Abstract
Introduction
Type 1 diabetes mellitus is associated with early atherosclerosis and enhanced cardiovascular mortality. The relationship between carotid IMT (cIMT), a marker of subclinical atherosclerosis and left ventricular (LV) mass, an independent predictor of cardiovascular morbidity has not been previously studied in type 1 diabetics.
Methods
The Epidemiology of Diabetes Interventions and Complications (EDIC) study is a multicenter observational study designed to follow up the Diabetes Control and Complications Trial (DCCT) cohort. LV mass was measured with cardiac MRI at EDIC year 15 and common cIMT was assessed using B-mode ultrasound at EDIC year 12. Multivariable linear regression models were used to assess the relationship between cIMT at year 12 and LV mass at year 15.
Results
A total of 889 participants had both cardiac MRI and cIMT measures available for these analyses. At EDIC year 15, the mean age of the participants was 49 (±7) years; mean diabetes duration was 28 (±5) years and 52% were males. Spearman correlation coefficient (r) between LV mass and cIMT was 0.33 (p<0.0001). After adjusting for basic covariates (machine, reader, age and gender), a significant association between LV mass and cIMT (estimate 2.0 g/m2 per 0.1 mm cIMT increment, p < 0.0001) was observed. This association was diminished by the addition of systolic blood pressure in particular 1.15 g/m2 per 0.1 mm cIMT increment, p<0.0001) and to a lessor extent other cardiovascular disease (CVD) risk factors. The relationship observed between LV mass and cIMT was stronger (HOW MUCH) in patients with shorter diabetes duration.
Conclusion
In a well characterized population with type 1 diabetes, cIMT was an independent predictor of higher LV mass. These findings suggest a common pathway, possibly mediated by blood pressure dependent mechanisms, for vascular and myocardial structural change in T1DM.
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is characterized by an increased prevalence of cardiovascular disease (CVD) risk factors, accelerated atherosclerosis, microvascular disease and increased burden of CVD events1. Increased carotid intima-media thickness (cIMT) measured with B mode ultrasound is regarded as a measure of generalized atherosclerosis2, 3 and has been shown to have prognostic significance for CVD events including myocardial infarction, stroke, coronary revascularization and death4–6. Likewise, left ventricular hypertrophy (LVH) determined by ECG, echocardiography and magnetic resonance imaging (MRI) has been shown to be an independent risk factor for coronary heart disease, stroke and heart failure7–11. Even without hypertrophy, continuous measures of left ventricular (LV) mass within the normal range are also predictive of cardiovascular morbidity and mortality12. LVH is frequently seen in T1DM and correlates with the duration of diabetes and prevalence of other diabetic complications13, 14.
Parallel relationships have been demonstrated between cIMT and LV mass in population studies15 and particularly well documented in hypertensive populations16, 17. In this report, we examine the relationship between cIMT and LV mass in a cohort of patients with T1DM. We further explore how traditional CVD risk factors and diabetic factors affect the association between cIMT and LV mass. We hypothesized that delineation of these interrelationships would provide a better understanding of the mechanism of end organ remodeling in T1DM while also offering enhanced prognostic information for risk stratification.
METHODS
Study design
The study designs for the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) study have been described elsewhere18, 19. Briefly, 1441 patients with T1DM aged 13–39 years without cardiovascular disease, hypertension, or hypercholesterolemia at baseline were recruited and randomly assigned to intensive or conventional diabetes therapy. More than 95% of the surviving DCCT cohort (1375 subjects) agreed to be followed up in the Epidemiology of Diabetes Interventions and Complications (EDIC) study which was designed as a prospective observational follow-up study of the DCCT cohort. The study was approved by the institutional review boards of all participating centers and all participants gave written informed consent.
Cardiac Magnetic Resonance Imaging
1028 participants from the EDIC cohort consented and were eligible for cardiac magnetic resonance imaging (MRI) exam. Cardiac MRI exam was done at EDIC year 15. The details of the cardiac MRI protocol have been previously described20. Briefly, participants were scanned with 1.5 Tesla magnets in all but one center which had a 3 Tesla magnet. Cardiac cine images were acquired in two-chamber, four-chamber and short axis planes with breath holds using an ECG triggered steady-state free-precession (SSFP) pulse sequence (TR/TE: <3.8/minimized msec; flip angle: maximized; spatial resolution: 2.5×2×8 mm; slice gap: 2 mm; temporal resolution: 30–50 msec). All cardiac MRI studies were evaluated and quantified at a single reading center by readers who were blinded to the patients’ clinical information. Left ventricular (LV) mass, volumes and functional parameters were determined from short axis cine images covering the heart from base to apex throughout the cardiac cycle using the QMASS software (version 6, Medis, Leiden, the Netherlands). Left ventricular endocardial and epicardial contours were traced manually at both end-diastole and end-systole by one physician reader and checked by a second cardiac magnetic resonance physician. Papillary muscles were included in the LV end-diastolic volume determinations and LV end-systolic volume but excluded from LV mass measurements. LV mass was determined by the sum of the myocardial area (thedifference between endocardial and epicardial contour) timesslice thickness plus image gap in the end-diastolic phase multipliedby the specific gravity of myocardium (1.05 g/mL. Intra-observer variability was assessed by repeating the measurements of LV mass and volumes on 100 DCCT/EDIC participants.
