Abstract
Over 300 amino acids are found in proteins in nature, yet typically only 20 are genetically encoded. Reassigning stop codons and use of quadruplet codons emerged as the main avenues for genetically encoding non-canonical amino acids (NCAAs). Canonical aminoacyl-tRNAs with near-cognate anticodons also read these codons to some extent. This background suppression leads to ‘statistical protein’ that contains some natural amino acid(s) at a site intended for NCAA. We characterize near-cognate suppression of amber, opal and a quadruplet codon in common Escherichia coli laboratory strains and find that the PylRS/tRNAPyl orthogonal pair cannot completely outcompete contamination by natural amino acids.
Keywords: 4-base codon, Boc-lysine, orthogonal translation systems, pyrrolysyl-tRNA synthetase, tRNA, synthetic biology
1. Introduction
Expanding protein synthesis to site-specifically introduce amino acids beyond the canonical 20 has impacted broad areas of molecular biology. Nearly 100 non-canonical amino acids (NCAAs) have been added to the genetic code [1–3]. Among these newly encoded NCAAs are useful biophysical probes [1,2] and post-translation modifications that play critical roles in basic processes ranging from cellular communication to oncogenesis [4–6]. Code expansion is made possible by employing an ‘extra’ aminoacyl-tRNA synthetase (AARS)/tRNA pair that does not cross react those of the host system and is, therefore, ‘orthogonal’ in that host cell. The AARS/tRNA pair is engineered to ligate a desired NCAA to its cognate tRNA which then directs co-translational site-specific insertion of the NCAA in protein by use of an ‘open’ codon. The vast majority of NCAAs are encoded by the UAG (amber) codon. To a lesser extent UAA (ochre), UGA (opal) and more rarely quadruplet codons (e.g., AGGA in [7]) have been used for code expansion.
Biosynthesis of biochemically homogenous proteins containing multiple non-canonical amino acids is a major goal of synthetic biology [8], yet it remains to be seen just how far the genetic code can be stretched. Current techniques enable simultaneous encoding of two NCAAs in the same polypeptide in vivo using stop [9,10] and quadruplet codons [7]. Quadruplet codons are of great interest, promising an astounding 256 ‘open’ codons. Since the initial discoveries of +1 frameshift suppressors [11,12], some progress has been made to enhance translation of quadruplet codons [7], but 4-base codons give rise to diverse translational products (e.g., [13]). Using supplementation incorporation, 3 analogs of canonical amino acids were encoded in a protein by the native E. coli aaRS/tRNA pairs using their cognate sense codons [14]. In vitro cell-free systems have incorporated up to 3 NCAAs [15].
Although in most organisms UAG, UGA and UAA signal translational stop, it is long known [16] that mutations resulting in premature stop codons can be suppressed by naturally evolved tRNAs that encode canonical amino acids with stop codons by virtue of anticodon mutation (e.g., glutamine amber suppressor supE) or by other mutations that stimulate near-cognate decoding of the stop codon (reviewed in [17]). Pyrrolysyl-tRNA synthetase and tRNAPyl are the only known system to have naturally evolved to promote amber codon re-assignment to a NCAA, pyrrolysine [18]. For this reason, ‘un-engineered’ PylRS and tRNAPyl were able to directly expand the genetic code of E. coli [19–21], and this pair has become a critical vehicle for genetic code expansion (reviewed in [3]). Biochemical [19,22] and structural [21] characterization of PylRS revealed that the enzyme does not recognize the anticodon bases, indicating that genetic code expansion beyond the amber codon is possible with PylRS and tRNAPyl. As a PylRS/tRNAPylUUA ochre codon decoding pair was recently characterized [10], we were prompted to evaluate the opal and quadruplet codon decoding capacity of PylRS/tRNAPyl in vivo in E. coli.
2. Materials and Methods
2.1 Plasmids, strains, chemicals, and protein purification
E. coli strains TOP10, BL21(DE3), and DH5α were from Invitrogen; MG1655 was from the American Type Culture Collection (ATCC). In vivo suppression experiments require two plasmids as detail previously [23]. pKTS, encodes the Methanosarcina mazei PylRS (pKTS-pylS). pTech, contains both the lacZ reporter and a tRNA expression cassette with the M. mazei pylT (pTech-pylT). The pTech plasmid with wild type lacZ reporter (lacZ(WT)) was subject to quick-change mutagenes is (Agilent) to produce the M 3 to TAG a nd M 3 to TGA variants and to convert the tRNAPylCUA to tRNAPylUCA in the opal reporter. pETtrio-pylT(CUA)-PylRS-sfGFP134TAG [24] (K.A.O. & W.R.L unpublished data) was quick-changed to pETtrio-pylT( UCCU)-sfGFP134AGGA, encoding tRNAPylUCCU and a C-terminal His6-tagged superfolder green fluorescent protein (sfGFP) with AGGA at codon 134. tRNAs were cloned into pUC18 (XbaI/BamHI) for in vitro transcription, which was performed as described [25]. His6-tagged PylRS (in pet15b) was over-expressed and purified as before [26]. E. coli ArgRS and TrpRS were purified from the ASKA collection strains JW1865 and JW3347 [27], respectively. His-tagged proteins were purified over Ni-NTA (Qiagen) according to manufacture’s instructions. Oligonucleotide primers (Integrated DNA Technologies) and Pyl analogs, N-ε-(tert-butyloxycarbonyl)- L-lysine (BocK) (ChemImpex) and N-ε-cyclopentyloxycarbonyl-L-lysine (Cyc) (Sigma), were purchased.
