Abstract
Lipoprotein(a) (Lp[a]) is a LDL-like particle consisting of an ApoA moiety linked to one molecule of ApoB100. Recent data from large-scale prospective studies and genetic association studies provide highly suggestive evidence for a potentially causal role of Lp(a) in affecting risk of cardiovascular disease (CVD) in general populations. Patients with Type 2 diabetes display clustered metabolic abnormalities and elevated risk of CVD. Lower plasma Lp(a) levels were observed in diabetic patients in several recent studies. Epidemiology studies of Lp(a) and CVD risk in diabetic patients generated inconsistent results. We recently found that Lp(a)-related genetic markers did not predict CVD in two diabetic cohorts. The current data suggest that Lp(a) may differentially affect cardiovascular risk in diabetic patients and in the general population. More prospective studies, Mendelian randomization analysis and functional studies are needed to clarify the causal relationship of Lp(a) and CVD in diabetic patients.
Keywords: cardiovascular disease, diabetes, lipoprotein(a), Mendelian, randomization, prospective studies
Since the discovery of lipoprotein(a) (Lp[a]) in 1963 by Berg [1], numerous studies have assessed its relationship with cardiovascular diseases (CVDs). Recent data from large-scale meta-analyses and genetic association studies using Mendelian randomization analyses provide highly suggestive evidence for a potentially causal role of Lp(a) in affecting CVD risk in general populations [2–5], promoting the discussion regarding utility of Lp(a) in clinical practice [6–8]. Patients with Type 2 diabetes are featured by clustered metabolic abnormalities and an approximately two- to four-fold increased risk of CVD compared with nondiabetics [9, 10]. It remains unclear as to whether Lp(a) causally affects increased CVD risk in diabetic patients. Interestingly, it was recently found that blood Lp(a) levels might be inversely related to risk of Type 2 diabetes [11, 12], making it more intriguing to understand the role of Lp(a) in the development of CVD, especially among diabetic patients. In this article, we focus on relationships of Lp(a), diabetes and CVD and, in particular, we discuss the issue of Lp(a) as a risk factor for CVD in diabetic patients.
Lp(a) & genetic determinants
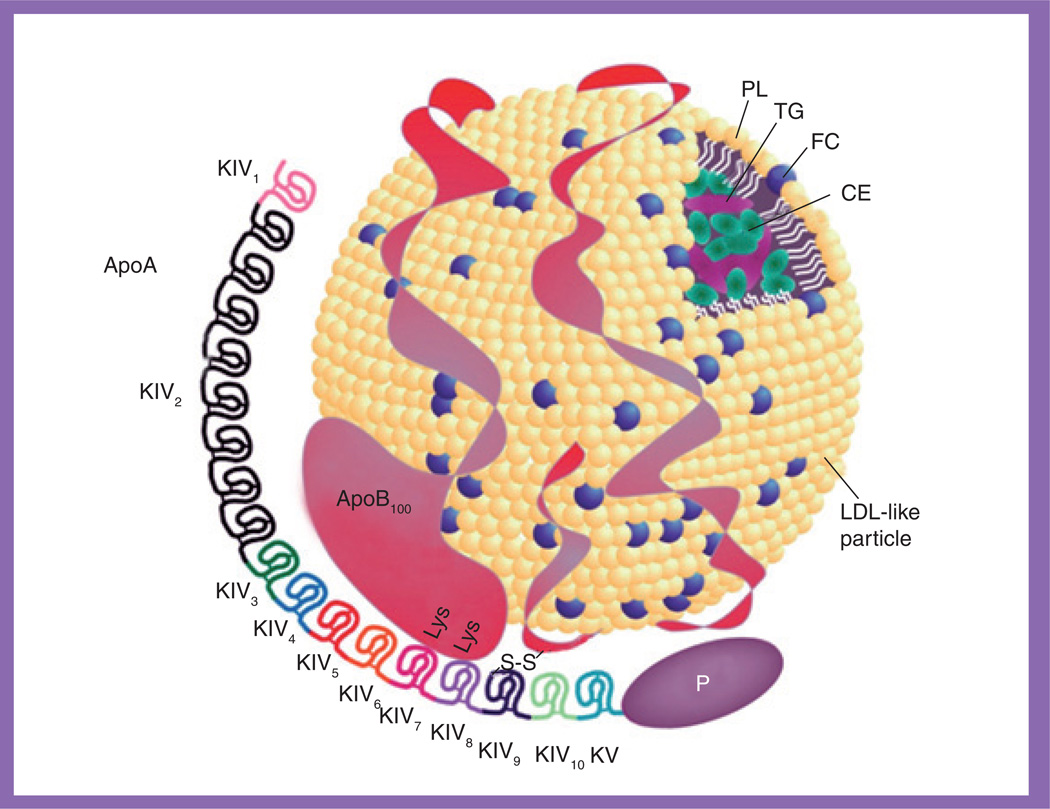
Lp(a) is a LDL-like particle that consists of an ApoA moiety linked to one molecule of apoB via a disulphide bond (Figure 1) [13, 14]. The LDL-like moiety is composed of a central core of cholesteryl esters and triglycerides surrounded by phospholipids, free cholesterol and a single molecule of apoB [13, 14]. Lp(a) is secreted by the liver [15] and circulates in blood, and plasma concentrations are highly heritable and mainly determined by the ApoA gene (LPA), which is located on chromosome 6q26–27 [16, 17]. It has been documented that a copy number variation, the kringle IV type 2 (KIV2) size polymorphism [18], determines the differently sized isoforms of ApoA. A high number of KIV2 repeats is correlated with low plasma levels of Lp(a), which is likely to be the result of the lower rate of production and secretion of the larger isoforms [19, 20]. In addition, several recent genome-wide association studies identified multiple single nucleotide polymorphisms (SNPs) in the chromosome 6q26–27 region associated with plasma Lp(a) levels in both general and diabetic populations [21–23].
Figure 1. Structure of lipoprotein(a).
Lipoprotein(a) is a LDL-like particle that consists of an ApoA moiety linked to one molecule of ApoB100.
CE: Cholesteryl ester; FC: Free cholesterol; KIV: Kringle IV domain; KV: Kringle V domain; PL: Phospholipid; TG: Triglyceride.
Modified with permission from [14].
Lp(a) as a cardiovascular risk factor in general populations
A larger number of studies have investigated the relationship between Lp(a) and CVD risk in general populations. A meta-analysis of 126,634 participants from 36 prospective studies, published in 2009, demonstrated that plasma Lp(a) level is continuously associated with an increased risk of coronary heart disease (CHD) and stoke [2]. Each 3.5-fold higher than usual Lp(a) level was associated with a 13% increased risk of CHD and a 10% increased risk of stroke after adjustment for age, sex, lipids and other conventional risk factors. In addition, a recent meta-analysis of 40 studies reported that smaller ApoA isoforms, which correlated with higher plasma Lp(a) levels, were associated with an approximately twofold increased risk of CVD in general populations [5]. These findings suggest that Lp(a) is an independent but moderate risk factor for CVD in general populations. Concurrently, a genetic-association study examined the association between genetically elevated levels of plasma Lp(a) and CHD risk by using the Mendelian randomization approach based on three pieces of data, including plasma Lp(a) levels, the LPA KIV2 size polymorphism and myocardial infarction (MI) events [3]. It was found that elevated plasma Lp(a) levels were associated with an increased risk of MI, the number of KIV2 repeats was inversely associated with Lp(a) levels (KIV2 size polymorphism explained 20–30% of variation in Lp(a) levels) and a lower number of KIV2 repeats was associated with increased risk of MI. In instrumental variable analysis, the risk ratio for MI for a doubling of genetically elevated Lp(a) levels was 1.22 (95% CI: 1.09–1.37). Later in the same year, a further important study on this topic identified two LPA SNPs in LD with the KIV2 size polymorphism, rs3798220 and rs1045872, to be strongly associated with both an increased level of Lp(a) and an increased risk of CHD, and the association with CHD risk was abolished after adjustment for Lp(a) levels [4]. These associations were confirmed in a recent meta-analysis [24], in which each copy of the risk allele of rs3798220 (minor C-allele) and rs1045872 (minor G-allele) was associated with a 57% (risk ratio: 1.57 [95% CI: 1.38–1.79]) and 42% (risk ratio: 1.42 [95% CI: 1.22–1.65]) increased risk of CHD, respectively. These findings provide evidence of a potentially causal relationship between Lp(a) and CVD because genetic variants are generally independent of behavioral and environmental factors, thus yielding associations that are largely unaffected by confounding and reverse causation.
