Abstract
In mammals, rewarding properties of drugs depend on their capacity to activate a dopamine-mediated appetitive motivational seeking state—a system that allows animals to pursue and find all kinds of objects and events needed for survival. With such states strongly conserved in evolution, invertebrates have recently been developed into a powerful model in addiction research, where a shared ancestral brain system for the acquisition of reward can mediate drug addiction in many species. A conditioned place preference paradigm has illustrated that crayfish seek out environments that had previously been paired with psychostimulant and opioid administration. The present work demonstrates that the administration of d-amphetamine stimulates active explorative behaviors in crayfish through the action of the drug within their head ganglion. Crayfish, with a modularly organized and experimentally accessible, ganglionic nervous system offers a unique model to investigate (1) the fundamental, biological mechanisms of addictive drug reward; (2) how an appetitive/seeking disposition is implemented in a simple neural system, and (3) how it mediates the rewarding actions of major drugs of abuse.
Keywords: amphetamine, addiction, invertebrate, reward, exploration, crayfish
1. Introduction
Invertebrates have emerged as useful animal models of addiction (see review in Wolf & Heberlin, 2003). With comparatively simple nervous systems and amenability to genetic manipulations, such models have advanced studies of the molecular underpinnings of behavioral responses to drugs, including acute responses, tolerance, withdrawal and sensitization (Morgan and Sedensky, 1995, McClung & Hirsh, 1999, Singh & Heberlein, 2000, Schafer, 2004, Scholtz et al., 2005, Nichols, 2006, Morozova et al., 2006, Raffa et al., 2006, Feng et al., 2006, Huber et al., 2011).
Many invertebrates are susceptible to developing drug-seeking behaviors as they show a preference for a variety of human drugs of abuse. Rewarding properties have been demonstrated across different invertebrate taxa for psychostimulants (Wolf, 1999, Kusayama & Watanabe, 2000, Panksepp & Huber, 2004), opioids (Srivastava & Singh, 2006, Nathaniel et al., 2009, 2010), alcohol (Parson, 1979, Bellen, 1998, Cadieu et al., 1999, Abramson et al., 2000, 2004), nicotine (Singaravelan et al., 2005), and caffeine (Singaravelan et al., 2005). Drugs of abuse also promote unconditioned behavioral responses similar to those in mammals, including stereotypical movements, increased locomotor activity, and consummatory behaviors (Wong et al., 1991, Morgan and Sedensky, 1995, Mc Clung & Hirish, 1998, Singh and Heberlein, 2000; Bainton et al., 2000, Rothenfluh & Heberlein, 2002, Dimitrijevic et al., 2004, Raffa & Martley, 2005, Feng et al., 2006). Many of these responses, following repeated drug administrations, are susceptible to sensitization (McClung & Hirsh, 1998, Wolf, 1999, Wolf & Heberlein, 2003, Hou et al., 2004, Dimitrijevic et al., 2004, Scholz, 2005).
Among the wide range of drug-elicited behavior, exploration and approach signify an appetitive motivational state evident when animals seek natural rewards such as food, water, sexual stimuli, and secure environments (Glickman & Schiff, 1967; Trowill et al., 1969; Panksepp, 1981, 1998; Ikemoto & Panksepp, 1999; Alcaro et al., 2007). In mammals, it has been suggested that the rewarding properties of drugs arise out of an abnormal stimulation of the neural processes involved in the activation of appetitive dispositions - the SEEKING system (Wise & Bozarth, 1987, Robinson & Berridge, 1993; Panksepp et al., 2002). Invertebrates, which exhibit similar dispositions during naturalistic contexts, represent useful models for identifying and manipulating the neural circuits responsible for exploration and investigation (from sensory input to motor output), and to characterize how drugs of abuse may affect such activities. The neural circuitry of forward locomotion in the brain of Caenorhabditis elegans has recently been identified, along with some of the external stimuli that influence movements toward favorable conditions of chemotaxis, thermotaxis, and aerotaxis (Gray et al., 2005). Dopamine and glutamate serve as key modulators of such circuits (Hills et al., 2004), and it is possible that some of the rewarding properties of drugs derive from their capacity to stimulate their relevant, underlying networks (Panksepp, 1998; Ikemoto & Panksepp, 1999; Alcaro et al., 2007).