2.2 In vivo suppression assays
BL21(DE3) cells were co-transformed with pKTS (empty or bearing pylS) and pTECH vectors (empty or containing a tRNAPyl amber or opal variant and lacZ with a cognate amber or opal codon at M3). β-galactosidase activity was measured as before [28]. Cells were grown in LB (37°C) to early stationary phase (A600 = 2.0); 20 μl of culture was used for each replicate and 6 independent cultures were measured in duplicate reactions. For each strain, intrinsic β-galactosidase activity (~3% of the activity of the LacZ(WT) reporter) in cells carrying empty pKTS and pTech plasmids is subtracted from all activity measurements.
BL21(DE3) cells were transformed with pETtrio-pylT(UCCU)-PylRS-sfGFP134AGGA, and sfGFP was expressed with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) or 1 mM IPTG and 5 mM BocK.
2.3 Aminoacylation assay
Aminoacylation assays were performed for all AARS/tRNA pairs as described [25]. Enzyme, tRNA, amino acid, and ATP were included in excess concentrations to detect low activity reactions. For PylRS, ArgRS and TrpRS, aminoacylation of tRNAs was performed (37°C) in 100 mM Na-HEPES (pH 7.2), 30 mM KCl (or 60 mM KCl for PylRS only), 10 mM MgCl2 (or 25 mM MgCl2 for PylRS only), 10 μM AARS, 5 μM α-[32P]-labeled tRNA, 10 mM amino acid, 2 mM ATP, and 4 mM dithiothreitol. The reaction was stopped with 1.5 volumes of 0.66 μg/μL nuclease P1 (Sigma) in 100 mM sodium citrate, pH 5.49. Because the tRNA is labeled only at A76, the radiolabeled reaction products after digestion are AMP (representing unaminoacylated tRNA) and aminoacyl-AMP (representing aminoacylated tRNA). The reaction products are separated on PEI-cellulose plates in 0.1 M ammonium acetate and 5% acetic acid then visualized, and quantified using phosphorimaging.
2.4 Mass spectrometry
Electrospray ionization mass spectrometry (ESI-MS) of sfGFP134AGGA was on an Applied Biosystems QSTAR Pulsar (Concord, ON, Canada) equipped with a nanoelectrospray ion source. The data were acquired in positive ion mode (500–2000 Da); spray voltage of +2000 V; flow rate of 700 nL/min. Spectra were analyzed with BioAnalyst software (Applied Biosystems). A mass range of m/z 500–2000 was used for spectral deconvolution and the output range was 20000 to 30000 Da (resolution of 0.1 Da and S/N threshold of 20).
3. Results
In evaluating PylRS/tRNAPyl pair for opal and 4-base decoding, we are interested in how and to what extent natural amino acids can be inserted at a protein locus intended for NCAA insertion. In the experiments below, we characterize background suppression of amber and opal codons, show aminoacylation activity of tRNAPyl variants, and attempt to install BocK with an AGGA decoding PylRS system.
3.1 Amber and opal background suppression in common E. coli strains
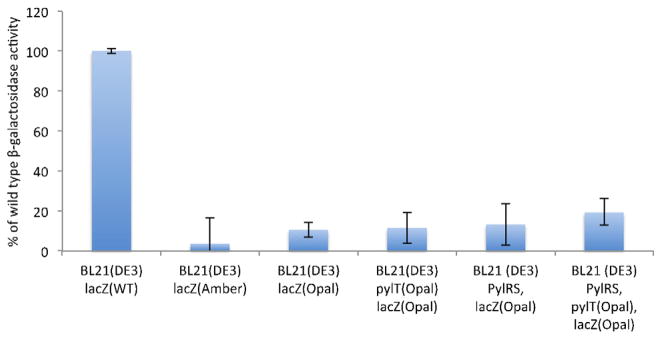
Given that directed evolution of AARS/tRNA orthogonal pairs requires a strain with high transformation efficiency to maintain library diversity (e.g., TOP10), while expression of NCAA-containing protein can be enhanced in a strain optimized for protein expression (e.g., BL21), we characterized amber and opal background suppression in common laboratory E. coli strains (Fig. 1). We also tested DH5α, a common cloning strain, and MG1655, which is the parent of an E. coli strain in which TAG was globally recoded to TAA for enhanced NCAA incorporation [29]. M3 of β-galactosidase was mutated to a TAG or TGA codon to generate amber and opal reporter constructs, which were separately transformed into different E. coli strains. Activity of the enzyme reports on translational read-through of amber or opal codons.
Fig. 1.
Relative amber (UAG) and opal (UGA) codon background suppression in different E. coli strains. β-galactosidase, containing either Methionine 3 (WT), M3UAG (Amber), or M3UGA (Opal), activities were calculated in Miller units. Since absolute activity varies between strains, WT activity for each strain was set to 100% to show relative suppression levels.