Carotid IMT measures
The measurement of carotid intima–media thickness (IMT) has been described in detail21. Carotid IMT was measured 3 times during the EDIC study, at baseline, year 6 and year 12. A single longitudinal lateral view of the distal right and left common carotid arteries was obtained. Studies were performed by certified technicians at the clinical centers, recorded on videotapes, and read in a central unit (Tufts University, Boston) by two single readers, who were unaware of the subjects’ diagnostic groups, treatment assignments and the time of the studies. The mean of the maximum intima–media thickness of the common carotid artery was defined as the mean of the maximum value for the near and far walls on both the right and left sides. Since there was only a single measure available for cardiac MRI, we decided to use the carotid IMT measure from EDIC year 12 as this was obtained closest to the time of the cardiac MRI study.
Covariates
During DCCT, participants underwent an annual medical history and physical examination, electrocardiography and laboratory testing for fasting lipid levels, serum creatinine values and other risk factors for cardiovascular disease18. Glycated hemoglobin values (HbA1c) were measured quarterly22. Hypertension was defined as blood pressure ≥ 140/90 mmHg or use of anti-hypertensive medications19. Hyperlipidemia was defined as low density lipoprotein levels ≥ 130 mg/dl or use of lipid lowering medication. Macroalbuminuria was defined by urinary excretion of albumin equal to or greater than 300 mg during a 24 hour period or end stage renal disease (ESRD). During the EDIC follow up study, the same methods used in DCCT were continued, but glycated hemoglobin (HbA1c) was measured annually, and fasting lipid levels and renal function indices were measured in alternate years.
Statistical Analysis
Clinical characteristics of DCCT/EDIC participants, measured immediately before or at the time of cardiac magnetic resonance scan, are reported as mean ± SD or percentage as appropriate. Differences between participants and non-participants or men and women were compared using Wilcoxon rank-sum test for quantitative variables and chi-square test for categorical variables.
Adjustment for body size was made by dividing LV mass by body surface area (LV mass index). The relationship between carotid IMT (cIMT) and LV mass index was evaluated using the Spearman correlation coefficient. Multivariable linear regression models were used to study the association between LV mass and the preceding cIMT, after adjusting for basic covariates; age, gender, MRI scan, primary vs. secondary cohort, and IMT reader and machine. Further models included CVD risk factors; current smoking, mean systolic blood pressure (SBP) over DCCT/EDIC, mean LDL cholesterol, mean HbA1c, attained duration of diabetes, and history of macroalbuminuria. Covariate mean values were weighted means where the values at each visit were weighted by the interval between values, owing to differences in visit schedules during DCCT and EDIC. The most significant factor for the multivariate association among similar variables (e.g. systolic and diastolic blood pressure) was employed. The strength of a covariate effect was measured by the semi-partial type II R2 that is the change in model R2 when that covariate is dropped from the model, expressed as a percentage. The two-way interaction between cIMT and each risk factor was assessed, and the interaction of cIMT and attained diabetes duration was significant in the final full model. cIMT and duration are continuous variables so the slope for regression of LV mass on cIMT over the range of attained duration is presented. All analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC). P values <0.05 were considered statistically significant.
RESULTS
Study participants
Of the 1441 EDIC participants, both cardiac MRI and carotid IMT (cIMT) measures were available in 889 participants. Comparing the subjects included as compared to those not included (Table 1), there were no significant differences in age, gender, diabetes duration, treatment assigned, renal function, body mass index, weighted systolic and diastolic blood pressure or total cholesterol levels. However, HbA1c levels, the percentage of participants who were current smokers and total triglyceride levels were significantly lower in the subjects included in the current study versus the values in those not included.
Table 1.
Clinical characteristics of participants with both CMRI and cIMT measures available (included in the present study) vs. overall study population
| Clinical Characteristics | Included (n=889) | Excluded (n=552) | * p-value |
|---|---|---|---|
| Female (%) | 47.5 | 46.7 | 0.787 |
| Primary (%) | 49.0 | 52.5 | 0.197 |
| Intensive (%) | 51.2 | 46.4 | 0.076 |
| DCCT Baseline | |||
| Age (years) | 27 ± 7 | 27 ± 8 | 0.775 |
| Adult (%) | 88.3 | 83.5 | 0.010 |
| Type1 duration (years) | 5.8 ± 4.2 | 5.4 ± 4.1 | 0.072 |
| Smoking (%) | 16.1 | 22.3 | 0.003 |
| BMI (kg/m2) | 23.3 ± 2.7 | 23.5 ± 3.0 | 0.411 |
| HbA1c (%) | 8.7 ± 1.5 | 9.1 ± 1.7 | < 0.0001 |
| Cholesterol | |||
| Total (mg/dl) | 175 ± 33 | 178 ± 34 | 0.309 |
| HDL (mg/dl) | 50 ± 12 | 51 ± 12 | 0.466 |
| LDL (mg/dl) | 109 ± 29 | 110 ± 30 | 0.910 |
| Triglycerides(mg/dl) | 79 ± 45 | 85 ± 51 | 0.029 |
| Blood pressure (mm-Hg) | |||
| Systolic | 114 ± 11 | 115 ± 12 | 0.080 |
| Diastolic | 73 ± 8 | 73 ± 9 | 0.570 |
| Renal Function | |||
| Serum Creatinine+ | 0.81 ± 0.15 | 0.80 ± 0.15 | 0.344 |
| Log (AER) (mg/24 hr) | 2.39 ± 0.78 | 2.47 ± 0.80 | 0.098 |
| AER ≥ 30 mg/24 hr (%) | 9.8 | 12.7 | 0.086 |
| eGFR | 112 ± 26 | 114 ± 28 | 0.183 |
| eGFR < 60 ml/min/1.73m2 (%) | 0 | 0.4 | 0.073 |
| Weight Gain (DCCT baseline to DCCT Close out) | 6.9 ± 7.8 | 8.3 ± 9.4 | 0.061 |
p-value is based on Chi-Square test for categorical variables and Wilcoxon Rank Sum test for continuous variables.