On the basis of recent evidence from the large-scale epidemiological association studies and Mendelian randomization analyses, it has been widely considered that Lp(a) may causally affect CVD risk in general populations. The European Atherosclerosis Society Consensus Panel recommended screening for elevated Lp(a) in individuals at intermediated or high CVD risk, and a desirable level of below the 80th percentiles (<~50 mg/dl) as a cut-off for global cardiovascular risk was decided on [7]. However, Gudnason and Dube et al. suggested that the clinical practice regarding Lp(a) might still be of limited use because of Lp(a)’s moderate effect on CVD risk and unknown functional mechanisms underlying the association [6, 8].
Lp(a) & diabetes
Results of studies investigating Lp(a) levels in patients with Type 1 diabetes are inconsistent. Several studies have reported that Lp(a) levels were increased in patients with Type 1 diabetes [25–28], while in others, a significant relationship was not observed [29–33]. It is also controversial as to whether Lp(a) levels are related to glycemic control in Type 1 diabetes. Although some studies suggested that Lp(a) levels decreased with improved metabolic and/or glycemic control [25, 26, 33, 34], others reported that improved metabolic control did not change plasma Lp(a) levels in patients with Type 1 diabetes [35, 36]. For example, in the Diabetes Control and Complications Trial [33], there was no difference in plasma Lp(a) levels between 1299 patients with Type 1 diabetes and 2158 nondiabetic controls at baseline. Patients receiving intensive insulin therapy showed lower Lp(a) levels compared with those receiving conventional insulin therapy, but Lp(a) levels was not correlated with glycemic control estimated by HbA1c levels [33].
Similarly, highly conflicting results were reported by studies examining the relationship between Lp(a) and Type 2 diabetes, in which unchanged [32, 37], increased [38] or decreased [39, 40] Lp(a) levels were observed in patients with Type 2 diabetes compared with healthy controls. The cross-sectional analysis of plasma Lp(a) levels from 36 studies showed that Lp(a) levels were 11% (95% CI: 4–17) lower in people with diabetes than in those without [2]. Although the type of diabetes was unknown, it is likely that most of these diabetic patients in the included populations (~90%) were affected by Type 2 diabetes. Lp(a) levels were also inversely associated with fasting insulin, and insulin and glucose levels measured by the 2-h glucose challenge test in both diabetic and nondiabetic subjects [41]. An in vitro study reported that insulin suppressed ApoA production in primary cynomolgus monkey hepatocytes [42]. These data may partly account for the decreased Lp(a) levels in patients with Type 2 diabetes (characterized by hyperinsulinemia and insulin resistance), and increased Lp(a) levels in patients with Type 1 diabetes (characterized by insulin deficiency). However, this hypothesis needs to be tested in further studies.
Recently, Mora et al. reported prospective data on plasma Lp(a) levels and incident Type 2 diabetes (n = 1670) in 26,746 women from the WHS trial, and replicated their findings in 9652 Danish men and women with prevalent diabetes (n = 419) from the CCHS trial [11]. In both the WHS and CCHS trials, Lp(a) levels were significantly lower in diabetes cases than in nondiabetics. In the WHS trial, Lp(a) levels were inversely associated with incident Type 2 diabetes, with fully adjusted hazard ratios (95% CIs) for quintiles 2–5 versus quintile 1 (the lowest) of 0.87 (95% CI: 0.75–1.01), 0.80 (95% CI: 0.68–0.93), 0.88 (95% CI: 0.76–1.02) and 0.78 (95% CI: 0.67–0.91), respectively; p = 0.002. These findings were replicated in a case–control analysis of the CCHS trial. Notably, the authors adjusted for correlates of insulin resistance, such as triglycerides, HDL-cholesterol, HbA1c and CRP, and results were not changed. This is the first prospective study yielding an inverse association between Lp(a) levels and risk of Type 2 diabetes. Of note, such an association is contrary to that with risk of CVD, although it has long been known that these two conditions may share a common soil in etiology [43]. Although it needs to be verified in further studies, this surprising finding suggests a more complicated role of Lp(a) in cardiometabolic diseases than we expected. Mendelian randomization studies using plasma Lp(a)-related genetic variants as instrumental variables (such as rs3798220 and rs1045872) would be useful in testing whether there is a causal association between decreased Lp(a) levels and increased risk of Type 2 diabetes.
Lp(a) & CVD in diabetic patients
Plasma Lp(a) levels & CVD
A number of cross-sectional studies [44–52] and prospective studies [23, 40, 53–57] have investigated the relationship between plasma Lp(a) levels and CVD risk in diabetic patients and reported inconsistent results. Table 1 summarizes conflicting results from prospective studies in diabetic patients. Haffner et al. reported that plasma Lp(a) levels were not associated with CVD in a prospective nested case–control study of 22 patients with Type 1 diabetes and 48 patients with Type 2 diabetes (35 CVD cases and 35 controls) [53]. Similarly, in a prospective study in 449 patients with Type 2 diabetes after a mean of 13 years follow-up, the cumulative probability of CHD or stroke was not significantly different between patients with high Lp(a) levels and those with low Lp(a) levels [55]. By contrast, two prospective studies reported positive associations between Lp(a) levels and risk of CVD in patients with Type 2 diabetes, but the risk ratio for CVD by comparing high and low Lp(a) levels might be overestimated (relative risk [RR]: 7.67 [95% CI: 1.10–53.3] and 6.6 [95% CI: 1.6–26.8]) owing to the small number of cases (n = 8 and 23, respectively) [54, 55]. In 429 consecutive unrelated patients with Type 1 diabetes during the observation period of up to 10.7 years, Kollerits et al. reported Lp(a) levels of >30 mg/dl as a strong and independent predictor of CVD (RR: 2.23 [95% CI: 1.28–3.87]) [57].
Table 1.
Summary of prospective studies addressing the association between circulating lipoprotein(a) levels and cardiovascular disease risk in diabetic patients.