Amber suppression in DH5α is highly efficient, directed by the suppressor tRNA (supE44) encoded in its genome. The SupE gene product, tRNAGlnCUA, is aminoacylated by GlnRS and leads to Gln incorporation at UAG [30]. In MG1655, BL21 and TOP10 strains, no suppressor tRNA is present in their genomes. Despite this fact, background amber suppression is found in all three strains. Although low in Top 10 (1%, not significantly above background), greater suppression is seen in BL21 (3%) and MG1655 (17%). In both BL21 and MG1655, therefore, using the amber codon to encode NCAAs may lead to greater contaminating incorporation of canonical amino acids compared to TOP10. In BL21(DE3) and derived strains, near-cognate incorporation of Trp, Gln, and Tyr in response to UAG has also been reported [31], and code expansion systems for phosphoserine [5] and BocK (K.A.O. & W.R., unpublished data) displayed contamination by Gln and Trp at the UAG encoded locus, respectively.
We find that, in the absence of a suppressor tRNA, UGA displays generally higher background suppression than UAG (Fig. 1). Even when background amber suppression is low (e.g., 3% in BL21(DE3)), contamination of an NCAA encoded site with natural amino acids has been observed in BL21(DE3) and derived strains [5,31] (K.A.O. & W.R.L, unpublished data). We anticipate that UGA-based code expansion systems may encounter yet greater contamination by natural amino acids than amber codon systems.
3.2 Orthogonality of PylRS/tRNAPylUCA for opal codon decoding
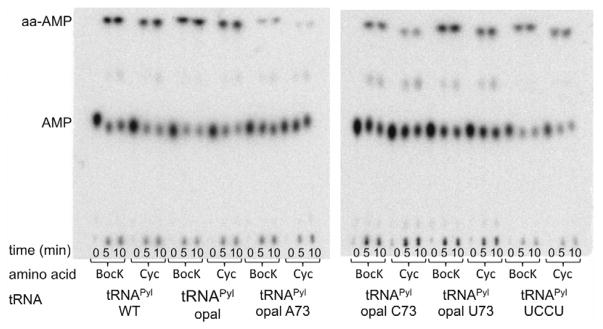
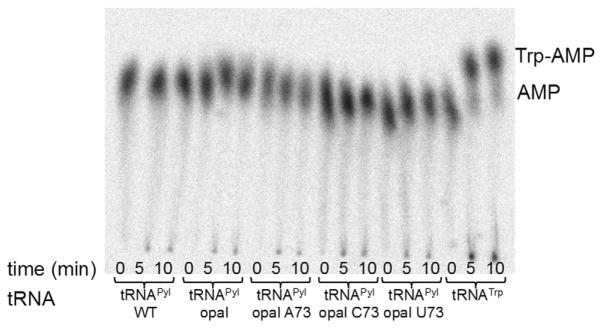
It was recently shown that the PylRS/tRNAPyl can support incorporation of BocK in response to the opal codon in E. coli BL21(DE3) (K.A.O. & W.R.L, unpublished data). Mass spectroscopic analysis revealed that the sfGFP reporter contained both Trp and BocK at the position encoded by UGA. In the absence of BocK, only the Trp containing peptide was detected, but the source of Trp read-through was not confirmed. To rule out the possibility that Trp could be ligated to tRNAPylUCA, we assayed PylRS and TrpRS in vitro with α-[32P]-labeled tRNA substrates (Fig. 2–3). Commercially available Pyl analogs (BocK and Cyc) are used for the assays. Plateau aminoacylation by PylRS with tRNAPylUCA and either BocK (52 ±11 %) or Cyc (46 ± 3%) is similar to that with wild type tRNAPylCUA and BocK (45 ± 5 %) or Cyc (43 ± 9 %) (Table 1, Fig. 2). The E. coli TrpRS, although active with tRNATrp, fails to ligate Trp to any tRNAPyl variant (Fig. 3).
Fig. 2.
In vitro aminoacylation of tRNAPyl variants by M. mazei PylRS with Pyl analogs N-ε-(tert-butyloxycarbonyl)- L-lysine (BocK) or N-ε-cyclopentyloxycarbonyl-L-lysine (Cyc). An additional spot (representing < 5% of total radiolabel) between the aa-AMP and AMP is an unidentified possibly degraded reaction product, often seen in aminoacylation assays (e.g., [53]).
Fig. 3.
In vitro aminoacylation of tRNAPyl variants and tRNATrp by E. coli TrpRS with Trp.
Table 1.
Plateau aminoacylation levels.
| enzyme | amino acid | tRNAPyl | tRNAPyl opal | tRNAPyl opalA73 | tRNAPyl opal C73 | tRNAPyl opal U73 | tRNAPyl UCCU | tRNATrp | tRNAArg |
|---|---|---|---|---|---|---|---|---|---|
| PylRS | BocK | 45 ± 5% | 52 ± 11% | 13 ± 2% | 25 ± 2% | 40 ± 2% | 40 ± 6% | ||
| Cyc | 43 ± 9% | 46 ± 3% | 6 ± 3% | 13 ± 4% | 29 ± 3% | 40 ± 7% | |||
| TrpRS | Trp | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 77 ± 7% | |
| ArgRS | Arg | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 89 ± 1% |
Values are in % of total tRNA aminoacylated at plateau level (reached before the 10 min time point) of the reaction.
n.d. – no aminoacylation detected.
