors. The sample were washed with PBS, blocked with 1% bovine serum albumin, and incubated with primary antibodies against EPC marker CD34, EC marker CD31, peripheral blood mononuclear cell marker CD45 and SDF-1α receptor CXCR7, and then incubated with Alexa-Fluor 488 or Alexa-Fluor 546 labeled secondary antibodies, followed by confocal microscopy.
2.7. Mechanical testing
The freshly explanted vascular grafts (n ≥ 3) were cut into 1-mm-wide ring segments. Approximate wall thickness of all samples was 120 um. The tensile strength in the circumferential direction of these rings was tested by using a custom-built soft tissue tester. Two 0.016-inch-diameter stainless steel rods were inserted into the lumen of the ring segment and fixed on mechanical loading grips. The sample was then placed onto the mechanical tester, and the applied deformation (strain rate was 0.1 mm/sec) and force were recorded. The elastic modulus was calculated based on the applied force, graft deformation, and the dimensions (thickness and width) of the rings. The slope of stress-strain curve was quantified in the linear region of the curve between 5–10% strain.
2.8. Statistical analysis
For two-sample comparison, Student’s t-test was used. For multiple-sample comparison, analysis of variance (ANOVA) was performed to detect whether a significant difference existed between groups with different treatments, and a multiple comparison procedure Holm’s t-test was used for post-analysis. A p-value of 0.05 or less will indicate significant difference between samples in comparison.
3. Results
3.1. Fabrication of microfibrous vascular grafts with SDF-1α
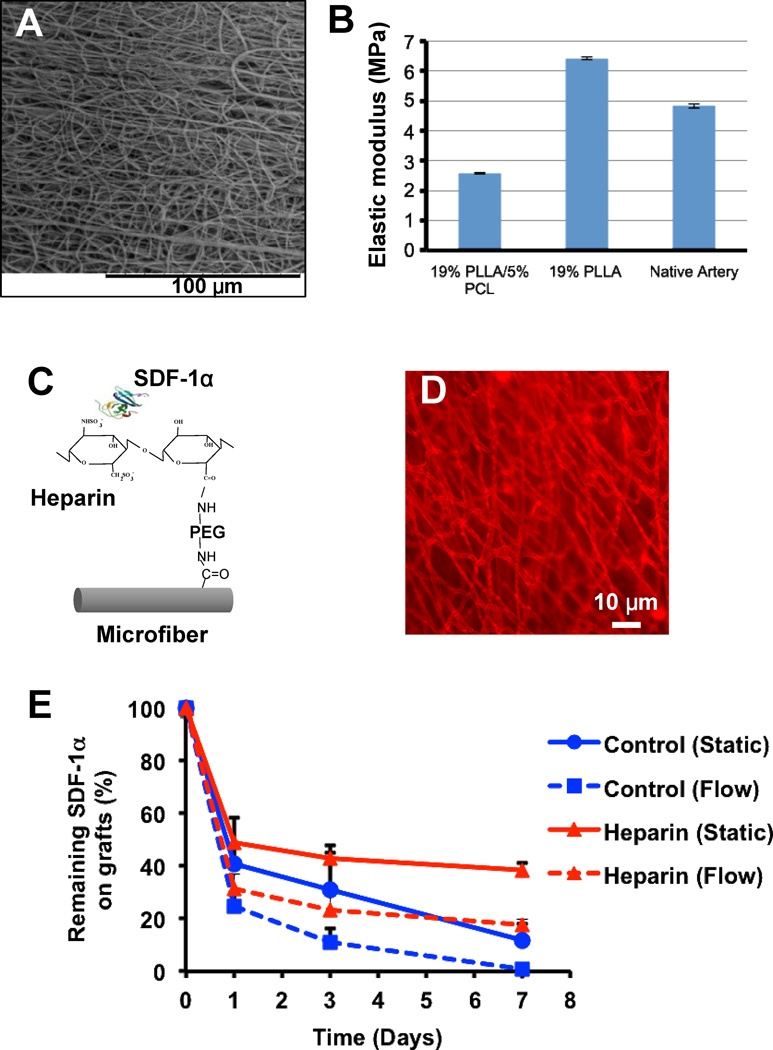
Microfibrous tubular grafts were fabricated by electrospinning poly-L-lactide (PLLA) and poly-caprolactone (PCL) polymer blends onto a rotating mandrel. Scanning electron microscopy (SEM) images showed that the electrospun grafts had a porous structure of microfibers (Fig. 1A). The addition of low molecular weight PCL decreased the elastic modulus of the scaffolds (from 6.4+0.06 to 2.6±0.03 MPa, n=3) and increased the conjugation sites (carboxylic groups) on the microfibers (Henry J et al., unpublished observation). The elastic modulus of the grafts was in the same order of magnitude as the native arteries, (carotid arteries from Sprague Dawley rats) which was 4.8 ± 0.07 MPa (n=3) (Fig. 1B).
Figure 1.
Characterization of the microstructure and surface chemistry of microfibrous vascular grafts. (A) Structure of microfibers in the luminal surface of the vascular grafts. (B) Mechanical property of a vascular graft and a rat carotid artery. (C) Schematic illustration of chemical modification of microfibers with SDF-1α. (D) Immunostaining for SDF-1α immobilized on the microfibers of a graft. (E) The time course of SDF-1α release from the grafts. The release kinetics of the control group (passively adsorbed SDF-1α) and heparin-bound SDF-1α under static and flow conditions was compared. The amount of SDF-1α remaining on the grafts is shown (n=3).