No one had serum creatinine ≥ 2 mg/dl at DCCT baseline
Clinical characteristics of the study participants at the time of cardiac MRI stratified by gender are presented in Table 2. The majority of the participants were Caucasian (> 96%) and 53% were men. The mean age was slightly higher for men than women (50 vs. 49 years; p<0.01). Men had higher body mass index than women (28.2 vs. 27.9 kg/m2; p<0.05), higher systolic blood pressure (120 vs. 115 mmHg; p<0.0001), higher diastolic blood pressure (76 vs. 72 mm Hg; p<0.0001), more likely to be on antihypertensive medications (p<0.05), had higher triglyceride levels (89 vs. 78 mg/dl; p<0.001), lower HDL cholesterol (50 vs. 60 mg/dl; p<0.0001), and were more likely to be on lipid lowering medications (p<0.0001). Men were also more likely to have albuminuria (11.4% vs. 6.6%; p<0.05). Both men and women had similar attained duration of T1DM, weighted HbA1c levels and LDL cholesterol levels. A lower proportion of the women than the men were on ACE-inhibitors or ARBs (49% vs 59%,p<0.01).
Table 2.
Clinical characteristics of the participants immediately before, or at the time of the CMRI scan, by gender (numbers are presented as mean ± SD or percentage as appropriate).
| Variable | Female (n=422) | Male (n=467) | * p value |
|---|---|---|---|
| Race(%White) | 96.7 | 96.6 | 0.857 |
| Attained Age (years) | 49 ± 7 | 50 ± 6 | 0.005 |
| Attained Duration of IDDM (years) | 27.8 ± 4.9 | 27.4 ± 4.8 | 0.207 |
| Current cigarette smokers (%) | 10.7 | 12.0 | 0.533 |
| Current drinker (%) | 37.2 | 52.9 | < 0.0001 |
| Body Mass Index | 27.9 ± 5.3 | 28.2 ± 4.1 | 0.043 |
| BMI >= 25 (%) (Overweight) | 67.1 | 75.8 | 0.004 |
| BMI >= 30 (%) (Obese) | 30.3 | 31.1 | 0.817 |
| Natural waist-to-hip ratio | 0.80 ± 0.07 | 0.91 ± 0.07 | < 0.0001 |
| Weighted systolic blood pressure (mm Hg) | 115 ± 8 | 120 ± 7 | < 0.0001 |
| Weighted diastolic blood pressure (mm Hg) | 72 ± 5 | 76 ± 5 | < 0.0001 |
| Hypertensive+ (%) | 45.0 | 52.9 | 0.019 |
| Anti-Hypertensive Medication (%) | 36.3 | 44.5 | 0.012 |
| Hemoglobin A1c (%) | |||
| Prior to CMRI | 8.0 ± 1.3 | 7.8 ± 1.2 | 0.063 |
| Weighted HbA1C | 8.0 ± 1.0 | 7.9 ± 1.0 | 0.282 |
| Weighted Lipid-HDL Cholesterol (mg/dl) | 60 ± 12 | 50 ± 11 | < 0.0001 |
| Weighted Lipid-LDL Cholesterol (mg/dl) | 110 ± 20 | 111 ± 21 | 0.523 |
| Weighted Lipid-Total Cholesterol (mg/dl) | 186 ± 23 | 178 ± 24 | < 0.0001 |
| Weighted Lipid Triglyceride (mg/dl) | 78 ± 35 | 89 ± 45 | 0.0006 |
| Hypercholesterolemia# (%) | 54.3 | 72.4 | < 0.0001 |
| Lipid lowering medication (%) | 47.6 | 67.5 | < 0.0001 |
| AER >= 30 mg/24h(%) or ESRD (sustained) | 23.7 | 28.3 | 0.121 |
| AER >= 300 mg/24h(%) or ESRD (ever) | 6.6 | 11.4 | 0.015 |
| eGFR < 60 | 7.8 | 4.9 | 0.075 |
| Left Ventricular Mass/BSA (g/m2) | 64.0 ± 10.3 | 76.5 ± 12.2 | < 0.0001 |
| Common IMT Year 12 (mm) | 0.65 ± 0.10 | 0.71 ± 0.16 | < 0.0001 |
| Any ACE or ARB | 49.1 | 58.7 | 0.004 |
| Any Beta Blocker | 8.1 | 7.9 | 0.941 |
p-value based on Chi-Square test for categorical variables and Wilcoxon rank sum test for continuous variables.
