Abstract
Neutralizing (nAbs) and high affinity binding antibodies may be critical for an efficacious HIV-1 vaccine. We characterized virus-specific nAbs and binding antibody responses over 21 months in eight HIV-1 subtype C chronically infected individuals with heterogeneous rates of disease progression. Autologous nAb titers at study exit were significantly higher compared to contemporaneous responses at study entry (p=0.002) and exit (p=0.01). NAb IC50 titers correlated inversely with V1-V2 length (p=0.04). Significant differences in breadth and potencies were noted against subtype C compared to subtype A (p= 0.03 and p=0.01) or subtype B (p= 0.03; p=0.05) viruses respectively. IgG binding affinity for gp41 was higher than for gp120 (p=0.0002). IgG-FcγR1 affinity was significantly higher than FcγRIIIa (p<0.005) at study entry and FcγRIIb (p<0.05) or FcγRIIIa (p<0.005) at study exit. Evolving IgG binding suggests alteration of immune function mediated by binding antibodies. Evolution of nAbs was a potential marker of HIV-1 disease progression.
Keywords: HIV-1 subtype C, neutralizing antibodies, binding antibodies, chronic infection
Introduction
In 2010 alone, there were an estimated 2.4 to 2.9 million new HIV-1 infections worldwide with 70% of these new infections occurring in sub-Saharan Africa (UNAIDS, 2011). This high HIV incidence makes the development of a protective vaccine a global public health priority. Such a vaccine will likely need to elicit antiviral antibodies, similar to the successful vaccines against other viral infections such as hepatitis B, measles, mumps and polio that are thought to mediate their effects primarily through antibody mechanisms (Plotkin, 2008). However, despite intense efforts in the study of HIV envelope structure and immunogen design, the development of an efficacious vaccine able to induce broadly effective antibody responses remains elusive.
During natural HIV infection, only a subset of individuals, less than 30%, develop broad, cross-neutralizing antibodies after many years (Gray et al., 2011; Li et al., 2009; Sather et al., 2009; Simek et al., 2009; Stamatatos et al., 2009). Such individuals have been an important source of new neutralizing monoclonal antibodies (nmAbs) against the HIV envelope that have enhanced our understanding of HIV pathogenesis, envelope structure and provided clues for rational immunogen design (Pejchal et al., 2011; Walker et al., 2010; Wu et al., 2010). However, clinical benefit of anti-HIV antibodies has not yet been definitively demonstrated. Given that HIV-1C is the predominant circulating and most rapidly spreading subtype worldwide (Esparza, 2005; Hemelaar et al., 2011), screening, characterizing and understanding the types of nAbs produced by HIV-1C-infected individuals; and defining the potencies and breadth of these nAbs may contribute to the design of the next generation of envelope immunogens.
While only some people develop cross-neutralizing antibodies, autologous nAbs (AnAbs) appear in almost all HIV-infected individuals usually within the first year. A number of studies have shown that contemporaneous viruses are less sensitive to AnAbs than earlier autologous viruses suggesting that viral evolution and escape occurs rapidly and this remains a significant obstacle to HIV vaccine development (Delwart et al., 1997; Moore et al., 2009; Richman et al., 2003; Rong et al., 2009; Wei et al., 2003). Several mechanisms of viral escape have been documented; these include insertions and deletions of amino acids, amino acid substitutions and shifting the position of N-linked glycans in Env (Frost et al., 2005; Lynch et al., 2011; Moore et al., 2009; Rong et al., 2009). Further understanding of HIV antibody escape patterns and mechanisms may help to inform better immunogen design to overcome Env diversity and immune escape.
Although the major focus of the HIV vaccine field is the development of immunogens able to induce broadly neutralizing antibodies, V1-V2 binding antibodies appear to have played some role against HIV-1 acquisition in the RV144 vaccine trial (Haynes et al., 2012). The role of binding or non-neutralizing antibodies in inhibiting virus replication through an Fc (fragment crystallizable) receptor (FcR)-dependent mechanism has been demonstrated (Peressin et al., 2011). FcRs are part of the immunoglobulin (Ig) superfamily and bind to the Fc portion of antibodies forming a bridge between the cell bearing the target antigens and the effector cell (Nimmerjahn and Ravetch, 2007). FcγRs have either an activating or inhibitory function with relative IgG binding affinities. FcγRI (high affinity), FcγRlla and FcγRllla (medium-low affinity) - all have activating functions; and FcγRllb has an inhibitory function (medium-low affinity) (Forthal and Moog, 2009; Siberil et al., 2007). The effector cell subsequently mediates virus killing through antibody-dependent cell mediated cytotoxicity (ADCC) or through antibody-dependent cell-mediated viral inhibition (ADCVI). Some studies have shown that the strength of binding interaction between the Fc region of the antibody and the FcγRIlla and/or FcγRlla potently increases ADCC activity and Fc binding affinity could be altered through deglycosylation or site-specific mutagenesis, abrogating downstream ADCC (Jefferis, 2009; Lazar et al., 2006; Richards et al., 2008; Shields et al., 2001). Thus Fc-binding interaction may be crucial to improving the efficacy of antibodies (Lazar et al., 2006). These studies therefore provide a rationale for assessing the binding affinities of the IgG-Fc regions to their cognate FcγRs as an indication of putative antiviral activity of binding antibodies in vivo. Previously, ADCC activity was reported to be significantly higher in elite controllers compared to chronic progressors (Lambotte et al., 2009). These data on HIV are consistent with earlier studies suggesting a role for non-neutralizing antibodies in viral control and disease attenuation (Alsmadi et al., 1997; Baum et al., 1996). More recently, it was demonstrated that non-neutralizing antibodies conferred protection, albeit limited, to macaques against vaginal SHIV challenge (Burton et al., 2011). The binding affinities of IgGs to various HIV-1 antigens and to FcγRs have rarely been studied together and no studies have reported on the characterization of these antibodies in chronic HIV-1 subtype C infections.
In this study, we used standard high throughput neutralization assays to evaluate the potency and breadth of neutralizing activity in patient plasma and map potential Env targets associated with nAb breadth. We profiled autologous and cross-reactive (against a standard panel of 20 subtype A, B and C viruses) nAb titers in eight chronically HIV-1C infected individuals with heterogeneous rates of disease progression. Envelope genotypic characteristics (amino acid length and numbers of potential N-linked glycosylation sites (PNGs) associated with autologous nAb titers were investigated. Binding affinities of total immunoglobulins (IgGs) to HIV-1 antigens (gp41, gp120 and p24) and FcγRs (FcγRI, FcγRlla, FcγRllb and FcγRIIIa) were quantified. Associations between autologous nAb titers with markers of disease progression (namely CD4 T-cell counts and viral loads) were investigated.
Materials and Methods
Participants
Participant samples were retrospectively identified from the Sinikithemba cohort, which is a prospective natural history study of HIV-1 infected individuals based at McCord Hospital, Durban, South Africa as previously reported (Kiepiela et al., 2004). Human subjects for this study were eight chronically-infected individuals with heterogenous rates of clinical disease progression as previously described (Archary et al, 2010). The median period of follow-up overall was 21 months. Ethical approval for this study was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee and all participants gave written informed consent to participate in the study.
Sample Collection, CD4 T-cell counts and Plasma Viral Load
CD4 T-cell counts were performed at three-month intervals whereas viral loads were done at six-month intervals as previously described (Archary et al., 2010). Blood was drawn from each subject into EDTA tubes and plasma was separated by centrifugation and stored at −80°C until use. Viral load was measured using the Amplicor Version 1.5 assay (Roche, Alameda CA, USA). CD4 T-cell counts were enumerated by Trucount technology on a four colour FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, New Jersey, USA).