| Study (year) | Country | Participants (n) | Outcomes | Results | Comments | Ref. |
|---|---|---|---|---|---|---|
| Cohorts with only diabetic patients | ||||||
| Haffner et al. (1992) | USA | 70 | 35 CHD deaths | No significant difference in the distribution (p = 0.70) and mean levels (p = 0.78) of Lp(a) between cases and controls; RR not provided | No association | [53] |
| Hiraga et al. (1995) | Japan | 215 | Eight CVD events | For high Lp(a) vs low Lp(a) levels (difference ≥20 mg/dl), the RR of CVD was 7.67 (95% CI: 1.10–53.3; p = 0.0395) | Positive association | [54] |
| Abu-Lebdeh et al. (2001) | USA | 449 | 216 CHD and 115 stroke events | For high Lp(a) vs low Lp(a) levels, there was no significant difference in the cumulative probability of CHD (p = 0.19) or stroke (p = 0.44); RR not provided | No association | [55] |
| Hernández et al. (2005) | Spain | 100 | 23 CVD deaths | For Lp(a) ≥20 vs <20 mg/dl, the RR of CVD death was 6.6 (95% CI: 1.6–26.8; p = 0.008) | Positive association | [56] |
| Kollerits et al. (2006) | Austria | 429 | 60 CVD events | For Lp(a) >30 vs ≤30 mg/dl, the RR of CVD was 2.23 (95% CI: 1.28–3.87; p = 0.004) | Positive association | [57] |
| Qi et al. (2012)† | USA | 1686 | 561 CVD events | For 1-SD higher log-transformed Lp(a), the RRs of CHD, CVD and CVD death were 1.05 (95% CI: 0.95–1.15; p = 0.35), 1.05 (95% CI: 0.96–1.15; p = 0.32) and 1.21 (95% CI: 0.99–1.47; p = 0.06), respectively | No association | [23] |
| Cohorts or general populations with both diabetic & nondiabetic individuals | ||||||
| Saely et al. (2006) | Austria | 136 diabetics; 451 nondiabetics | 42 CVD events in diabetics | For 1-SD higher log-transformed Lp(a), the RRs of CVD were 0.81 (95% CI: 0.54–1.22; p = 0.32) in diabetic patients and 1.46 (95% CI: 1.12–1.90; p = 0.005) in nondiabetic individuals (p = 0.008) | No association | [40] |
| Kamstrup et al. (2008) | Denmark | 375 diabetics (4% of overall population) | 103 CHD events in diabetics | For 10 mg/dl-higher Lp(a), the RRs of CHD were 1.08 (95% CI: 1.01–1.16) in diabetic patients and 1.05 (95% CI: 1.03–1.07) in nondiabetic individuals (p = 0.25) | Positive association | [59] |
| Erqou et al. (2009) | Meta-analysis in general populations | 6372 diabetics (6% of overall population) | 942 CHD events in diabetics | For 1-SD higher log-transformed Lp(a), the RRs of CHD were 1.09 (95% CI: 1.01–1.18) in diabetic patients and 1.14 (95% CI: 1.09–1.19) in nondiabetic individuals (p = 0.79) | Positive association | [2] |
This is an updated analysis expanding a previous study conducted by Shai et al. [57], which is not included in this table.
CHD: Coronary heart disease; CVD: Cardiovascular disease; Lp(a): Lipoprotein(a); RR: Relative risk; SD: Standard deviation.
The most recent prospective analysis was conducted in 926 women and 760 men with Type 2 diabetes from the NHS and HPFS trials [23]. In the two cohorts of women (16-year follow-up) and men (12-year follow-up) with Type 2 diabetes, there was a marginal association between plasma Lp(a) levels and CVD incidence and mortality in age-adjusted models (RRs per 1-standard deviation higher log-transformed Lp(a) levels for CHD, CVD and CVD death: 1.08 [95% CI: 0.98–1.19], 1.08 [95% CI: 0.98–1.18] and 1.17 [95% CI: 0.98–1.40], respectively). After further adjustment for lipids and other conventional risk factors, the RRs for CHD and CVD were reduced to 1.05 (95% CI: 0.95–1.15) and 1.05 (95% CI: 0.96–1.15), respectively, while the RR for CVD death slightly increased to 1.21 (95% CI: 0.99–1.47). This is the largest prospective study in diabetic patients to date, expanding a previous study by having longer follow-up and more CVD events [58]. Shai et al. previously reported a borderline association between Lp(a) levels and CHD (p = 0.035) in diabetic women from the NHS [58].
Several studies have compared the association between Lp(a) levels and CVD risk in diabetic and nondiabetic populations. In 587 consecutive patients undergoing coronary angiography (including 136 patients with Type 2 diabetes), Saely et al. found that Lp(a) was a strong and independent predictor of CVD events in nondiabetic individuals (RR per 1-standard deviation higher log-transformed Lp(a) levels: 1.461 [95% CI: 1.121–1.904]), but not in diabetic patients (RR: 0.812 [95% CI: 0.539–1.223]) [40], and there was a significant difference in the association of Lp(a) with CVD risk between nondiabetic individuals and diabetic patients (p = 0.008). However, a recent 10-year follow-up prospective study in a general population (n = 9330; 375 diabetics) reported that there was no significant difference in the association between Lp(a) and CHD stratified by diabetes (RR: 1.08 [95% CI: 1.01–1.16] in diabetic patients and 1.05 [95% CI: 1.03–1.07] in nondiabetic individuals; p = 0.25) [59]. In the earlier-mentioned meta-analysis data from general populations [2], the RRs for CHD also did not vary significantly due to diabetes status (p = 0.79), but the RR for CHD in people with diabetes was slightly smaller than that in those without diabetes (RR: 1.09 [95% CI: 1.01–1.18] vs 1.14 [95% CI: 1.09–1.19]).
In summary, most published prospective studies of plasma Lp(a) levels and CVD risk in diabetic patients are limited by their relatively small sample sizes, and the results are inconclusive. Although meta-analysis in general populations suggested that there was no significant difference in the association between Lp(a) and CHD stratified by diabetes status, larger prospective studies in clearly defined diabetic populations are required to evaluate the effect of Lp(a) on CVD risk in diabetic patients.
Genetic & Mendelian randomization analyses
Despite that a large body evidence has shown that Lp(a)-related genetic variants including KIV2 size polymorphism and SNPs are associated with CVD risk in general populations [3, 4, 22, 24, 60, 61], evidence of associations between Lp(a)-related genetic variants and CVD risk in diabetic patients is sparse. Recently, the authors conducted a genome-wide association study for plasma Lp(a) levels in 2308 patients with Type 2 diabetes and identified six SNPs in a region encompassing LPA, PLG, SLC22A3 and LPAL2 genes on chromosome 6q26–27 that were independently associated with Lp(a) levels [23]. Consistent with the results in the general populations [4], the SNP rs10455872 in LPA showed the strongest association with Lp(a), with a similar effect size (1.28 vs 1.08 for 1-standard deviation log-transformed Lp[a]) on plasma Lp(a) levels in the diabetic and general populations (Table 2). However, this SNP was not associated with risk of CHD, CVD or CVD death in diabetic patients [23]. The association between SNP rs10455872 and CHD risk showed significant heterogeneity (p = 0.006) between the diabetic patients (odds ratio: 0.94; 95% CI: 0.69–1.28) [23] and general populations (odds ratio: 1.47; 95% CI: 1.35–1.60) [4]. It should be noted that the SNP rs10455872 accounted for a much smaller proportion of the variation in plasma Lp(a) levels in diabetic patients (~3–4%) compared with the general populations (~25%) [4, 23]. In order to test whether the relatively small proportion of the variation in plasma Lp(a) levels explained by this SNP may account for the null association with CVD risk in the diabetic population, a genotype score calculated based on the six SNPs that jointly explained approximately 20% variation of plasma Lp(a) levels was used. Similarly, the genotype score was not associated with CVD risk in diabetic patients [23]. These data regarding Lp(a)-related genetic variants, together with inconsistent results of plasma Lp(a) and CVD risk, from the diabetic patients suggest that Lp(a) may contribute less to cardiovascular risk in diabetic patients than in the general population. However, given the moderate size of the genetic effect, studies with larger sample sizes are needed to provide further verification.
Table 2.
Single nucleotide polymorphism rs10455872, plasma lipoprotein(a) levels and risk of coronary heart disease in the diabetic and general populations.
| Populations | Risk allele frequency |
Plasma Lp(a) levels (mg/dl) | Risk of CHD | Ref. | |||
|---|---|---|---|---|---|---|---|
| β (95% CI)† | p-value |
Variance explained (%) |
Odds ratio (95% CI) |
p-value | |||
| Diabetic patients | 0.07 | 1.28 (1.08–1.48) | 4.6 × 10−39 | 3–4 | 0.94 (0.69–1.28) | 0.36 | [23] |
| General populations | 0.07 | 1.08 (1.02–1.13) | 3.6 × 10−166 | 25 | 1.47 (1.35–1.60) | 3.6 × 10−15 | [4] |
| ‡p-value for heterogeneity | – | – | 0.06 | – | – | 0.006 | – |
Regression coefficients were estimated as each one copy effect of a risk allele (G-allele) for 1-standard deviation log-transformed Lp(a).
