Abstract
Archaea assemblages from the Arctic Ocean and Antarctic waters were compared by PCR-denaturing gradient gel electrophoresis (DGGE) analysis of 16S rRNA genes amplified using the Archaea-specific primers 344f and 517r. Inspection of the DGGE fingerprints of 33 samples from the Arctic Ocean (from SCICEX submarine cruises in 1995, 1996, and 1997) and 7 Antarctic samples from Gerlache Strait and Dallman Bay revealed that the richness of Archaea assemblages was greater in samples from deep water than in those from the upper water column in both polar oceans. DGGE banding patterns suggested that most of the Archaea ribotypes were common to both the Arctic Ocean and the Antarctic Ocean. However, some of the Euryarchaeota ribotypes were unique to each system. Cluster analysis of DGGE fingerprints revealed no seasonal variation but supported depth-related differences in the composition of the Arctic Ocean Archaea assemblage. The phylogenetic composition of the Archaea assemblage was determined by cloning and then sequencing amplicons obtained from the Archaea-specific primers 21f and 958r. Sequences of 198 clones from nine samples covering three seasons and all depths grouped with marine group I Crenarchaeota (111 clones), marine group II Euryarchaeota (86 clones), and group IV Euryarchaeota (1 clone). A sequence obtained only from a DGGE band was similar to those of the marine group III Euryarchaeota.
The widespread distribution and significance of members of the domain Archaea in antarctic waters has been evident since the early 1990s (14, 26, 29, 30). In contrast, little is known about the composition or distribution of this group in the Arctic Ocean. One recent study (43) demonstrated the numerical significance of Archaea in coastal waters of the Arctic Ocean basin and concluded that they tended to be associated with particles. Because it was based on fluorescent in situ hybridization (FISH), the study provided only limited phylogenetic resolution. Another study of arctic (Svalbard) sediment microbial communities, also based on FISH, showed that up to 5% of the cells hybridized to an archaeal probe (36). A recent study of the phylogenetic composition of Arctic Ocean bacterioplankton (5) did not include Archaea. As a result, there is a significant gap in our knowledge of the richness of prokaryotes in the Arctic Ocean, and we cannot compare arctic and antarctic prokaryotes on an equal basis.
The physical environments of the two polar oceans differ in a number of important ways. The Arctic Ocean is perennially ice covered; is surrounded by continents with wide, shallow continental shelves; and receives ∼10% of the freshwater flowing into the world ocean. Freshwater inflow, brine exclusion during sea ice formation, the annual cycle of ice formation and melting, and the effective absence of wind mixing result in a very stable, highly stratified water column consisting of three major water masses (1, 2). The upper layer (to a depth of ∼60 m) consists of relatively well-mixed, low-salinity (<34‰) water with a seasonal, shallow halocline. The intermediate layer (between 75 and 150 m) contains high-salinity (>34‰) cold (<−1.5°C) water separated from the surface layer by an extremely stable, essentially permanent halocline. Relatively warm (2 to 4°C) Atlantic Ocean water is found below this intermediate layer. In contrast, the Southern Ocean surrounds a continent (Antarctica) with narrow, deep continental shelves and is bounded by an oceanic front. The water column is not strongly stratified. Coastal waters stratify during periods of meltwater formation when the pack ice retreats during spring and summer. The Southern Ocean receives virtually no freshwater inflow, no terrigenous organic matter, and only small amounts of terrestrially derived micronutrients (e.g., Fe) (20, 23, 42).
Archaea is divided into two kingdoms, the Euryarchaeota and the Crenarchaeota, with the possibility of a third kingdom, the Korarchaeota (6, 39). Cultivation-based analysis of the distribution of Archaea led to the belief that Archaea were restricted to extreme environments. Recent studies based on analyses of 16S rRNA gene sequences have demonstrated that Archaea are much more diverse and widespread than previously suspected (11, 18, 24). Karner et al. (19) concluded that there are 1.3 × 1028 archaeal cells (of which ∼20% are Crenarchaeota) and 3.1 × 1028 bacterial cells in the world ocean. Most Crenarchaeota 16S rRNA gene sequences from the marine environment are closely related and are identified as group I Crenarchaeota. The other predominantly marine group is affiliated with the Euryarchaeota and is identified as marine group II Euryarchaeota. This group has greater phylogenetic diversity among its sequenced representatives than the marine group I Crenarchaeota.
We have investigated the spatial and temporal distribution of Archaea in the Arctic Ocean to complement previous work on the Bacteria (5) and to obtain a more robust assessment of the diversity and richness of prokaryotes in the Arctic Ocean. We were also interested in comparing the results of our assessment of Archaea community composition with similar studies of Archaea in antarctic waters to identify differences, if they exist, in the compositions of these assemblages, with the ultimate goal of relating them to differences in the environmental conditions of the two polar oceans.
MATERIALS AND METHODS
Sampling and DNA extraction.
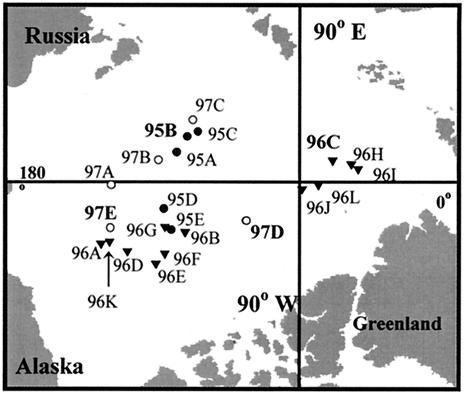
The samples we used in this study were collected from the central Arctic Ocean during the SCICEX 95 (8 to 16 April 1995), SCICEX 96 (8 to 17 October 1996), and SCICEX 97 (5 September to 2 October 1997) cruises of the U.S. Navy nuclear submarines Cavalla, Pogy, and Archerfish (see references 5 and 16 for details of sample collection and processing). The locations of the Arctic Ocean stations chosen for this study are shown in Fig. 1. Physical, chemical, and biological characteristics of the samples are given in Table 1. The depths sampled corresponded to the bottom of the surface mixed layer (55 m), the middle of the halocline (131 m), and the maximum depth at which sampling was possible (“deep water”; 235 m). We selected 10 representative samples from SCICEX 95 (5 each from 55 and 131 m), 18 samples from SCICEX 96 (5 from 55 m, 11 from 131 m, and 2 from 235 m), and 5 from SCICEX 97 (2 from 55 m, 2 from 131 m, and 1 from 235 m) for analysis by denaturing gradient gel electrophoresis (DGGE). We also selected three samples from 55 m (one from each cruise), four samples from 131 m (one each from SCICEX 95 and 96 and two from SCICEX 97), and two samples from 235 m (one each from SCICEX 96 and SCICEX 97) for cloning and sequencing.