We conducted in vivo and in vitro assays to further characterize the PylRS/tRNAPyl sys tem for opal codon decoding. E. coli BL21 (DE3) cells were transformed with empty plasmids or with two vectors (pKTS and pTECH) for independent expression of PylRS or tRNAPylUCA, respectively (Fig. 4). These experiments lack the amino acid substrate for PylRS as here we evaluate background opal suppression, possible endogenous charging of tRNAPylUCA or canonical amino acid charging by PylRS. We observe an opal codon background suppression of 10% in BL21, and addition of tRNAPylUCA (11%) or the PylRS (13%) does not significantly enhance suppression. The tRNAPylUCA is, therefore, not a substrate for endogenous E. coli synthetases. Addition of both tRNAPylUCA and PylRS (19%) appears to marginally increase activity, but this is within the error of the background measurement for opal suppression.
Fig. 4.
Opal codon background suppression in E. coli BL21(DE3) is not significantly increased by the addition of tRNAPyl or PylRS.
Base 73 of the tRNAPylUCA, the so-called discriminator base, was mutated recently (K.A.O. & W.R.L, unpublished data) to find a tRNAPyl variant better able to compete with Trp-tRNATrp read-through of UGA. In vivo, these mutants did not significantly affect read-though of UGA, and we found in vitro that these substrates aminoacylated to a significantly lower level than tRNAPylUCA with the wild type G73 (Table 1, Fig. 2).
Taken together, the data show that background opal codon suppression in E. coli BL21 exists at a level of ~10% and this leads to Trp insertion (K.A.O. & W.R.L, unpublished data) in response to the UGA codon. It is well established [21,26,32], and demonstrated again in Fig. 4, that PylRS is not active with any of the canonical 20 amino acids, so there is no possibility of PylRS forming Trp-tRNAPyl or aminoacylating any tRNAPyl variant with natural amino acids. Since TrpRS has no activity towards tRNAPylUCA, Trp insertion likely results from near-cognate (one codon anti-codon mismatch) suppression of UGA by tRNATrpCCA. PylRS/tRNAPylUCA can promote assignment of UGA to encode a NCAA, such as BocK, but BocK-tRNAPylUCA fails to completely outcompete background suppression by Trp-tRNATrp.
3.3 Near-cognate suppression outcompetes BocK-tRNAPylUCCU for quadruplet codon decoding
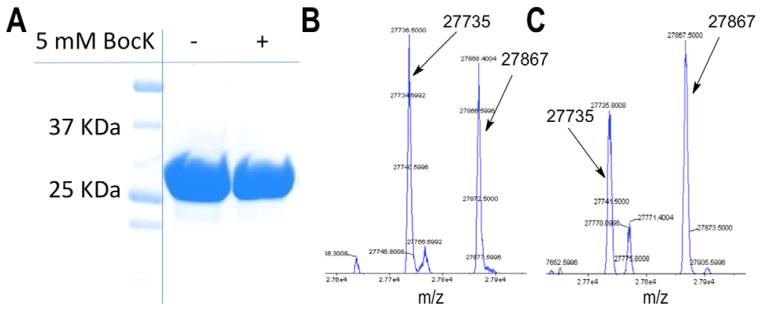
Since quadruplet codons could provide many new openings for genetic code expansion, we characterized PylRS/tRNAPylUCCU for its capacity to decode the 4-base codon AGGA. We used a sfGFP reporter with AGGA encoded at position N134. BL21(DE3) cells transformed with pETtrio-pylT(UCCU)-PylRS-sfGFP134AGGA showed significant and similar sfGFP expression levels both in the absence (122 mg/L culture) and in the presence of 5 mM BocK (138 mg/L culture). Although we suspected BocK should be incorporated into the sfGFP reporter to some extent, the ESI-MS analysis only detected Arg at position 134 (Table 2, Fig. 5). Addition of BocK to the medium did not change the ESI-MS patterns. Interestingly, we found about half as much background AGGA suppression in TOP10 cells transformed with this sfGFP reporter (70 mg/L compared to ~130 mg/L in BL21(DE3)).
Table 2.
ESI-MS characterization of sfGFP.
| Protein | Calculated mass /Da | Detected mass /Da |
|---|---|---|
| sfGFPN134AGGAa | 27867c | 27868 |
| 27735d | 27737 | |
| sfGFPN134AGGAb | 27867c | 27867 |
| 27735d | 27736 |
sfGFP expressed in cells with pETtrio-pylT(UCCU)-PylRS-sfGFP134AGGA, 1 mM IPTG.
sfGFP expressed in cells with pETtrio-pylT(UCCU)-PylRS-sfGFP134AGGA, 1 mM IPTG and 5 mM BocK.
Full length sfGFP proteins.
Full length sfGFP proteins without N-terminal Met1.
Fig. 5.
Quadruplet codon AGGA encodes arginine in vivo in E. coli. (A) Protein produced by BL21(DE3) cells transformed with pETtrio-pylT(UCCU)-sfGFP134AGGA, grown with or without BocK. (B) ESI-MS of sfGFP produced by cells grown with (B) or without (C) BocK.