The p-value was tested for heterogeneity in the association of single nucleotide polymorphism rs10455872 with plasma Lp(a) levels and risk of CHD between the diabetic and general populations.
CHD: Coronary heart disease; Lp(a): Lipoprotein(a).
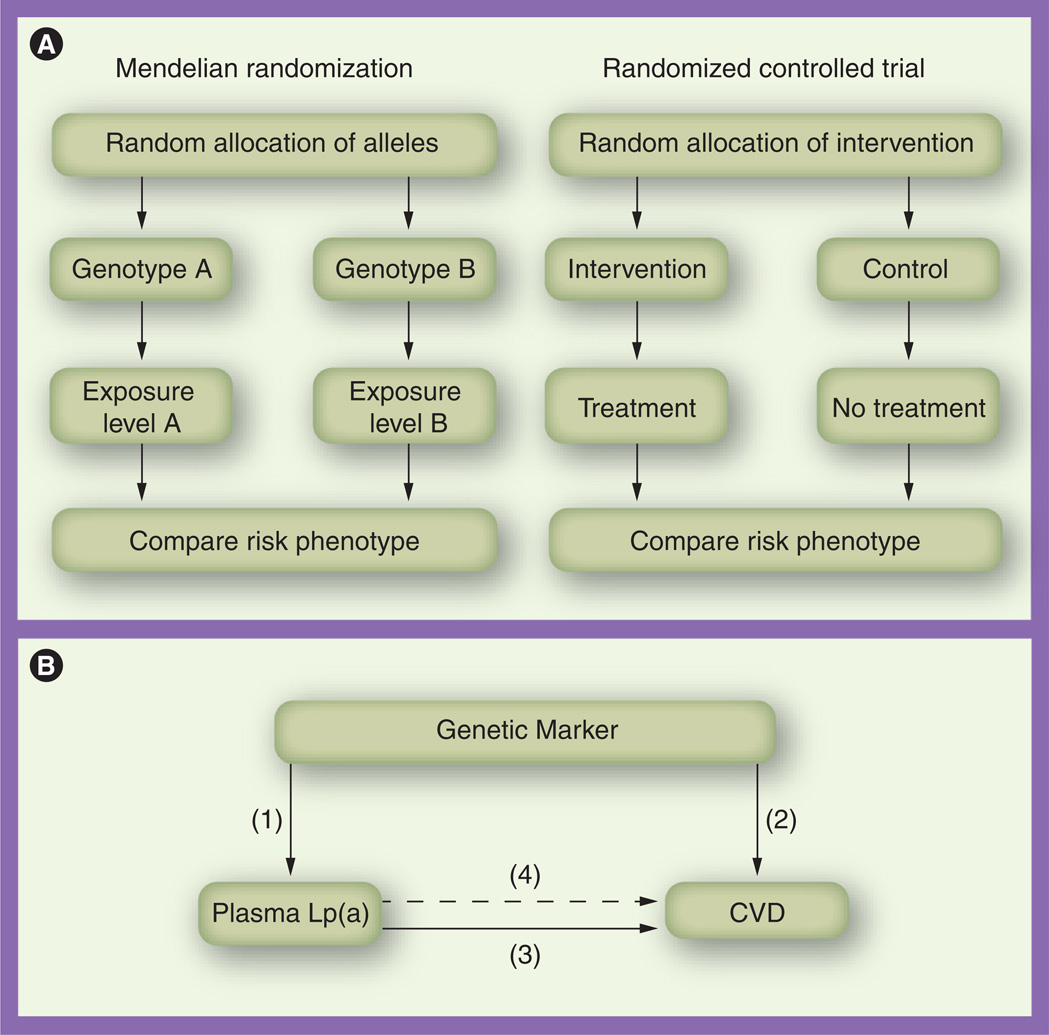
Mendelian randomization is a recent development in genetic epidemiology [62–65], and its principle originates from ‘The Law of Independent Assortment’, also known as ‘Mendel’s second law’, which states that assortments of alleles/genotypes are randomly transferred from parent to offspring at the time of gamete formation and the inheritance of one trait is independent of other traits. The independent distribution of alleles/genotypes means that a study relating health outcome to genetic variation would not be affected by confounders that often distort the interpretation of findings. In addition, because the random assignment to genotype takes place at conception, the association between a genetic variant and disease is free of reverse causation. Thus, Mendelian randomization is particularly intended to address these two major biases affecting observational associations. Mendelian randomization analysis mimics a randomized controlled trial (Figure 2A) [62, 66]. The method of using the genetic markers related to the exposure of interest as instrumental variables (robust proxies for modifiable exposures) is a formal test of Mendelian randomization [63, 67, 68]. This method estimates the predicted effect of genetically determined levels of exposure on outcome, based on the observed associations between genotype and exposure and between genotype and outcome.
Figure 2. Mendelian randomization and randomized controlled trial in the case of lipoprotein(a) and cardiovascular disease.
(A) Parallel relationship between Mendelian randomization and randomized controlled trial. (B) Application of Mendelian randomization to the case of Lp(a) and CVD. Relationships include (1) genetic marker and plasma Lp(a) levels; (2) genetic marker and CVD risk; (3) plasma Lp(a) levels and CVD risk; and (4) the predicted relationship between genetically elevated Lp(a) levels and CVD risk (derived from the first two estimates), which is then compared with the observed estimate (3).
CVD: Cardiovascular disease; Lp(a): Lipoprotein(a).
Modified with permission from [65].
In the case of Lp(a) and CVD risk in diabetic patients, using Mendelian randomization analysis may avoid the influence of some diabetes-specific metabolic changes (such as hyperinsulinemia and insulin resistance) on the plasma levels of Lp(a) as well as the potential reverse causation of CVD onset. Moreover, because plasma levels of Lp(a) are largely determined by genetic factors, genetic variants strongly associated with Lp(a) levels, for example, SNP rs10455872 and a genotype score combining all the independently associated SNPs, could be robust proxies in Mendelian randomization analysis. The application of Mendelian randomization, using an instrumental variable method in this case, requires three observed relationships and a fourth predictive relationship to be estimated:
-
■
A relationship between a genetic marker and plasma Lp(a) levels;
-
■
Association between the selected genetic marker and CVD risk;
-
■
Association between plasma Lp(a) levels and CVD risk;
-
■
The predicted relationship between genetically elevated Lp(a) levels and CVD risk (derived from the first two estimates), which is then compared with the third estimate (Figure 2B).
Since the predicted association between genetically elevated Lp(a) levels and CVD risk is unbiased and nonconfounded, if the association between plasma Lp(a) and CVD risk is true (causal effect), the observed and predicted Lp(a)–CVD associations will be similar.
Since the effect size of most associations between genetic variants and diseases is small–modest, a very large sample size (>10,000 individuals) is usually required to estimate reliable genetic associations for adequately powered Mendelian randomization studies, and most of the individual studies are underpowered [64, 65]. Although the study conducted by Qi et al. simultaneously investigated the relationships among plasma Lp(a) levels, genetic variants and CVD risk in diabetic patients, the sample size was inadequate for performing Mendelian randomization analysis [23]. Since the association data among Lp(a)-related genetic variants, plasma Lp(a) and CVD risk can be obtained from different study samples for Mendelian randomization analysis [65, 69, 70], a meta-analysis based on large-scale collaboration of multiple studies with related data would be a promising next step to investigate causal inference for Lp(a) and CVD risk in diabetic patients in the future.