FIG. 1.
Locations of Arctic Ocean stations where the samples used in this study were collected. The locations of samples used to generate clone libraries are shown in boldface and larger.
TABLE 1.
Environmental data for Arctic Ocean samplesa
| Lane or library ID | SCICEX stn | Date | Temp (°C) | Salinity (PSU) | NOx (μM) | PO4 (μM) | NH4 (μM) | SiO4 (μM) | Cells (109 liter−1) | Chl (ng liter−1) | Phaeo (ng liter−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCICEX 95 | |||||||||||
| 95A-55 | 1.19.6.b | 16 Apr | −1.84 | 33.77 | 4.55 | 0.75 | 2.03 | 5.93 | 0.07 | 10.80 | 27.30 |
| 95A-131 | 1.19.6.c | 16 Apr | −0.95 | 34.29 | 9.59 | 1.02 | 1.82 | 5.14 | 0.11 | 0.60 | 7.90 |
| 95B-55 | 1.18.30.b | 15 Apr | −1.88 | 33.70 | 5.27 | 0.81 | 1.14 | 3.29 | 0.09 | 8.50 | 23.90 |
| 95B-131 | 1.18.30.c | 15 Apr | −1.14 | 34.27 | 9.66 | 1.03 | 1.04 | 4.42 | 0.06 | 0.90 | 10.90 |
| 95C-55 | 1.16.2.b | 11 Apr | −1.78 | 33.72 | 4.85 | 0.90 | 1.01 | 3.57 | 0.13 | 8.70 | 20.90 |
| 95C-131 | 1.16.2.c | 11 Apr | −0.83 | 34.33 | 10.11 | 1.13 | 1.39 | 5.00 | 0.07 | 0.70 | 8.90 |
| 95D-55 | 1.15.1.b | 10 Apr | −1.73 | 31.61 | 1.53 | 1.60 | 1.04 | 10.43 | 0.15 | 12.50 | 23.20 |
| 95D-131 | 1.15.1.c | 10 Apr | −1.52 | 34.09 | 6.51 | 1.45 | 0.97 | 11.15 | 0.08 | 1.30 | 10.90 |
| 95E-55 | 1.13.1.b | 8 Apr | −1.69 | 31.15 | 3.09 | 1.62 | 1.20 | 10.85 | 0.10 | 6.20 | 28.10 |
| 95E-131 | 1.13.1.c | 8 Apr | −1.46 | 34.17 | 8.72 | 1.18 | 1.06 | 6.93 | 0.10 | 1.20 | 13.30 |
| SCICEX 96 | |||||||||||
| 96A-55 | 76 | 10 Oct | −1.285 | 32.248 | 9.78 | 0.84 | 1.99 | 26.51 | 0.02 | ND | ND |
| 96A-131 | 76 | 10 Oct | −1.587 | 33.059 | 16.44 | 0.86 | 0.90 | 42.13 | 0.01 | ND | ND |
| 96B-55 | 61 | 9 Oct | −1.559 | 31.178 | 6.63 | 0.14 | 2.86 | 17.70 | 0.11 | ND | ND |
| 96B-131 | 61 | 9 Oct | −1.61 | 33.838 | 11.42 | 0.18 | 2.79 | 7.76 | 0.22 | ND | ND |
| 96B-235 | 61 | 9 Oct | −1.34 | 34.611 | 12.60 | ND | 0.22 | ND | 0.09 | ND | ND |
| 96C-55 | 110 | 17 Oct | −1.852 | 33.988 | 0.25 | 0.46 | 2.76 | 3.00 | 0.12 | ND | ND |
| 96C-131 | 110 | 17 Oct | −1.733 | 34.279 | 0.25 | 0.46 | 2.76 | 3.00 | 0.08 | ND | ND |
| 96C-235 | 110 | 17 Oct | 1.028 | 34.789 | 0.35 | 0.38 | 2.00 | 3.52 | 0.04 | ND | ND |
| 96D-55 | 77 | 11 Oct | −1.511 | 32.109 | 8.10 | 0.46 | 1.13 | 5.38 | 0.11 | ND | ND |
| 96E-131 | 73 | 10 Oct | −1.611 | 32.828 | 18.36 | 0.77 | 0.42 | 48.01 | 0.31 | ND | ND |
| 96F-131 | 65 | 9 Oct | −1.458 | 33.311 | 13.90 | 0.19 | 2.34 | 34.62 | 0.08 | ND | ND |
| 96G-131 | 58 | 8 Oct | −1.553 | 33.892 | 9.84 | 0.21 | 2.14 | 12.99 | 0.11 | ND | ND |
| 96H-131 | 108 | 16 Oct | ND | ND | 6.93 | 0.25 | 2.02 | 3.80 | 0.07 | ND | ND |
| 96I-131 | 104 | 15 Oct | ND | ND | 6.67 | 0.25 | 1.36 | 3.38 | 0.04 | ND | ND |
| 96J-131 | 98 | 14 Oct | ND | ND | 0.37 | 0.39 | 1.24 | 6.51 | 0.07 | ND | ND |
| 96K-55 | 68 | 9 Oct | ND | ND | 6.23 | 1.26 | 2.89 | 17.21 | 1.26 | ND | ND |
| 96K-131 | 68 | 9 Oct | ND | ND | 16.09 | 2.01 | 2.25 | 41.11 | 2.01 | ND | ND |
| 96L-131 | 99 | 14 Oct | −1.128 | 34.296 | 0.37 | 0.41 | 1.58 | 5.91 | 0.41 | ND | ND |
| SCICEX 97 | |||||||||||
| 97A-55 | 2.155.1 | 20 Sep | −1.66 | 33.69 | 4.50 | 0.41 | 1.08 | 5.29 | 0.12 | ND | ND |
| 97A-131 | 2.155.4 | 20 Sep | −1.40 | 34.39 | 6.63 | 0.51 | 1.07 | 5.25 | 0.11 | ND | ND |
| 97B-235 | 2.120.8 | 18 Sep | 1.02 | 34.78 | 11.42 | 0.96 | 1.34 | 5.92 | 0.05 | ND | ND |
| 97C-55 | 2.88.1 | 16 Sep | −1.66 | 32.58 | 10.62 | 1.80 | 1.55 | ND | 0.21 | ND | ND |
| 97C-131 | 2.88.5 | 16 Sep | −1.60 | 34.20 | 9.50 | 1.34 | 1.37 | 6.87 | 0.11 | ND | ND |
| 97D-55 | 1.6.1 | 5 Sep | −1.52 | 32.19 | 11.15 | 1.31 | 0.63 | ND | 0.10 | ND | ND |
| 97D-131 | 1.6.4 | 5 Sep | −1.58 | 34.04 | 8.30 | 0.54 | 0.88 | ND | 0.05 | ND | ND |
| 97D-235 | 1.6.7 | 5 Sep | −0.22 | 34.64 | 11.95 | 0.63 | 0.84 | ND | 0.04 | ND | ND |
| 97E-131 | 5.6.4 | 2 Oct | −1.72 | 33.06 | 14.81 | 1.69 | 0.92 | ND | 0.16 | ND | ND |
The column “Lane or library ID” gives the sample identifier used in this study; the first number indicates the cruise (SCICEX 95, 96, or 97), and the last number indicates the nominal sample depth (thus, 95A-55 indicates that the sample was collected on the SCICEX 95 cruise, it was used for lane or library A, and it was collected at 55-m nominal depth). Other column headings are as follows: SCICEX stn, the station identifier used in SCICEX cruise reports; Date, date the sample was collected; Temp, water temperature; NOx, nitrate plus nitrite concentration; PO4, reactive-phosphorus concentration; NH4, ammonium concentration, SiO4, reactive-silicate concentration; Cells, bacterioplankton abundance; Chl, chlorophyll concentration; Phaeo, phaeopigment concentration. ND, no data. Temperature and salinity were taken from an expendable conductivity-temperature-depth recorder cast made when the submarine came onto station. Nutrient and pigment data courtesy of T. Whitledge, University of Alaska, Fairbanks; CTD data courtesy of T. Boyd, Oregon State University. Apr, April; Sep, September; Oct, October.