We conducted in vitro aminoacylation assays to determine if tRNAPylUCCU is a competent substrate for PylRS (Fig. 2). With BocK, aminoacylation reached a plateau of 40 ± 6% for tRNAPylUCCU, compared to 45 ± 5% for tRNAPylCUA (the wild type substrate). The aminoacylation level for tRNAPylUCCU should be similar to amber and opal decoding tRNAPyl variants that support BocK incorporation in vivo [33] (K.A.O. & W.R.L unpublished data). To test if misacylation might contribute to the signal seen in Fig. 5, we assayed tRNAPyl variants for Arg accepting activity. Although, the E. coli ArgRS is highly active with its native substrate, no tRNAPyl variants could be aminoacylated with Arg (Fig. 6). In summary, the data indicate that the PylRS/tRNAPylUCCU system, although competent in BocK aminoacylation, cannot outcompete AGGA suppression by E. coli’s native Arg-tRNAArg.
Fig. 6.

In vitro aminoacylation of tRNAPyl variants and tRNAArg by E. coli ArgRS.
4. Discussion
In the experiments above, we showed that amber (5–10%) and opal codon (10–25%) background suppression vary in different commonly used laboratory E. coli strains, and this will likely lead to mis-incorporation of natural amino acid in many of the currently available orthogonal translation systems. Background amino acid incorporation at amber and opal codons has not been extensively described or quantified, but in certain cases mass spectrometry has detected the presence of TAG encoding both the desired NCAA and contaminant natural amino acid (e.g., [5]). Incorporation of BocK in response to UGA by PylRS/tRNAPyl is accompanied by significant Trp incorporation (K.A.O. & W.R.L unpublished data). It is well known that the ribosome is most permissive to codon anti-codon mismatch at the third nucleotide of the codon, so near-cognate suppression of UGA by Trp-tRNATrpCCA is not unexpected [34–37].
Several engineered E. coli strains survive without release factor 1 (RF1), which is responsible for stimulating ribosome dissociation when the amber codon is encountered in the RNA message [38]. RF1 deletion leads to extension of natural E. coli proteins beyond their normal TAG stop codon, and some of these extensions are detrimental to cellular fitness [39,40]. These defective phenotypes can be alleviated either by compensating mutations in RF2 (possibly allowing RF2 to resolve ribosomes stalled at UAG) [31,41–43], or by reassignment of at least 7 critical TAG stop codons to TAA [39]. These strains are of great utility for recoding UAG to a sense codon because the RF1 deletion strains provide a higher level of UAG read-through [39,43], but background suppression has not been thoroughly characterized in these strains. In a ΔRF1 E. coli strain with a minimized genome (lacking ~500 genes), Johnson et al. [31] reported background suppression of UAG with Trp, Tyr, and Gln by single-base mismatch near-cognate suppression. In the presence of an evolved TyrRS/tRNATyr orthogonal pair, incorporation of p-acetyl- L-phenylalanine was observed in the mass spectrum, but peptides resulting from background suppression with Trp, Tyr or Gln were not detected. They also observe a marked increase in background suppression upon RF1 deletion [31]. It is likely that for some NCAAs, expression of protein in a RF1 deletion strain will lead to higher rates of NCAA incorporation, but enhanced near-cognate suppression could yield greater natural amino acid contamination.
We found the situation is exacerbated when considering quadruplet codons. The background suppression of AGGA by Arg-tRNAArg is so efficient that it completely outcompetes AGGA reading by BocK-tRNAPylUCCU, even though we found tRNAPylUCCU is a competent substrate for PylRS. To address generally inefficient quadruplet decoding of the E. coli ribosome, Neumann et al. evolved an orthogonal ribosome for increased suppression of AGGA [7]. Using a TyrRS/tRNATyr orthogonal pair, enhanced p-azido-L-phenylalanine (AzF) incorporation by the evolved ribosome was confirmed by mass spectrometry. The evolved ribosome allowed significantly higher background insertion of (presumably) natural amino acid when AzF was absent, but the level of natural amino acid contamination at the AGGA encoded locus in the presence of the orthogonal pair and AzF was not quantified.
Quadruplet decoding is still not completely understood (reviewed in [44,45]). Some data favor a 3-base decoding model followed by ribosomal slipping to the +1 frame, but almost all 4-base suppressors do have extended anticodon loops. In many 4-base decoding systems, establishing 4 W-C pairs is not essential for suppression activity, but it enhances suppression in certain cases. For AGGA, both Arg-tRNAArgCCU and Bock-tRNAPylUCCU could make at least 3 W-C base pairs in the decoding center, and it may be that the ribosome is showing its natural tendency to favor Arg-tRNAArgCCU over any other aa-tRNAs for AGG decoding and then ‘slipping’ to the +1 frame. Although quadruplet codons potentially allow incorporation of vastly more NCAAs simultaneously, competition with natural aminoacyl-tRNA will be a significant challenge. Designing efficient and accurate 4-base decoding systems is still an important direction for future work.
Current genetic code expansion technology is generally unable to completely eliminate contamination by natural amino acids. Fascinatingly, certain organisms can even derive a selective advantage from the production of such ‘statistical proteins’ [46]. For many structural, functional, and enzymological studies of NCAA-containing proteins, it is critical to produce homogenous product. Hyper-accurate ribosomes, with mutations in rpsL, may limit near-cognate suppression of amber and opal codons [47,48], providing a valuable genetic background for future studies.