Hypertension was defined as blood pressure ≥ 140/90 mmHg or use of anti-hypertensive medications.
Hypercholesterolemia was defined as low density lipoprotein levels ≥ 130 mg/dl or use of lipid lowering medication
Left ventricular mass indexed for body surface area was significantly higher in men than women (76.5 g/m2 vs. 64 g/m2; p<0.0001). Likewise, common cIMT was significantly higher in men as compared to women (0.71 mm vs. 0.65 mm; p<0.0001).
Complications
Table 3 presents the presence of diabetes associated complications in the study cohort. One or more clinical and/or subclinical complications were present in 43% of the current study participants. Clinical or silent myocardial infarction had occurred in 3.4% of participants, coronary calcium score >200 was prevalent in 6.8% participants, retinopathy in 20.4%, microalbuminuria in 26.1%, autonomic neuropathy in 31.3% and peripheral neuropathy in 28.9% of the participants.
Table 3.
Characterization of Complications1
| CMRI screened participants with current EDIC data (N=1240) | Participants with CMRI (N=1017) | Participants with CMRI & IMT (N=889) | |
|---|---|---|---|
| Cardiovascular Disease | N (%) | N (%) | N (%) |
| Clinical or Silent MI | 52 (4.2) | 37 (3.6) | 30 (3.4) |
| Adjudicated Clinical MI Events | 24 (1.9) | 14 (1.4) | 10 (1.1) |
| Silent MI | 30 (2.4) | 23 (2.3) | 20 (2.3) |
| CAC score > 0 (%) (year 7–9) | 343 (30.6) | 282 (30.3) | 244 (29.4) |
| CAC score > 200 (%) (year 7–9) | 88 (7.9) | 67 (7.2) | 56 (6.8) |
| Retinopathy | |||
| PDR or worse | 252 (20.3) | 206 (20.3) | 181 (20.4) |
| Nephropathy | |||
| Macroalbuminuria/ESRD2 | 124 (10.0) | 98 (9.6) | 81 (9.1) |
| Sustained Microalbuminuria/ESRD3 | 338 (27.3) | 269 (26.5) | 232 (26.1) |
| Neuropathy4 | |||
| Autonomic Neuropathy | 377 (32.2) | 310 (31.7) | 270 (31.3) |
| Peripheral Neuropathy | 342 (30.0) | 282 (29.4) | 245 (28.9) |
| All Complications5 | |||
| Participants with 0 complication | 694 (56.0) | 578 (56.8) | 507 (57.0) |
| Participants with 1 complication | 351 (28.3) | 277 (27.2) | 241 (27.1) |
| Participants with 2 complications | 138 (11.1) | 116 (11.4) | 104 (11.7) |
| Participants with ≥ 3 complications | 57 (4.6) | 46 (4.5) | 37 (4.2) |
All complications were cumulative from DCCT to EDIC year 14–16 except for neuropathy.
AER ≥ 300 mg/24hr or ESRD
AER ≥ 30 mg/24hr consecutive two visits or ESRD
Neuropathy data was obtained once at EDIC year 13/14.
All complications were defined having clinical or silent MI, PDR or worse, macroalbuminuria/ESRD, or autonomic neuropathy
Relationship between LV mass and common cIMT
Carotid IMT (cIMT) was significantly correlated with LV mass (r=0.33; p<0.0001) and LV mass indexed to body surface area (r=0.28; p<0.0001). In the basic adjusted model, LV mass index was greater by 2g/m2 for every 0.1mm higher common cIMT with a semi-partial R2 = 3.8%, p<0.0001 (Table 4; basic model). In a model further adjusted for current smoking, presence of macroalbuminuria/ESRD, attained diabetes duration, mean weighted systolic blood pressure, mean weighted LDL cholesterol and HbA1c, the association between LV mass and cIMT was attenuated but remained statistically significant with a 1.22g/m2 higher LV mass index corresponding to a 0.1mm higher cIMT (R2 = 1.2%, p<0.0001). The proportion of variability explained by the covariates included in the basic model was 30% and that by the fully adjusted model was 40%. To assess the effect of individual risk factors on the relationship between cIMT and LV mass index, we further developed a series of regression models over the basic model where each risk factor was added individually to the basic model. We compared the R2 of the new model thus generated with the R2 of the basic model and also the change if any for the beta coefficient between cIMT and LV mass index. The association between cIMT and LV mass index after adjusting for each of the individual risk factors separately over the basic model is shown in Table 5. The greatest changes in the model R2 and the semi-partial R2 for the cIMT were seen after adding either mean systolic blood pressure or a history of end stage renal disease to the basic model, while other risk factors only minimally changed these R2 Values. While the 2 models including either SBP or ESRD were comparable in the extent of variability explained for LV mass, the model obtained by adding SBP reduced the beta estimate for cIMT by 42.5% percent while ESRD only reduced the beta estimate for cIMT by 16.5%.