Envelope clones
Briefly, single genome PCR amplification derived env amplicons were directionally T/A cloned into the CMV-driven expression plasmid pcDNA3.1-V5 HisTOPO-TA and screened for biological function as pseudoviruses following co-transfection with an env-deficient subtype B proviral plasmid (SG3ΔEnv) into 293T cells as described previously (Derdeyn et al., 2004; Haaland et al., 2009). A total of 20 standard reference envelope clones were obtained from the NIH AIDS Research and Reference Reagent Program and used in the heterologous neutralization assays. These included five subtype A, seven subtype B and eight subtype C envelope pseudoviruses as depicted in Table 2. Tier 1, 2 and 3 viruses were stratified previously based on a continuum of patterns of neutralization sensitivity- with tier 1A being the most sensitive, tier 1B above average, tier 2 displaying moderate to low sensitivity and tier 3 displaying the lowest sensitivity to neutralization (Seaman et al., 2010). The ConC plasmid based on the consensus of all the HIV-1 subtype C sequences from the Los Alamos database by 2001 was obtained from Dr Feng Gao (Kothe et al., 2006). The envelope plasmids containing single point mutations are described in Gray et al (2011).
Table 2. HIV-1 Env pseudovirus panel of subtype C, B and A reference strains.
| HIV Isolate | Viral Subtype | Tiered Category | Reference |
|---|---|---|---|
| MW965.25 | C | 1A | NIH ARRRP# |
| ZM197 M.PB7 | C | 1B | (Li et al., 2006) |
| ConC | C | 2 | (Kothe et al., 2006) |
| DU156.12 | C | 2 | (Li et al., 2006) |
| DU172.17 | C | 2 | (Li et al., 2006) |
| ZM214 M.PL15 | C | 2 | (Li et al., 2006) |
| CAP45.G3 | C | 2 | (Li et al., 2006) |
| CAP239.G3 | C | 2 | (Gray et al., 2007) |
| SF162.LS | B | 1A | (Stamatatos and Cheng-Mayer, 1998) |
| 6535.3 | B | 1B | (Li et al., 2005) |
| AC10.0.29 | B | 2 | (Li et al., 2005) |
| WITO 4160.33 | B | 2 | (Li et al., 2005) |
| TRO.11 | B | 2 | (Li et al., 2005) |
| QH0692.42 | B | 2 | (Li et al., 2005) |
| PVO.4 | B | 3 | (Li et al., 2005) |
| Q23ENV17 | A | 2 | (Blish et al., 2007) |
| Q842ENVd12 | A | 2 | (Blish et al., 2007) |
| Q168ENVa2 | A | 2 | (Blish et al., 2007) |
| Q461ENVe2 | A | 2 | (Blish et al., 2007) |
| Q769ENVd22 | A | 2 | (Blish et al., 2007) |
NIH ARRRP# - National Institutes of Health AIDS Research and Reference Reagent Program
Neutralization assays
Patient plasma samples were evaluated for nAb activity against virions pseudotyped with autologous patient-derived viral Envs using a single reporter assay as described previously (Derdeyn et al., 2004; Gray et al., 2007; Li et al., 2005; Li et al., 2006; Wei et al., 2003). Figure 1A illustrates the schema used for autologous neutralizing antibody challenge assays where the study entry and exits Envs were tested for neutralization using the study entry and study exit plasma nAb samples for eight study participants. A total of 37 autologous Envs were tested against their study entry plasma and study exit plasma (range: 2-6 env clones per participant). Neutralization was measured as a reduction in luciferase gene expression after a single round of infection of TZM-bl cells (NIH AIDS Research and Reference Reagent Program). Two thousand infectious units of each pseudovirus was combined with five-fold dilutions of heat-inactivated participant plasma and incubated for 1 hour at 37°C as previously described (Rong et al., 2009). Subsequently, the virus and antibody reaction was added to the plated TZM-bl cells, and left to incubate at 37°C for 48 hours. The cells were then lysed and the luciferase activity was determined using a BioTek Synergy HT (BioTek, USA). The background luminescence of the uninfected wells was subtracted from the test wells. The percentage of infectivity was calculated by dividing the number of luciferase units at each plasma dilution by the value in the well containing no test plasma. The dilution that yielded 50% inhibitory activity against the virus, known as the nAb IC50 titer was determined on Microsoft Excel. Each experiment was performed in duplicate and independently at least twice for replicability. The nAb IC50 titer was calculated as the reciprocal plasma dilution causing a 50% reduction of relative light units (IC50). Heterologous assays were done as previously reported (Montefiori, 2004). Briefly, positive neutralization was scored as an IC50 titer above 1:45. The nAb breadth and potency was determined as a score based on the median IC50 titer for each virus as reported in Blish et al. (2008).
Figure 1.
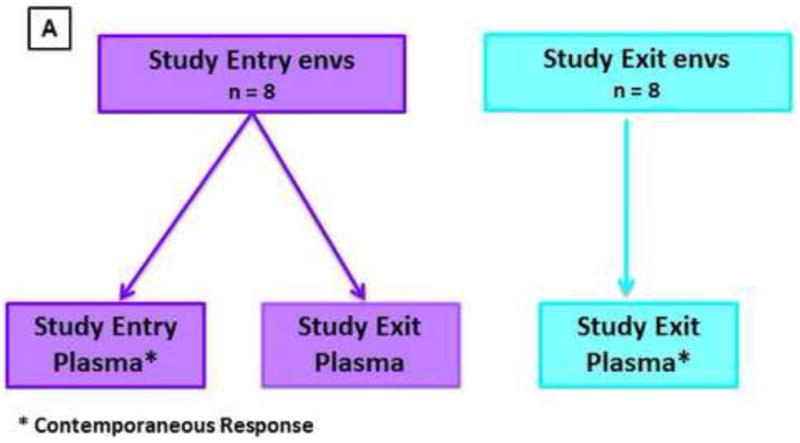
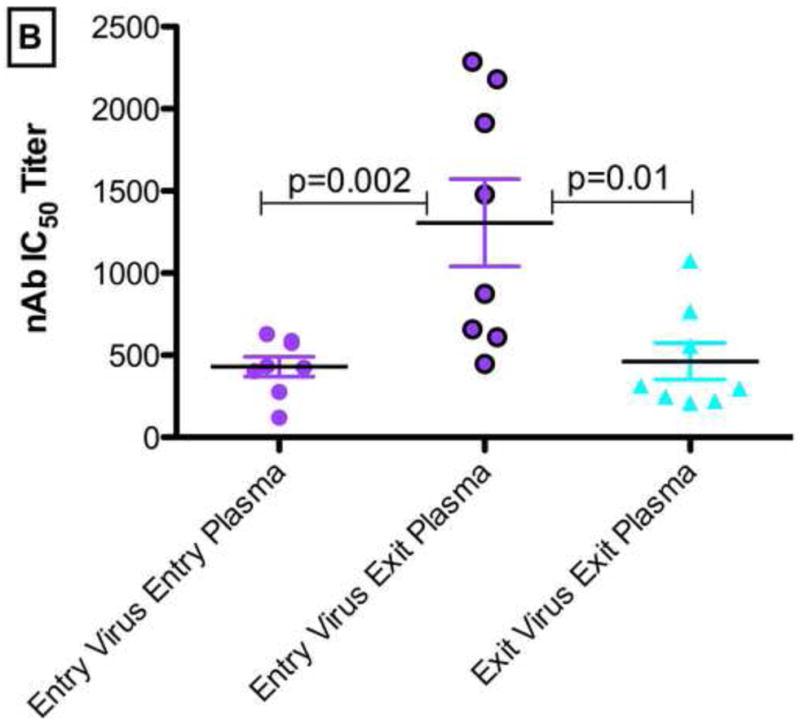
(A). Schematic illustrating the autologous neutralizing antibody challenge assays in all participants over a median of 21 months from study entry to study exit. The average nAb IC50 titers for study entry and exit Envs used in the assays are shown. The study entry Envs were tested against nAbs from the study entry (contemporaneous) and study exit plasma samples. Likewise the study exit Envs were tested against nAbs from the study entry and study exit (contemporaneous) plasma samples. Figure 1(B) depicts the autologous nAb IC50 titers in study participant entry and exit plasma samples for all participants. Figure 1(C) and 1(D) shows the CD4 T-cell decline rate and the log viral load changes over time respectively. P-values <0.05 were considered significant. P value was calculated using the two-tailed Mann-Whitney non-parametric test overall. All the p values (p<0.0125) remained statistically significant after Bonferroni adjustment for multiple comparisons.