The molecular mechanism of tRNAPyl decoding, in Methanosarcina or in the heterologous E. coli background, is not yet characterized. It is, therefore, not obvious why tRNAPyl does not compete more effectively against near-cognate suppression at UGA and AGGA. Since EF-Tu recognizes tRNAPyl as a ‘normal’ tRNA [49] and EF-Tu does not contact the anticodon, incompatibility with EF-Tu cannot explain the differential levels of near-cognate suppression permitted by amber, opal, and 4-base decoding tRNAPyl variants. Either there is an insufficient concentration of aminoacyl-tRNAPyl in the cell, or perhaps tRNAPyl is not optimally suited to decoding and translocation on the E. coli ribosome. In support of the latter view, we observed more efficient translation of Pyl-tRNAPyl in Methanosarcina acetivorans cells compared to E. coli, which shows significant levels of premature termination at the Pyl-encoding UAG [50,51]. Although mechanistic details have also not been established for other orthogonal pairs, it is already clear that for many orthogonal translation systems improved aminoacylation efficiency [52], or compatibility with EF-Tu [5] will be required to synthesize and deliver sufficient noncanonical aminoacyl-tRNA to the ribosome to outcompete near-cognate suppression by natural amino acids.
Highlights.
Nonsense suppression by natural amino acids hinders genetic code expansion.
Higher levels of near-cognate suppression found in opal versus amber codons.
E. coli BL21 and MG1655 display higher background suppression than TOP10.
ylRS/tRNAPylUCA cannot outcompete Trp incorporation at opal codons.
PylRS/tRNAPylUCCU is outcompeted by Arg-tRNAArg in quadruplet decoding.
Acknowledgments
This work was supported by grants from the NIH (GM22854 to D.S.; CA161158 to W.R.L.), the Welch Foundation (A-1715 to W.R.L.), and a DARPA contract (N66001-12-C-4020 to D.S.). We thank Dr. Yohannes H. Rezenom from the Laboratory for Biological Mass Spectrometry at Texas A&M University for characterizing our proteins using ESI-MS, and Dr. Yuchen Liu and Dr. Li-Tao Guo for discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu CC, Schultz PG. Adding new chemistries to the genetic code. Annu Rev Biochem. 2010;79:413–44. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]
- 2.Davis L, Chin JW. Designer proteins: applications of genetic code expansion in cell biology. Nat Rev Mol Cell Biol. 2012;13:168–82. doi: 10.1038/nrm3286. [DOI] [PubMed] [Google Scholar]
- 3.Neumann H. Rewiring translation - Genetic code expansion and its applications. FEBS Lett. 2012;586:2057–64. doi: 10.1016/j.febslet.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Neumann H, Peak-Chew SY, Chin JW. Genetically encoding N-ε-acetyllysine in recombinant proteins. Nat Chem Biol. 2008;4:232–4. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]
- 5.Park HS, Hohn MJ, Umehara T, Guo LT, Osborne EM, Benner J, Noren CJ, Rinehart J, Söll D. Expanding the genetic code of Escherichia coli with phosphoserine. Science. 2011;333:1151–4. doi: 10.1126/science.1207203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidal CJ. Post-translational modifications in health and disease. Springer Science; New York, NY: 2011. [Google Scholar]
- 7.Neumann H, Wang K, Davis L, Garcia-Alai M, Chin JW. Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome. Nature. 2010;464:441–4. doi: 10.1038/nature08817. [DOI] [PubMed] [Google Scholar]
- 8.Hoesl MG, Budisa N. In vivo incorporation of multiple noncanonical amino acids into proteins. Angew Chem Int Ed Engl. 2011;50:2896–902. doi: 10.1002/anie.201005680. [DOI] [PubMed] [Google Scholar]
- 9.Köhrer C, Sullivan EL, RajBhandary UL. Complete set of orthogonal 21st aminoacyl-tRNA synthetase-amber, ochre and opal suppressor tRNA pairs: concomitant suppression of three different termination codons in an mRNA in mammalian cells. Nucleic Acids Res. 2004;32:6200–11. doi: 10.1093/nar/gkh959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan W, Huang Y, Wang Z, Russell WK, Pai PJ, Russell DH, Liu WR. A facile system for genetic incorporation of two different noncanonical amino acids into one protein in Escherichia coli. Angew Chem Int Ed Engl. 2010;49:3211–4. doi: 10.1002/anie.201000465. [DOI] [PubMed] [Google Scholar]
- 11.Riddle DL, Roth JR. Frameshift suppressors. 3. Effects of suppressor mutations on transfer RNA. J Mol Biol. 1972;66:495–506. doi: 10.1016/0022-2836(72)90429-9. [DOI] [PubMed] [Google Scholar]
- 12.Roth JR. Frameshift mutations. Annu Rev Genet. 1974;8:319–46. doi: 10.1146/annurev.ge.08.120174.001535. [DOI] [PubMed] [Google Scholar]
- 13.Moore B, Nelson CC, Persson BC, Gesteland RF, Atkins JF. Decoding of tandem quadruplets by adjacent tRNAs with eight-base anticodon loops. Nucleic Acids Res. 2000;28:3615–24. doi: 10.1093/nar/28.18.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepthien S, Merkel L, Budisa N. In vivo double and triple labeling of proteins using synthetic amino acids. Angew Chem Int Ed Engl. 2010;49:5446–50. doi: 10.1002/anie.201000439. [DOI] [PubMed] [Google Scholar]