To suppress thrombogenic events, microfibers were functionalized with heparin by using di-amino-poly (ethylene glycol) (di-NH2-PEG) as a linker molecule. Di-NH2-PEG was covalently attached to the carboxylic groups on the microfibers by using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (Sulfo-NHS) (Fig. 1C). Heparin was then covalently attached to the free amines on the di-NH2-PEG molecules via carbodiimide chemistry. Since heparin binds to and stabilizes SDF-1α, we used heparin as a molecular linker to sequester recombinant SDF-1α. Immunostaining for SDF-1α showed that SDF-1α was evenly conjugated onto the microfibers (Fig. 1D). A non-immunogenic immunoglobulin of the same species was used as the primary antibody for the negative control and it showed negative staining (data not shown). The stability and in vitro release of immobilized SDF-1α was evaluated by ELISA and the results were normalized and shown in Fig. 1E. The amount of SDF-1α at Day 0 in the control and in the heparin-bound group was 87.2 ± 3.7 ng/cm3 and 268.3 ± 13.8 ng/cm3 (n=6), respectively. It showed that SDF-1α was stably immobilized on the microfibrous scaffolds and a slow release of SDF-1α was achieved for more than a week in vitro (Fig. 1E). In contrast, passively adsorbed SDF-1α on microfibers was not stable and was released rapidly. This was further accelerated by flow as the passively adsorbed SDF-1α was completely washed off within a week. However, after 1 week, 40% and 20% of heparin-bound SDF-1α remained on the grafts under static and flow conditions respectively.
3.2. Patency of vascular grafts
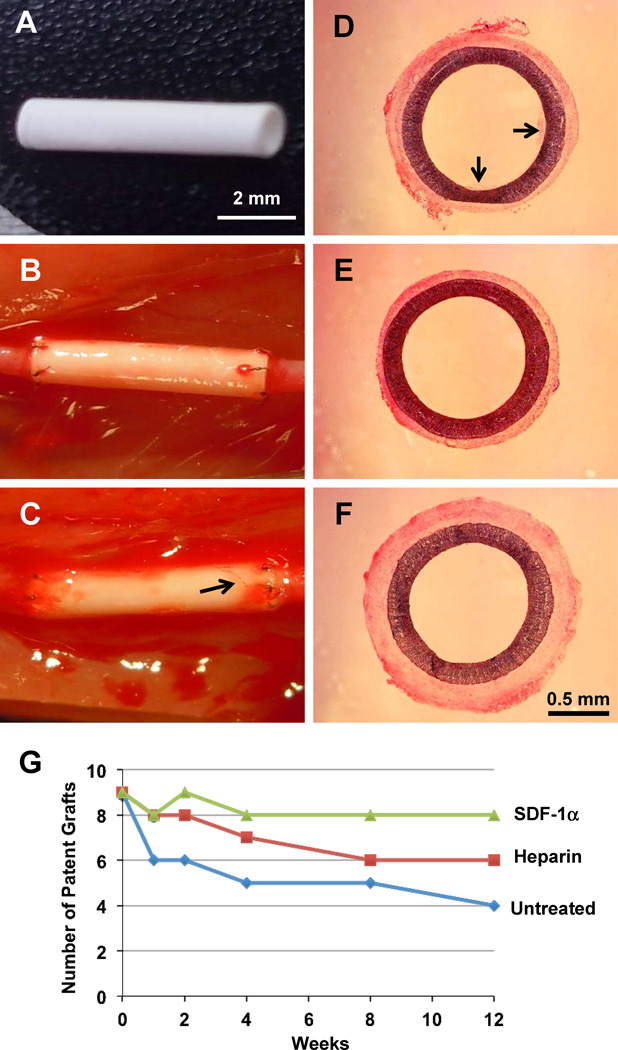
To evaluate the effects of surface modification on the patency of vascular grafts in vivo, untreated grafts, heparin-treated grafts and heparin-SDF-1α-treated grafts with a length of 6 mm and an inner diameter of 1 mm (Fig. 2 A) were implanted into the left common carotid artery of rats by anastomosis (Fig. 2B) and examined at various time points for up to 12 weeks, with 9 animals per group at each time point. Untreated and heparin-treated grafts were used as controls for comparison to determine the effect of immobilized SDF-1α. After surgery, blood flow was observed immediately at both the proximal and distal ends of the grafts. After 4 weeks, visible angiogenesis could be observed in the tissue around the graft (Fig. 2C), suggesting the integration of the vascular grafts with the surrounding tissues. All animals (n= 9×3×5=135) survived after the artery replacement procedure. Hematoxylin and eosin (H&E) staining of the cross-sections in the middle portion of the grafts was performed to characterize the morphology of the explanted grafts at different time points. At 4 weeks, the patent grafts in the three experimental groups are exemplified in Fig. 2D–F. In general, all patent grafts had wide open lumen and little thrombus formation because significant amount of thrombus would cause clogging in these small-diameter (1-mm inside diameter) grafts. Occasionally, small thrombus could be observed in some untreated grafts (Fig. 2D). Significant thrombus formation, but not neointimal formation, was found in all clogged grafts, as exemplified in fig S1, suggesting that thrombus formation is a major mechanism for graft clogging and failure in small-diameter grafts.
Figure 2.
Macroscale characterization and patency of the grafts. (A) A graft before implantation. (B) A graft right after implantation. (C) A heparin-SDF-1α-treated graft at 4 weeks after implantation. Arrow indicates microvessels in the wall of the graft. (D–F) H&E staining of an untreated graft (D), a heparin-treated graft (E) and a heparin-SDF-1α-treated graft (F) at 4 weeks after implantation. Arrows in D indicate thrombus formation. Scale bar = 2 mm in A–C; scale bar = 0.5 mm in D–F. (G) The patency of the grafts was examined at various time points. Each group at each time point included 9 animals.
Necropsy showed that 67% (6 of 9) of untreated grafts, 89% (8 of 9) of heparin-treated grafts, and 89% of (8 of 9) heparin-SDF-1α-treated grafts were patent at 1 week after implantation, suggesting that heparin modification improved short-term patency (Fig. 2G). At 4 weeks, the patency decreased slightly for untreated grafts (5 of 9; 56%) and heparin-treated grafts (7 of 9; 78%), but did not change for heparin-SDF-1α-treated grafts (8 of 9; 89%). At 12 weeks, the patency further decreased for untreated grafts (4 of 9; 44%) and heparin-treated grafts (6 of 9; 67%), while the heparin-SDF-1α-treated grafts maintained higher patency (8 of 9; 89%) (Fig. 2G).