Table 4.
Association of Carotid IMT with Left Ventricular Mass/BSA after minimal adjustment (the Basic Model) and adjustment for other risk factors (theFully Adjusted Model).
| Variable | Left Ventricular Mass (g/m2)
|
|||||
|---|---|---|---|---|---|---|
| Basic Model1 (R2=30%) | Fully Adjusted Model (R2 =40%) | |||||
|
| ||||||
| Semi-partial R2 (%) | Estimate of β ± SE | p-value | Semi-partial R2 (%) | Estimate of β ±SE | p-value | |
| Age | 1.41 | −0.25 ± 0.06 | < 0.0001 | 1.14 | −0.23 ± 0.06 | <0.0001 |
| Gender (male vs. female) | 19.33 | 11.84 ± 0.77 | < 0.0001 | 13.34 | 10.06 ± 0.73 | <0.0001 |
| Cohort2 (secondary vs. primary) | 0.35 | −1.56 ± 0.75 | 0.0365 | 0.001 | −0.15 ± 1.04 | 0.8880 |
| Smoking | 1.10 | 4.34 ± 1.09 | <0.0001 | |||
| Attained duration (per year) | 0.36 | −0.24 ± 0.11 | 0.0239 | |||
| Mean SBP3 | 2.86 | 0.32 ± 0.05 | <0.0001 | |||
| Mean LDL3 | 0.74 | −0.06 ±0.02 | 0.0011 | |||
| Mean HbA1c3 | 0.00008 | 0.01 ± 0.40 | 0.9737 | |||
| AER ≥300/ESRD | 2.82 | 8.27 ± 1.30 | <0.0001 | |||
| Common IMT (per 0.1mm) | 3.79 | 2.00 ± 0.29 | < 0.0001 | 1.22 | 1.19 ± 0.29 | <0.0001 |
Basic Model was also adjusted for MRI machine type and IMT reader.
Cohort: secondary cohort that had diabetes for 1–15 years with mild to moderate non-proliferative retinopathy and urinary albumin excretion rate < 200 mg/dl at the DCCT baseline versus primary cohort had diabetes for 1–5 years with no related complications
The weighted mean of the covariate values over the period of the DCCT and EDIC combined up to the time of the cardiac MRI, where the individual visit values are weighted by the interval of time between visits that differed during DCCT and EDIC.
The units are missing for SBP, LDL and Hba1c
Table 5.
Cardiovascular risk factors and the association between cIMT and LV mass after minimal adjustment and adjustment for each risk factor separately.
| Model | Model R2 (%) | Semi-partial R2 (%) | Estimate of β±SE | p-value |
|---|---|---|---|---|
| Basic model + smoking | 30.9 | 1.08 | 4.31 ± 1.17 | 0.0002 |
| Common IMT (per 0.1mm) | 3.31 | 1.88 ± 0.29 | < 0.0001 | |
| Basic model +attained diabetes duration | 31.4 | 0.54 | −0.30 ± 0.12 | 0.0094 |
| Common IMT (per 0.1mm) | 3.52 | 1.94 ± 0.29 | < 0.0001 | |
| Basic model + mean SBP | 36.5 | 5.14 | 0.42 ± 0.05 | < 0.0001 |
| Common IMT (0.1mm) | 1.11 | 1.15 ± 0.30 | < 0.0001 | |
| Basic model + mean LDL | 32.3 | 0.43 | −0.04 ± 0.02 | 0.0200 |
| Common IMT (per 0.1mm) | 3.69 | 1.97 ± 0.29 | < 0.0001 | |
| Basic model + mean HbA1C | 32.1 | 0.66 | 1.13 ± 0.39 | 0.0039 |
| Common IMT (per 0.1mm) | 3.00 | 1.81 ± 0.29 | < 0.0001 | |
| Basic model + Any AER≥300/ESRD(ever) | 37.0 | 5.64 | 10.90 ± 1.24 | < 0.0001 |
| Common IMT (per 0.1mm) | 2.58 | 1.67 ± 0.28 | < 0.0001 |
Basic model adjusted for attained age, gender, study cohort, MRI machine type, IMT reader and machine type,
The units are missing for SBP, LDL and Hba1c
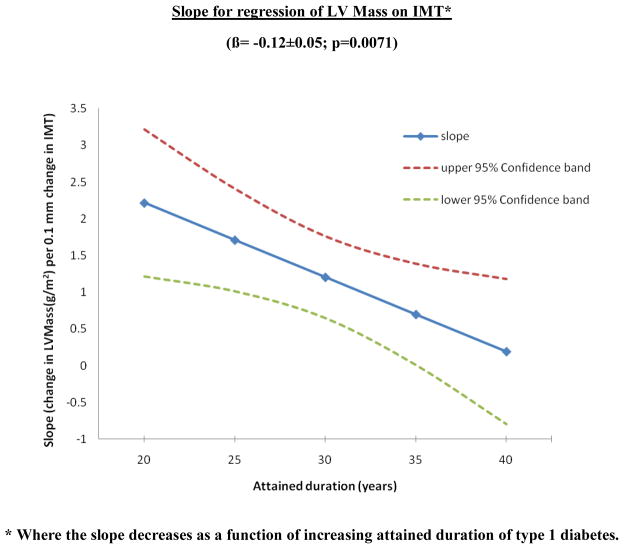
We further assessed possible interactions between individual risk factors in the final model and carotid IMT in relationship to LV mass. After correcting for multiple tests, the interaction of attained diabetes duration with cIMT on LV mass was significant. Interestingly, an inverse interaction was observed such that patients with shorter disease duration had a stronger association between cIMT and LV mass as compared to those who have had diabetes for a longer time (interaction β = −0.12±0.05, p=0.0071) (Figure 1). There were no significant interactions between other CVD risk factors and the effect of cIMT on LV mass.