Influence of pharmacologic agents on Lp(a) levels
Several pharmacologic agents have been demonstrated to influence plasma Lp(a) levels. For example, it has been found that niacin (nicotinic acid or vitamin B3), a drug with a CVD-reducing effect [71], lowered Lp(a) levels by up to 30–40% in a dose-dependent manner [7, 72]. Regular aspirin use has also been reported to lower CVD risk and ApoA mRNA expression by up to 85% [73]. Two kinds of well-known CVD agents, statins and fibrates, were reported to influence Lp(a) levels but with minimal effects [7, 72, 74, 75]. Very recently, the LPA SNP rs10455872 was reported to be associated with a LDL-cholesterol response to atorvastatin therapy [76]. Other agents have been reported to lower Lp(a) levels, including l-carnitine, ascorbic acid combined with l-lysine, calcium antagonists, angiotensin-converting enzyme inhibitors, androgens, estrogen and its replacements (e.g., tibolone), antiestrogens (e.g., tamoxifen) and thyroxine replacement [7, 8, 72, 77]. It is unknown whether reduction of Lp(a) levels by pharmacologic agents directly reduce CVD risk, as most Lp(a)-lowering drugs also modulate other lipoproteins. For example, in addition to lowering Lp(a), niacin has apparently beneficial effects on LDL-cholesterol, total cholesterol, HDLcholesterol, triglycerides, apoB-containing lipoprotein particles and remnant cholesterol levels [7, 8, 72]. Thus, the benefit of niacin on CVD might not be solely attributed to Lp(a) reduction.
In patients with Type 2 diabetes, treatment of insulin led to higher plasma Lp(a) levels than that with diet or oral hypoglycemic agents [30]. Similarly, insulin administration resulted in a significant increase in Lp(a) levels in patients with Type 1 diabetes [78]. Metformin, an antihyperglycemic drug, has been reported to lower Lp(a) levels in nondiabetic individuals, while a study in patients with Type 2 diabetes found that Lp(a) levels were not influenced by metformin administration. In addition, troglitazone, a withdrawal antidiabetic drug owing to its adverse side effects, has been reported to increase Lp(a) levels in patients with Type 2 diabetes and obese subjects with insulin resistance [79–81]. It seems that some antidiabetic treatments may increase Lp(a) levels, although more studies are needed to confirm these observations and elucidate the underlying mechanisms. This raises a critical question as to whether elevated Lp(a) levels by pharmacologic agents increase risk of cardiovascular complications in diabetic patients. If this is true, an evaluation of the effect of diabetes management and treatment on Lp(a) levels will be necessary in the future.
Conclusion & future perspective
During past decades, great efforts have been devoted to clarifying the relationship between Lp(a) and CVD, and recent advances in large epidemiological studies and genetic-association studies has materially expanded our knowledge in this area. Lp(a) has been established to be an independent risk factor for CVD in general populations, raising a number of discussions on the potential usefulness of Lp(a) in clinical practice. Since diabetic patients have a significantly increased risk of CVD compared with nondiabetic individuals, it is of particular interest to investigate the role of Lp(a) in the development of CVD in diabetic patients. Current data suggest that the roles of Lp(a) in affecting CVD risk among diabetic patients are more complicated owing to the presence of various metabolic abnormalities, and a clear role of Lp(a) in diabetic patients is yet to be demonstrated. Several issues need to be further clarified in future studies prior to evaluation of the use of Lp(a) in clinical practice in diabetic patients.
More prospective studies of plasma Lp(a) levels and CVD risk with larger samples of diabetic patients are needed. A large-scale meta-analysis based on individual data would be useful to clarify the relationship between Lp(a) levels and CVD risk in diabetic patients. The question of whether Lp(a) has a linear or threshold effect on CVD risk in diabetic patients will be particularly motivating, as it is possible that only extreme levels of Lp(a) may exert adverse effects – either highly increased levels (in the case of CVD) or very low levels (in the case of diabetes) [11, 12]. Whether the association is the same in diabetic patients remains unknown. Studies in diabetic patients among different ethnic groups will provide additional useful information because plasma Lp(a) levels vary with two- to three-fold differences among various ethnic groups [7, 82].
Large-scale Mendelian randomization studies of Lp(a) and CVD risk in diabetic patients will help to clarify the casual relationship between them, in which Lp(a)-related genetic makers, such as KIV2 size polymorphism, individual SNPs (e.g., rs10455872) and a genotype score [23, 83, 84] could be analyzed as instrumental variables. In addition, randomized controlled intervention trials as the gold standard of evidence may be ultimately required to demonstrate that a reduction in Lp(a) levels leads to reduced CVD in causal inference. Despite this, functional studies have implicated various mechanisms underlying the association between elevated Lp(a) and increased CVD risk, such as through promoting thrombosis, atherogenesis, inflammation, endothelial dysfunction and foam cell formation [85–88]. However, little is known in the setting of the diabetic condition, and the related data are urgently needed.
In conclusion, the current evidence does not support Lp(a) as a causal factor for CVD in diabetic patients. It remains elusive regarding whether Lp(a) is a useful maker in the practice of medicine and public health in relation to cardiometabolic diseases. Such discussions will continue until the relationships between Lp(a), diabetes and CVD are fully understood.
Executive summary.
Background
-
■
Lipoprotein(a) (Lp[a]) is a LDL-like particle consisting of an ApoA moiety linked to one molecule of ApoB100.
-
■
Plasma Lp(a) concentrations are highly heritable and mainly determined by the LPA gene.
Lp(a) & cardiovascular disease in general populations
-
■
Large-scale prospective studies demonstrate that Lp(a) is an independent but moderate risk factor for cardiovascular disease (CVD) in general populations.
-
■
Mendelian randomization studies suggest that Lp(a) may causally affect CVD risk in general populations.
Lp(a) & diabetes
-
■
Plasma Lp(a) levels are decreased in patients with Type 2 diabetes compared with nondiabetics in a cross-sectional analysis.
-
■
Plasma Lp(a) levels are inversely associated with risk of Type 2 diabetes in a prospective study.
Lp(a) & CVD in diabetic patients
-
■
Most published prospective studies of Lp(a) levels and CVD risk in diabetic patients are limited by their relatively small sample size and the results are inconclusive.
-
■
Plasma Lp(a) levels and related genetic variants are not significantly associated with CVD risk in the largest prospective study in diabetic patients to date.
Treatments on Lp(a)
-
■
Several pharmacologic agents (e.g., niacin) have been demonstrated to lower plasma Lp(a) levels, but it is unknown whether reduction of Lp(a) levels by pharmacologic agents directly reduces CVD risk.
-
■
Some antidiabetic treatments (e.g., insulin) may increase Lp(a) levels in diabetic patients.
Conclusion & future perspective
-
■
Current evidence does not support Lp(a) as a causal factor for CVD in diabetic patients.
-
■
More prospective studies, Mendelian randomization analyses and functional studies are needed in order to clarify the relationships between Lp(a), diabetes and CVD.
Acknowledgments
This study was supported by grant HL071981 from the NIH. L Qi was a recipient of the American Heart Association Scientist Development Award (0730094N).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■ ■ of considerable interest
- 1.Berg K. A new serum type system in man – the Lp system. Acta Pathol. Microbiol. Scand. 1963;59(3):369–382. doi: 10.1111/j.1699-0463.1963.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 2. Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–423. doi: 10.1001/jama.2009.1063. ■ Demonstrates that lipoprotein(a) (Lp[a]) is an independent but moderate risk factor for cardiovascular disease in general populations by a large-scale meta-analysis of 36 prospective studies.
- 3. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331–2339. doi: 10.1001/jama.2009.801. ■ Suggests a causal association between elevated Lp(a) levels and increased risk of coronary heart disease in general populations using Mendelian randomization analysis.