Plasma IgG Isolation
Total IgGs were purified from the participant's plasma samples using the Melon Gel (Thermo Scientific, Surrey, United Kingdom) method according to the manufacturer's instructions. Briefly, 500 μl of a 1:10 dilution of plasma to buffer was added to the column. The tubes were rotated for 5 minutes in order to ensure maximum capture of the IgGs and were then spun down for 1 minute at 5,000 × g to elute the IgGs. IgG concentrations were measured on the NanoDrop 2000 Spectrophotometer (Thermo Scientific, Surrey, United Kingdom) and stored at 4°C for downstream ELISA assays.
FcGamma (γ) Receptor Binding ELISAs
Binding affinities of the bulk population of IgGs purified from plasma were measured for the various activating- FcγRI, FcγRIIa and FcγRIIIa- (R&D Systems, Minneapolis, USA) and inhibitory receptors - FcγRIIb.
Each ELISA plate (Ni-NTA HisSorb™ Plate Qiagen, Dusseldorf, Germany) was coated with 100 μl/well of diluted FcγR (FcγRI, FcγRIIa, FcγRIIb, and FcγRIIIa) at a concentration of 5 μg/ml using PBS. One receptor type per plate was added and incubated overnight (O/N) at room temperature (RT). The plate was washed three times with PBS-Tween (0.05%) and blocked with 250 μl/well 5% PBS-Bovine Serum Albumin (BSA) (Sigma-Aldrich, St Louis, USA) and then incubated for 1 hour at RT. The plate was washed three times with 0.05% PBS-Tween. 100 μl/well of antibodies were added (the IgGs derived from the test plasma were diluted serially starting at 100 μg/μl diluted down to 1.6 μg/μl) to the respective wells and was left to incubate for 1 hour at RT. The plate was washed three times with 0.05% PBS-Tween. 100 μl per well of anti-human IgG antibody, Fab fragment, peroxidase labeled was added (KPL Protein Research Products, Maryland, USA). 20 μl of HRP Fab (1 mg/ml) was added to 10 mls PBS 1× for one plate. The plate was incubated for 1 hour at RT and covered to protect it from light. The plate was washed three times with 0.05% PBS-Tween. The colour was resolved with 100 μl/well of O-phenylenediamine (OPD) (Sigma-Aldrich, St Louis, USA) - one OPD tablet was added to 11 mls of StrepHRP substrate), the reactions were stopped by adding 100 μl/well of 2.5N sulphuric acid (Sigma-Aldrich, St Louis, USA). The plate was read at 490 nm using the Tecan Sunrise 16039400 plate reader (Tecan Group, Switzerland). The optical density data was exported to Microsoft Excel worksheet to calculate the 50% effective concentration (EC50) of each batch of total IgGs needed to bind HIV-specific gp41, gp120 and p24 antibodies and various FcγR using an ELISA-based assay. EC50 (μg/ml) corresponds to the concentration of antibody needed to achieve half of the maximal binding to FcγR. The EC50 binding titer of the IgGs was used as a surrogate indicator of how strongly or poorly the IgGs' Fc portion may bind to the FcγR to recruit effector cells like phagocytic (macrophages) or non-phagocytic (natural killer cells) cells to initiate either ADCVI or ADCC activity. Essentially, the higher the EC50 the more antibody is needed for antiviral activity like ADCC or ADCVI to occur, the lower the affinity of antibody binding to the respective target cells bearing the antigens e.g. the FcγRs to antibodies to initiate effector functions.
Gp120, gp41, and p24 Binding ELISAs
Binding affinities of the bulk population of IgGs purified from plasma were measured for the various HIV-1 specific antigens- gp120, gp41 and p24. ELISA plates were coated with 80 μl/well of 250ng/ml gp120 (Immune Technology, New Jersey, USA) or gp41 (Immune Technology, New Jersey, USA) or p24 (Immune Technology, New Jersey, USA) and then incubated O/N at 4°C. The plates were washed three times with PBS Tween 0.05% and blocked with 5% PBS-BSA for 2 hours at RT (100 μl/well). IgGs isolated previously were diluted in PBS and added to respective wells according to a ten times serial dilution to respective wells and incubated for 2 hours at RT. The plates were washed three times with 0.05% PBS Tween. 100 μl anti-human IgG antibody, Fab fragment, peroxidase labeled was added to each well (KPL Protein Research Products, Maryland, USA) and left for 1 hour at RT (1:500 diluted-per plate: 20 μl HRP in 10 ml PBS) and covered in tinfoil. The plate was washed 3 times with 0.05% PBS-Tween. One OPD tablet was added to 11 mls of phosphate citrate buffer + 4.4 μl Hydrogen Peroxide (H2O2); and 50 μl/well were added. The reaction was stopped by adding 50 μl 2.5 N H2SO4 per well. The plate was read at 490 nm on a microplate reader and the data analyzed using GraphPad Prism 5 software.
Statistical analyses
Comparisons of different parameters including nAb IC50 titers for both the autologous and heterologous responses, and binding affinities of the various HIV-specific antigens and FcγRs to IgGs was performed. Intra-group comparisons between study entry and study exit time-points were done using the Mann-Whitney U test and the Spearman rank correlation test. ANOVA was used to compare three or more groups and the Dunn's post-test was used to perform pairwise comparisons. A general linear model was used to estimate the rate of change in viral load and CD4 T-cell count over time. Viral load was log-transformed to ensure normality. All reported p values are two-sided and are considered significant if less than 0.05. The statistical analysis was performed using GraphPad Prism 5 and SAS version 9.3 (SAS Institute Inc., Cary).
Results
Study Participants
There were eight participants in this study, seven female and one male. Table 1 depicts the clinical profiles of the participants for this retrospective study. All participants were of African descent. All participants were antiretroviral treatment naïve for a median of 21 months. The median age was 31 years (range: 22-59 years). The median CD4 T-cell count at study entry was 578 cells/μl (range: 503-936 cells/μl) and at study exit was 369 cells/μl (range: 218-881 cells/μl). The median viral load at study entry was 6,140 copies/ml (range: 2,210-14,360 copies/ml) and at study exit was 14,750 (range: 2,440-345,000 copies/ml). The mean CD4 T-cell decline rate was −7 cells/μl per month (standard error 1.50 cells/μl). Since data on the length of time that participants were infected was unavailable, sequence analysis was performed by using the Bayesian evolutionary analysis by sampling trees (BEAST) to estimate the approximate time of infection in both groups of participants. Participants were estimated to be infected for a mean period of 5.1 years (range: 9 months-15 years).
Table 1.