3.3. EPC recruitment and endothelialization
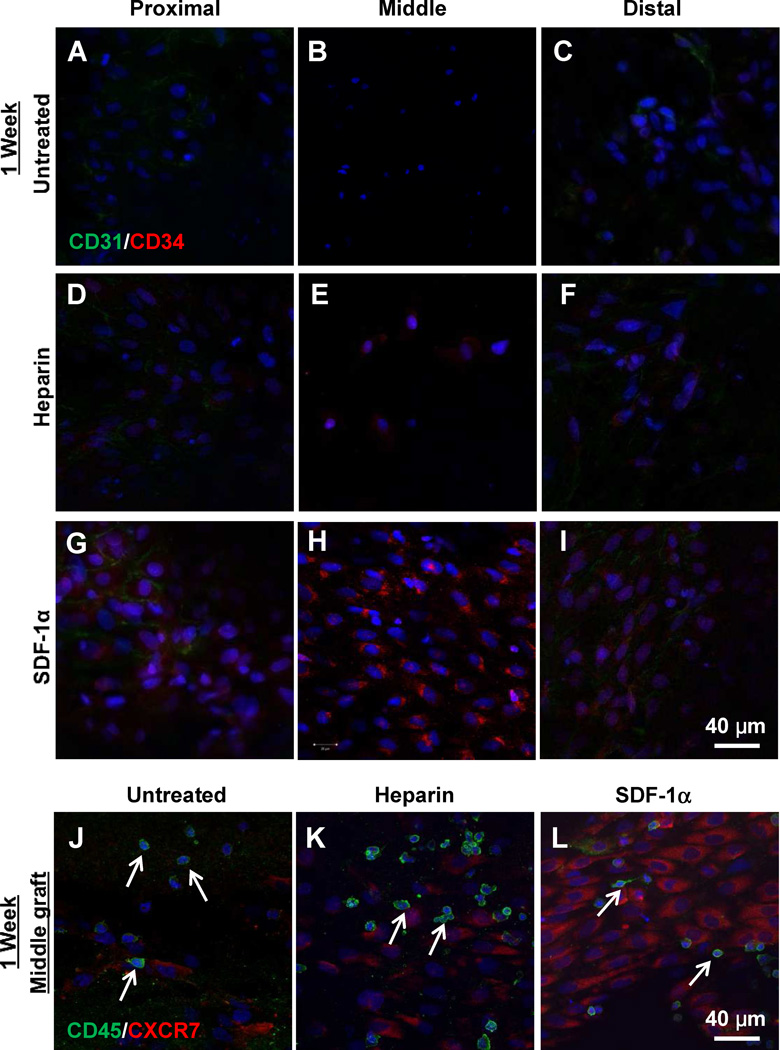
We then investigated whether the maintenance of the long-term patency in heparin-SDF-1α-treated grafts was related to the endothelialization on the luminal surface of the grafts. At 1 week, cells were mostly observed near the proximal end of the grafts in untreated and heparin-treated grafts (Fig. S2A–B). In contrast, heparin-SDF-1α-treated grafts showed much more cells in the middle portion of the grafts (Fig. S2C). Cell number at different portions of the grafts was quantified, which showed that the cell density in the middle portion of the graft was significantly (p < 0.05) higher in the heparin-SDF-1α-treated grafts (1570±413 per mm2, n=3) than the untreated (661±268 per mm2, n=3) and heparin-treated grafts (847±392 per mm2, n=3).
To characterize the cells on the luminal surface of the grafts, en face and cross-section stainings were performed to detect EPC markers CD34, EC marker CD31, peripheral blood mononuclear cell marker CD45 and SDF-1α receptor CXCR-7. Untreated and heparin-treated grafts showed none or few cells positive for CD34 (Fig. 3A–F; Fig. S3A–B) or CXCR7 (Fig. 3J–K). Patches of ECs from the adjacent carotid arteries were found at the proximal and distal end of the grafts (Fig. S2A–B). There were a small number of cells in the middle portion of the grafts that were negative for EPC and EC markers. Further characterization showed that they were mostly peripheral blood mononuclear cells positive for CD45 (Fig. 3J–K).
Figure 3.
En face immunostaining for CD31, CD34, CD45 and CXCR7 of the explanted grafts at 1-week after implantation. (A–I) En face immunostaining for CD31 (green) and CD34 (red) of proximal, middle and distal portions of untreated grafts (A–C), heparin-treated grafts (D–F) and heparin-SDF-1α-treated grafts (G–I). (J–L) En face immunostaining for CD45 (green) and CXCR7 (red) of the untreated control grafts (J), heparin-treated grafts (K) and heparin-SDF-1α-treated grafts (L). Arrows indicate CD45+ cells. Nuclei were stained by DAPI (blue). Scale bar = 40 µm.
In contrast, heparin-SDF-1α-treated grafts recruited many cells to the luminal surface in the middle portion of the grafts, with most of the cells positive for CD34 (Fig. 3H, Fig. S3C) and CXCR7 (Fig. 3L) at 1 week after implantation. These cells did not express CD31 (Fig. 3H), indicating that they were undifferentiated EPCs. At the proximal and distal ends of the grafts, CD31+ ECs migrated from adjacent carotid arteries, and few EPCs were found in these regions. Similar to untreated and heparin-treated grafts, some CD45+ peripheral blood mononuclear cells (Fig. 3L) and CD133+ cells (data not shown) from circulation were also found in heparin-SDF-1α-treated grafts. Quantification of CD45+ cells showed no significant difference among the three experimental groups. CD34+ cells recruited to the middle portion of the grafts were also positive for SDF-1α receptor CXCR-7 (Fig. S4), suggesting that these EPCs were specifically recruited by SDF-1α. The heparin-SDF-1α-treated grafts had significantly higher number of CXCR7 cells (1421±385 per mm2, n=3) than the untreated (144±103 per mm2, n=3) and heparin-treated grafts (309±185 per mm2, n=3).