We compared Arctic Ocean and antarctic Archaea assemblages using DGGE. The antarctic samples (purified DNA kindly provided by A. E. Murray) used in this study were collected on 10 October 1996 from Gerlache Strait (GER; 64.2°S, 61.8°W; depths, 5, 50, 125, 250, and 500 m) and on 8 January 1996 from Dallman Bay (DB; 64.1°S, 62.9°W; depths, 0 and 150 m), coastal waters near the Antarctic Peninsula. More detailed descriptions of the stations and collection methodologies for antarctic samples are given elsewhere (26, 29).
DGGE.
The v3 region of the Archaea 16S rRNA gene was amplified from genomic DNA using the Archaea-specific (35) forward primer at position 344 (ARC344f; 5′-ACGGGGCGCAGCAGGCGCGA-3′) and a universal reverse primer at position 517 (517r; 5′-ATTACCGCGGCTGCTGG-3′). All nucleotide positions given are in reference to the Escherichia coli 16S rRNA gene (9). A 40-bp GC clamp (5′-CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCCC-3′) (31) was added to the 5′ end of the ARC344f primer, and fluorescein was attached to the 5′ end of primer 517r. Each PCR mixture (100 μl) contained 1× PCR buffer (Promega), 2.5 mM MgCl2, 200 μM (each) deoxyribonucleoside triphosphate (dATP, dCTP, dGTP, and dTTP), 1.0 μM (each) primer, and 20 to 100 ng of template DNA. PCR amplification was performed under the following conditions: initial denaturation of the template DNA at 95°C for 5 min, a pause at 82°C to add Taq DNA polymerase (5 U; Promega), and then 35 cycles of denaturation, annealing, and extension using a touchdown program as described previously (16). The concentration of the resulting PCR product was estimated by the Hoechst dye assay (32), and then the mixed-template product was resolved by DGGE as described previously (4, 5). Bands of interest were excised from the gel, DNA was eluted from them into 50 μl of water by incubation at 55°C for 1 h, and then the eluted DNA was amplified as described above. The PCR products of the second amplification were resolved by DGGE, bands were excised, and DNA was eluted and amplified as described above. The PCR product was purified using Wizard PCR preps (Promega) and sequenced on an automated sequencer at the University of Georgia Molecular Genetics Instrument Facility with primer ARC344f to yield ∼120 bp of sequence information.
DGGE banding patterns were compared using Molecular Analyst software version 1.12 (Bio-Rad) essentially as described previously (16). The gel image was converted to a densitometry scan, standards run in the outside lanes of gels were used to normalize the gel lanes, the similarities of pairs of densitometry curves were quantified using Pearson's correlation coefficients, and cluster analysis was performed using the unweighted pair group method with averaging with fine alignment. Standard lanes from all gels were included in this analysis as controls; they clustered together at >90% similarity.
Clone libraries.
Because the sequences from DGGE bands were too short to provide adequate phylogenetic resolution, we sequenced a total of 198 cloned fragments of Archaea 16S rRNA genes amplified from nine samples covering three seasons and the range of depths sampled (Table 2). Archaea 16S rRNA genes were amplified using the Archaea-specific primers 21f (5′-TTCCGGTTGATCCYGCCGGA-3′) and 958r (5′-YCCGGCGTTGAMTCCAATT-3′). PCR mixtures were prepared as described above. PCR was performed under the following conditions: initial denaturation at 94°C for 5 min, followed by 25 cycles consisting of 94°C for 30 s, 55°C for 30 s, and 72°C for 60 s, and a final elongation step at 72°C for 10 min. To minimize the potential effect of PCR biases in single reactions (34), three or four independent PCRs were pooled. The combined PCR product was purified with QiaQuick PCR purification columns in accordance with the manufacturer's instructions. The amplicons were cloned using a PGEM T-Easy Vector cloning kit (Promega) as recommended by the manufacturer. Clones from each library were selected randomly and sequenced from either end at the Molecular Genetics Instrument Facility using plasmid-specific primers (or from both ends for a subset of representative cloned amplicons), yielding ∼600 bp of sequence per reading, or on an ABI 310 in the Department of Marine Sciences, yielding ∼425 bp of sequence per reading.
TABLE 2.
Number and percent of Crenarchaeota and Euryarchaeota found in clone libraries constructed from samples collected during the SCICEX 95, 96, and 97 cruises
| Library | No. (%) at sampling depth (m)a:
|
|||||
|---|---|---|---|---|---|---|
| 55
|
131
|
235
|
||||
| Eury | Cren | Eury | Cren | Eury | Cren | |
| 95B | 2 (15) | 11 (85) | 6 (46) | 7 (54) | ||
| 96C | 12 (63) | 7 (37) | 7 (44) | 9 (56) | 12 (52) | 11 (48) |
| 97D | 13 (32) | 28 (68) | 19 (44) | 24 (56) | 11 (52) | 10 (58) |
| 97E | 5 (55) | 4 (44) | ||||
| Overall | 27 (37) | 46 (63) | 37 (46) | 44 (54) | 23 (53) | 21 (47) |
Eury, Euryarchaeota; Cren, Crenarchaeota.
