Summary
Ingested dsRNAs trigger RNA interference (RNAi) in many invertebrates including the nematode Caenorhabditis elegans. Here we show that the C. elegans apical intestinal membrane protein SID-2 is required in C. elegans for the import of ingested dsRNA and, when expressed in Drosophila S2 cells, SID-2 enables the uptake of dsRNAs. SID-2-dependent dsRNA transport requires an acidic extracellular environment and is selective for dsRNAs with at least 50 base pairs. Through structure-function analysis, we identify several SID-2 regions required for this activity including three extracellular, positively-charged, histidines. Finally, we find that SID-2-dependent transport is inhibited by drugs that interfere with vesicle transport. Therefore, we propose that environmental dsRNAs are imported from the acidic intestinal lumen by SID-2 via endocytosis and are released from internalized vesicles in a secondary step mediated by the dsRNA-channel SID-1. Similar multistep mechanisms may underlie the widespread observations of environmental RNAi.
Introduction
Double-stranded RNAs (dsRNAs) ingested from the environment can directly manipulate gene expression in some animals by initiating cellular RNA interference (RNAi) silencing mechanisms (Huvenne and Smagghe, 2010; Tabara et al., 1998; Timmons and Fire, 1998; Whangbo and Hunter, 2008). This process, termed environmental RNAi, was initially discovered in the nematode Caenorhabditis elegans and has subsequently been observed in many invertebrates, including vectors of human disease (kissing bugs, tsetse flies, ticks), research models (planaria, hydra), and animals both essential (honey bees) and detrimental (western corn rootworms, cotton bollworms, aphids, root-knot nematodes) to agriculture (Huvenne and Smagghe, 2010; Whangbo and Hunter, 2008). While the endogenous role of this process is unclear, its widespread occurrence among invertebrates suggests that silencing genes in response to ingested dsRNA is advantageous. Despite our poor mechanistic understanding of how these dsRNAs are recognized and imported, environmental RNAi has rapidly become an important and established technique enabling many novel applications of RNAi-mediated gene silencing. For example, high-throughput, genome-wide screens using feeding RNAi have successfully identified new genes required for diverse biological processes including lifespan regulation, stem cell biology, and even RNAi itself (Whangbo and Hunter, 2008). In addition, the specificity of environmental RNAi has facilitated the development of non-chemical agricultural pesticides that utilize plants engineered to express dsRNAs targeting essential genes in insect or nematode parasites (Huvenne and Smagghe, 2010; Whangbo and Hunter, 2008). Furthermore, preliminary experiments indicate that feeding large animal populations with dsRNA containing viral sequences is sufficient to confer protection against the corresponding disease (Hunter et al., 2010; Liu et al., 2010; Maori et al., 2009; Sarathi et al., 2008). It is possible that environmental RNAi similarly functions as an immune defense in nature; the uptake of extracellular dsRNA is necessary for efficient antiviral immunity in Drosophila (Saleh et al., 2009) and in many plants (Li and Ding, 2006). Here we investigate the mechanistic basis of environmental RNAi using C. elegans.
Before it can trigger RNAi, ingested dsRNAs must be transported from the intestinal lumen into the cytosol where the dsRNA-processing and RNAi-silencing proteins reside. To identify proteins required for this process, our lab previously carried out a genetic screen using C. elegans and isolated mutants completely insensitive to environmental dsRNAs. These mutants mapped to two membrane proteins, sid-1 and sid-2 (systemic RNAi defective) (Winston et al., 2002; Winston et al., 2007). Further analysis determined that SID-1 is expressed in all non-neuronal cells (Winston et al., 2002) whereas SID-2 is largely present only in the intestine (Winston et al., 2007). Moreover, a rescuing SID-2::GFP fusion protein localized to the apical (lumenal) membrane of intestinal cells, suggesting that SID-2 has a direct role in the uptake of ingested dsRNAs (Winston et al., 2007).