Clinical description of study participants.
| Patient Identification | Sex | Age at Baseline | Viral Load study entry (copies/ml) | Viral Load study exit (copies/ml) | CD4 T-cell count study entry (cells/μl) | CD4 T-cell count study exit (cells/μl) | CD4 T-cell decline rate (cells/μl/ per month) |
|---|---|---|---|---|---|---|---|
| SK010 | F | 31 | 6,480 | 345,000 | 649 | 268 | −10 |
| SK035 | F | 31 | 5,800 | 2,950 | 680 | 322 | −7 |
| SK036 | M | 32 | 5,100 | 10,600 | 936 | 575 | −7 |
| SK169 | F | 30 | 2,210 | 2,440 | 561 | 437 | −4 |
| SK200 | F | 40 | 14,360 | 24,900 | 595 | 416 | −7 |
| SK221 | F | 30 | 9,740 | 23,700 | 503 | 297 | −3 |
| SK233 | F | 22 | 11,800 | 18,900 | 547 | 218 | −8 |
| SK312 | F | 59 | 3,460 | 3,630 | 545 | 881 | −9 |
Autologous Neutralizing Antibody Responses become potent over time in chronic infection
To better understand autologous neutralization during chronic infection we tested the participants' plasma against their plasma-derived Envs using a pseudovirus-based assay (see Figure 1A). The titers represented on Figure 1B were the average nAbIC50 titers of two or three Envs per participant.
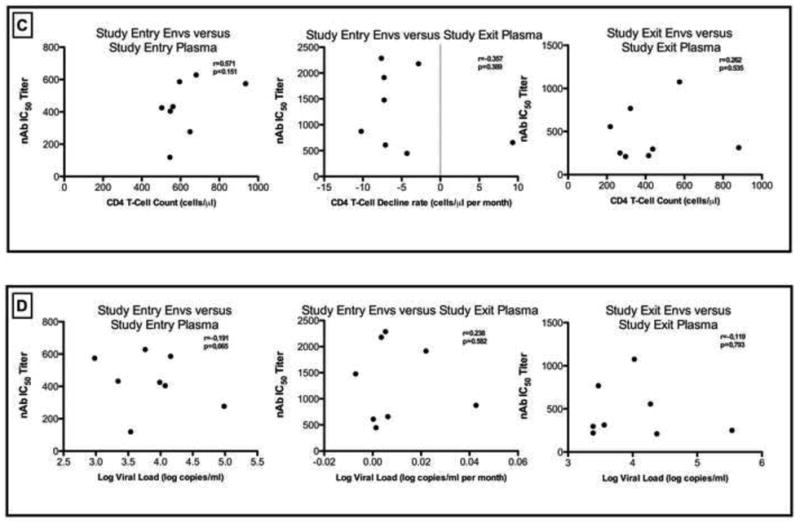
NAb IC50 titers for the study entry and study exit Envs differed when the study entry and study exit plasma antibody responses were compared. As shown in Figure 1B, there were significant differences when the study entry Envs were tested against the study exit plasma (median nAb IC50 of 1,178; range: 610-2,286) compared to the contemporaneous responses at study entry (median nAb IC50 of 429; range: 119-628; p=0.002) and study exit (median nAb IC50 of 305; range: 210-1,076; p=0.01). There were no correlations between the rates of CD4 T-cell decline or viral loads with for the nAb IC50 titers of study entry Envs against the study exit plasma or between contemporaneous nAb IC50 titers at study entry or study exit- Figure 1C and1D.
Autologous neutralization titers correlated with the length of the hypervariable regions V1-V2
Previous studies have shown an inverse association between neutralizing antibody titers and length of variable loops (V1-V2) and numbers of potential N-linked glycosylation sites (PNGs) (Chackerian et al., 1997; Gray et al., 2007; Pinter et al., 2004; Rong et al., 2007; Sagar et al., 2006). To better understand the relationship between genotypic characteristics and neutralization sensitivity, the average amino acid length of the hypervariable loops of Env and PNGs were correlated to average autologous nAb IC50 titers (Figure 2). Significant inverse correlation was found between the lengths of V1-V2 and nAb IC50 titers (r=−0.80; p=0.02) for the entry viruses tested against the study exit plasma. There was a trend towards C2-V5 length correlation with nAb IC50 titers (r=−0.67; p=0.08) and no significant correlations for V1-V5 (r=0.52; p=0.2). The same analysis was extended for nAb IC50 titers and the numbers of potential N-linked glycosylation sites (PNGs) in the various hypervariable loops and there were no significant correlations observed.
Figure 2.
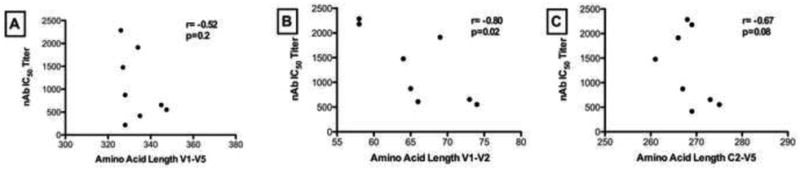
Correlation between the amino acid length of (A) V1-V5, (B) V1-V2 and (C) C2-V5 of Env and autologous neutralization titers (nAb IC50 titer) when study entry viruses were tested against study exit plasma samples. The Spearman r-coefficient and p-values are shown and a correlation was considered significant when p<0.05.
Neutralization breadth scores and potency scores indicate subtype-specific responses in chronic infection
To assess neutralization breadth in this chronic infection cohort, we investigated changes in plasma neutralization activity over a median of 21 months. Figure 3 depicts the profile of plasma neutralizing activity against a panel of heterologous Envs for the eight study participants at study entry and study exit. A total of 20 reference pseudoviruses from subtype A, B and C (as depicted in Table 2) were used to determine breadth and potency scores of neutralization according to the criteria previously described (Blish et al., 2008). Results are presented as a breadth score per subtype virus panel, and a total breadth score including all the subtype A, B and C viruses. Results are presented as potency scores per subtype, and total potency scores including all the subtype A, B and C viruses.
Figure 3.
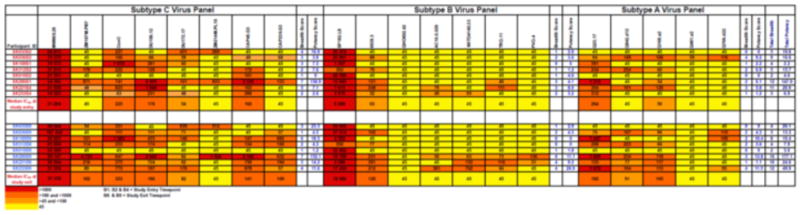
Heterologous responses of participant plasma tested at study entry and study exit against 20 Env pseudoviruses from a standard reference panel including subtypes C, B and A of tier 1A, 1B, tier 2 and 3 categories over a median of 21 months. The neutralization titer is shown as reciprocal plasma dilution required to inhibit 50% of virus infectivity when the virus is challenged with the participant's plasma. The highest titer (>1,000) is shown in red, and the lowest in light orange, yellow depicts a titer of <1:45 that is below detection as shown above.
There was a wide range of variation in neutralization titers with most of the sera from the participants displaying preferential heterologous activity against the subtype C panel (Figure 3). Significantly higher breadth scores were observed against subtype C viruses (median: 3; range: 1-7) compared to subtypes A (median: 2; range: 0-4; p= 0.03) or B (median: 2; range: 0-6; p=0.01) - Figure 3. Likewise, significantly higher potency scores were observed against subtype C viruses (median: 7.6; range: 0-152.1) compared to subtypes A (median: 4; range: 0-13.8; p= 0.03) or B (median: 3.9; range: 0-26.9; p=0.05). Three of the participants (SK200, SK221 and SK233) showed a consistent increase of neutralization breadth scores and potency scores from study entry to study exit for subtypes C, B and A respectively translating into overall increase in total breadth and potency scores as well. Analysis of these participants indicated significantly higher viral load at study entry compared to study exit (p=0.04); suggesting that increasing breadth may be a result of higher antigenic stimulation.