Phylogenetic analysis.
Sequences were checked for chimeras using the Ribosomal Database Project CHECK-CHIMERA program. Sequences were aligned using the Genetics Computer Group, Inc., package (Wisconsin package version 10.0, 1999) and compared to known sequences by using BLAST (3). Phylogenetic analysis used positions 536 to 941 (group I Crenarchaeota) or 512 to 937 (group II Euryarchaeota). Phylogenetic trees were inferred and bootstrap analysis (100 replicates) was performed with the PHYLIP package (15) using evolutionary distances (Jukes-Cantor distances) and the neighbor-joining method.
Nucleotide sequence accession numbers.
The sequences of cloned fragments have been deposited in GenBank (see Fig. 5 for the accession numbers).
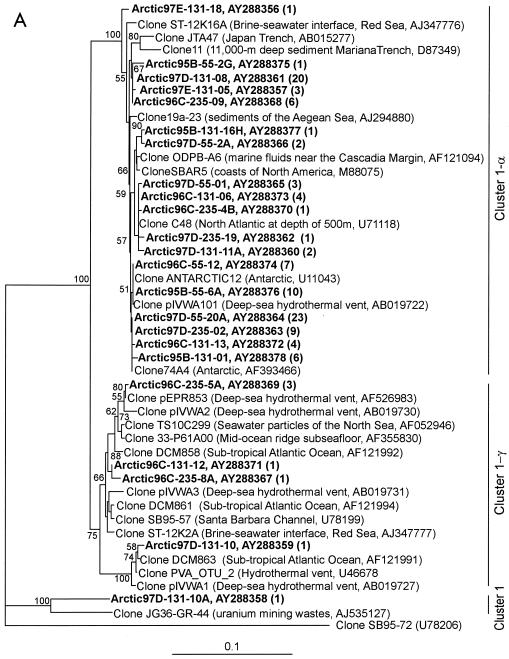
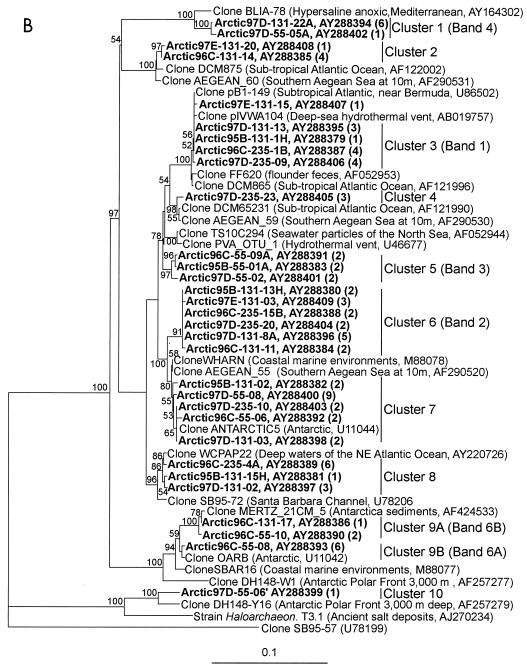
FIG. 5.
Neighbor-joining trees showing phylogenetic affiliations of representative partial 16S rRNA gene sequences retrieved from Arctic Ocean samples to closely related database sequences. Only one representative from each clone library of sequences that are ≥99% similar is shown; the total number of clones represented by a sequence is given in parentheses. Clones from this study are indicated in boldface. Bootstrap values higher than 50% are shown. The tree is unrooted, and the bar indicates a Jukes-Cantor distance of 0.1. (A) Marine group I Crenarchaeota (positions 536 to 941); clone SB95-72 is used as an outgroup; clusters are named as in reference 24. (B) Marine group II and IV Euryarchaeota (positions 512 to 937); clone SB95-57 is used as an outgroup.
RESULTS AND DISCUSSION
DGGE.
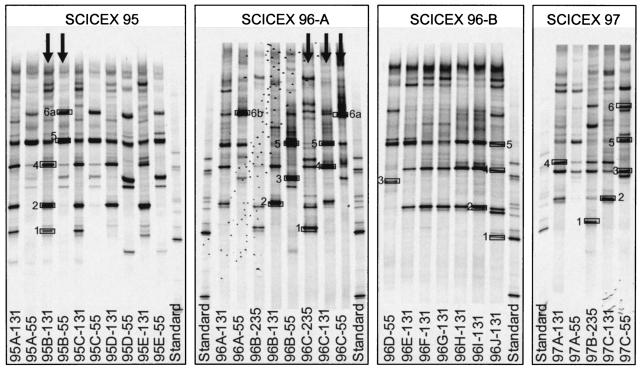
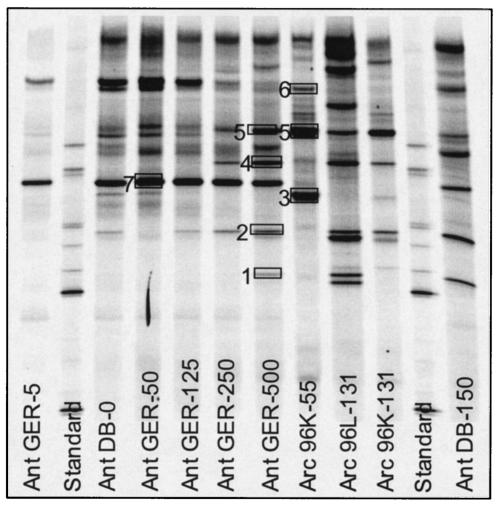
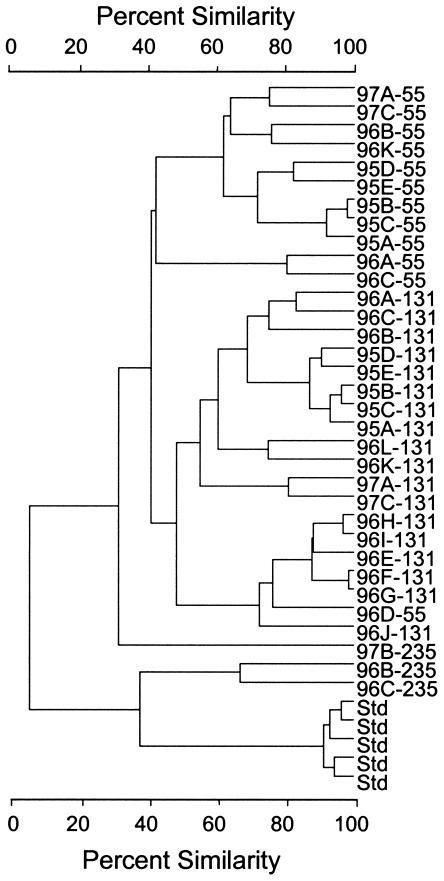
Comparison of DGGE fingerprints (Fig. 2 and 3) of samples from both polar oceans revealed that the richness of the Archaea assemblage was greater in samples from deep water than in those from the upper water column. In addition, most of the DGGE bands were common to both Arctic Ocean and antarctic samples. However, band 3, a dominant band in the Arctic Ocean surface mixed layer, was not detected at any depth in antarctic samples, whereas band 7, a dominant band present at all depths in antarctic samples, was not found in any Arctic Ocean samples. DNA sequences obtained from DGGE bands were compared to longer sequences from cloned ribosomal gene amplicons and to closely related sequences from GenBank. The sequences of amplicons from DGGE bands with the same melting points were identical, confirming that the same ribotypes were present in arctic and antarctic deep-water samples (Fig. 3). Cluster analysis of DGGE banding patterns (Fig. 4) revealed that the Arctic Ocean samples collected at different times of the year clustered by depth rather than by cruise (season).