- 4. Clarke R, Peden JF, Hopewell JC, et al. Genetic variants in Lp(a) lipoprotein and coronary disease. N. Engl. J. Med. 2009;362(12):1146–1148. ■ Identifies two LPA genetic variants associated with both Lp(a) levels and increased risk of coronary heart disease in general populations.
- 5.Erqou S, Thompson A, Di Angelantonio E, et al. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J. Am. Coll. Cardiol. 2010;55(19):2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 6.Gudnason V. Lipoprotein(a): a causal independent risk factor for coronary heart disease? Curr. Opin Cardiol. 2009;24(5):490–495. doi: 10.1097/HCO.0b013e32832f0a5b. [DOI] [PubMed] [Google Scholar]
- 7. Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur. Heart J. 2010;31(23):2844–2853. doi: 10.1093/eurheartj/ehq386. ■ Evaluates Lp(a) as a cardiovascular risk factor in general populations on the basis of recent evidence, and recommends screening for elevated Lp(a) in individuals at intermediated or high cardiovascular disease risk.
- 8.Dube JB, Boffa MB, Hegele RA, Koschinsky ML. Lipoprotein(a): more interesting than ever after 50 years. Curr. Opin Lipidol. 2012;23(2):133–140. doi: 10.1097/MOL.0b013e32835111d8. [DOI] [PubMed] [Google Scholar]
- 9.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis. JAMA. 2002;287(19):2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 10.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of Type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch. Intern. Med. 2004;164(13):1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 11. Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein(a) and risk of Type 2 diabetes. Clin. Chem. 2010;56(8):1252–1260. doi: 10.1373/clinchem.2010.146779. ■■ Reports a significant inverse association between plasma Lp(a) levels and risk of Type 2 diabetes in prospective cohorts.
- 12.Karakas M, Koenig W. Lipoprotein(a) and cardiometabolic diseases: the mystery continues. Clin. Chem. 2010;56(8):1207–1209. doi: 10.1373/clinchem.2010.150953. [DOI] [PubMed] [Google Scholar]
- 13.Koschinsky ML, Marcovina SM. Structurefunction relationships in apolipoprotein(a): insights into lipoprotein(a) assembly and pathogenicity. Curr. Opin Lipidol. 2004;15(2):167–174. doi: 10.1097/00041433-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Albers JJ, Koschinsky ML, Marcovina SM. Evidence mounts for a role of the kidney in lipoprotein(a) catabolism. Kidney Int. 2007;71(10):961–962. doi: 10.1038/sj.ki.5002240. [DOI] [PubMed] [Google Scholar]
- 15.Kraft HG, Menzel HJ, Hoppichler F, Vogel W, Utermann G. Changes of genetic apolipoprotein phenotypes caused by liver transplantation. Implications for apolipoprotein synthesis. J. Clin. Invest. 1989;83(1):137–142. doi: 10.1172/JCI113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J. Clin. Invest. 1992;90(1):52–60. doi: 10.1172/JCI115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mooser V, Scheer D, Marcovina SM, et al. The Apo(a) gene is the major determinant of variation in plasma Lp(a) levels in African Americans. Am. J. Hum. Genet. 1997;61(2):402–417. doi: 10.1086/514851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mclean JW, Tomlinson JE, Kuang WJ, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330(6144):132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- 19.Brunner C, Lobentanz EM, Petho-Schramm A, et al. The number of identical kringle IV repeats in apolipoprotein(a) affects its processing and secretion by HepG2 cells. J. Biol. Chem. 1996;271(50):32403–32410. doi: 10.1074/jbc.271.50.32403. [DOI] [PubMed] [Google Scholar]
- 20.White AL, Guerra B, Lanford RE. Influence of allelic variation on apolipoprotein(a) folding in the endoplasmic reticulum. J. Biol. Chem. 1997;272(8):5048–5055. doi: 10.1074/jbc.272.8.5048. [DOI] [PubMed] [Google Scholar]
- 21.Ober C, Nord AS, Thompson EE, et al. Genome-wide association study of plasma lipoprotein(a) levels identifies multiple genes on chromosome 6q. J. Lipid Res. 2009;50(5):798–806. doi: 10.1194/jlr.M800515-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tregouet DA, Konig IR, Erdmann J, et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2–LPA gene cluster as a risk locus for coronary artery disease. Nat. Genet. 2009;41(3):283–285. doi: 10.1038/ng.314. [DOI] [PubMed] [Google Scholar]
- 23. Qi Q, Workalemahu T, Zhang C, Hu FB, Qi L. Genetic variants, plasma lipoprotein(a) levels, and risk of cardiovascular morbidity and mortality among two prospective cohorts of Type 2 diabetes. Eur. Heart J. 2012;33(3):325–334. doi: 10.1093/eurheartj/ehr350. ■■ Reports a genome-wide association study of Lp(a) levels in two prospective cohorts of Type 2 diabetes and shows that plasma Lp(a) and related genetic variants are not associated with cardiovascular disease risk in diabetic patients.
- 24.Li Y, Luke MM, Shiffman D, Devlin JJ. Genetic variants in the apolipoprotein(a) gene and coronary heart disease. Circ. Cardiovasc. Genet. 2011;4(5):565–573. doi: 10.1161/CIRCGENETICS.111.959601. [DOI] [PubMed] [Google Scholar]
- 25.Bruckert E, Davidoff P, Grimaldi A, et al. Increased serum levels of lipoprotein(a) in diabetes mellitus and their reduction with glycemic control. JAMA. 1990;263(1):35–36. [PubMed] [Google Scholar]
- 26.Haffner SM, Tuttle KR, Rainwater DL. Decrease of lipoprotein(a) with improved glycemic control in IDDM subjects. Diabetes Care. 1991;14(4):302–307. doi: 10.2337/diacare.14.4.302. [DOI] [PubMed] [Google Scholar]
- 27.Couper JJ, Bates DJ, Cocciolone R, et al. Association of lipoprotein(a) with puberty in IDDM. Diabetes Care. 1993;16(6):869–873. doi: 10.2337/diacare.16.6.869. [DOI] [PubMed] [Google Scholar]
- 28.Salzer B, Stavljenic A, Jurgens G, Dumic M, Radica A. Polymorphism of apolipoprotein E, lipoprotein(a), and other lipoproteins in children with Type I diabetes. Clin. Chem. 1993;39(7):1427–1432. [PubMed] [Google Scholar]
- 29.Klausen IC, Schmidt EB, Lervang HH, Gerdes LU, Ditzel J, Faergeman O. Normal lipoprotein(a) concentrations and apolipoprotein(a) isoforms in patients with insulin-dependent diabetes mellitus. Eur. J. Clin. Invest. 1992;22(8):538–541. doi: 10.1111/j.1365-2362.1992.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 30.Heller FR, Jamart J, Honore P, et al. Serum lipoprotein(a) in patients with diabetes mellitus. Diabetes Care. 1993;16(5):819–823. doi: 10.2337/diacare.16.5.819. [DOI] [PubMed] [Google Scholar]
- 31.Hirata K, Kikuchi S, Saku K, et al. Apolipoprotein(a) phenotypes and serum lipoprotein(a) levels in maintenance hemodialysis patients with/without diabetes mellitus. Kidney Int. 1993;44(5):1062–1070. doi: 10.1038/ki.1993.349. [DOI] [PubMed] [Google Scholar]
- 32.Csaszar A, Dieplinger H, Sandholzer C, et al. Plasma lipoprotein (a) concentration and phenotypes in diabetes mellitus. Diabetologia. 1993;36(1):47–51. doi: 10.1007/BF00399092. [DOI] [PubMed] [Google Scholar]
- 33.Purnell JQ, Marcovina SM, Hokanson JE, et al. Levels of lipoprotein(a), apolipoprotein B, lipoprotein cholesterol distribution in IDDM. Results from follow-up in the diabetes control and complications trial. Diabetes. 1995;44(10):1218–1226. doi: 10.2337/diab.44.10.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez LC, Arauz-Pacheco C, Lackner C, Albright G, Adams BV, Raskin P. Lipoprotein (a) levels in diabetes mellitus: relationship to metabolic control. Ann. Intern. Med. 1992;117(1):42–47. doi: 10.7326/0003-4819-117-1-42. [DOI] [PubMed] [Google Scholar]