Figure 1.
Interaction between attained diabetes duration and the relationship between cIMT and LV mass
* Where the slope decreases as a function of increasing attained duration of type 1 diabetes.
DISCUSSION
In this study, we found that in a population of well-characterized patients with T1DM, there was a strong association between ultrasound measures of carotid wall thickness and MRI determined left ventricular mass. The association was most significantly attenuated (almost 50%) after the addition of weighted systolic blood pressure and changed minimally with the addition of other risk factors suggesting that this relationship is predominantly mediated through blood pressure dependent mechanisms. Furthermore, patients with shorter duration of T1DM exhibited a more marked association between carotid IMT and left ventricular mass in comparison with those with longer duration of diabetes. These findings suggest that parallel vascular and cardiac structural changes occur in patients with T1DM.
Both LV mass and cIMT share common determinants such as age, gender, body size and are also affected by CVD risk factors which occur with greater frequency in the type 1 diabetes mellitus (T1DM) population compared with age-matched non-diabetics. While these subclinical target organ changes could be the result of common pathophysiologic pathways such as hemodynamic factors, neurohormonal influences, or the atherosclerotic process, it is not known whether the association between LV mass and cIMT is an interdependent bidirectional association or an actual cause effect relationship. Vascular remodeling is thought to be a composite of adaptive and atherosclerotic response to altered hemodynamic load and accumulation of risk factors23. The association between LVmass and cIMT could represent a compensatory adaptation to increased hemodynamic load in both end organs and studies have shown that the relationship is mediated mainly through hypertrophic responses24. Findings from previous studies and the current study show that the relationship between LVmass and cIMT persists even after adjusting for established risk factors, supporting an independent direct relationship.
The relationship between cIMT and LV mass has been previously studied in a healthy population25, a community dwelling older population15, primary and secondary hypertension26–28, patients with prior myocardial infarction29, in patients with type 2 diabetes mellitus30 and in patients with end stage renal disease31. In these studies, the strength of the association (r) between LV mass and cIMT measures ranged from 0.20 to 0.62 (VERSUS 0.19 IN THE CURRENT STUDY? ARE THOSE ADJUSTED OR RAW ASSOCIATIONS. IF 0.19 IS RIGHT, WE COULD ADD THE STATEMENT BELOW IN YELLOW). A weaker relationship was seen in patients with more atherosclerotic burden (older population, patients with prior MI) and stronger relationship was observed in hypertensive populations. The weaker relationship observed in older individuals and those with previous MI could be due to the use of lipid lowering medications and ACE inhibitors which modify the structural changes in arterial walls and cardiac structure. In the current study, the long duration of diabetes (27 years) may have also resulted in lower associations (r = 0.19) between LV mass and cIMT that in prior reports. Although a direct comparison is not possible, the relationship between LV mass and cIMT may mediated through a complex interplay of several different factors representing diabetic, atherosclerotic and hemodynamic alterations in this population.
We found that the slope of the relationship between cIMT and LV mass was stronger for those with shorter duration of diabetes than those with longer duration of diabetes. This could mean that the association is stronger early in the course of diabetes and shows a plateau effect with longer disease duration or that there is dissociation between these two parameters later in the course of diabetes. One possible explanation is the initiation over time of more aggressive CVD risk factor reduction, especially with drugs such as ACE inhibitors and beta blockers which have protective effects on ventricular remodeling. In the current study, more than 50% of the participants were treated with ACE inhibitors. Such drugs are being increasingly prescribed early in the course of the diabetes possibly modifying the natural progression of the disease. This is further heightened by the fact that patients with T1DM now have an exceptionally improved survival and life expectancy than that seen some decades ago. Moreover, our subjects were extensively monitored and treated for risk factors and complications like nephropathy, neuropathy, retinopathy, had strictly controlled HbA1c levels (all <8), and relatively well controlled risk factors such as blood pressure and cholesterol levels (table 2). Another possible mechanism of this observed relationship with disease duration could be the opposite effects that diabetes duration has on cIMT and LVmass. In the DCCT/EDIC study, diabetes duration has been shown to have an independent positive relationship with cIMT32 and an inverse relationship with LV mass (fully adjusted model in table 4). While no other risk factor had a significant interaction in the relationship between cIMT and LV mass, it could also be true that the small variation in risk factors in the present study might also limit the power to observe additional interactions between cIMT and these risk factors.