FIG. 2.
DGGE fingerprints of Archaea amplicons. Clone libraries were constructed from the samples marked by arrows and from two other SCICEX 97 samples; however, these samples were not analyzed by DGGE. The numbered boxes indicate bands that were excised for sequencing.
FIG. 3.
Denaturing gradient gel comparing antarctic and arctic samples. The numbered boxes indicate bands that were excised for sequencing.
FIG. 4.
Dendrogram showing similarities of DGGE banding patterns for Arctic Ocean samples shown in Fig. 2. Std, standards.
Relative abundance in clone libraries.
All sequences of cloned 16S rRNA gene fragments grouped with marine group I Crenarchaeota (111 clones), marine group II Euryarchaeota (86 clones), and group IV Euryarchaeota (1 clone). The relative abundances of group I Crenarchaeota and group II Euryarchaeota in different libraries are given in Table 2. Clones containing inserts with sequences affiliated with group I Crenarchaeota dominated libraries constructed from surface mixed-layer samples collected during SCICEX 95 and 97, but most sequences from the library constructed from a SCICEX 96 surface mixed-layer sample were affiliated with group II Euryarchaeota. The highest proportion of clones containing inserts affiliated with group I Crenarchaeota (85%) was found in a library constructed from a surface mixed-layer sample collected during SCICEX 95, a late-winter-early-spring cruise. The SCICEX 96 and 97 cruises took place during the late summer and fall at the end of the productive season; therefore, this difference may reflect a seasonal shift in the composition of the Archaea community, with group I Crenarchaeota dominating in the winter, as has been reported by others (10).
We also found that clones containing inserts affiliated with group I Crenarchaeota and group II Euryarchaeota were equally abundant below the surface mixed layer in Arctic Ocean samples. Several reports have indicated a distinct partitioning of the marine water column between Crenarchaeota and Euryarchaeota, with Euryarchaeota dominant in the photic zone and Crenarchaeota dominant in deep water, for example, in the Santa Barbara Channel and Monterey Bay, California (13, 25, 33); the Mediterranean Sea (24); the North Atlantic Ocean and North Sea (24, 33); and west of the Antarctic Peninsula (10). Massana et al. (24) concluded that group I Crenarchaeota are ubiquitous and abundant and are often the dominant prokaryotes in marine waters, whereas group II Euryarchaeota are dominant only at the surface in temperate waters. In the Southern Ocean, group I Crenarchaeota phylotypes were dominant throughout the water column and group II Euryarchaeota were almost absent from deep-water samples (12, 24). Pernthaler et al. (33) demonstrated seasonal variation in the relative abundance of Euryarchaeota, with the largest populations occurring in surface waters during spring and summer. Karner et al. (19) conducted a quantitative analysis of the vertical distribution of Archaea in the Pacific Ocean using FISH. In contrast to previous reports of the dominance of Euryarchaeota in surface waters, they found the same low abundances of Euryarchaeota throughout the water column (surface to 4,750 m). In accordance with other studies, they also found that Crenarchaeota were dominant in deep water. In another study, only Euryarchaeota sequences were retrieved from 3,000 m in the antarctic Polar Front (21).
Crenarchaeota.
The majority of the group I Crenarchaeota sequences we obtained (104 of 111) form a closely related, monophyletic clade with sequence similarities of 98 to 100% (Fig. 5A). This clade is identified as cluster 1-α, following the designation given by Massana et al. (24). Sequences in this clade are closely related (99 to 100% similarity) to sequences cloned from various environments, such as the antarctic (7), the North Atlantic at a depth of 500 m (27), marine geothermal fluids sampled near the Cascadia Margin (8), a deep-sea hydrothermal vent (38), sediments of the Aegean Sea (GenBank description), and coastal marine environments (11). All of the Crenarchaeota clones we obtained from surface mixed-layer samples belonged to cluster 1-α, which is consistent with previous reports (24).
Cluster 1-γ contained sequences from deep water that were closely related (99%) to cloned sequences obtained from an East Pacific Rise deep-sea hydrothermal-vent sample (GenBank description) and particles from the North Sea (40), the subtropical Atlantic Ocean (GenBank description), and the Santa Barbara Channel (25). Our results are in agreement with previous reports and suggest that members of cluster 1-γ are adapted to live in the aphotic zone (24).
Despite the overall dominance (56%) of group I Crenarchaeota sequences in our clone libraries, none of the sequences we obtained from DGGE bands were related to this group. This may be explained by a mismatch at the 3′ end of the putatively universal 517r primer. There are two other mismatches in the middle of the primer against the sequences of both group I Crenarchaeota and group II Euryarchaeota. Despite these mismatches, this primer set amplified a diverse Euryarchaeota population (41). It has been shown that a single mismatch at or near the terminal 3′ base of a primer affects PCR more dramatically than single mismatches located internally or at the 5′ end of a primer (37). Vetriani et al. (41) also used primer 517r for amplifying Archaea and identified only three Crenarchaeota bands in their DGGE profiles, even though they detected much greater Crenarchaeota diversity in clone libraries from the same samples. They also concluded that this discrepancy is due to the mismatch at the 3′ end of primer 517r.
Euryarchaeota.