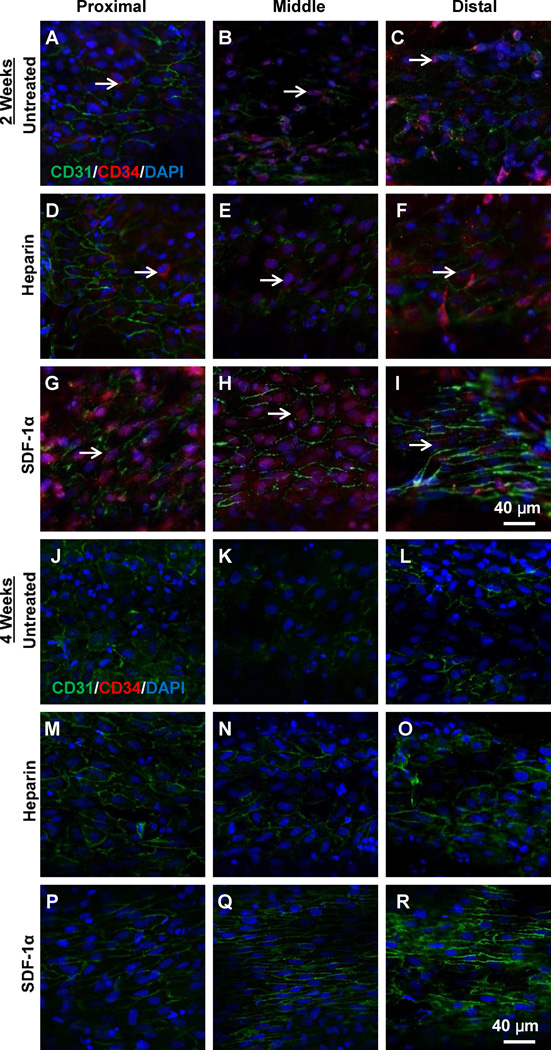
At 2 weeks after implantation, if the grafts remained patent, most areas of luminal surface in the grafts were covered by cells, with the best cell coverage in heparin-SDF-1α-treated grafts (Fig. S5). Further migration of ECs at the proximal and distal ends of grafts was observed (Fig. 4A–I). In the middle portion of the grafts, patches of ECs without mature cell-cell boundaries were found in untreated and heparin-treated grafts (Fig. 4B, E), while an EC monolayer with well-defined cell-cell boundaries had formed in heparin-SDF-1α-treated grafts (Fig. 4H). Few cells were positive for EPC markers in untreated and heparin-treated grafts. In contrast, in heparin-SDF-1α treated grafts, more than 70% of cells in the middle portion of the grafts were positive for both CD31 and CD34, suggesting that these ECs were derived from EPCs. The staining of cross sections also showed EC and EPC staining on the luminal surface in the middle portion of the grafts (Fig. S3D–F), with better endothelialization in heparin-SDF-1α-treated grafts.
Figure 4.
En face immunostaining for CD31 (green) and CD34 (red) of the explanted grafts at 2-week (A–I) and 4-week (J–R) after implantation. Nuclei were stained by DAPI (blue). Scale bar = 40 µm.
At 4 weeks after implantation, the luminal surfaces of all grafts were almost completely covered by cells (Fig. S6). Most cells were CD31+, but were negative for CD34 (Fig. 4J–R; Fig. S3G–I), suggesting that EPCs, if any, already differentiated into ECs. In untreated and heparin-treated grafts, cells were not fully confluent; ECs had random, disorganized morphology and the cell-cell boundary was not well defined in many areas, indicating that ECs were still in the process of remodeling and a stable monolayer had not formed. In contrast, ECs on heparin-SDF-1α grafts showed continuous EC monolayer with well-organized structure and cell alignment in the direction of blood flow, similar to that in native blood vessels (Fig. 4P–R), indicating the maturation of the endothelium. At 12 weeks, mature endothelium was noticed on the luminal surface of the patent grafts in all three groups (Fig. S7). Immnunostaining of the cross sections for endothelial marker VE-Cadherin confirmed the complete coverage of endothelium on the luminal surface of the patent grafts (Fig. S8).
3.4. Recruitment of SMPCs and vascular wall remodeling
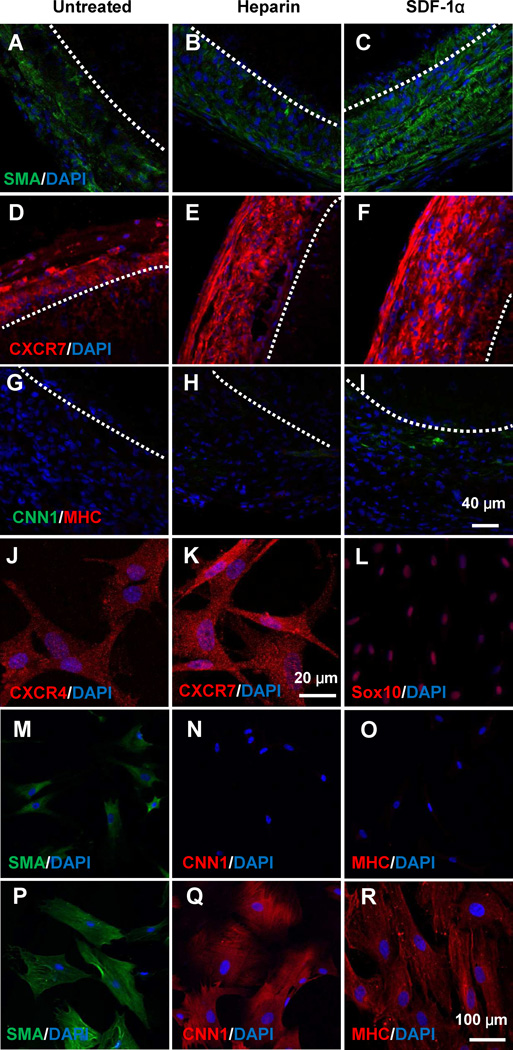
In addition to endothelialization, vascular wall remodeling and thus the mechanical properties of the grafts are critical for the maturation of blood vessels. Therefore, we determined the recruitment of mesenchymal cells to the grafts and the change of mechanical property of grafts with time. At macroscopic level, the tissue layer around heparin-SDF-1α-treated grafts appeared thicker (Fig. 2F). At 1 week, cells positive for smooth muscle α-actin (SMA) and CXCR7 were recruited to the outer layer of the grafts (Fig. 5A–F), and heparin-SDF-1α treated grafts increased the recruitment of SMA+ cells (Fig. 5A–F). However, only a few cells were positive for calponin 1 (CNN1), an intermediate marker of SMC differentiation [31], in heparin-SDF-1α treated grafts, and all cells were negative for smooth muscle myosin heavy chain (MHC) (Fig. 5G–I), suggesting that these cells were not mature SMCs. Immunostaining for SDF-1α receptor CXCR7 showed that more CXCR7+ cells were recruited to the outside of the grafts in the heparin-SDF-1α-treated grafts than the untreated grafts and heparin-treated grafts (Fig. 5D–F). Double staining for SMA and CXCR7 showed that the cells recruited by SDF-1α were positive for both CXCR7 and SMA (Fig. S9). Interestingly, CD68+ macrophages, CD11b+ inflammatory cells (Fig. S10) and CD3+ T cells (data not shown) were hardly found in the graft.