- 15.Ohtsuki T, Manabe T, Sisido M. Multiple incorporation of non-natural amino acids into a single protein using tRNAs with non-standard structures. FEBS Lett. 2005;579:6769–74. doi: 10.1016/j.febslet.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Brenner S, Stretton AO, Kaplan S. Genetic code: the ‘nonsense’ triplets for chain termination and their suppression. Nature. 1965;206:994–8. doi: 10.1038/206994a0. [DOI] [PubMed] [Google Scholar]
- 17.Eggertsson G, Söll D. Transfer ribonucleic acid-mediated suppression of termination codons in Escherichia coli. Microbiol Rev. 1988;52:354–74. doi: 10.1128/mr.52.3.354-374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao B, Gong W, Ferguson TK, James CM, Krzycki JA, Chan MK. A new UAG-encoded residue in the structure of a methanogen methyltransferase. Science. 2002;296:1462–6. doi: 10.1126/science.1069556. [DOI] [PubMed] [Google Scholar]
- 19.Ambrogelly A, Gundllapalli S, Herring S, Polycarpo C, Frauer C, Söll D. Pyrrolysine is not hardwired for cotranslational insertion at UAG codons. Proc Natl Acad Sci USA. 2007;104:3141–6. doi: 10.1073/pnas.0611634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longstaff DG, Larue RC, Faust JE, Mahapatra A, Zhang L, Green-Church KB, Krzycki JA. A natural genetic code expansion cassette enables transmissible biosynthesis and genetic encoding of pyrrolysine. Proc Natl Acad Sci USA. 2007;104:1021–6. doi: 10.1073/pnas.0610294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nozawa K, O’Donoghue P, Gundllapalli S, Araiso Y, Ishitani R, Umehara T, Söll D, Nureki O. Pyrrolysyl-tRNA synthetase-tRNAPyl structure reveals the molecular basis of orthogonality. Nature. 2009;457:1163–7. doi: 10.1038/nature07611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herring S, Ambrogelly A, Polycarpo CR, Söll D. Recognition of pyrrolysine tRNA by the Desulfitobacterium hafniense pyrrolysyl-tRNA synthetase. Nucleic Acids Res. 2007;35:1270–8. doi: 10.1093/nar/gkl1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umehara T, Kim J, Lee S, Guo LT, Söll D, Park HS. N-acetyl lysyl-tRNA synthetases evolved by a CcdB-based selection possess N-acetyl lysine specificity in vitro and in vivo. FEBS Lett. 2012;586:729–33. doi: 10.1016/j.febslet.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Peeler JC, Woodman BF, Averick S, Miyake-Stoner SJ, Stokes AL, Hess KR, Matyjaszewski K, Mehl RA. Genetically encoded initiator for polymer growth from proteins. J Am Chem Soc. 2010;132:13575–7. doi: 10.1021/ja104493d. [DOI] [PubMed] [Google Scholar]
- 25.O’Donoghue P, Sheppard K, Nureki O, Söll D. Rational design of an evolutionary precursor of glutaminyl-tRNA synthetase. Proc Natl Acad Sci USA. 2011;108:20485–90. doi: 10.1073/pnas.1117294108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polycarpo C, Ambrogelly A, Berube A, Winbush SM, McCloskey JA, Crain PF, Wood JL, Söll D. An aminoacyl-tRNA synthetase that specifically activates pyrrolysine. Proc Natl Acad Sci USA. 2004;101:12450–4. doi: 10.1073/pnas.0405362101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2005;12:291–9. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Bremer H. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J Biol Chem. 1995;270:11181–9. doi: 10.1074/jbc.270.19.11181. [DOI] [PubMed] [Google Scholar]
- 29.Isaacs FJ, Carr PA, Wang HH, Lajoie MJ, Sterling B, Kraal L, Tolonen AC, Gianoulis TA, Goodman DB, Reppas NB, Emig CJ, Bang D, Hwang SJ, Jewett MC, Jacobson JM, Church GM. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science. 2011;333:348–53. doi: 10.1126/science.1205822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inokuchi H, Yamao F, Sakano H, Ozeki H. Identification of transfer RNA suppressors in Escherichia coli. I. Amber suppressor su+2, an anticodon mutant of tRNA2Gln. J Mol Biol. 1979;132:649–62. doi: 10.1016/0022-2836(79)90380-2. [DOI] [PubMed] [Google Scholar]
- 31.Johnson DB, Wang C, Xu J, Schultz MD, Schmitz RJ, Ecker JR, Wang L. Release Factor One Is Nonessential in Escherichia coli. ACS Chem Biol. 2012 doi: 10.1021/cb300229q. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blight SK, Larue RC, Mahapatra A, Longstaff DG, Chang E, Zhao G, Kang PT, Green-Church KB, Chan MK, Krzycki JA. Direct charging of tRNACUA with pyrrolysine in vitro and in vivo. Nature. 2004;431:333–5. doi: 10.1038/nature02895. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi T, Yanagisawa T, Sakamoto K, Yokoyama S. Recognition of non-alpha-amino substrates by pyrrolysyl-tRNA synthetase. J Mol Biol. 2009;385:1352–60. doi: 10.1016/j.jmb.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 34.Ogle JM, Ramakrishnan V. Structural insights into translational fidelity. Annu Rev Biochem. 2005;74:129–77. doi: 10.1146/annurev.biochem.74.061903.155440. [DOI] [PubMed] [Google Scholar]