The present paper aimed to characterize the effects of amphetamine on invertebrate appetitive/exploratory behaviors. In a conditioned place preference (CPP) paradigm, crayfish preferred environments that had been associated with cocaine or amphetamine (Panksepp & Huber, 2004, Huber, 2005). However, it has remained unclear whether amphetamine really activates a behaviorally evident, appetitive motivational state. Following a characterization of exploratory behavior of crayfish (experiment 1), we tested whether amphetamine enhanced the exploration of surrounding cues. Since novelty-induced behavioral activation could mask the effects of drugs, a subset of experiments administered amphetamine in an environment to which crayfish had previously been habituated. Past CPP experiments utilized a single 5mg/kg application of amphetamine, which represents a high dose1. Accordingly, we obtained a dose response curve for systemic amphetamine infusions (experiment 2). To determine the role of head ganglia in the orchestration of different exploratory patterns, we tested for changes in extent and time course of behavioral effects following amphetamine administration into the brain rather than the general circulation (experiment 3).
2. Materials and methods
2.1. Animals
Crayfish (Orconectus rusticus) were wild-caught in the Portage River (near Bowling Green, OH, USA) and maintained in a community tank inside the laboratory. Prior to each experiment, intermolt individuals were isolated for a minimum of 3 days in individual plastic containers (160mm diameter, 95mm depth) at a 16:8h light:dark cycle containers and maintained on holding trays with a continuous low of filtered, aerated water at 20°C. Crayfish were fed twice a week with a combination of fish, earthworms and rabbit chow.
2.2 Experimental procedure
Experiment 1 characterised the behaviour patterns that crayfish exhibit in novel environments. Towards this goal the types of active exploratory behaviour were described and their frequencies quantified. Following 3 days of isolation, 2 experimental groups were formed. Individuals of the control group (N=6) were transferred to a test arena (Plexiglas aquarium (0.6 × 0.4 × 0.25m) with a gravel substrate) and left undisturbed for a 4 hour habituation period. At the end of this period, each animal was captured and immediately placed back into the experimental arena. Individuals were videotaped for 40 minutes with a digital camcorder (XL1, Canon, Japan) mounted above the aquarium. Crayfish of the experimental group (N=6) were placed in the arena and videotaped immediately after transfer (without waiting for the 4h habituation period). Any consistent differences in behaviour between the two groups are interpreted as an unconditioned response to novelty.
Experiments 2 and 3 were designed to monitor changes in these exploratory behavioural effects that result from infusion of different doses of d-amphetamine. One day prior to the experiment, a cannula was implanted either into the pericardial sinus (experiment 2) or the head ganglion (experiment 3). On the test day, 0.5m of deactivated, fine-bore, fused silica needle material (Agilent, i.d. = 100μm) was connected to the canulated crayfish with Tygon microbore tubing (Fisher Scientific, i.d. = 250μm). The infusion canula was connected to a microdialysis pump (CMA/102) via to a microdialysis swivel mounted above the aquarium. The cannula was primed to fill its void volume to insure that drug (or saline) was delivered immediately when the pump was turned on. Crayfish of different dose-response groups were placed in a white Plexiglas aquarium (0.6 × 0.4 × 0.25m) with a gravel substrate and left undisturbed for 4 hours. Following the habituation phase, crayfish behaviour was videotaped for 40 minutes. A range of doses of d-amphetamine sulfate (FW: 368.5; Sigma, St. Louis: A 5880) were administered systemically into the pericardial sinus of crayfish in experiment 2 (0.1, 0.5, 1, and 5 mg/kg of body weight), and directly into the brain in experiment 3 (at 0.1, 0.5, and 1 mg/kg). A vehicle-injected (125mM saline) group served as control in both experiments. The total injection volume for experiment 2 was adjusted to not exceed 1/50 of the estimated hemolymph volume for each animal (Panksepp & Huber, 2004). In experiment 3, the total injection volume was 0.5 μl for all animals. Crayfish (10.9 – 31.5g) were randomly assigned to 5 groups in experiment 2 (saline control [C], amphetamine 0.1mg/kg [A01], 0.5mg/kg [A05], 1mg/kg [A1], and 5mg/kg [A5]) and to 4 groups in the experiment 3 (saline control [C], amphetamine 0.1mg/kg [A01], 0.5mg/kg [A05], and 1mg/kg [A1]). The 5 mg/kg dose of amphetamine was excluded for the brain infusion study as it produced repetitive tail-flips and convulsions. Each group contained 7 animals at the outset, but a few individuals had to be excluded due to death from surgery, insufficient evidence for effective substance delivery, or lack of staining (see below). A total of 4–7 valid individuals remained in each treatment group.
2.3. Surgery
Animals were anaesthesized in crushed ice for 20min. In experiment 2, a deactivated, fine-bore, fused silica cannula (Agilent, i.d. = 250μm) was implanted into the pericardial sinus (allowing 3mm to extend into the sinus) and secured with cyanoacrylate (Fig. 1). All animals were allowed to recover overnight within their holding containers. A successful cannula implant was confirmed via behavioral consequences of a major cocaine injection (20-60μg) following the conclusion of the experiment. Inclusion in this study was conditional on a strong reaction to this treatment.