Figure 5.
Identification and characterization of SMPCs recruited by SDF-1α. Immunostaining for SMA (A–C, green), CXCR7 (D–F, red), CNN1 (green) and SM-MHC (red) of the cross sections (G–I) in the middle portion of the grafts was performed at 1 week after implantation. Dashed lines indicate the border between the graft (top part) and the outer layer. SMPCs isolated from explanted grafts were stained for CXCR4, CXCR7, Sox10, SMA, CNN1 and SM-MHC (J–O). After 4-weeks of spontaneous differentiation, SMPCs were stained for SMA, CNN1 and SM-MHC (P–R). Nuclei were stained by DAPI (blue). Scale bar = 40 µm in A–L; scale bar = 100 µm in J–R.
To characterize the cells recruited to the grafts, the grafts after 1-week implantation were used for explant culture. The cells derived from all three groups showed the same characteristics. As exemplified in Figure 5J–O, the isolated cells were positive for SDF-1α receptor CXCR4 (Fig 5J) and CXCR7 (Fig 5K), and expressed Sox10 (Fig 5L). These cells were also positive for general mesenchymal cell markers CD29 and CD44 (Fig. S11). The isolated cells were SMA+CNN1−MHC− after 1-week culture (Fig 5M–O). Following 1-month culture, the cells spontaneously differentiated into SMA+CNN1+MHC+ cells (Fig. 5P–R), and the differentiated cells had larger spreading area and larger nucleus. These results suggest that the cells recruited to the grafts are SMPCs. However, these SMPCs were different from previously identified SMPCs because they were negative for markers such as Sca-1, c-kit, CD34 and CD146 (data not shown).
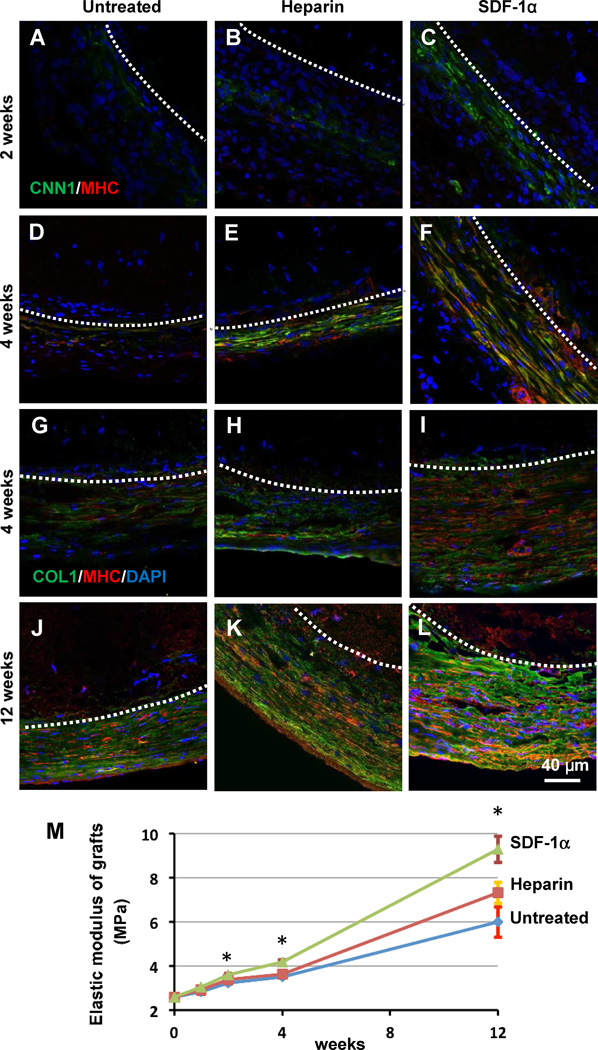
To investigate the differentiation of SMPCs and the remodeling and extracellular matrix (ECM) deposition in the regenerated blood vessels, smooth muscle markers and matrix protein markers were examined at a series of time points. At 2 weeks, cells in the outer layer became CNN1+MHC−, with more CNN1+MHC− cells found in heparin-SDF-1α treated grafts (Fig. 6A–C). At 4 weeks, CNN1+MHC+ were present in the outer layer (Fig. 6D–F), suggesting the differentiation of the cells into SMCs. Among all three groups, heparin-SDF-1α treated grafts had the most SMCs. Immunostaining of collagen-I and SM-MHC was performed to study the remodeling of the grafts in vivo. At 4 weeks and 12 weeks post implantation, heparin-SDF-1α-treated grafts showed more extensive and denser collagen-I deposition surrounding the vascular grafts than the other two groups (Fig.6 G–L). Mechanical tests demonstrated that the elastic modulus of the grafts of all three groups increased significantly over time after implantation. SDF-1α significantly increased the elastic modulus at 2 weeks, 4 weeks and 12 weeks (Fig. 6M). At 4 weeks and 12 weeks, heparin-SDF-1α-treated grafts had higher elastic modulus (4.2 ± 0.1 MPa (n=4) and 9.3 ± 0.6 MPa (n=4), respectively) than that of untreated group (3.5 ± 0.03 MPa (n=3) and 6.0 ± 0.7 MPa (n=3), respectively) and heparin-treated group (3.6 ± 0.04 MPa (n=3) and 7.3 ± 0.5 MPa (n=3), respectively) (Fig. 6M), indicating that immobilized SDF-1α improved the mechanical property of the grafts. In addition, the ultimate tensile strength of the grafts was also significantly higher in heparin-SDF-1α-group (7.6 ± 1.7 MPa; n=3) than heprin-treated group (4.6 ± 1.0 MPa (n=3) and untreated group (4.6 ± 1.4 MPa (n=5).