SK200 displayed the highest breadth scores and also had potent nAb IC50 scores (Figure 3) to six of the eight subtype C viruses tested including five of six tier 2 viruses. SK200 had a total neutralization breadth score of 16 (range: 2-16) and the overall potency score was 177.1 (range: 3-177.1) at study exit. SK200 neutralized Subtype C- tier 2 viruses -CAP45.G3 and CAP239.G3 at study entry and exit plasmas, and CAP45.G3 in particular, was neutralized at nAb IC50 titers of >2,000 (range: 45-5,326). SK200 also displayed the highest titers >1,000 at study entry (range: 45-1,316) and study exit (range: 45-1,829) to a subtype A tier 1 virus- Q23ENV17.
CD4 T-cell count and viral load are not correlated with neutralization breadth
A number of studies have shown an inverse relationship between neutralization breadth and CD4 T-cell counts and a positive correlation with viral loads (Euler et al., 2010; Gray et al., 2011; Piantadosi et al., 2009; Sather et al., 2009). To better understand the relationship between neutralization and clinical disease markers in this subtype C chronic cohort, we investigated the association of CD4 T-cell counts and viral loads with neutralization breadth or potency over a median of 21 months. However, there were no significant correlations between CD4 T-cell counts or viral loads and total breadth and potency scores (data not shown). A larger sample size may have been able to detect significant correlations between breadth or potency and markers of disease progression.
Mapping of potential epitopes targeted by cross-neutralizing antibodies
The putative targets of neutralization in participants displaying the highest breadth scores for neutralization at study exit (SK200, SK221 and SK233) against the reference panel viruses were further investigated (using study exit plasma samples). Single point mutations in V2 (N160A, K165E or L165A) and C3 (N332A) were introduced into CAP45.G3, ConC, Du156.12, TRO.11 and Q23.17 and tested for loss of sensitivity against the three plasmas.
As depicted in Table 3, 5.6-fold and 4-fold drops in neutralization titers (relative to the wild type neutralization titers) were seen when plasma samples from SK221 and SK233, respectively, were tested against the K169E mutation in the CAP45 backbone. This suggested that the broadly neutralizing antibodies in these two participants targeted the V2 region and that a charge change from a lysine (K) to glutamic acid (E) at position 169 resulted in a disruption to the nAb epitope. In addition SK221 plasma also showed a 4.2 fold drop in neutralization titers to N332A made in the TRO.11 envelope indicating that the asparagine (N), or perhaps a potential N-linked glycosylation at this position, was essential for the antibody activity. Together, these results suggested that SK221 most likely had cross-neutralizing antibodies that target the V2 and C3 regions on Env. SK200, who displayed the highest neutralization breadth score, neutralized the wild-type and mutant viruses equivalently suggesting that this particular participant may have antibodies directed to other regions of Env, a profile that warrants further investigation.
Table 3. Effects of single point mutations on neutralization sensitivity and summary of antibody specificities.
| Plasma sample identity | Fold effect of mutationa | Antibody specificity conferring breadth | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ConC N160A | ConC I165A | ConC K169E | CAP45 N160A | CAP45 I165A | CAP45.G3 K169E | Du156.12 N332A | Q23.17 N332A | Tro.11 N332A | ||
| SK200 | 1.1 | 0.5 | 0.7 | 1.0 | 0.6 | 1.9 | 1.2 | 1.5 | 0 | Unknown |
| study exit SK221 | 0.4 | 0.3 | 1.1 | 2.0 | 0.4 | 5.6 | 1.4 | 0.6 | 4.2 | Quaternary, PG9/PG16 like, N332 |
| study exit SK233 study exit | 0.1 | 0 | 0.6 | 0.8 | 0.9 | 4 | 0.7 | 1.2 | 0 | Quaternary, PG9/PG16 like |
Calculated as wild type IC50/mutant IC50 for the plasma. Changes in titer of >3 fold are shown in bold.
HIV-specific IgG binding titers for gp41, gp120 or p24 in chronic infection over 21 months
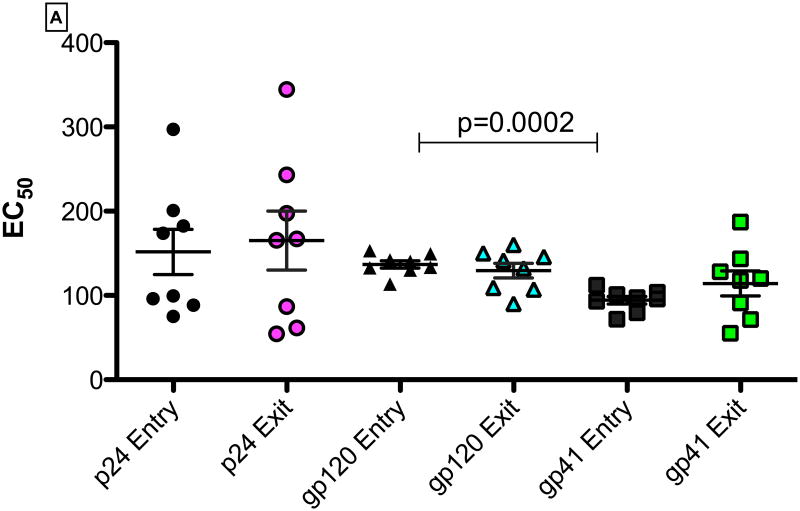
We isolated total immunoglobulins (IgGs) from study participants at study entry and study exit time points and measured the specificity and the binding affinity of IgGs to HIV-1 specific antigens. There were no significant differences in the median EC50 binding affinities between study entry and study exit for gp41, gp120 or p24. Interestingly, at study entry, the median IgG binding affinities for gp120 (median: 137; range: 11.3-153.0) was significantly lower than for gp41 (median: 96.8; range: 71.9-112.1; p=0.0002). Binding affinity for gp120 compared to p24 trended to be higher (median: 154.3; range: 57.9-293.9; p=0.09)- Figure 4. These findings may suggest that binding IgGs were selectively binding gp41compared to gp120 during the chronic infection stage. In addition, there were no significant correlations between the CD4 T-cell counts or viral loads at study entry or exit with the EC50 of p24, gp120 or gp41 over the study period.
Figure 4.
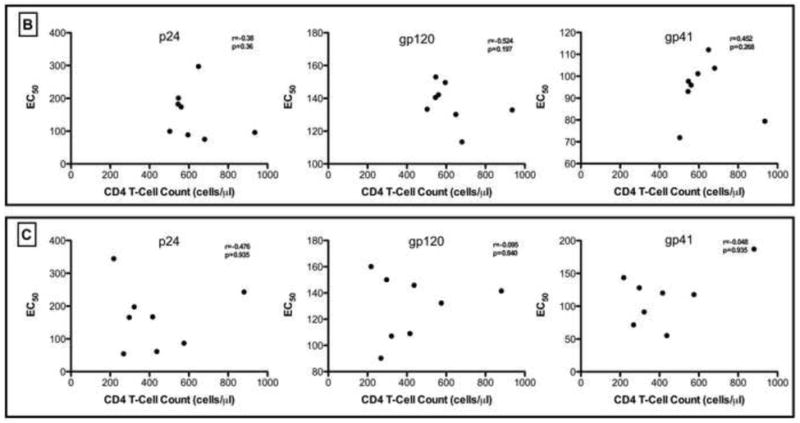
(A). IgG EC50 binding titers for gp41, gp120 and p24-specific antibodies at study entry and exit time points. Panels for Figure 4(B) and (C) show the correlation of EC50 binding titers for gp41, gp120 and p24-specific antibodies with CD4-T cell counts at study entry and study exit respectively. None of the correlations were statistically significant (p< 0.05).