Marine group II Euryarchaeota sequences were more diverse than group I Crenarchaeota sequences (Fig. 5B), consistent with previous studies (24). Sequences in this group were assigned to nine different clusters, depending on sequence similarities and tree topology. Another cluster (Fig. 5B, cluster 10) contained sequences affiliated with marine group IV Euryarchaeota. Shorter sequences from DGGE bands were contained in sequences representing five of these clusters. The discussion below is ordered by the number of the DGGE band as identified in Fig. 2 and 3, with clusters that did not contain sequences from DGGE bands discussed last.
Sequences in cluster 3 (Fig. 5B) contained the sequence obtained from DGGE band 1 (100% similarity). Figures 2 and 3 show that band 1 was intense in fingerprints of samples collected below the halocline (235 m) on SCICEX 96 and 97 and in the antarctic samples GER 500 m and DB 150 m but was faint or absent in fingerprints of samples from shallower depths. In contrast, this band was intense in halocline samples from SCICEX 95. Sequences in cluster 3 were obtained from 131- and 235-m Arctic Ocean samples and were 100% identical over 520 bp to a sequence cloned from a sample collected at 1,000 m in the subtropical Atlantic near Bermuda (GenBank description). These sequences were also 98 to 99% similar to sequences cloned from deep-sea hydrothermal-vent fluids (38) and from flounder feces (40).
Cluster 6 was dominated by sequences from the halocline. Sequences in cluster 6 contained (100% similarity) the shorter sequence obtained from DGGE band 2. Band 2 was intense in fingerprints of Arctic Ocean halocline samples (Fig. 2) and was found in fingerprints of antarctic samples (GER 125 m and below; DB 0 and 150 m). Sequences in cluster 6 were distantly related to sequences in GenBank. The most closely related sequence (96% similarity) was cloned from a coastal marine environment (11). Cluster 5 contained only sequences obtained from mixed-layer samples. Sequences in cluster 5 contained (100% similarity) the shorter sequence obtained from DGGE band 3. Band 3 was intense in DGGE fingerprints from Arctic Ocean surface mixed-layer samples (Fig. 2). This band was faint or absent in fingerprints from antarctic samples (Fig. 3). Sequences in cluster 5 were 97% similar to sequences retrieved from 10 m in the southern Aegean Sea (28).
Cluster 1 contained six sequences from the halocline and one from the mixed layer. Sequences in cluster 1 contained (100% similarity) the shorter sequence obtained from DGGE band 4. Band 4 was intense in fingerprints from samples of the Arctic Ocean halocline (Fig. 2). In antarctic samples, band 4 was intense in DGGE fingerprints from deep-water samples (GER 500), but it was faint or absent in fingerprints from surface water samples (Fig. 3). Although we did not retrieve this sequence from clone libraries constructed from SCICEX 95 and 96 samples, DGGE analysis indicated that the ribotype represented by band 4 was present in all three seasons. Cluster 1 sequences are 97% similar to a sequence cloned from samples collected in hypersaline, anoxic basins in the Mediterranean Sea (GenBank description).
All (except one) of the sequences in cluster 9 were from the mixed-layer samples, and they formed two subclusters (9A and 9B) that were 98% similar to each other. Sequences in subclusters 9A and 9B contained (100% similarity) the shorter sequence obtained from DGGE bands 6B and 6A, respectively, suggesting that the small differences between them are not due to cloning or sequencing errors. DGGE analysis showed that bands 6A and 6B were intense in fingerprints from Arctic Ocean mixed-layer samples. None of the sequences cloned from SCICEX 95 and 97 samples fell into this cluster; however, bands 6A and 6B were present in DGGE fingerprints of samples from these cruises (Fig. 2, verified by sequencing DNA extracted from bands), indicating the year-round presence of these ribotypes. Bands 6A and 6B were also intense in DGGE fingerprints of antarctic surface water samples, but they were absent from fingerprints of deep-water samples (Fig. 3). Bands 6A and 6B had similar electrophoretic mobilities in DGGE and were not always distinct. Sequences in clusters 9A and 9B were 99% similar to a sequence cloned from continental shelf sediments collected off Antarctica (GenBank description) and from marine surface waters of Antarctica (14), respectively. Massana et al. (24) reported that 25 clones from surface water and only 1 clone from deep water fell into their cluster II-α (the same as our cluster 9a and b) and concluded that organisms represented by sequences in this cluster were adapted to surface waters.
We were not able to establish correspondence between the remaining cloned sequences and sequences from DGGE bands. Sequences in clusters 2 and 4 were retrieved from halocline and deep-water samples, respectively, and were 97 and 99% similar, respectively, to sequences retrieved from samples collected in the southern Aegean Sea at 10-m depth (28) and from the subtropical Atlantic (GenBank description). Sequences that corresponded to cluster 7 were retrieved from all depths but dominated libraries from Arctic Ocean mixed-layer samples. A previous study by Massana et al. (24) of the distribution of Archaea in different oceanic regions also found that sequences in this cluster (our cluster 7 corresponds to a subcluster of their group II-β) were retrieved primarily from surface samples, suggesting that this organism is adapted to surface conditions. We were unable to identify a DGGE band corresponding to these sequences in our Arctic Ocean samples. However, they were 99% similar to a sequence retrieved from an antarctic sample by DeLong et al. (14). We also found a DGGE band sequence (band 7) in our antarctic samples that was 97% similar to cluster 7 sequences. Band 7 was intense in DGGE fingerprints of samples from all depths in the antarctic (Fig. 3). Sequences in cluster 8 were 98 to 99% similar to sequences obtained from deep water of the northeast Atlantic (GenBank description) and the Santa Barbara Channel (25).
One clone from the surface mixed layer was assigned to cluster 10. This sequence was 96% similar to the sequence of clone DH148-Y16, a newly identified lineage of Archaea, marine group IV Euryarchaeota, related to Haloarchaeon (22). Group IV Euryarchaeota sequences were detected in antarctic samples and in the Atlantic and Mediterranean using group-specific primers (22). Prior to our study, group IV Euryarchaeota sequences had been retrieved solely from samples collected at bathypelagic depths and had not been reported in surface waters.
The sequence from DGGE band 5 was not found in any of the clones we sequenced. This band was present in fingerprints of Arctic Ocean samples from both the mixed layer and the halocline but was faint or absent from 235-m samples. In antarctic samples, the band was intense in fingerprints from deep-water samples (GER 500 m) and faint or absent at shallower depths (GER 250 m and shallower). The sequence we obtained from it was 95% similar to sequences of group III Euryarchaeota environmental clones: AEGEAN_62, retrieved from the southern Aegean Sea at 10-m depth (28), and ME-450_P9, retrieved from the Mediterranean Sea at 450 m (24). Group III Euryarchaeota have not previously been reported in antarctic coastal water samples; however, a sequence that was 88% similar to our band 5 sequence was retrieved from a water sample collected at 3,000 m from the polar front just north of the antarctic peninsula (21). Previous studies retrieved marine group III Euryarchaeota sequences primarily from mesopelagic samples (17, 24). Massana et al. (24) screened 2,328 Archaea clones from different oceanic provinces and found only one group III Euryarchaeota clone (retrieved from 450 m in the Mediterranean Sea). In contrast, Moeseneder et al. (28) reported that 12% of the clones retrieved from the photic zone at 10-m depth in the eastern Mediterranean Sea belonged to marine group III Euryarchaeota.