Figure 6.
SMPC differentiation and matrix remodeling of the explanted grafts. (A–F) Immunostaining for CNN1 (green) and SM-MHC (red) of the cross sections of untreated grafts (control), heparin-treated grafts and heparin-SDF-1α-treated grafts (middle portion), respectively, at 2 weeks and 4 weeks after implantation. (G–L) Immunostaining for collagen I (green) and SM-MHC (red) of the cross sections of untreated grafts (control), heparin-treated grafts and heparin-SDF-1α-treated grafts, respectively, at 4 weeks and 12 weeks after implantation. Dashed lines indicate the border between the graft (top part) and the outer layer. Nuclei were stained by DAPI (blue). Scale bar = 40 µm in A–L. (M) Elastic modulus of the grafts at 1, 2, 4 and 12 weeks after implantation. The data were shown as mean ± standard deviation (SD) (n=4). * indicates significant difference compared to untreated group (control) at the same time point.
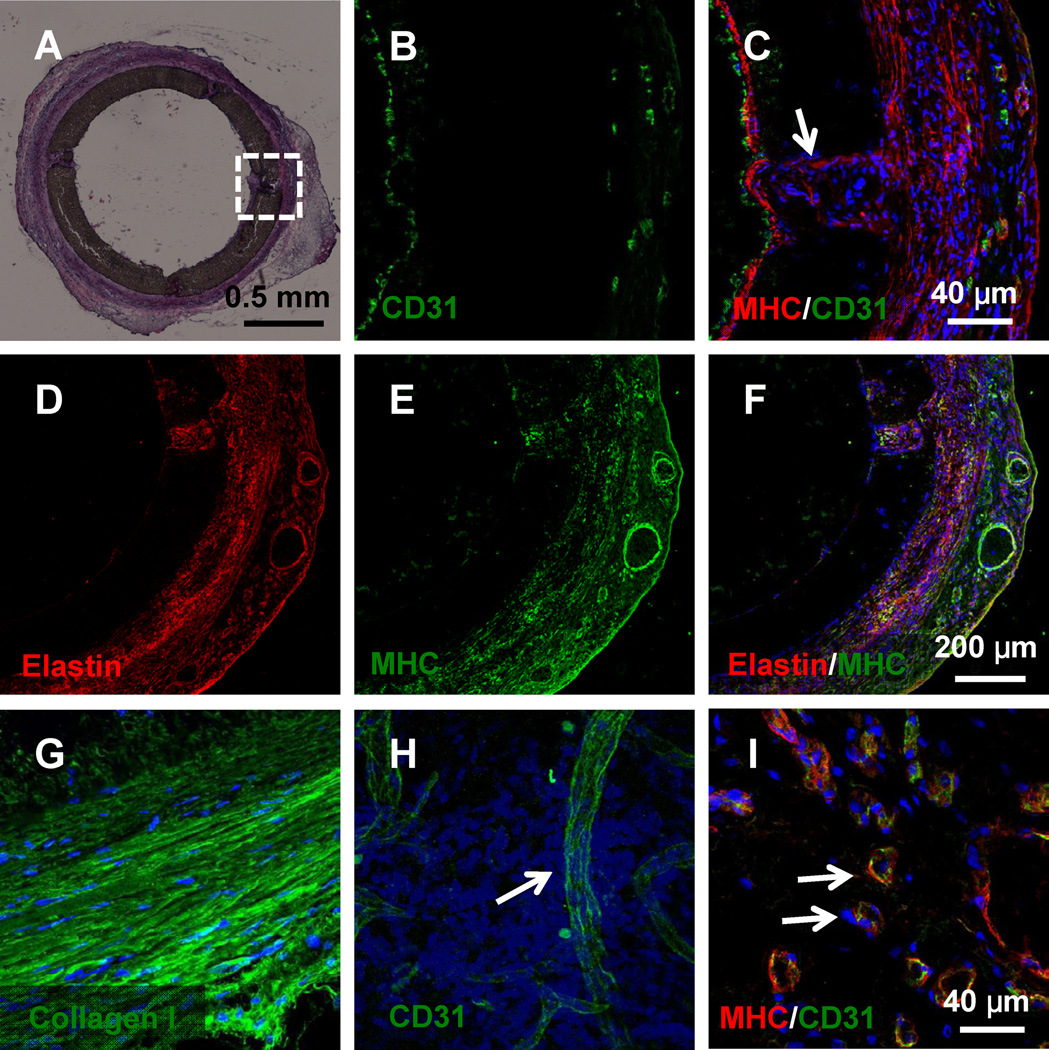
Since the grafts were biodegradable, to determine the effect of polymer degradation on graft remodeling, heparin-SDF-1α-treated grafts were examined as a representative at 6 months post implantation. As shown in Fig. 7A, H&E staining of the cross-sections in the middle portion of the grafts showed that the polymer had been partially degraded at certain sites. However, there was no sign of aneurysm formation and mechanical weakening in the wall. The endothelium was maintained intact in the lumen surface of the partially degraded grafts, while some SMCs migrated into the scaffolds (Fig. 7B,C) and deposited significant amount of elastin and collagen (Fig. 7D and G). Many microvessels were found in the outer part of the scaffolds indicated that the implanted grafts had been well vascularized and integrated with the surrounding tissue (Fig. 7H–I).
Figure 7.
Long-term remodeling of the biodegradable vascular grafts after 6 months of implantation. (A) H&E staining of the cross-sections in the middle portion of the grafts. Immunostaining of the region in the square is shown in B and C. (B–G) The graft remodeling was characterized by immunostaining for EC marker CD31, SMC marker MHC and ECM protein elastin. Arrow in C indicates the cells infiltrated into the region of polymer degradation. (H–I) Neovascularization and tissue integration on the outer surface of the grafts were characterized by staining for CD31 and MHC. Nuclei were stained by DAPI (blue). Arrows in H and I indicate microvessels in the vascular wall. Scale bar = 0.5 mm in A; scale bar = 40 µm in B–C and G–I; scale bar = 200 µm in D–F.