IgG binding affinities differ for the various FcγR I, IIa, IIb and IIIa
To determine if the binding affinities of the IgGs with various activating and inhibitory FcγRs were different, we measured and compared the EC50 binding titers over time between study entry and study exit time-points, and overall (Figure 5A). IgG binding for FcγRI at study entry was significantly higher than for FcγRIIIa (p<0.0005). Likewise at study exit, the binding affinity of IgG for the FcγRI were consistently higher compared with FcγRIIb (p<0.05) and FcγRIIIa (p<0.0005). Significantly higher binding affinities for FcγRIIa at study entry were noted when compared to FcγRIIIa at study entry (p<0.05). Likewise at study exit, the binding affinity of IgG for the FcγRIIa was higher compared to FcγRIIIa (p<0.05) in chronic HIV-1 C disease. Collectively, these results indicated that there was differential kinetics of binding affinities of the IgG during chronic infection, which may then impact downstream non-neutralizing activity through ADCC. Sustained high affinity IgG binding to FcγRI may be preserved during chronic infection. There were no significant correlations between viral loads or CD4 T-cell counts with the EC50 IgG binding titers to the various receptors (FcγR I, IIa, IIb and IIIa)- Figures 5B and 5C.
Figure 5.
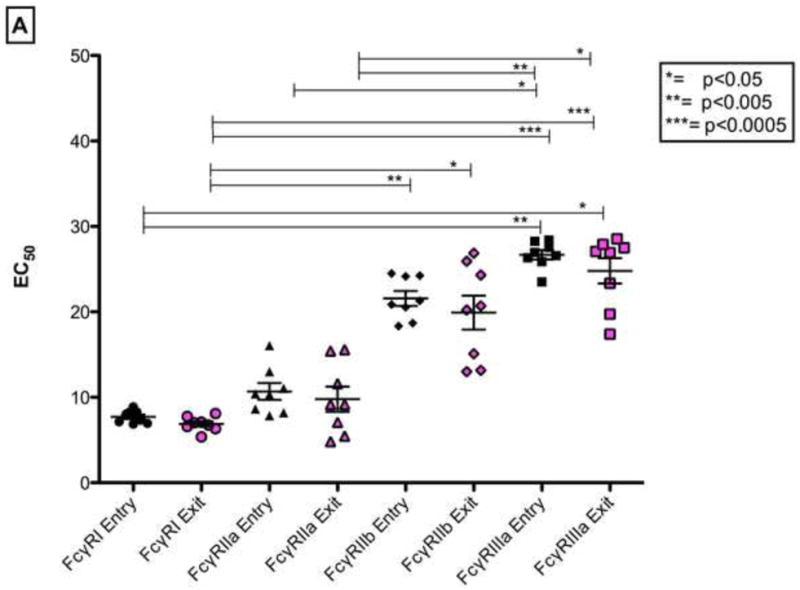
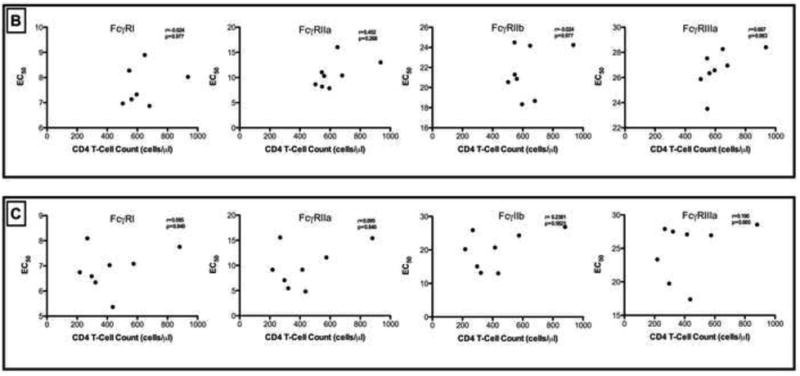
(A). EC50 binding titers of IgGs for FcγRI, FcγRIIa, FcγRIIb and FcγRIIIa at study entry and exit time points. Intra- or inter-group comparisons were statistically different i.e. p< 0.05. Figure 5(B) and (C). Correlation of CD4 T-cell counts at study entry and study exit with FcγRI, FcγRIIa, FcγRIIb and FcγRIIIa (EC50) are shown. Only p-values <0.05 are shown.
Discussion
Neutralizing and binding antibodies may play a role in HIV disease progression but these parameters have rarely been investigated concurrently. We therefore undertook this study in individuals chronically infected with HIV-1 subtype C, the most abundant subtype worldwide, to characterize neutralizing and binding antibody patterns in chronic disease, and to determine whether certain genotypic env characteristics are associated with autologous or heterologous nAb responses. Our data indicated that nAbs did not appear to protect against disease progression, rather, greater type-specific neutralization breadth and potency against subtype C Envs and increasing autologous nAb titers were associated with chronic disease progression. NAb IC50 titers were correlated with env genotypic characteristics, including increased amino acid length in hypervariable regions V1-V2 of gp120.
In this study, nAb potency or breadth did not predict disease progression rate in individuals with chronic HIV-1 subtype C infection. However, a number of interesting findings were apparent. Firstly, high-level neutralizing titers to contemporaneous autologous virus were not observed in most of the participants. Instead, we observed significantly higher autologous responses over time (when the study entry viruses were tested against the study exit plasma) compared to contemporaneous responses, which suggests that nAb are continuously evolving during chronic infection. These findings also argue that an increased nAb titer per se is not effective at attenuating disease progression but rather is a marker of disease progression. Secondly, autologous nAb IC50 titers correlated inversely with longer amino acid length for V1-V2 length indicating that increased length in this domain may be masking key neutralization epitopes. Indeed, evidence for V2 dependent epitopes was observed in SK221 and SK233. This data suggests that V1-V2 of Env may be persistently targeted by nAb in the chronic infection stage. Alternatively, there may be other intrinsic genetic differences of env that dictate neutralization potency and breadth that need to be further defined. Lastly, the dynamic nature of IgG binding affinity to various FcγRs and the selective high affinity binding to HIV-1 gp41 may be indicative of chronic disease progression.
The selective increase in neutralization breadth over time suggests that this parameter could be a marker of disease progression in chronic subtype C infection (Euler et al., 2010). Neutralization breadth did not significantly correlate with markers of disease progression (viral loads and CD4 T-cell counts in this chronic HIV-1C infection cohort, although the small sample size and short time-frame may have severely limited our statistical power to address this issue. Gray and colleagues (2011) found significant correlations between CD4 T-cell count and viral load with neutralization breadth at six months post-infection only and not at later time points (Gray et al., 2011). These observations suggest that higher antigenic stimulation may dictate the breadth of heterologous antibody responses (Doria-Rose et al., 2010; Fraser et al., 2007; Goujard et al., 2006; Mellors et al., 1997; Piantadosi et al., 2009; Sather et al., 2009). Although increased neutralization antibody breadth may not be protective against disease progression, they may be effective against super-infection as has been suggested by some studies (Smith et al, 2006 and Deeks et al, 2006), although conflicting data exists (Blish et al., 2008). Together, these studies have implications for HIV vaccine design, as vaccine immunogens may need to be given over long periods of time to stimulate the B cell response, and to facilitate affinity maturation which appears to be necessary for antibodies to acquire cross-neutralizing activity (Pancera et al., 2010; Scheid et al., 2009). For the participants who displayed potent cross-neutralizing antibody responses, their nAbs likely targeted quaternary V2 epitopes similar to PG9/PG16-like antibodies (Moore et al., 2011). In addition, nAbs that targeted the N332 glycan in C3 suggest that the asparagine in that position is essential for neutralization activity. In subtype C infection, the V1-V2 and C3 regions are the immunodominant regions commonly targeted by AnAbs particularly during the early stage of infection (Lynch et al., 2011; Moore et al., 2008; Moore et al., 2009; Rong et al., 2009). Our results indicate the V1-V2 and C3 regions remain immunodominant and the focus of the nAb response resulting in broadly cross-neutralizing antibodies even during chronic progressive HIV-1 disease.