Our study shows that although there appears to be significant overlap in the compositions of deep-water Archaea assemblages of the two polar oceans, there appear to be significant differences among assemblages in surface water samples. Previous studies have reported that antarctic planktonic Archaea assemblages were dominated by group I Crenarchaeota and that group II Euryarchaeota were a minor fraction of the Archaea assemblage (29, 30). In contrast, analysis of our Arctic Ocean clone libraries suggests that the abundances of the two groups are similar there. In addition, most of the group II Euryarchaeota sequences we retrieved were <97% similar to previously reported antarctic sequences. Only clusters 7 and 9 contained Arctic Ocean sequences that were similar (>99%) to previously reported antarctic sequences.
The biogeography of polar Archaea appears to differ from that of polar Bacteria in two significant ways. The first is that the richness of Archaea sequences encountered in this study appears to be lower than the richness of Arctic Ocean Bacteria (5) reported previously. The Bacteria study (5) used a similar combination of DGGE and cloning to examine the distribution of Bacteria in the Arctic Ocean, so the difference in apparent richness is not likely to be due to differences in methodology. However, differences in the robustness of primers for Bacteria versus Archaea, as discussed above, may contribute to the apparent discrepancy. Murray et al. (29) reported a similar discrepancy in the antarctic, and Massana et al. (24), who screened >2,000 clones from all over the world, concluded that the diversity of marine planktonic Archaea is low and is comparable to that detected in previous studies based on fewer samples. Alternatively, differences in richness, and presumably also diversity, may represent a real difference in the population genetics of these two groups. Secondly, in contrast to Arctic Ocean Bacteria sequences, which in general were related to polar or deep-sea clones or isolates (5), more of the Archaea we detected in this study seem to be affiliated with ribotypes found in extreme environments, warm seas, and temperate surface waters—habitats that are very unlike cold polar oceans. This may simply be a function of the relative abundance of Bacteria versus Archaea sequences in GenBank and of the distribution of sampling efforts.
Acknowledgments
We express our appreciation to the U.S. Navy, particularly to the officers and crew of the USSN Cavalla, Pogy, and Archerfish; to Arctic Submarine Laboratory personnel; and to the scientists who collected samples for us on the SCICEX 95, 96, and 97 cruises, especially T. DeLaca, D. Stockwell, T. Whitledge, R. Sambrotto, G. Steward, J. Gossett, M. Mosher, and B. Campbell. These studies would not have been possible without their willing collaboration. We are also indebted to A. Murray for providing the samples of genomic DNA from antarctic waters used in this study and for her advice and suggestions during their analysis.
This work was supported by NSF awards OPP 95-00237, OPP 96-25131 (reissued as OPP 97-96261), and OPP 98-09971 to J.T.H. S.R. was supported by a CURO Internship from the UGA Honors Program, and B.R. was supported by REU supplements to NSF grant MCB 99-77886.
REFERENCES
- 1.Aagaard, K., L. K. Coachman, and E. C. Carmack. 1981. On the halocline of the Arctic Ocean. Deep Sea Res. 28:529-545. [Google Scholar]
- 2.Aagaard, K., J. H. Swift, and E. C. Carmack. 1995. Thermohaline circulation in the Arctic Mediterranean Sea. J. Geophys. Res. 90:4833-4846. [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Bano, N., and J. T. Hollibaugh. 2000. Diversity and distribution of DNA sequences with affinity to ammonia-oxidizing bacteria of the β-subdivision of the class Proteobacteria in the Arctic Ocean. Appl. Environ. Microbiol. 66:1960-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bano, N., and J. T. Hollibaugh. 2002. Phylogenetic composition of bacterioplankton assemblages from the Arctic Ocean. Appl. Environ. Microbiol. 68:505-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barns, S. M., C. F. Delwiche, J. D. Palmer, and N. R. Pace. 1996. Perspectives on archaeal diversity, thermophily and monophily from environmental rRNA sequences. Proc. Natl. Acad. Sci. USA 93:9188-9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beja, O., E. V. Koonin, L. Aravind, L. T. Taylor, H. Seitz, J. L. Stein, D. C. Bensen, R. A. Feldman, R. V. Swanson, and E. F. DeLong. 2002. Comparative genomic analysis of archaeal genotypic variants in a single population and in two different oceanic provinces. Appl. Environ. Microbiol. 68:335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bidle, K. A., M. Kastner, and D. H. Bartlett. 1999. A phylogenetic analysis of microbial communities associated with methane hydrate-containing marine fluids and sediments in the Cascadia margin (ODP site 892B). FEMS Microbiol. Lett. 177:101-108. [DOI] [PubMed] [Google Scholar]
- 9.Brosius, J., M. L. Palmer, J. K. Poindexter, and H. F. Noller. 1978. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 75:4801-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Church, M. J., E. F. DeLong, H. W. Ducklow, M. B. Karner, C. M. Preston, and D. M. Karl. 2003. Abundance and distribution of planktonic Archaea and Bacteria in the waters west of the Antarctic Peninsula. Limnol. Oceanogr. 48:1893-1902. [Google Scholar]
- 11.DeLong, E. F. 1992. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 89:5685-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLong, E. F., L. L. King, R. Massana, H. Cittone, A. E. Murray, C. Schleper, and S. G. Wakeham. 1998. Dibiphytanyl ether lipids in non-thermophilic crenarchaeotes. Appl. Environ. Microbiol. 64:1133-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLong, E. F., L. T. Taylor, T. L. Marsh, and C. M. Preston. 1999. Visualization and enumeration of marine planktonic archaea and bacteria by using polyribonucleotide probes and fluorescent in situ hybridization. Appl. Environ. Microbiol. 65:5554-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLong, E. F., K. Y. Wu, B. B. Prezelin, and R. V. Jovine. 1994. High abundance of Archaea in Antarctic marine picoplankton. Nature 371:695-697. [DOI] [PubMed] [Google Scholar]
- 15.Felsenstein, J. 1993. PHYLIP (Phylogeny Inference Package), v. 3.5 ed. University of Washington, Seattle.