- 35.Torres-Tamayo M, Perez-Pasten LE, Barron-Uribe C, et al. Improved metabolic control does not change plasma lipoprotein(a) levels in adolescents with Type 1 diabetes mellitus. Arch. Med. Res. 1998;29(4):307–312. [PubMed] [Google Scholar]
- 36.Pérez A, Carreras G, Caixãs A, et al. Plasma lipoprotein(a) levels are not influenced by glycemic control in Type 1 diabetes. Diabetes Care. 1998;21(9):1517–1520. doi: 10.2337/diacare.21.9.1517. [DOI] [PubMed] [Google Scholar]
- 37.Haffner SM, Morales PM, Stern MP, Gruber MK. Lp(a) concentrations in NIDDM. Diabetes. 1992;41(10):1267–1272. doi: 10.2337/diab.41.10.1267. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins AJ, Steele JS, Janus ED, Santamaria JD, Best JD. Plasma apolipoprotein (a) is increased in Type 2 (non-insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1992;35(11):1055–1059. doi: 10.1007/BF02221681. [DOI] [PubMed] [Google Scholar]
- 39.Rainwater DL, Maccluer JW, Stern MP, Vandeberg JL, Haffner SM. Effects of NIDDM on lipoprotein(a) concentration and apolipoprotein(a) size. Diabetes. 1994;43(7):942–946. doi: 10.2337/diab.43.7.942. [DOI] [PubMed] [Google Scholar]
- 40.Saely CH, Koch L, Schmid F, et al. Lipoprotein(a), Type 2 diabetes and vascular risk in coronary patients. Eur. J. Clin. Invest. 2006;36(2):91–97. doi: 10.1111/j.1365-2362.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- 41.Rainwater DL, Haffner SM. Insulin and 2-hour glucose levels are inversely related to Lp(a) concentrations controlled for LPA genotype. Arterioscler. Thromb. Vasc. Biol. 1998;18(8):1335–1341. doi: 10.1161/01.atv.18.8.1335. [DOI] [PubMed] [Google Scholar]
- 42.Neele DM, De Wit EC, Princen HM. Insulin suppresses apolipoprotein(a) synthesis by primary cultures of cynomolgus monkey hepatocytes. Diabetologia. 1999;42(1):41–44. doi: 10.1007/s001250051110. [DOI] [PubMed] [Google Scholar]
- 43.Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995;44(4):369–374. doi: 10.2337/diab.44.4.369. [DOI] [PubMed] [Google Scholar]
- 44.Winocour PH, Durrington PN, Bhatnagar D, et al. A cross-sectional evaluation of cardiovascular risk factors in coronary heart disease associated with Type 1 (insulin-dependent) diabetes mellitus. Diabetes Res. Clin. Pract. 1992;18(3):173–184. doi: 10.1016/0168-8227(92)90143-f. [DOI] [PubMed] [Google Scholar]
- 45.Maser RE, Usher D, Becker DJ, Drash AL, Kuller LH, Orchard TJ. Lipoprotein(a) concentration shows little relationship to IDDM complications in the Pittsburgh epidemiology of diabetes complications study cohort. Diabetes Care. 1993;16(5):755–758. doi: 10.2337/diacare.16.5.755. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz J, Thillet J, Huby T, et al. Association of elevated lipoprotein(a) levels and coronary heart disease in NIDDM patients. Relationship with apolipoprotein(a) phenotypes. Diabetologia. 1994;37(6):585–591. doi: 10.1007/BF00403377. [DOI] [PubMed] [Google Scholar]
- 47.James RW, Boemi M, Sirolla C, Amadio L, Fumelli P, Pometta D. Lipoprotein (a) and vascular disease in diabetic patients. Diabetologia. 1995;38(6):711–714. doi: 10.1007/BF00401844. [DOI] [PubMed] [Google Scholar]
- 48.Watts GF, Ap Gwilym RM, Mazurkiewicz J, Coltart J. Independent correlation between plasma lipoprotein(a) and angiographic coronary artery disease in NIDDM. Diabetes Care. 1995;18(2):234–236. doi: 10.2337/diacare.18.2.234. [DOI] [PubMed] [Google Scholar]
- 49.Mohan V, Deepa R, Haranath SP, et al. Lipoprotein(a) is an independent risk factor for coronary artery disease in NIDDM patients in South India. Diabetes Care. 1998;21(11):1819–1823. doi: 10.2337/diacare.21.11.1819. [DOI] [PubMed] [Google Scholar]
- 50.Pedreno J, Fernandez R, Ballester A, et al. Lack of association of serum lipoprotein (a) levels with Type-2 diabetes mellitus in patients with angiographically defined coronary artery disease. Int. J. Cardiol. 2000;74(2–3):159–167. doi: 10.1016/s0167-5273(00)00304-1. [DOI] [PubMed] [Google Scholar]
- 51.Gazzaruso C, Garzaniti A, Falcone C, Geroldi D, Finardi G, Fratino P. Association of lipoprotein(a) levels and apolipoprotein(a) phenotypes with coronary artery disease in Type 2 diabetic patients and in non-diabetic subjects. Diabet. Med. 2001;18(7):589–594. doi: 10.1046/j.1464-5491.2001.00536.x. [DOI] [PubMed] [Google Scholar]
- 52.Murase T, Okubo M, Amemiya-Kudo M, Ebara T, Mori Y. Impact of elevated serum lipoprotein (a) concentrations on the risk of coronary heart disease in patients with Type 2 diabetes mellitus. Metabolism. 2008;57(6):791–795. doi: 10.1016/j.metabol.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 53.Haffner SM, Moss SE, Klein BE, Klein R. Lack of association between lipoprotein (a) concentrations and coronary heart disease mortality in diabetes: the Wisconsin epidemiologic study of diabetic retinopathy. Metabolism. 1992;41(2):194–197. doi: 10.1016/0026-0495(92)90152-z. [DOI] [PubMed] [Google Scholar]
- 54.Hiraga T, Kobayashi T, Okubo M, et al. Prospective study of lipoprotein(a) as a risk factor for atherosclerotic cardiovascular disease in patients with diabetes. Diabetes Care. 1995;18(2):241–244. doi: 10.2337/diacare.18.2.241. [DOI] [PubMed] [Google Scholar]
- 55.Abu-Lebdeh HS, Hodge DO, Nguyen TT. Predictors of macrovascular disease in patients with Type 2 diabetes mellitus. Mayo Clin. Proc. 2001;76(7):707–712. doi: 10.4065/76.7.707. [DOI] [PubMed] [Google Scholar]
- 56.Hernández C, Francisco G, Chacón P, Simó R. Lipoprotein(a) as a risk factor for cardiovascular mortality in Type 2 diabetic patients. Diabetes Care. 2005;28(4):931–933. doi: 10.2337/diacare.28.4.931. [DOI] [PubMed] [Google Scholar]
- 57.Kollerits B, Auinger M, Reisig V, et al. Lipoprotein(a) as a predictor of cardiovascular disease in a prospectively followed cohort of patients with Type 1 diabetes. Diabetes Care. 2006;29(7):1661–1663. doi: 10.2337/dc06-0546. [DOI] [PubMed] [Google Scholar]
- 58.Shai I, Schulze M, Manson J, Stampfer M, Rifai N, Hu F. A prospective study of lipoprotein(a) and risk of coronary heart disease among women with Type 2 diabetes. Diabetologia. 2005;48:1469–1476. doi: 10.1007/s00125-005-1814-3. [DOI] [PubMed] [Google Scholar]
- 59.Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population. Circulation. 2008;117(2):176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 60.Shiffman D, O’Meara ES, Bare LA, et al. Association of gene variants with incident myocardial infarction in the cardiovascular health study. Arterioscler. Throm. Vasc. Biol. 2008;28(1):173–179. doi: 10.1161/ATVBAHA.107.153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chasman DI, Shiffman D, Zee RY, et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203(2):371–376. doi: 10.1016/j.atherosclerosis.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davey Smith G, Ebrahim S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ. 2005;330(7499):1076–1079. doi: 10.1136/bmj.330.7499.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat. Methods Med. Res. 2007;16(4):309–330. doi: 10.1177/0962280206077743. [DOI] [PubMed] [Google Scholar]
- 64.Thanassoulis G, O’Donnell CJ. Mendelian randomization. JAMA. 2009;301(22):2386–2388. doi: 10.1001/jama.2009.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qi L. Mendelian randomization in nutritional epidemiology. Nutr. Rev. 2009;67(8):439–450. doi: 10.1111/j.1753-4887.2009.00218.x. ■ Introduces Mendelian randomization and its application in nutritional epidemiology.