Fig. 1.

(a) Crayfish are chilled in ice. A small hole is drilled through the dorsal carapace within the caudal third of the pericard, a few millimeters to the right of the midline to avoid damaging the underlying heart. Ten centimeters of fused silica tubing (J&W Scientific, #160-2644) are cut, pre-rinsed with 125mM NaCl, and fitted with a small piece of paper towel 3mm from one end to restrict how far the tube will protrude into the sinus. (b) The tubing is then placed into the hole and secured with small drops of cyanoacrylate and pieces of paper towel. Deactivated needle material (J&W Scientific, #160-1010) is cut to 0.5m length with both ends reinforced with 1cm of .250mm outer diam., fused silica material (J&W Scientific, #160-2255). A small piece of 0.28mm ID polyethylene tubing connects this tubing to a blunt-tipped, hypodermic needle (26g × 1/2”) attached to a 1ml syringe. After pre-rinsing the system with NaCl, the flexible canula is connected with PE tubing material to the short piece of stiff, fused silica already attached to the animal.
In experiment 3, 15mm of deactivated, fine-bore, fused silica was implanted into the brain. Crayfish were anaesthesized and mounted on a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The coordinates were calculated from a conjunction point (P) between three lines evident in the head exoskeleton. The coordinates were as follows (in mm): -1 antero-posterior, ±0 lateral and -3 ventral. After insertion, the fused silica cannula was fixed in place with cyanoacrylate. Animals were allowed to recover overnight in their holding containers. Successful placement of cannulae was confirmed via methylene blue staining. At the end of the experiments, individuals were injected with the dye and decapitated. The brains were dissected, stored in 4% paraformaldehyde for 24h, and then placed in 20% sucrose solution before sectioning. Only animals with unambiguous methylene blue stained brains were used in statistical analyses.
2.4. Behavioral Analysis
Initial observations, revealed several conspicuous behavioural patterns during a crayfish’ exposure to a novel Plexigas aquarium. A number of behavioural differences distinguished the crayfish that were acclimated from those that were not, including locomotion (all forms of walking), antenna movements (whipping antennae back and forth), rearing (standing on its last pair of walking legs, reaching upwards along the wall), site building (excavating a depression in the gravel), grooming (cleaning and maintaining parts of the body), and tail-flip (escape behavior). The pharmacological part of this study quantified changes in these behaviours as a result of amphetamine infusion. The 40 minute test session was recorded on video and the duration of time spent in each (mutually exclusive) behaviour was quantified with the aid of JWatcher software (http://www.jwatcher.ucla.edu/).
2.5. Statistical Analyses
Statistical analyses were applied to relative frequencies of each behavioural category and analyzed using one-way ANOVAs. The independent variables were experience (habituated or novel) for experiment 1, treatment dose (C, A01, A05, A1 and A5) for experiment 2, and treatment dose (C, A01, A05, and A1) for experiment 3. All post-hoc comparisons used Duncan’s test with the level of significance (p) set at <0.05.
3. Results
In experiment 1, one-way ANOVA revealed significant effects of an acclimation period with a reduction in locomotion (F[1,9]=183.142; P<0.001), and antenna movements (F[1,9]=49.429; P<0.001). The exposure to a novel environment increased the proportion of time spent in the two active exploratory behaviors (Fig. 2) with a robust increase in locomotion. Novelty did not affect other behavioral patterns such as site building (F[1,9]=0.113; P>0.05ns), or grooming (F[1,9]=0.179; P>0.05ns). Tailflips were rare in both group, precluding a statistical analysis.
Fig. 2.
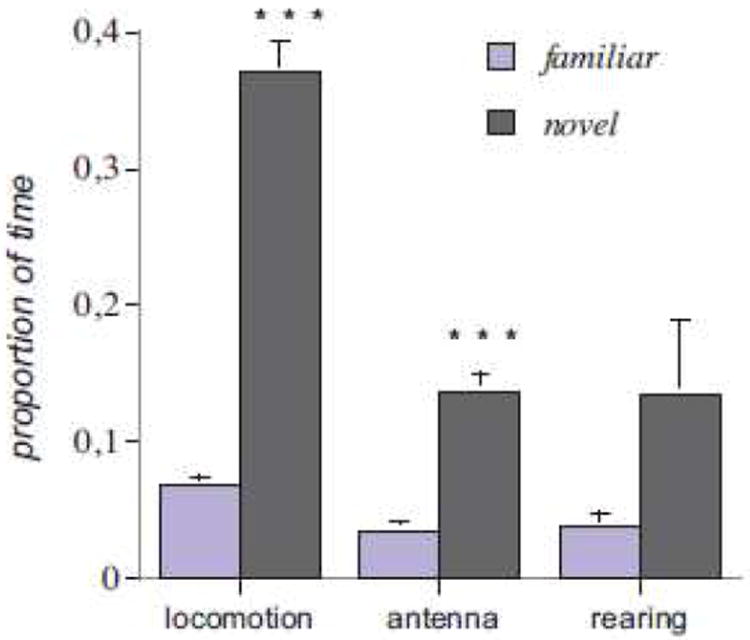
Effects of environmental conditions (familiar vs novel) on crayfish exploratory behaviors during a 40 minute period. Results are expressed as the mean percentage of time spent performing each behavioral category. *P< 0.05, **P<0.01, ***P<0.001 compared to behavior in the familiar environment.