Strengths and limitations
This study is the first to describe the parallel relationship between carotid wall thickness and left ventricular mass in a large population of patients with T1DM patients, taking into account the association of risk factors on these target organs. LV mass was determined using MRI which is the gold standard for accurate estimation of cardiac parameters. Potential limitations of this study are that the study population might not be representative of the general diabetes population as this is a long term observational study of a clinical trial cohort where participants received close monitoring and follow up. Carotid wall area, lumen diameter and carotid plaque measures were not available in the present study. Furthermore, we did not take into account patterns of LVH (concentric, eccentric vs. concentric remodeling). Finally, this is a cross sectional study since cardiac MRI was available at only one time point; hence it is not possible to draw causal inferences.
Clinical implications
Cardiovascular disease is the leading cause of premature mortality in T1DM, over and above that estimated from traditional CVD risk factors. Accurate risk estimation and subsequent modifications with therapy are important goals for improved clinical outcomes in such patients. This study shows that even with well controlled risk factors, subclinical alterations in cIMT and LV mass, both of which are independent predictors of CVD events, occur in parallel to each other. While there is a significant relationship between these measurements, the association is not absolute warranting the clinical evaluation of each individual component for better risk profiling. Further studies in type 1 diabetes populations utilizing both carotid and cardiac imaging markers for risk reclassification should be evaluated.
CONCLUSION
An association between carotid IMT, a measure of atherosclerosis, and left ventricular mass has been demonstrated in a cohort of patients with T1DM. The association persisted even after controlling for CVD and other diabetes-associated risk factors. These findings suggest a potential contribution of arterial remodeling on LV mass and might help to explain additional risk for CVD seen in these patients. The role of improving risk stratification in patients with long standing diabetes using both imaging methods should be explored further.
Acknowledgments
FUNDING SOURCES
The DCCT/EDIC project is supported by contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, National Eye Institute, National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program and the Clinical and Translation Science Centers Program, National Center for Research Resources, and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases.
A complete list of the members of the DCCT/EDIC Research Group can be found in Archives of Ophthalmology, 2008;126(12):1707–1715.
The participating radiologists and technologists are listed in the online-only Data Supplement in Circulation (2011 Oct 18;124(16):1737–1746). Contributors of free or discounted supplies and/or equipment: Lifescan, Roche, Aventis, Eli Lilly, OmniPod, Can-Am, B–D, Animas, Medtronic, Medtronic Minimed, Bayer (donation one time in 2008), Omron.
The authors acknowledge the data processing and technical assistance of Wanyu Hsu at the Biostatistics Center, the George Washington University.
Footnotes
A list of the participating radiologists and technologists is shown in the online supplemental material 1 available at Circulation web site.
DISCLOSURES
None
References
- 1.Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, et al. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111(25):3489–93. doi: 10.1161/CIRCULATIONAHA.104.529651. [DOI] [PubMed] [Google Scholar]
- 2.Amato M, Montorsi P, Ravani A, Oldani E, Galli S, Ravagnani PM, et al. Carotid intima-media thickness by B-mode ultrasound as surrogate of coronary atherosclerosis: correlation with quantitative coronary angiography and coronary intravascular ultrasound findings. Eur Heart J. 2007;28(17):2094–101. doi: 10.1093/eurheartj/ehm244. [DOI] [PubMed] [Google Scholar]
- 3.Grobbee DE, Bots ML. Carotid artery intima-media thickness as an indicator of generalized atherosclerosis. J Intern Med. 1994;236(5):567–73. doi: 10.1111/j.1365-2796.1994.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 4.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96(5):1432–7. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 5.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128(4):262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 6.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 7.Bikkina M, Levy D, Evans JC, Larson MG, Benjamin EJ, Wolf PA, et al. Left ventricular mass and risk of stroke in an elderly cohort. The Framingham Heart Study. JAMA. 1994;272(1):33–6. [PubMed] [Google Scholar]
- 8.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) Study. J Am Coll Cardiol. 2008;52(25):2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox ER, Alnabhan N, Penman AD, Butler KR, Taylor HA, Jr, Skelton TN, et al. Echocardiographic left ventricular mass index predicts incident stroke in African Americans: Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2007;38(10):2686–91. doi: 10.1161/STROKEAHA.107.485425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110(2):101–7. doi: 10.7326/0003-4819-110-2-101. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham Study. Ann Intern Med. 1970;72(6):813–22. doi: 10.7326/0003-4819-72-6-813. [DOI] [PubMed] [Google Scholar]