- 66.Nitsch D, Molokhia M, Smeeth L, Destavola BL, Whittaker JC, Leon DA. Limits to causal inference based on mendelian randomization: a comparison with randomized controlled trials. Am. J. Epidemiol. 2006;163(5):397–403. doi: 10.1093/aje/kwj062. [DOI] [PubMed] [Google Scholar]
- 67.Greenland S. An introduction to instrumental variables for epidemiologists. Int. J. Epidemiol. 2000;29(4):722–729. doi: 10.1093/ije/29.4.722. [DOI] [PubMed] [Google Scholar]
- 68.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 69.Minelli C, Thompson JR, Tobin MD, Abrams KR. An integrated approach to the meta-analysis of genetic association studies using Mendelian randomization. Am. J. Epidemiol. 2004;160(5):445–452. doi: 10.1093/aje/kwh228. [DOI] [PubMed] [Google Scholar]
- 70.Chen L, Davey Smith G, Harbord RM, Lewis SJ. Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Med. 2008;5(3):e52. doi: 10.1371/journal.pmed.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruckert E, Labreuche J, Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210(2):353–361. doi: 10.1016/j.atherosclerosis.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 72.Tziomalos K, Athyros VG, Wierzbicki AS, Mikhailidis DP. Lipoprotein a: where are we now? Curr. Opin Cardiol. 2009;24(4):351–357. doi: 10.1097/HCO.0b013e32832ac21a. [DOI] [PubMed] [Google Scholar]
- 73.Kagawa A, Azuma H, Akaike M, Kanagawa Y, Matsumoto T. Aspirin reduces apolipoprotein(a) (apo(a)) production in human hepatocytes by suppression of apo(a) gene transcription. J. Biol. Chem. 1999;274(48):34111–34115. doi: 10.1074/jbc.274.48.34111. [DOI] [PubMed] [Google Scholar]
- 74.Cobbaert C, Jukema JW, Zwinderman AH, Withagen AJ, Lindemans J, Bruschke AV. Modulation of lipoprotein(a) atherogenicity by high density lipoprotein cholesterol levels in middle-aged men with symptomatic coronary artery disease and normal to moderately elevated serum cholesterol. J. Am. Coll. Cardiol. 1997;30(6):1491–1499. doi: 10.1016/s0735-1097(97)00353-7. [DOI] [PubMed] [Google Scholar]
- 75.Gonbert S, Malinsky S, Sposito AC, et al. Atorvastatin lowers lipoprotein(a) but not apolipoprotein(a) fragment levels in hypercholesterolemic subjects at high cardiovascular risk. Atherosclerosis. 2002;164(2):305–311. doi: 10.1016/s0021-9150(02)00072-2. [DOI] [PubMed] [Google Scholar]
- 76.Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J. Am. Coll. Cardiol. 2008;52(2):124–131. doi: 10.1016/j.jacc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simo R, Hernandez C, Chacon P, Marti R, Mesa J. Effect of insulin administration on serum lipoprotein(a) and its phenotypes in new-onset IDDM patients. Diabetes Care. 1998;21(5):866–867. doi: 10.2337/diacare.21.5.866. [DOI] [PubMed] [Google Scholar]
- 78.Matsumoto K, Miyake S, Yano M, Ueki Y, Tominaga Y. Increase of lipoprotein (a) with troglitazone. Lancet. 1997;350(9093):1748–1749. doi: 10.1016/S0140-6736(05)63572-6. [DOI] [PubMed] [Google Scholar]
- 79.Ovalle F, Bell DS. Troglitazone’s effect on lipoprotein(a) levels. Diabetes Care. 1999;22(5):859–860. doi: 10.2337/diacare.22.5.859a. [DOI] [PubMed] [Google Scholar]
- 80.Tack CJ, Smits P, Demacker PN, Stalenhoef AF. Effect of troglitazone on lipoprotein(a) levels in obese subjects. Diabetes Care. 1999;22(10):1752–1753. doi: 10.2337/diacare.22.10.1752. [DOI] [PubMed] [Google Scholar]
- 81.Lanktree MB, Anand SS, Yusuf S, Hegele RA. On Behalf of the SHARE Investigators: comprehensive analysis of genomic variation in the LPA locus and its relationship to plasma lipoprotein(a) in south Asians, Chinese, and European Caucasians. Circ. Cardiovasc. Genet. 2010;3(1):39–46. doi: 10.1161/CIRCGENETICS.109.907642. [DOI] [PubMed] [Google Scholar]
- 82.Li S, Zhao J, Luan J, et al. Genetic predisposition to obesity leads to increased risk of Type 2 diabetes. Diabetologia. 2010;54(4):776–782. doi: 10.1007/s00125-011-2044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Silva NM, Freathy RM, Palmer TM, et al. Mendelian randomization studies do not support a role for raised circulating triglyceride levels influencing Type 2 diabetes, glucose levels, or insulin resistance. Diabetes. 2011;60(3):1008–1018. doi: 10.2337/db10-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boonmark NW, Lou XJ, Yang ZJ, et al. Modification of apolipoprotein(a) lysine binding site reduces atherosclerosis in transgenic mice. J. Clin. Invest. 1997;100(3):558–564. doi: 10.1172/JCI119565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poon M, Zhang X, Dunsky KG, Taubman MB, Harpel PC. Apolipoprotein(a) induces monocyte chemotactic activity in human vascular endothelial cells. Circulation. 1997;96(8):2514–2519. doi: 10.1161/01.cir.96.8.2514. [DOI] [PubMed] [Google Scholar]
- 86.Nielsen LB, Juul K, Nordestgaard BG. Increased degradation of lipoprotein(a) in atherosclerotic compared with nonlesioned aortic intima-inner media of rabbits: in vivo evidence that lipoprotein(a) may contribute to foam cell formation. Arterioscler. Thromb. Vasc. Biol. 1998;18(4):641–649. doi: 10.1161/01.atv.18.4.641. [DOI] [PubMed] [Google Scholar]
- 87.Schlaich MP, John S, Langenfeld MR, Lackner KJ, Schmitz G, Schmieder RE. Does lipoprotein(a) impair endothelial function? J. Am. Coll. Cardiol. 1998;31(2):359–365. doi: 10.1016/s0735-1097(97)00497-x. [DOI] [PubMed] [Google Scholar]
- 88.Boffa MB, Marcovina SM, Koschinsky ML. Lipoprotein(a) as a risk factor for atherosclerosis and thrombosis: mechanistic insights from animal models. Clin. Biochem. 2004;37(5):333–343. doi: 10.1016/j.clinbiochem.2003.12.007. [DOI] [PubMed] [Google Scholar]