In experiment 2, one-way ANOVAs revealed significant effects of amphetamine treatment for all active exploratory behaviors, including antenna movements (F[4,20]=3.816; P<0.05), rearing (F[4,20]=5.435; P<0.01), and locomotion (F[4,20]= 12.390; P<0.001). These effects were dose-dependent. Compared with saline, post-hoc analysis identified significant differences in rearing for doses of 0.5 mg/kg (P<0.05), 1mg/kg (P<0.001), and 5mg/kg (P<0.01). Amphetamine significantly enhanced antenna movements and locomotion at doses of 1mg/kg (P<0.01), and 5mg/kg (P<0.01). In sum, systemic amphetamine injections consistently increased the amount of time crayfish pursued active exploration of the observation arena in dose-dependent fashion (Fig. 3). Compared to the 1mg/kg dose, 5mg/kg amphetamine increased rearing and decreased locomotion, suggesting a certain level of motivational competition between these behaviors at high arousal levels. One-way ANOVA revealed significant effects of amphetamine in tail flip behavior (F[4,20]=3.276; P<0.05), with post-hoc analysis showing a significant increase at the 5mg/kg dose (P<0.01) (data not shown). Systemic amphetamine did not change the frequencies of site building (F[4,20]=0.115; P>0.05ns) or grooming (F[4,20]=0.048; P>0.05ns).
Fig. 3.
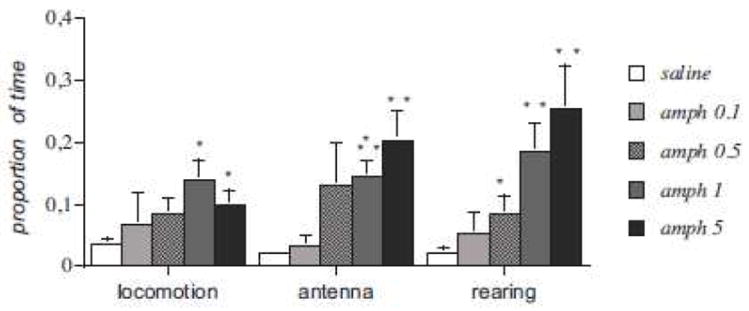
Effects of different doses of systemic amphetamine injections on crayfish exploratory behaviors during a 40 minute period. Results are expressed as the mean percentage of time spent in each behavioral category. *P<0.05, **P<0.01, ***P<0.001 compared to saline.
In experiment 3, one-way ANOVAs revealed significant effects of amphetamine in locomotion (F[3,16]= 9.316; P<0.001), antenna movements (F[3,16]= 17.082; P<0.001), and, to a lesser degree, for rearing (Fig. 4). Post-hoc analysis demonstrated a significant increase in locomotion for amphetamine at the 0.5mg (P<0.05) and 1.0mg dose (P<0.001). Post-hoc analysis showed significant differences in antenna movements for crayfish receiving 0.1mg (P<0.05), 0.5 (P<0.01) and 1.0mg amphetamine (P<0.001) compared to saline. In rearing behavior, only animals receiving the 0.5mg amphetamine dose (P<0.05) differed from saline controls. Compared to 0.5mg/kg, a 1mg/kg dose increased locomotion and antenna movements, while reducing rearing. This finding supports the idea of a possible motivational competition between locomotion and rearing. When injected into the brain, amphetamine did not significantly influence tail flips (F[3,16]=1.018; P=0.412), grooming (F[3,16]=0.753; P=0.537) or site building behaviors (F[3,16]=0.901; P=0.463). Direct application of amphetamine to the brain selectively enhanced exploratory behaviors in dose-dependent fashion as it did with systemic infusions - a primary site of action in the supraesophageal ganglion is thus suggested.
Fig. 4.