- 16.Ferrari, V. C., and J. T. Hollibaugh. 1999. Distribution of microbial assemblages in the central Arctic Ocean basin studied by PCR/DGGE: analysis of a large data set. Hydrobiology 401:55-68. [Google Scholar]
- 17.Fuhrman, J. A., and A. A. Davis. 1997. Widespread Archaea and novel Bacteria from the deep sea as shown by 16S rRNA gene sequences. Mar. Ecol. Prog. Ser. 150:275-285. [Google Scholar]
- 18.Fuhrman, J. A., K. McCallum, and A. A. Davis. 1992. Novel major Archaebacterial group from marine plankton. Nature 356:148-149. [DOI] [PubMed] [Google Scholar]
- 19.Karner, M. B., E. F. DeLong, and D. M. Karl. 2001. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 25:507-510. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, N., R. F. Anderson, R. A. Mortlock, P. N. Froelich, P. Kubik, B. Dittrichhannen, and M. Suter. 1995. Increased biological productivity and export production in the glacial Southern Ocean. Nature 378:675-680. [Google Scholar]
- 21.López-García, P., A. Lopez-Lopez, D. Moreira, and F. Rodriguez-Valera. 2001. Diversity of free-living prokaryotes from a deep-sea site at the Antarctic Polar Front. FEMS Microbiol. Ecol. 36:193-202. [DOI] [PubMed] [Google Scholar]
- 22.López-García, P., D. Moreira, A. López-López, and F. Rodríguez-Valera. 2001. A novel haloarchaeal-related lineage is widely distributed in deep oceanic regions. Environ. Microbiol. 3:72-78. [DOI] [PubMed] [Google Scholar]
- 23.Martin, J. H. 1990. Global-interglacial CO2 change: the iron hypothesis. Paleooceanography 5:1-13. [Google Scholar]
- 24.Massana, R., E. F. DeLong, and C. Pedros-Alio. 2000. A few cosmopolitan phylotypes dominate planktonic archaeal assemblages in widely different oceanic provinces. Appl. Environ. Microbiol. 66:1777-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massana, R., A. E. Murray, C. M. Preston, and E. F. DeLong. 1997. Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara Channel. Appl. Environ. Microbiol. 63:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massana, R., L. T. Taylor, A. E. Murray, K. Y. Wu, W. H. Jeffrey, and E. F. DeLong. 1998. Vertical distribution and temporal variation of marine planktonic Archaea in the Gerlache Strait, Antarctica, during early spring. Limnol. Oceanogr. 43:607-617. [Google Scholar]
- 27.McInerney, J. O., M. Mullarkey, M. E. Wernecke, and R. Powell. 1997. Phylogenetic analysis of group I marine archaeal rRNA sequences emphasizes the hidden diversity within the primary group Archaea. Proc. R. Soc. Lond. Ser. B 264:1663-1669. [Google Scholar]
- 28.Moeseneder, M. M., C. Winter, J. M. Arrieta, and G. J. Herndl. 2001. Terminal-restriction fragment length polymorphism (T-RFLP) screening of a marine archaeal clone library to determine the different phylotypes. J. Microbiol. Methods 44:159-172. [DOI] [PubMed] [Google Scholar]
- 29.Murray, A. E., C. M. Preston, R. Massana, L. T. Taylor, A. Blakis, K. Y. Wu, and E. F. DeLong. 1998. Seasonal and spatial variability of bacterial and archaeal assemblages in the coastal waters near Anvers Island, Antarctica. Appl. Environ. Microbiol. 64:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray, A. E., K. Y. Wu, C. L. Moyer, D. M. Karl, and E. F. DeLong. 1999. Evidence for circumpolar distribution of planktonic Archaea in the Southern Ocean. Aquat. Microbiol. Ecol. 18:263-273. [Google Scholar]
- 31.Myers, R. M., S. G. Fischer, L. S. Lerman, and T. Maniatis. 1985. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 13:3131-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul, J. H., and B. Myers. 1982. Fluorometric determination of DNA in aquatic microorganisms by use of Hoechst 33258. Appl. Environ. Microbiol. 43:1393-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pernthaler, A., C. M. Preston, J. Pernthaler, E. F. DeLong, and R. Amann. 2002. Comparison of fluorescently labeled oligonucleotide and polynucleotide probes for the detection of pelagic marine bacteria and archaea. Appl. Environ. Microbiol. 68:661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polz, M. F., and C. M. Cavanaugh. 1998. Bias in template-to-product ratios in multitemplate PCR. Appl. Environ. Microbiol. 64:3724-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raskin, L., J. M. Stromley, B. E. Rittmann, and D. A. Stahl. 1994. Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl. Environ. Microbiol. 60:1232-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravenschlag, K., K. Sahm, and R. Amann. 2001. Quantitative molecular analysis of the microbial community in marine arctic sediments (Svalbard). Appl. Environ. Microbiol. 67:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simsek, M., and H. Adnan. 2000. Effect of single mismatches at 3′-end of primers on polymerase chain reaction. Med. Sci. 2:11-14. [PMC free article] [PubMed] [Google Scholar]
- 38.Takai, K., and K. Horikoshi. 1999. Genetic diversity of Archaea in deep-sea hydrothermal vent environments. Genetics 152:1285-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takai, K., and Y. Sako. 1999. A molecular view of archaeal diversity in marine and terrestrial hot water environments. FEMS Microbiol. Ecol. 28:177-188. [Google Scholar]
- 40.Van der Maarel, M. J. E. C., R. R. E. Artz, R. Haanstra, and L. J. Forney. 1998. Association of marine Archaea with the digestive tracts of two marine fish species. Appl. Environ. Microbiol. 64:2894-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vetriani, C., H. W. Jannasch, B. J. MacGregor, D. A. Stahl, and A. L. Reysenbach. 1999. Population structure and phylogenetic characterization of marine benthic archaea in deep-sea sediments. Appl. Environ. Microbiol. 65:4375-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson, A. J., D. C. E. Bakker, A. J. Ridgwell, P. W. Boyd, and C. S. Law. 2000. Effect of iron supply on Southern Ocean CO2 uptake and implications for glacial atmospheric CO2. Nature 407:730-733. [DOI] [PubMed] [Google Scholar]
- 43.Wells, L. E., and J. W. Deming. 2003. Abundance of bacteria, the Cytophaga-Flavobacterium cluster and Archaea in cold oligotrophic waters and nepheloid layers of the Northwest Passage, Canadian Archipelago. Aquat. Microbiol. Ecol. 31:19-31. [Google Scholar]