4. Discussion
Here we developed small diameter microfibrous vascular grafts that could harness the endogenous regeneration potential by recruiting both endothelial and smooth muscle progenitor cells, thereby addressing two critical issues of vascular grafts: endothelialization and the remodeling of the vascular wall. The accelerated endothelialization and the increase of SMPC recruitment resulted in the improvement of long-term patency and the increase of the mechanical strength of the vascular grafts respectively. These results demonstrate that endogenous progenitor cells are capable of regenerating vascular tissues if vascular grafts are engineered to harness this potential. This in situ tissue engineering approach by recruiting endogenous progenitor cells breaks new grounds in vascular tissue engineering, and demonstrates the feasibility of making bioactive vascular grafts available off-the-shelf. On the other hand, cell-seeded grafts can also enhance the biocompatibility and non-thrombogenic property of the grafts, and possibly promote cell recruitment by paracrine signaling. However, there are still several barriers to overcome to scale up the clinical treatment, e.g., cell characterization and manipulation, cell survival in the grafts during the surgery, and potential cell detachment under flow.
Despite extensive research on engineering the luminal surfaces, prosthetic vascular grafts still lack endothelialization in human. Pre-seeding ECs on luminal surfaces with sufficient pre-implant culture can improve clinical outcomes [32], but the procedure still requires extensive graft preparation before the surgery.. Heparin possesses excellent anticoagulant and antithrombogenic properties and it has been widely used as a coating reagent for blood contacting surfaces. Immobilized heparin may decrease the fibrin formation on the scaffold. It has been reported that fibrin may facilitate remodeling of vascular grafts [33]. However, EC seeding is required to cover the luminal surface of the grafts with fibrin to avoid coagulation and clogging, and thus autologous fibrin treatment may only be used for cell-seeded grafts. Although heparin coating could suppress acute thrombus formation and improve short-term patency, oral anticoagulant and anti-platelet therapies might be needed to maintain the long-term patency of vascular grafts. Therefore, accelerated in situ EC regeneration is desirable for vascular grafts.
It has also been shown that heparin binds to SDF-1α and stabilizes SDF-1α while maintaining its binding capability to its receptor [34–37]. Our data indicated that heparin-SDF-1α binding was more stable than passively adsorbed SDF-1α under both static and flow conditions. A recent work showed that adsorbed SDF-1α could also enhance endothelialization [38], but the stability of SDF-1α might not be optimal; in addition, the effect of immobilized SDF-1α on SMPCs is not known. We showed that EPC recruitment by heparin-bound SDF-1α was highly effective, with the majority of attached cells positive for EPC markers. This result demonstrates the feasibility of recruiting circulating EPCs under the flow condition in arteries. Besides chemotactic recruitment of EPCs, SDF-1α plays an important role in many aspects of EPC functions. For example, SDF-1α is a mediator of CD34+ EPCs trafficking between peripheral circulation and bone marrow through its receptor on EPC surface [39]. SDF-1α could also induce EC proliferation and differentiation [30, 40]. It is likely that immobilized SDF-1α plays an important role in EPC recruitment and subsequent cell proliferation and EPC differentiation.
Our results suggest that the transanastomotic migration of ECs is the major mechanism of endothelialization for untreated and heparin-treated grafts. In contrast, endothelialization in SDF-1a-treated grafts involves both the transanatomotic migration of ECs at the two ends and the recruitment of EPCs in the middle portion of the grafts. The time course studies showed that SDF-1α-recruited EPCs differentiated into ECs and accelerated the endothelialization process, which was correlated with the maintenance of long-term patency of heparin-SDF-1α-treated vascular grafts between 4 and 12 weeks. In contrast, the patency of untreated and heparin-treated grafts still decreased after 4 weeks, possibly due to the incomplete endothelialization. Our results also suggest that endothelialization by the proliferation and migration of ECs from anastomotic sites at the two ends of vascular grafts was not efficient, which took more than 4 weeks to cover the 6-mm long grafts in untreated and heparin-treated grafts. Because human ECs have slower proliferation and migration rate than rat ECs and the vascular grafts used in human are generally longer, it would take more time to achieve endothelialization in the vascular grafts, and thus EPC recruitment is necessary.
Another important finding is that SDF-1α increased the recruitment of SMPCs and improved the mechanical property of the grafts. The recruited SMPCs were capable of differentiating into SMCs in vivo and in vitro. Interestingly, these SMPCs were negative for the markers previously identified for SMPCs. Our preliminary studies suggest that these SMPCs express Sox10, an important marker for multipotent vascular stem cells [41] and can differentiate into SMCs, implicating a novel cell type and a new mechanism involved in vascular regeneration. These SMPCs are positive for SDF-1α receptors CXCR7 and CXCR4. It has been shown that during the wound-healing process of skin, CXCR4+ cells migrating around vessels release MMP-9 to enhance angiogenesis [42]. Whether SDF-1α regulates the differentiation and matrix synthesis of SMPCs remains to be determined. Nevertheless, we have shown that SDF-1α has dual functions to recruit the progenitor cells of both ECs and SMCs and that endogenous progenitor cells are sufficient to regenerate blood vessels. These findings lay down a foundation for in situ tissue engineering of blood vessels. It is also noted that SMPCs formed tissues similar to the media layer of native arteries on the outer surface of grafts, including SMCs and extensive matrix synthesis, suggesting an important role of SMPCs in vascular remodeling. These SMPCs are likely recruited from the surrounding tissues. Therefore, one could also fabricate non-degradable vascular grafts with similar mechanical property to the native arteries, and achieve the maturation and integration of the grafts in situ effectively. Since vascular remodeling in small animals does not recapitulate all aspects in human [43], preclinical and clinical studies will be performed to further translate this technology into clinical treatment.
5. Conclusions
We have successfully engineered and characterized small-diameter bioactive microfibrous vascular grafts. Heparin can stabilize immobilized SDF-1α on microfibers. The engineered vascular grafts have the capability of self-regeneration by recruiting both endothelial and smooth muscle progenitor cells, thereby addressing two critical issues of vascular grafts: endothelialization and the remodeling of the vascular wall. The accelerated endothelialization and the increase of SMPC recruitment resulted in the improvement of long-term patency and the increase of the mechanical strength of the vascular grafts respectively. This in situ tissue engineering approach breaks new grounds in vascular tissue engineering, and demonstrates the feasibility of making bioactive vascular grafts available off-the-shelf.