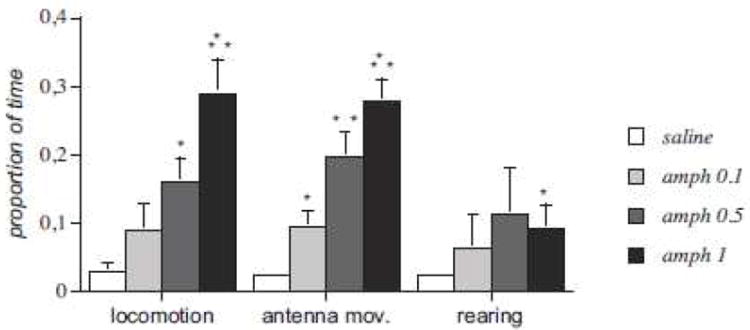
Effects of different doses of direct brain amphetamine injections on crayfish exploratory behaviors during a 40 minute period. Results are expressed as the mean percentage of time spent in each behavioral category. *P<0.05, **P<0.01, ***P<0.001 compared to saline.
4. Discussion
Collectively, these findings demonstrate that d-amphetamine enhances exploration in crayfish, with increased locomotion and exploratory sampling of the environment. Active exploration, as highlighted by patterns for locomotion and antenna movements, is enhanced in crayfish which are placed into a novel environment, compared to others who had already habituated to the test arena. Novel stimuli appear to directly influence brain networks that facilitate exploratory behaviors that are normally used in pursuit of natural rewards. It is likely that these appetitive motivational states may be recruited by psychostimulants such as amphetamine.
Crayfish, habituated to their environments, tended to settle into a location along an edge or corner. Systemic administration of amphetamine induced strong arousal, locomotion, and increased exploration of the test arena. The most effective dose of amphetamine (1mg/kg) enhanced locomotion by 383%, antenna movements by 725% and rearing by 876%. Such behavioral responses to amphetamine suggest increased brain monoaminergic transmission (Fleckenstein et al., 2007). It is possible that amphetamine simply causes a generalized arousal state, and that the observed behaviors are merely the consequence of such activation. However, other behaviors transiently exhibited in habituated environments (e.g., activity directed towards site building or grooming) were unaffected by amphetamine, suggesting selective effects towards those behavioral patterns that are specifically associated with exploration.
Systemic administration of amphetamine at the highest dose (5mg/kg) also increased the occurrence of tail flips. This is not surprising since monoamines play a modulatory role in the escape circuit of crayfishes (Glanzman and Krasne, 1983) and preliminary observations in our lab have demonstrated that amphetamine facilitates escape patterns. However, tail flip was stimulated when amphetamine was injected in an acute treatment of less than 5 minutes. In contrast, injection utilizing longer time-frames of around 20 minutes resulted in considerably fewer tail flips, with minimized escape, and amplified SEEKING.
Administration of amphetamine directly into the brain was more effective than peripheral drug application in enhancing the above behaviors; for instance, central application of 1mg/kg amphetamine increased locomotion by +1000%, antenna movements by +1210% but produced only modest effects on rearing (+385%). Moreover, other behavioral patterns such as tail flips, grooming, and nest building were unaffected by amphetamine administration directly to the brain. Since locomotor activity and antenna movements are largely exploratory, we surmise that the aroused appetitive states were mainly related to the effect of amphetamine within the crayfish head ganglion. Enhanced rearing, which in contrast is mainly directed towards escaping from the arena, may have been mediated by more distal ganglionic actions of the drug. Site-specific, pharmacological experiments are needed to further pinpoint the precise anatomical sites of such behavioral effects.
Exploratory behaviors in crayfish are driven by tactile and olfactory information (Kraus-Epley & Moore, 2002, McMahon et al., 2005, Patullo & Macmillan, 2006), detected primarily via antennae and antennules, and conveyed mainly to the olfactory lobe of the brain (Mellon, 2000, Sullivan & Beltz, 2005). Modulated by serotonin and dopamine transmission (Sandeman & Sandeman, 1987, Sandeman et al., 1995, Schmidt, 1997), the olfactory lobe of crayfish represents a site of action of amphetamine, and perhaps other drugs as well. The perception of the external environment is not a passive process, but is characterized by active movements as animals orient their sensory organs towards specific sources of stimulation, and move in specific directions to localize and interact with objects of interest. Locomotion and antenna tactile exploration act in concert with other sensory information to produce whole-body adaptive responses. For instance, navigation of C. elegans in resource-rich environments is controlled by central neural integrators that span primary sensory neurons for olfaction and taste, interneurons, and motor neurons (Gray et al., 2005). The presence of similar amphetamine-sensitive circuits within the crayfish brain suggests the presence of evolutionarily conserved processes that promote exploratory and approach behavior patterns through increased incentive salience of surrounding stimuli or enhance exploratory motor patterns - a distinction that needs further investigation.