A decline of p24 antibody titers as disease progresses has previously been reported (Allain et al., 1987; Binley et al., 1997; Forster et al., 1987; Lange et al., 1986), and it is therefore not surprising that the p24-specific IgGs may lose their affinity over time. Chargelegue and colleagues found that low p24 IgG affinity correlated with HIV-1 disease progression (Chargelegue et al., 1995). Low affinity IgG HIV-1 specific binding antibodies may be indicative of chronic disease progression. It is plausible that a relative decrease in the binding affinity for activating FcγRIIa and FcγRIIIa may be consequent to changes in the Fc portion of the IgGs which do not affect FcγRI binding. Polymorphisms in the FcγRIIa (Forthal and Moog, 2009), and the extent of glycosylation of the antibodies (Forthal et al., 2010) affect binding affinity. Additionally, a decrease in binding affinity of FcγRIIa was associated with dysfunction in the expression of these receptors on the cells of the innate immune system (Dugast et al., 2011). Dugast and colleagues purport that HIV infection is associated with a number of changes in FcR expression on phagocytic cells that are associated with changes in their ability to respond to antibody-opsonized targets, leading to a failure in viral clearance in chronic infection (Dugast et al., 2011). We found a consistently high affinity binding of IgG to FcγRI over time which may suggest that the non-neutralizing pathway augmenting effector cell activity may be maintained during the chronic infection stage. However, in the absence of a functional assay, our data do not provide definitive answers on whether high affinity binding IgG to FcγRI is biologically significant in chronic infection and what the profiles of the various FcγR-IgG binding were during the initial HIV-1 infection stages.
Some limitations of our study are worth noting. Firstly, we only tested a limited number of viral clones from each patient, and therefore may have biased the results of the study to the cloned variants only. Secondly, we do not know the exact time of infection for the study participants and relied on short-term median (21 months) follow-up immunological data, which may be an unrepresentative snap-shot of the entire natural history of disease progression for these participants. Thirdly, the small sample size meant that our study was underpowered to reach definitive conclusions regarding the significance of breadth of neutralization and autologous responses in attenuating disease. Fourthly, we used binding affinity assays as an indication of the strength of affinity of the Fc portion of IgG to the FcγRs which may affect downstream in vivo effector cell activity. Lastly, the dynamic nature of IgG binding affinity to various FcγRs and the selective high affinity binding to HIV-1 gp41 may be indicative of differences and evolution of the antibody mediated innate antiviral immune response in progressive HIV-1 subtype C infection, although this will require additional functional assays to prove.
Despite these caveats, the findings suggest that an ongoing de novo nAb response does not directly protect against disease progression during chronic HIV-1 subtype C infection, corroborating data from previous studies. Our results are also consistent with data from non-human primate studies showing that high titer neutralizing antibody titers are not found in sooty mangabeys (Li et al., 2010) suggesting that autologous nAb are not part of the protection against disease progression in HIV and SIV infections. Furthermore, overall nAb breadth increased over time in most subjects, regardless of their disease status. The mechanism by which nAb breadth increased in these subjects is of interest. Thus, it will be important to determine which Env epitopes in chronically infected individuals elicit broadly neutralizing antibodies and whether these provide any clinical benefit to the patient. In addition, animal studies suggest that anti-HIV antibodies can protect against HIV-1 infection (Burton et al., 2011; Hessell et al., 2009; Hessell et al., 2010) and vaccine-induced V1-V2 binding antibodies may be able to prevent infection (Haynes et al., 2012). Future studies will need to address whether neutralizing and binding antibodies from HIV infected individuals may be used to halt or reduce HIV acquisition.
Acknowledgments
This work was supported by the Hasso Plattner Foundation and the South African Department of Science and Technology/National Research Foundation Research Chair Initiative. D.A. was supported by the Columbia University-Southern African Fogarty AIDS International Training and Research Programme funded by the Fogarty International Center, NIH (grant#D43TW00231) and NIH R01 AI-58706 to C.A.D.
Footnotes
Competing interests: The author(s) of this paper declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allain JP, Laurian Y, Paul DA, Verroust F, Leuther M, Gazengel C, Senn D, Larrieu MJ, Bosser C. Long-term evaluation of HIV antigen and antibodies to p24 and gp41 in patients with hemophilia. Potential clinical importance. N Engl J Med. 1987;317:1114–1121. doi: 10.1056/NEJM198710293171804. [DOI] [PubMed] [Google Scholar]
- Alsmadi O, Herz R, Murphy E, Pinter A, Tilley SA. A novel antibody-dependent cellular cytotoxicity epitope in gp120 is identified by two monoclonal antibodies isolated from a long-term survivor of human immunodeficiency virus type 1 infection. J Virol. 1997;71:925–933. doi: 10.1128/jvi.71.2.925-933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archary D, Gordon ML, Green TN, Coovadia HM, Goulder PJ, Ndung'u T. HIV-1 subtype C envelope characteristics associated with divergent rates of chronic disease progression. Retrovirology. 2010;7:92. doi: 10.1186/1742-4690-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–2173. [PubMed] [Google Scholar]
- Binley JM, Klasse PJ, Cao Y, Jones I, Markowitz M, Ho DD, Moore JP. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blish CA, Dogan OC, Derby NR, Nguyen MA, Chohan B, Richardson BA, Overbaugh J. Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses. J Virol. 2008;82:12094–12103. doi: 10.1128/JVI.01730-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A. 2011;108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian B, Rudensey LM, Overbaugh J. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J Virol. 1997;71:7719–7727. doi: 10.1128/jvi.71.10.7719-7727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargelegue D, Stanley CM, O'Toole CM, Colvin BT, Steward MW. The affinity of IgG antibodies to gag p24 and p17 in HIV-1-infected patients correlates with disease progression. Clin Exp Immunol. 1995;99:175–181. doi: 10.1111/j.1365-2249.1995.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwart EL, Pan H, Sheppard HW, Wolpert D, Neumann AU, Korber B, Mullins JI. Slower evolution of human immunodeficiency virus type 1 quasispecies during progression to AIDS. J Virol. 1997;71:7498–7508. doi: 10.1128/jvi.71.10.7498-7508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- Doria-Rose NA, Klein RM, Daniels MG, O'Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, Mascola JR, Connors M. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol. 2010;84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugast AS, Tonelli A, Berger CT, Ackerman ME, Sciaranghella G, Liu Q, Sips M, Toth I, Piechocka-Trocha A, Ghebremichael M, Alter G. Decreased Fc receptor expression on innate immune cells is associated with impaired antibody-mediated cellular phagocytic activity in chronically HIV-1 infected individuals. Virology. 2011;415:160–167. doi: 10.1016/j.virol.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza J. The global HIV vaccine enterprise. Int Microbiol. 2005;8:93–101. [PubMed] [Google Scholar]
- Euler Z, van Gils MJ, Bunnik EM, Phung P, Schweighardt B, Wrin T, Schuitemaker H. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J Infect Dis. 2010;201:1045–1053. doi: 10.1086/651144. [DOI] [PubMed] [Google Scholar]
- Forster SM, Osborne LM, Cheingsong-Popov R, Kenny C, Burnell R, Jeffries DJ, Pinching AJ, Harris JR, Weber JN. Decline of anti-p24 antibody precedes antigenaemia as correlate of prognosis in HIV-1 infection. Aids. 1987;1:235–240. [PubMed] [Google Scholar]