Consistent with its limited expression pattern, SID-2 is only required for environmental RNAi and not the subsequent transport of RNAi silencing throughout the organism, referred to as systemic RNAi. This is demonstrated by the ability of sid-2 mutants to systemically transport gene silencing if dsRNAs are introduced directly into the animal by injection or transgene expression. In addition, comparative analysis between C. elegans and the closely related nematode C. briggsae indicates that SID-2 has a specific, key function during environmental RNAi. C. briggsae is completely deficient for environmental RNAi even though it is capable of systemic RNAi and the C. briggsae SID-2 homolog is expressed in the intestine and localizes to the apical membrane. Since expressing C. elegans SID-2 in C. briggsae enables these animals to respond to ingested dsRNA, C. briggsae has all the necessary components to import environmental dsRNA except for the activity provided by C. elegans SID-2 (Winston et al., 2007). A recent paper has similarly shown that expressing C. elegans SID-2 in C. remanei is also sufficient to sensitize these nematodes to feeding RNAi (Nuez and Félix, 2012). Taken together, these observations suggest that SID-2 function is specialized to import ingested dsRNAs from the intestinal lumen.
In contrast to sid-2, sid-1 mutants do not initiate gene silencing from extracellular dsRNA, regardless of whether it is introduced by feeding, injection, or is expressed from a transgene. By expressing SID-1 in Drosophila S2 cells, we have previously shown that SID-1 is a dsRNA-selective channel that enables passive transport across the plasma membrane (Feinberg and Hunter, 2003; Shih et al., 2009; Shih and Hunter, 2011). S2 cells have proven to be an ideal heterologous system for investigating SID-1 transport properties partly because these cell lack identifiable SID protein homologs and, like C. elegans, are tolerant of long dsRNA. Untransfected Drosophila S2 cells can take up dsRNA from their growth media through a process at least partly dependent on endocytic transport mediated by the scavenging receptors SR-CL and eater (Saleh et al., 2006; Ulvila et al., 2006). The endogenous uptake of dsRNA in S2 cells, however, is relatively slow and requires high extracellular dsRNA concentrations. As a result, this baseline uptake can be easily differentiated from the rapid and efficient dsRNA transport mediated by SID-1.
Although SID-1 enables extracellular dsRNA uptake into S2 cells, the inability of C. elegans sid-2 mutants to respond to environmental dsRNA implies that SID-1 is not sufficient to deliver dsRNA from the nematode intestinal lumen to the cytoplasmic RNAi machinery. Therefore, to understand why environmental RNAi requires both SID-1 and SID-2, we have investigated the function of the poorly characterized SID-2 protein. Using C. elegans and Drosophila S2 cells, we show here that SID-2, like SID-1, can selectively import dsRNA. However, unlike for SID-1, SID-2-dependent transport requires an acidic extracellular pH that is comparable to the conditions in the intestinal lumen. Through structure-function analysis, we identify several SID-2 regions required for this dsRNA-uptake activity, including three extracellular histidines, which are positively charged at this pH and so may interact with negatively-charged dsRNA or may be important for the structure of SID-2. Finally, we determine that dsRNA transport by SID-2 further differs from that by SID-1 since it mediates a slow, energy-dependent process requiring endocytic machinery. Based on these results, we propose that SID-2 and SID-1 function sequentially during environmental RNAi with SID-2 mediating the initial internalization of dsRNA directly from the intestinal lumen and SID-1 functioning in a secondary step that transports this dsRNA into the cytoplasm. This type of multistep transport pathway may also explain the selective import of extracellular dsRNA observed in other metazoans.
Results
SID-2 is required for environmental dsRNA to accumulate in C. elegans intestinal cells
To determine whether SID-2 functions during the initial uptake of dsRNA from the intestinal lumen or for later processing/transport of already internalized dsRNA, we first tested whether C. elegans sid-2 mutants can import any extracellular dsRNA. We soaked sid-2(gk505) deletion mutants and positive-control animals expressing a rescuing translational fusion of SID-2 and GFP (SID-2::GFP) (Winston et al., 2007) in cy5-labeled 500 base pair (bp) dsRNA for one to two hours. Using confocal microscopy, we observed cy5-labeled structures within the intestinal cells of control animals but not in any of the sid-2(gk505) mutants (Figure 1). Therefore, SID-2 is required either for the initial uptake of dsRNA and/or its accumulation in distinct intestinal compartments. We did not observe any significant intracellular overlap of the cy5 signal and SID-2::GFP.
Figure 1.