- 12.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 13.Giunti S, Bruno G, Veglio M, Gruden G, Webb DJ, Livingstone S, et al. Electrocardiographic left ventricular hypertrophy in type 1 diabetes: prevalence and relation to coronary heart disease and cardiovascular risk factors: the Eurodiab IDDM Complications Study. Diabetes Care. 2005;28(9):2255–7. doi: 10.2337/diacare.28.9.2255. [DOI] [PubMed] [Google Scholar]
- 14.Sato A, Tarnow L, Parving HH. Prevalence of left ventricular hypertrophy in Type I diabetic patients with diabetic nephropathy. Diabetologia. 1999;42(1):76–80. doi: 10.1007/s001250051116. [DOI] [PubMed] [Google Scholar]
- 15.Kronmal RA, Smith VE, O’Leary DH, Polak JF, Gardin JM, Manolio TA. Carotid artery measures are strongly associated with left ventricular mass in older adults (a report from the Cardiovascular Health Study) Am J Cardiol. 1996;77(8):628–33. doi: 10.1016/s0002-9149(97)89319-8. [DOI] [PubMed] [Google Scholar]
- 16.Cuspidi C, Giudici V, Meani S, Negri F, Sala C, Zanchetti A, et al. Extracardiac organ damage in essential hypertensives with left ventricular concentric remodelling. J Hum Hypertens. 24(6):380–6. doi: 10.1038/jhh.2009.87. [DOI] [PubMed] [Google Scholar]
- 17.Shigematsu Y, Hamada M, Ohtsuka T, Hashida H, Ikeda S, Kuwahara T, et al. Left ventricular geometry as an independent predictor for extracardiac target organ damage in essential hypertension. Am J Hypertens. 1998;11(10):1171–7. doi: 10.1016/s0895-7061(98)00140-x. [DOI] [PubMed] [Google Scholar]
- 18.The DCCT Research Group. Design and methodologic considerations for the feasibility phase. The Diabetes Control and Complications Trial (DCCT) Diabetes. 1986;35(5):530–45. [PubMed] [Google Scholar]
- 19.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22(1):99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turkbey EB, Backlund JYC, Genuth S, Jain A, Miao C, Cleary PA, Lachin JM, Nathan DM, van der Geest RJ, Soliman EZ, Liu CY, Lima JAC, Bluemke DA. Myocardial structure, function and scar in patients with type 1 diabetes. Circulation. 2011;124(16):1737–1746. doi: 10.1161/CIRCULATIONAHA.111.022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Effect of intensive diabetes treatment on carotid artery wall thickness in the epidemiology of diabetes interventions and complications. Diabetes. 1999;48(2):383–90. doi: 10.2337/diabetes.48.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The DCCT Research Group. Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. Clin Chem. 1987;33(12):2267–71. [PubMed] [Google Scholar]
- 23.Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke. 1997;28(12):2442–7. doi: 10.1161/01.str.28.12.2442. [DOI] [PubMed] [Google Scholar]
- 24.Meijs MF, Doevendans PA, Cramer MJ, Vonken EJ, Velthuis BK, van der Graaf Y, et al. Relation of common carotid intima-media thickness with left ventricular mass caused by shared risk factors for hypertrophy. J Am Soc Echocardiogr. 2009;22(5):499–504. doi: 10.1016/j.echo.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Muiesan ML, Salvetti M, Zulli R, Pasini GF, Bettoni G, Monteduro C, et al. Structural association between the carotid artery and the left ventricle in a general population in Northern Italy: the Vobarno study. J Hypertens. 1998;16(12 Pt 1):1805–12. doi: 10.1097/00004872-199816120-00014. [DOI] [PubMed] [Google Scholar]
- 26.Cuspidi C, Mancia G, Ambrosioni E, Pessina A, Trimarco B, Zanchetti A. Left ventricular and carotid structure in untreated, uncomplicated essential hypertension: results from the Assessment Prognostic Risk Observational Survey (APROS) J Hum Hypertens. 2004;18(12):891–6. doi: 10.1038/sj.jhh.1001759. [DOI] [PubMed] [Google Scholar]
- 27.Muiesan ML, Rizzoni D, Salvetti M, Porteri E, Monteduro C, Guelfi D, et al. Structural changes in small resistance arteries and left ventricular geometry in patients with primary and secondary hypertension. J Hypertens. 2002;20(7):1439–44. doi: 10.1097/00004872-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 28.Rizzoni D, Muiesan ML, Porteri E, Salvetti M, Castellano M, Bettoni G, et al. Relations between cardiac and vascular structure in patients with primary and secondary hypertension. J Am Coll Cardiol. 1998;32(4):985–92. doi: 10.1016/s0735-1097(98)00322-2. [DOI] [PubMed] [Google Scholar]
- 29.Agewall S, Henareh L, Jogestrand T. Intima-media complex of both the brachial artery and the common carotid artery are associated with left ventricular hypertrophy in patients with previous myocardial infarction. J Hypertens. 2005;23(1):119–25. doi: 10.1097/00004872-200501000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Poppe KK, Whalley GA, Somaratne JB, Keelan S, Bagg W, Triggs CM, et al. The role of echocardiographic left ventricular mass and carotid intima-media thickness in the cardiovascular risk assessment of asymptomatic patients with type 2 diabetes mellitus. Intern Med J. doi: 10.1111/j.1445-5994.2010.02305.x. [DOI] [PubMed] [Google Scholar]
- 31.London GM, Guerin AP, Marchais SJ, Pannier B, Safar ME, Day M, et al. Cardiac and arterial interactions in end-stage renal disease. Kidney Int. 1996;50(2):600–8. doi: 10.1038/ki.1996.355. [DOI] [PubMed] [Google Scholar]
- 32.Polak JF, Backlund JY, Cleary PA, Harrington AP, O’Leary DH, Lachin JM, et al. Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 60(2):607–13. doi: 10.2337/db10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]