- Forthal DN, Gach JS, Landucci G, Jez J, Strasser R, Kunert R, Steinkellner H. Fc-glycosylation influences Fcgamma receptor binding and cell-mediated anti-HIV activity of monoclonal antibody 2G12. J Immunol. 2010;185:6876–6882. doi: 10.4049/jimmunol.1002600. [DOI] [PubMed] [Google Scholar]
- Forthal DN, Moog C. Fc receptor-mediated antiviral antibodies. Curr Opin HIV AIDS. 2009;4:388–393. doi: 10.1097/COH.0b013e32832f0a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci U S A. 2007;104:17441–17446. doi: 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SD, Wrin T, Smith DM, Kosakovsky Pond SL, Liu Y, Paxinos E, Chappey C, Galovich J, Beauchaine J, Petropoulos CJ, Little SJ, Richman DD. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci U S A. 2005;102:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujard C, Bonarek M, Meyer L, Bonnet F, Chaix ML, Deveau C, Sinet M, Galimand J, Delfraissy JF, Venet A, Rouzioux C, Morlat P. CD4 cell count and HIV DNA level are independent predictors of disease progression after primary HIV type 1 infection in untreated patients. Clin Infect Dis. 2006;42:709–715. doi: 10.1086/500213. [DOI] [PubMed] [Google Scholar]
- Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L. The Neutralization Breadth of HIV-1 Develops Incrementally over Four Years and Is Associated with CD4+ T Cell Decline and High Viral Load during Acute Infection. J Virol. 2011;85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ES, Moore PL, Choge IA, Decker JM, Bibollet-Ruche F, Li H, Leseka N, Treurnicht F, Mlisana K, Shaw GM, Karim SS, Williamson C, Morris L. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J Virol. 2007;81:6187–6196. doi: 10.1128/JVI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland RE, Hawkins PA, Salazar-Gonzalez J, Johnson A, Tichacek A, Karita E, Manigart O, Mulenga J, Keele BF, Shaw GM, Hahn BH, Allen SA, Derdeyn CA, Hunter E. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelaar J, Gouws E, Ghys PD, Osmanov S. Global trends in molecular epidemiology of HIV-1 during 2000-2007. Aids. 2011;25:679–689. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, Rathnavalu P, Moore C, Pfafferott KJ, Hilton L, Zimbwa P, Moore S, Allen T, Brander C, Addo MM, Altfeld M, James I, Mallal S, Bunce M, Barber LD, Szinger J, Day C, Klenerman P, Mullins J, Korber B, Coovadia HM, Walker BD, Goulder PJ. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 2004;432:769–775. doi: 10.1038/nature03113. [DOI] [PubMed] [Google Scholar]
- Kothe DL, Li Y, Decker JM, Bibollet-Ruche F, Zammit KP, Salazar MG, Chen Y, Weng Z, Weaver EA, Gao F, Haynes BF, Shaw GM, Korber BT, Hahn BH. Ancestral and consensus envelope immunogens for HIV-1 subtype C. Virology. 2006;352:438–449. doi: 10.1016/j.virol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, Haynes BF, Delfraissy JF. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange JM, Coutinho RA, Krone WJ, Verdonck LF, Danner SA, van der Noordaa J, Goudsmit J. Distinct IgG recognition patterns during progression of subclinical and clinical infection with lymphadenopathy associated virus/human T lymphotropic virus. Br Med J (Clin Res Ed) 1986;292:228–230. doi: 10.1136/bmj.292.6515.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, Vielmetter J, Carmichael DF, Hayes RJ, Dahiyat BI. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Stefano-Cole K, Kuhrt DM, Gordon SN, Else JG, Mulenga J, Allen S, Sodora DL, Silvestri G, Derdeyn CA. Nonpathogenic simian immunodeficiency virus infection of sooty mangabeys is not associated with high levels of autologous neutralizing antibodies. J Virol. 2010;84:6248–6253. doi: 10.1128/JVI.00295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, Mlisana K, Karim SA, Hong K, Greene KM, Bilska M, Zhou J, Allen S, Chomba E, Mulenga J, Vwalika C, Gao F, Zhang M, Korber BT, Hunter E, Hahn BH, Montefiori DC. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J Virol. 2006;80:11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Svehla K, Louder MK, Wycuff D, Phogat S, Tang M, Migueles SA, Wu X, Phogat A, Shaw GM, Connors M, Hoxie J, Mascola JR, Wyatt R. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J Virol. 2009;83:1045–1059. doi: 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch RM, Rong R, Boliar S, Sethi A, Li B, Mulenga J, Allen S, Robinson JE, Gnanakaran S, Derdeyn CA. The B cell response is redundant and highly focused on V1V2 during early subtype C infection in a Zambian seroconverter. J Virol. 2011;85:905–915. doi: 10.1128/JVI.02006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR., Jr Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- Montefiori D. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays. In: Coligan J, editor. Current protocols in immunology. John Wiley & Sons; New York, NY: 2004. pp. 12.11.11–12.11.15. [DOI] [PubMed] [Google Scholar]
- Moore PL, Gray ES, Choge IA, Ranchobe N, Mlisana K, Abdool Karim SS, Williamson C, Morris L. The c3-v4 region is a major target of autologous neutralizing antibodies in human immunodeficiency virus type 1 subtype C infection. J Virol. 2008;82:1860–1869. doi: 10.1128/JVI.02187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Gray ES, Sheward D, Madiga M, Ranchobe N, Lai Z, Honnen WJ, Nonyane M, Tumba N, Hermanus T, Sibeko S, Mlisana K, Abdool Karim SS, Williamson C, Pinter A, Morris L. Potent and broad neutralization of HIV-1 subtype C by plasma antibodies targeting a quaternary epitope including residues in the V2 loop. J Virol. 2011;85:3128–3141. doi: 10.1128/JVI.02658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Ranchobe N, Lambson BE, Gray ES, Cave E, Abrahams MR, Bandawe G, Mlisana K, Abdool Karim SS, Williamson C, Morris L. Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS Pathog. 2009;5:e1000598. doi: 10.1371/journal.ppat.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, Depetris R, Katpally U, Marozsan A, Cupo A, Maloveste S, Liu Y, McBride R, Ito Y, Sanders RW, Ogohara C, Paulson JC, Feizi T, Scanlan CN, Wong CH, Moore JP, Olson WC, Ward AB, Poignard P, Schief WR, Burton DR, Wilson IA. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peressin M, Holl V, Schmidt S, Decoville T, Mirisky D, Lederle A, Delaporte M, Xu K, Aubertin AM, Moog C. HIV-1 replication in Langerhans and interstitial dendritic cells is inhibited by neutralizing and Fc-mediated inhibitory antibodies. J Virol. 2011;85:1077–1085. doi: 10.1128/JVI.01619-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi A, Panteleeff D, Blish CA, Baeten JM, Jaoko W, McClelland RS, Overbaugh J. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J Virol. 2009;83:10269–10274. doi: 10.1128/JVI.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A, Honnen WJ, He Y, Gorny MK, Zolla-Pazner S, Kayman SC. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J Virol. 2004;78:5205–5215. doi: 10.1128/JVI.78.10.5205-5215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther. 2008;7:2517–2527. doi: 10.1158/1535-7163.MCT-08-0201. [DOI] [PubMed] [Google Scholar]
- Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Bibollet-Ruche F, Mulenga J, Allen S, Blackwell JL, Derdeyn CA. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J Virol. 2007;81:1350–1359. doi: 10.1128/JVI.01839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Li B, Lynch RM, Haaland RE, Murphy MK, Mulenga J, Allen SA, Pinter A, Shaw GM, Hunter E, Robinson JE, Gnanakaran S, Derdeyn CA. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog. 2009;5:e1000594. doi: 10.1371/journal.ppat.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar M, Wu X, Lee S, Overbaugh J. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J Virol. 2006;80:9586–9598. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, Fox JA, Presta LG. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- Siberil S, Dutertre CA, Fridman WH, Teillaud JL. FcgammaR: The key to optimize therapeutic antibodies? Crit Rev Oncol Hematol. 2007;62:26–33. doi: 10.1016/j.critrevonc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR, Koff WC. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- UNAIDS. UNAIDS World AIDS Day Report. Geneva, Switzerland: 2011. [Google Scholar]
- Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]