SID-2 is required to internalize environmental dsRNAs into C. elegans. 500 base pair (bp) cy5-labeled dsRNA fed to C. elegans could only be visualized intracellularly in sid-2(+) animals (observed in 6/15 sid-2(+) animals, 0/15 sid-2(−) animals).
SID-2 enables pH-dependent dsRNA uptake when expressed in Drosophila S2 cells
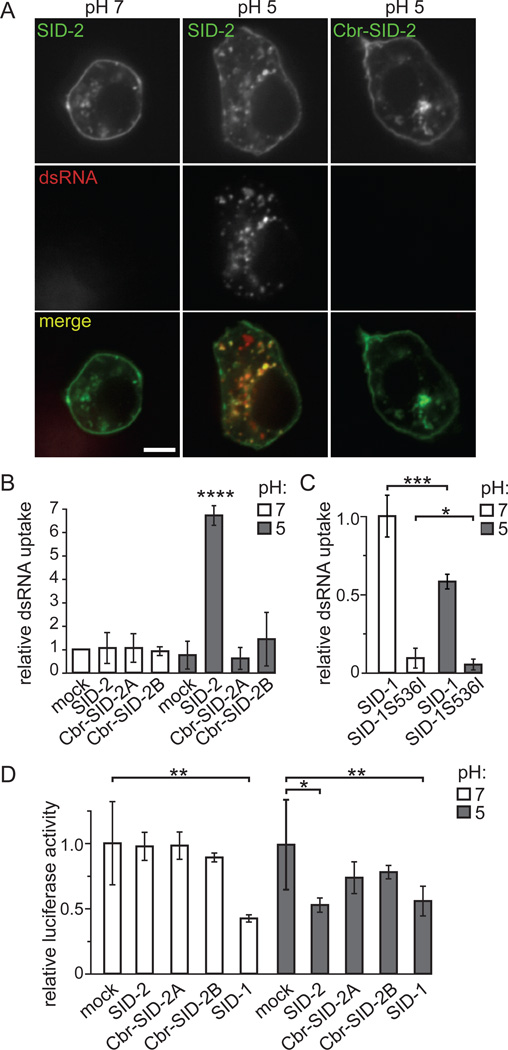
Because of the limitations of the C. elegans experiment described above, we further characterized the function of SID-2 by expressing it in Drosophila S2 cells. In these experiments, we transfected Drosophila S2 cells with a plasmid encoding a functional SID-2::GFP fusion (Winston et al., 2007). S2 cells expressing a SID-2::GFP transgene localized GFP to the plasma membrane and in punctate cytoplasmic structures (Figure 2A). The fluorescent pattern observed in S2 cells is similar to cultured intestinal cells isolated from a C. elegans strain expressing a rescuing SID-2::GFP construct (Figure S1A), indicating that SID-2::GFP is appropriately expressed and localized in the heterologous S2 cell system. As a negative control we transfected S2 cells with a plasmid encoding C. briggsae SID-2::GFP (Cbr-SID-2::GFP), which fails to rescue environmental RNAi when expressed in C. briggsae or C. elegans sid-2 mutants (Winston et al., 2007). While cloning this construct, we identified a splice variant of Cbr-SID-2 (Figure S1C); both Cbr-SID-2 proteins show the same GFP pattern as cells expressing SID-2::GFP (Figures 2A and S1B).
Figure 2.
SID-2 expression is sufficient for efficient dsRNA transport into Drosophila S2 cells at an acidic pH. (A) cy5-labeled dsRNA is internalized into and has extensive co-localization with SID-2::GFP expressed in S2 cells in media at pH 5 (42/57 cells contain at least three points of co-localization) but not with cells expressing Cbr-SID-2::GFP (n=48) or with SID-2::GFP expressed in cells at pH 7 (n=53). All images were taken at the same time using the same camera settings. (B) At pH 5, S2 cells expressing SID-2 internalize more 32P-labeled dsRNA than cells expressing Cbr-SID-2 or mock-transfected cells. Results shown are an average of 20 (SID-2, Cbr-SID-2B pH 5), 9 (Cbr-SID-2A pH 5), or 8 (remaining conditions) biological replicates. (C) Unlike SID-2, SID-1-dependent transport of 32P-labeled dsRNA is inhibited in media at pH 5 compared to pH 7. Results shown are an average of 6 biological replicates. (D) dsRNA internalized by SID-1 or SID-2 is processed into functional siRNAs as indicated by reduced luciferase expression in these cells following the addition of luciferase dsRNA to media at pH 5 (SID-2 and SID-1) or pH 7 (SID-1) as compared to cells not exposed to dsRNA. Results shown are an average of 5–8 biological replicates. *, p < 0.05; **, p < 0.005; ***, p < 0.0005, **** p < 1×10−6. Error bars represent 1 standard deviation. p values determined with a two-tailed paired t test. Scale bars,10 µm. See also Figure S1.