In sum, our data are consistent with rewarding properties of amphetamine in crayfish as determined by CPP procedures (Panksepp & Huber, 2004), and such reward components may arise from the activation of a disposition for exploration and approach within the crayfish brain. The brain’s integrative control over such adaptive responses may promote the organism’s ability to search for favorable, life-supporting environmental conditions. A comprehensive understanding of the causation of this behaviour beyond the intervening level of motivations requires that it ultimately is mapped onto their respective proximate mechanism. The neurochemistries and functional neuroanatomies of reward seeking brain circuits in crayfish remain to be detailed, but monoamines appear to play an important role. Monoamine systems are attractive candidates as they modify neural function at multiple levels and may thereby bring about coordinated responses to environmental perturbations. In this manner, amines are thought to alter the activity of neural decision-making centers (Nader et al. 1997). Rather than produce behavior per se, these substances appear to tune ongoing activity and, in a given context, promote the occurrence of adaptive behaviors (e.g. feeding, flight, fight, or mating) over contra-adaptive ones (Kravitz 1988). Among the monoaminergic systems present in the crayfish’s head ganglion (dopamine, serotonin, and octopamine), research on both invertebrates and mammals now suggest that exploration and approach may specifically depend on dopaminergic function (Hills et al., 2004; Alcaro, et al., 2007).
In mammals, the mesolimbic dopamine system has been indicated as a key component of complex appetitive-motivational neural circuits (Alcaro et al., 2007) which, to highlight its primary-process (i.e., instinctual) status, has been labeled the SEEKING emotional system in mammals (Panksepp, 1981, 1998). The primal function of this system is to sustain exploratory approach behaviors that optimize encounters with a large variety of conditions needed for survival. Certain drugs prove addictive when they activate this pro-survival mechanism, probably because the underlying action pattern itself is rewarding. Through learning, this system facilitates processing of the acquired affective incentive value for cues associated with natural and drug rewards (Di Chiara & Bassareo, 2007). The present work supports the conclusion that ancestral forms of a similar functional circuit exists in invertebrate brains. Further work towards characterizing this circuit, especially the role of dopamine and other neurochemicals within brain systems for cardinal survival functions, will allow us to better understand the relationships between ancestral appetitive motivational mechanisms and the fundamental brain sources of drug addiction.
Research highlights.
locomotion is a suitable measure of exploratory behavior in crayfish
exploratory behavior decreases as crayfish become familiar with their environment
amphetamine promotes exploratory behavior in a dose-dependent manner
Acknowledgments
We are thankful to the members of the Huber (Budhaditya Chowdhury, Udita Datta, Jeremy Didion, Beth Posta) and the van Staaden laboratory (Moira van Staaden, Angela Medina-Garcia) for helpful comments and suggestions on various versions of this manuscript. Studies reported in this paper were supported by a grant to R.H. and J.P. (NIH/NIDA 1R21DA016435-01A1) and the Hope for Depression Research Foundation to JP.
Footnotes
It was reported that at such high dose, amphetamine and cocaine reduced locomotor activity, as the animals spent most of the time in a corner of the arena with compulsive movements of their antennae (Panksepp & Huber, 2004). Such behavior may represent an equivalent to the drug-induced, stereotyped behavior commonly seen in mammals following high doses of psychostimulants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Abramson CI, Stone SM, Ortez RA, Luccardi A, Vann KL, Hanig KD, Rice J. The development of an ethanol model using social insects I: behavior studies of the honey bee (Apis mellifera L.) Alcohol Clin Exp Res. 2000;24(8):1153–66. [PubMed] [Google Scholar]
- Abramson CI, Kandolf A, Sheridan A, Donohue D, Bozic J, Meyers JE, Benbassat D. Development of an ethanol model using social insects: III. Preferences for ethanol solutions. Psychol Rep. 2004;94(1):227–39. doi: 10.2466/pr0.94.1.227-239. [DOI] [PubMed] [Google Scholar]
- Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 2007;56(2):283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10(4):187–94. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Bellen HJ. The fruit fly: a model organism to study the genetics of alcohol abuse and addiction? Cell. 1998;93:909–12. doi: 10.1016/s0092-8674(00)81195-2. [DOI] [PubMed] [Google Scholar]
- Cadieu N, Cadieu J-C, El Ghadraoui L, Grimal A, Lamboeuf Y. Conditioning to ethanol in the fruit fly—a study using and inhibitor of ADH. J Insect Physiol. 1999;45:579–586. doi: 10.1016/s0022-1910(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic N, Dzitoyeva S, Manev H. An automated assay of the behavioral effects of cocaine injections in adult Drosophila. J Neurosci Methods. 2004;37(2):181–4. doi: 10.1016/j.jneumeth.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell. 2006;127(3):621–33. doi: 10.1016/j.cell.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–98. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Krasne FB. Serotonin and octopamine have opposite modulatory effects on the crayfish’s lateral giant escape reaction. J Neurosci. 1983;311:2263–9. doi: 10.1523/JNEUROSCI.03-11-02263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman SE, Schiff BB. A biological theory of reinforcement. Psychol Rev. 1967;74(2):81–109. doi: 10.1037/h0024290. [DOI] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. PNAS. 2005;102(9):3184–91. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci. 2004;24(5):1217–25. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Kuromi H, Fukasawa Y, Ueno K, Sakai T, Kidokoro Y. Repetitive exposures to nicotine induce a hyper-responsiveness via the cAMP/PKA/CREB signal pathway in Drosophila. J Neurobiol. 2004;60(2):249–61. doi: 10.1002/neu.20021. [DOI] [PubMed] [Google Scholar]
- Huber R. Amines and motivated behaviors: a simpler systems approach to complex behavioral phenomena. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191(3):231–9. doi: 10.1007/s00359-004-0585-5. [DOI] [PubMed] [Google Scholar]
- Huber R, Panksepp JB, Nathaniel T, Alcaro A, Panksepp J. Drug-sensitive reward in crayfish: An invertebrate model system for the study of SEEKING, reward, addiction, and withdrawal. Neuroscience & Biobehavioral Reviews. 2011 doi: 10.1016/j.neubiorev.2010.12.008. 2010 Dec 21. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Kravitz EA. Hormonal control of behavior: amines and the biasing of behavioral output in lobsters. Science. 1988;241:1775–1781. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- Kraus-Epley KE, Moore PA. Bilateral and unilateral antennal lesions alter orientation abilities of the crayfish, Orconectes rusticus. Chem Senses. 2002;27(1):49–55. doi: 10.1093/chemse/27.1.49. [DOI] [PubMed] [Google Scholar]
- Kusayama T, Watanabe S. Reinforcing effects of methamphetamine in planarians. Neuroreport. 2000;11(11):2511–2513. doi: 10.1097/00001756-200008030-00033. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol. 1998;8(2):109–112. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr Biol. 1999;9(16):853–60. doi: 10.1016/s0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- McMahon A, Patullo BW, Macmillan DL. Exploration in a T-maze by the crayfish Cherax destructor suggests bilateral comparison of antennal tactile information. Biol Bull. 2005;208(3):183–8. doi: 10.2307/3593150. [DOI] [PubMed] [Google Scholar]
- Mellon D., Jr Convergence of multimodal sensory input onto higher-level neurons of the crayfish olfactory pathway. J Neurophysiol. 2000;84(6):3043–55. doi: 10.1152/jn.2000.84.6.3043. [DOI] [PubMed] [Google Scholar]
- Morgan PG, Sedensky MM. Mutations affecting sensitivity to ethanol in the nematode, Caenorhabditis elegans. Alcohol Clin Exp Res. 1995;19(6):1423–9. doi: 10.1111/j.1530-0277.1995.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Morozova TV, Anholt RR, Mackay TF. Transcriptional response to alcohol exposure in Drosophila melanogaster. Genome Biol. 2006;7(10):R95. doi: 10.1186/gb-2006-7-10-r95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Bechara A, van-der-Kooy D. Neurobiological constraints on behavioral models of motivation. Annu Rev Psychol. 1997;48:85–114. doi: 10.1146/annurev.psych.48.1.85. [DOI] [PubMed] [Google Scholar]
- Nathaniel TI, Panksepp J, Huber R. Drug-seeking behavior in an invertebrate system: evidence of morphine-induce reward, extinction an reinstatement in crayfish. Behavioural Brain Research. 2009;197:331–338. doi: 10.1016/j.bbr.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathaniel TI, Panksepp J, Huber R. Effects of a single and repeated morphine treatment on conditioned and unconditioned behavioral sensitization in Crayfish. Behavioural Brain Research. 2009;207:310–320. doi: 10.1016/j.bbr.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther. 2006;112(3):677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Hypothalamic integration of behavior: Rewards, punishments, and related psycho-biological process. In: Morgane PJ, Panksepp J, editors. Handbook of the Hypothalamus (vol 3, part A, Behavioral Studies of the Hypothalamus) Marcel Dekker; New York: 1981. pp. 289–487. [Google Scholar]