- 35.Johansson M, Lovmar M, Ehrenberg M. Rate and accuracy of bacterial protein synthesis revisited. Curr Opin Microbiol. 2008;11:141–7. doi: 10.1016/j.mib.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Zaher HS, Green R. Fidelity at the molecular level: lessons from protein synthesis. Cell. 2009;136:746–62. doi: 10.1016/j.cell.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodnina MV. Quality control of mRNA decoding on the bacterial ribosome. Adv Protein Chem Struct Biol. 2012;86:95–128. doi: 10.1016/B978-0-12-386497-0.00003-7. [DOI] [PubMed] [Google Scholar]
- 38.Scolnick E, Tompkins R, Caskey T, Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci USA. 1968;61:768–74. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukai T, Hayashi A, Iraha F, Sato A, Ohtake K, Yokoyama S, Sakamoto K. Codon reassignment in the Escherichia coli genetic code. Nucleic Acids Res. 2010;38:8188–95. doi: 10.1093/nar/gkq707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinemann IU, Rovner AJ, Aerni HR, Rogulina S, Cheng L, Olds W, Fischer JT, Söll D, Isaacs FJ, Rinehart J. Enhanced phosphoserine insertion during Escherichia coli protein synthesis via partial UAG codon reassignment and release factor 1 deletion. FEBS Lett. 2012 doi: 10.1016/j.febslet.2012.08.031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito K, Uno M, Nakamura Y. Single amino acid substitution in prokaryote polypeptide release factor 2 permits it to terminate translation at all three stop codons. Proc Natl Acad Sci USA. 1998;95:8165–9. doi: 10.1073/pnas.95.14.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uno M, Ito K, Nakamura Y. Functional specificity of amino acid at position 246 in the tRNA mimicry domain of bacterial release factor 2. Biochimie. 1996;78:935–43. doi: 10.1016/s0300-9084(97)86715-6. [DOI] [PubMed] [Google Scholar]
- 43.Johnson DB, Xu J, Shen Z, Takimoto JK, Schultz MD, Schmitz RJ, Xiang Z, Ecker JR, Briggs SP, Wang L. RF1 knockout allows ribosomal incorporation of unnatural amino acids at multiple sites. Nat Chem Biol. 2011;7:779–86. doi: 10.1038/nchembio.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkins JF, Björk GR. A gripping tale of ribosomal frameshifting: extragenic suppressors of frameshift mutations spotlight P-site realignment. Microbiol Mol Biol Rev. 2009;73:178–210. doi: 10.1128/MMBR.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang K, Schmied WH, Chin JW. Reprogramming the genetic code: from triplet to quadruplet codes. Angew Chem Int Ed Engl. 2012;51:2288–97. doi: 10.1002/anie.201105016. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Boniecki MT, Jaffe JD, Imai BS, Yau PM, Luthey-Schulten ZA, Martinis SA. Naturally occurring aminoacyl-tRNA synthetases editing-domain mutations that cause mistranslation in Mycoplasma parasites. Proc Natl Acad Sci USA. 2011;108:9378–83. doi: 10.1073/pnas.1016460108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorini L, Kataja E. Phenotypic repair by streptomycin of defective genotypes in E. coli. Proc Natl Acad Sci USA. 1964;51:487–93. doi: 10.1073/pnas.51.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal D, Gregory ST, O’Connor M. Error-prone and error-restrictive mutations affecting ribosomal protein S12. J Mol Biol. 2011;410:1–9. doi: 10.1016/j.jmb.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 49.Theobald-Dietrich A, Frugier M, Giege R, Rudinger-Thirion J. Atypical archaeal tRNA pyrrolysine transcript behaves towards EF-Tu as a typical elongator tRNA. Nucleic Acids Res. 2004;32:1091–6. doi: 10.1093/nar/gkh266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heinemann IU, O’Donoghue P, Madinger C, Benner J, Randau L, Noren CJ, Söll D. The appearance of pyrrolysine in tRNAHis guanylyltransferase by neutral evolution. Proc Natl Acad Sci USA. 2009;106:21103–8. doi: 10.1073/pnas.0912072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Namy O, Zhou Y, Gundllapalli S, Polycarpo CR, Denise A, Rousset JP, Söll D, Ambrogelly A. Adding pyrrolysine to the Escherichia coli genetic code. FEBS Lett. 2007;581:5282–8. doi: 10.1016/j.febslet.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 52.Nehring S, Budisa N, Wiltschi B. Performance analysis of orthogonal pairs designed for an expanded eukaryotic genetic code. PLoS One. 2012;7:e31992. doi: 10.1371/journal.pone.0031992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bullock TL, Uter N, Nissan TA, Perona JJ. Amino acid discrimination by a class I aminoacyl-tRNA synthetase specified by negative determinants. J Mol Biol. 2003;328:395–408. doi: 10.1016/s0022-2836(03)00305-x. [DOI] [PubMed] [Google Scholar]