Supplementary Material
Acknowledgements
This work was supported in part by the grants from National Institute of Health (EB012240 and HL083900 to S.L.), a postdoctoral training grant TG2-01164 from California Institute for Regenerative Medicine (to A.W.), a predoctoral training grant TG2-01164 from California Institute for Regenerative Medicine (to Z. T.), a Siebel predoctoral fellowship (to J.H.), an AHA Predoctoral Fellowship (to Y.Z.), and a NIH predoctoral fellowship (to J.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cleary MA, Geiger E, Grady C, Best C, Naito Y, Breuer C. Vascular tissue engineering: the next generation. Trends Mol Med. 2012;18:394–404. doi: 10.1016/j.molmed.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 2.L'Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 4.Nerem RM, Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225–243. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Henry JJ. Nonthrombogenic approaches to cardiovascular bioengineering. Annu Rev Biomed Eng. 2011;13:451–475. doi: 10.1146/annurev-bioeng-071910-124733. [DOI] [PubMed] [Google Scholar]
- 6.Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 7.Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao BS, Wang K, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104:11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) Faseb J. 2008;22:1635–1648. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. P Natl Acad Sci USA. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin'oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 11.Olausson M, Patil PB, Kuna VK, Chougule P, Hernandez N, Methe K, et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet. 2012;380:230–237. doi: 10.1016/S0140-6736(12)60633-3. [DOI] [PubMed] [Google Scholar]
- 12.Luttun A, Carmeliet G, Carmeliet P. Vascular progenitors: from biology to treatment. Trends Cardiovasc Med. 2002;12:88–96. doi: 10.1016/s1050-1738(01)00152-9. [DOI] [PubMed] [Google Scholar]
- 13.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 14.Hirschi KK, Goodell MA. Hematopoietic, vascular and cardiac fates of bone marrow-derived stem cells. Gene Ther. 2002;9:648–652. doi: 10.1038/sj.gt.3301722. [DOI] [PubMed] [Google Scholar]
- 15.Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sainz J, Al Haj Zen A, Caligiuri G, Demerens C, Urbain D, Lemitre M, et al. Isolation of "side population" progenitor cells from healthy arteries of adult mice. Arterioscler Thromb Vasc Biol. 2006;26:281–286. doi: 10.1161/01.ATV.0000197793.83391.91. [DOI] [PubMed] [Google Scholar]
- 18.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci. 2004;9:1422–1432. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 20.Stitzel J, Liu J, Lee SJ, Komura M, Berry J, Soker S, et al. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27:1088–1094. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 21.Soffer L, Wang X, Zhang X, Kluge J, Dorfmann L, Kaplan DL, et al. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. J Biomater Sci Polym Ed. 2008;19:653–664. doi: 10.1163/156856208784089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieponice A, Soletti L, Guan J, Deasy BM, Huard J, Wagner WR, et al. Development of a tissue-engineered vascular graft combining a biodegradable scaffold, muscle-derived stem cells and a rotational vacuum seeding technique. Biomaterials. 2008;29:825–833. doi: 10.1016/j.biomaterials.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashi CK, Derugin N, Janairo RR, Lee R, Schultz D, Lotz J, et al. Antithrombogenic modification of small-diameter microfibrous vascular grafts. Arterioscler Thromb Vasc Biol. 2010;30:1621–1627. doi: 10.1161/ATVBAHA.110.208348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo MM, et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122–2128. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 25.Dorigo W, Pulli R, Piffaretti G, Castelli P, Griselli F, Dorrucci V, et al. Results from an Italian multicentric registry comparing heparin-bonded ePTFE graft and autologous saphenous vein in below-knee femoropopliteal bypasses. J Cardiovasc Surg (Torino) 2012;53:187–194. [PubMed] [Google Scholar]
- 26.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 27.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, et al. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 29.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 31.Kurpinski K, Lam H, Chu JL, Wang AJ, Kim A, Tsay E, et al. Transforming Growth Factor-beta and Notch Signaling Mediate Stem Cell Differentiation into Smooth Muscle Cells. Stem Cells. 2010;28:734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- 32.Deutsch M, Meinhart J, Zilla P, Howanietz N, Gorlitzer M, Froeschl A, et al. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg. 2009;49:352–362. doi: 10.1016/j.jvs.2008.08.101. discussion 62. [DOI] [PubMed] [Google Scholar]
- 33.Koch S, Flanagan TC, Sachweh JS, Tanios F, Schnoering H, Deichmann T, et al. Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials. 2010;31:4731–4739. doi: 10.1016/j.biomaterials.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 34.Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, et al. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. Journal of Biological Chemistry. 1999;274:23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- 35.Murphy JW, Cho Y, Sachpatzidis A, Fan C, Hodsdon ME, Lolis E. Structural and functional basis of CXCL12 (stromal cell-derived factor-1 alpha) binding to heparin. Journal of Biological Chemistry. 2007;282:10018–10027. doi: 10.1074/jbc.M608796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadir R, Baleux F, Grosdidier A, Imberty A, Lortat-Jacob H. Characterization of the stromal cell-derived factor-1alpha-heparin complex. Journal of Biological Chemistry. 2001;276:8288–8296. doi: 10.1074/jbc.M008110200. [DOI] [PubMed] [Google Scholar]
- 37.Sadir R, Imberty A, Baleux F, Lortat-Jacob H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. Journal of Biological Chemistry. 2004;279:43854–43860. doi: 10.1074/jbc.M405392200. [DOI] [PubMed] [Google Scholar]
- 38.De Visscher G, Mesure L, Meuris B, Ivanova A, Flameng W. Improved endothelialization and reduced thrombosis by coating a synthetic vascular graft with fibronectin and stem cell homing factor SDF-1alpha. Acta Biomater. 2012;8:1330–1338. doi: 10.1016/j.actbio.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 40.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–2711. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 41.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, et al. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 43.Zilla P, Bezuidenhout D, Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28:5009–5027. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.