- Panksepp J. The foundation of human and animal emotions. Oxford University Press; New York: 1998. Affective Neuroscience. [Google Scholar]
- Panksepp JB, Huber R. Ethological analyses of crayfish behavior: a new invertebrate system for measuring the rewarding properties of psychostimulants. Behav Brain Res. 2004;153(1):171–80. doi: 10.1016/j.bbr.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of emotional brain systems in addictions: A neuro-evolutionary perspective. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Parsons PA. Larval responses to environmental ethanol in Drosophila melanogaster: variation within and among populations. Behav Genet. 1979;10:183–191. doi: 10.1007/BF01066268. [DOI] [PubMed] [Google Scholar]
- Patullo BW, Macmillan DL. Corners and bubble wrap: the structure and texture of surfaces influence crayfish exploratory behaviour. J Exp Biol. 2006;209(Pt 3):567–75. doi: 10.1242/jeb.02020. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Stagliano GW, Tallarida RJ. Subadditive withdrawal from cocaine/kappa-opioid agonist combinations in Planaria. Brain Res. 2006;1114(1):31–5. doi: 10.1016/j.brainres.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Martley AF. Amphetamine-induced increase in planarian locomotor activity and block by UV light. Brain Res. 2005;1031(1):138–40. doi: 10.1016/j.brainres.2004.10.051. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Heberlein U. Drugs, flies, and videotape: the effects of ethanol and cocaine on Drosophila locomotion. Curr Opin Neurobiol. 2002;12(6):639–45. doi: 10.1016/s0959-4388(02)00380-x. [DOI] [PubMed] [Google Scholar]
- Sandeman RE, Sandeman DC. Serotonin-like immunoreactivity of giant olfactory interneurons in the crayfish brain. Brain Res. 1987;403(2):371–4. doi: 10.1016/0006-8993(87)90078-3. [DOI] [PubMed] [Google Scholar]
- Sandeman RE, Watson AH, Sandeman DC. Ultrastructure of the synaptic terminals of the dorsal giant serotonin-IR neuron and deutocerebral commissure interneurons in the accessory and olfactory lobes of the crayfish. J Comp Neurol. 1995;361(4):617–32. doi: 10.1002/cne.903610406. [DOI] [PubMed] [Google Scholar]
- Schafer WR. Addiction research in a simple animal model: the nematode Caenorhabditis elegans. Neuropharmacology. 2004;47(S1):123–31. doi: 10.1016/j.neuropharm.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Distribution of centrifugal neurons targeting the soma clusters of the olfactory midbrain among decapod crustaceans. Brain Res. 1997;752(1-2):15–25. doi: 10.1016/s0006-8993(96)01441-2. [DOI] [PubMed] [Google Scholar]
- Scholz H. Influence of the biogenic amine tyramine on ethanol-induced behaviors in Drosophila. J Neurobiol. 2005;63(3):199–214. doi: 10.1002/neu.20127. [DOI] [PubMed] [Google Scholar]
- Singaravelan N, Nee’man G, Inbar M, Izhaki I. Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectars. J Chem Ecol. 2005;31(12):2791–804. doi: 10.1007/s10886-005-8394-z. [DOI] [PubMed] [Google Scholar]
- Singh CM, Heberlein U. Genetic control of acute ethanol-induced behaviors in Drosophila. Alcohol Clin Exp Res. 2000;24(8):1127–36. [PubMed] [Google Scholar]
- Srivastava HK, Singh D. Honeybees foraging response in genetically diversified opium poppy. Bioresour Technol. 2006;97(13):1578–81. doi: 10.1016/j.biortech.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Integration and segregation of inputs to higher-order neuropils of the crayfish brain. J Comp Neurol. 2005;481(1):118–26. doi: 10.1002/cne.20346. [DOI] [PubMed] [Google Scholar]
- Trowill JA, Panksepp J, Gandelman R. An incentive model of rewarding brain stimulation. Psychological Review. 1969;76:264–81. doi: 10.1037/h0027295. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–92. [PubMed] [Google Scholar]
- Wolf FW, Heberlein U. Invertebrate models of drug abuse. J Neurobiol. 2003;54(1):161–78. doi: 10.1002/neu.10166. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Cocaine addiction: clues from Drosophila on drugs. Curr Biol. 1999;9:R770–2. doi: 10.1016/S0960-9822(00)80009-3. [DOI] [PubMed] [Google Scholar]
- Wong M, Delaney K, Gelperin A. Opiate agonists activate feeding in Limax: comparison of in vivo and in vitro effects. Behav Neurosci. 1991;105(1):15–24. doi: 10.1037//0735-7044.105.1.15. [DOI] [PubMed] [Google Scholar]