To test whether expressing SID-2::GFP in S2 cells increases the uptake of extracellular dsRNA, we compared the amount of 500bp 32P-labeled dsRNA internalized by mock-transfected cells and cells expressing SID-2::GFP or a Cbr-SID-2::GFP variant. We initially found that cells expressing these constructs showed the same low levels of dsRNA uptake as mock-transfected cells (Figure 2B). However, the localization pattern of SID-2 in C. elegans predicts that it functions in the intestinal lumen (Winston et al., 2007), which is an acidic environment (Pfeiffer et al., 2008). When we mimicked this environment by adjusting the culture media from its normal pH of ~7 to pH 5, we observed a significant increase in SID-2-dependent dsRNA internalization (Figure 2B). In contrast, cells expressing either Cbr-SID-2::GFP construct did not internalize more dsRNA than mock-transfected cells at an acidic or neutral pH (Figure 2B), and so dsRNA internalized under these conditions presumably reflects the endogenous S2 uptake pathway (Saleh et al., 2006; Ulvila et al., 2006). It is important to note that, while additional Cbr-SID-2 splice variants may exist, they are unlikely to transport dsRNA since injection of genomic cbr-sid-2 failed to rescue the feeding RNAi defect of a C. elegans sid-2 mutant (Winston et al., 2007).
To determine whether this observed pH dependence is a non-specific effect of dsRNA uptake assays in S2 cells or is a result of the intrinsic and distinct mechanism of SID-2 function, we compared dsRNA transport in SID-1-expressing cells at a neutral or acidic pH. We found that reducing the pH from 7 to 5 reduced the amount of 32P-labeled dsRNA internalized by these cells by over 40% (Figure 2C). Therefore the effect of acidic pH is specific to the mechanism of SID-2-mediated transport.
As confirmation that SID-2 is sufficient to increase dsRNA uptake from acidic environments, we soaked transfected S2 cells in cy5-labeled dsRNA. Similar to our results using intact C. elegans shown in Figure 1, cy5-labeled dsRNA was readily detected in cells expressing SID-2::GFP (Figure 2A). This increased uptake was observed only when the cells and RNA were co-incubated at an acidic pH and was not detected in cells expressing Cbr-SID-2::GFP. In contrast to our C. elegans results, we found that cy5-containing structures co-localized with SID-2::GFP. For all conditions, some internalized dsRNA was detected that failed to overlap with GFP, which may indicate that this dsRNA is imported by the endogenous S2 cell pathway. Alternatively, for SID-2::GFP expressing cells, some dsRNA imported by SID-2 may ultimately accumulate in structures that lack SID-2. These compartments are potentially similar to the non-GFP, cy5-labeled structures observed in C. elegans. Another potential explanation is that the cy5 label may become separated from the dsRNA following SID-2-dependent internalization.
To test whether SID-2-internalized dsRNA is a substrate for RNAi, we co-expressed luciferase and SID proteins in S2 cells and added 500bp luciferase dsRNA to the extracellular media. SID-2-expressing cells exposed to luciferase dsRNA in pH 5 media demonstrated significantly less luciferase activity than cells not exposed to dsRNA. In contrast, Cbr-SID-2-expressing cells or SID-2-expressing cells at a neutral pH showed equivalent luciferase activity as mock treated cells (Figure 2D). These data are consistent with the dsRNA uptake data (Figures 2A and B) and demonstrate that dsRNA internalized by SID-2 can trigger RNAi.
SID-2 selectively transports dsRNA
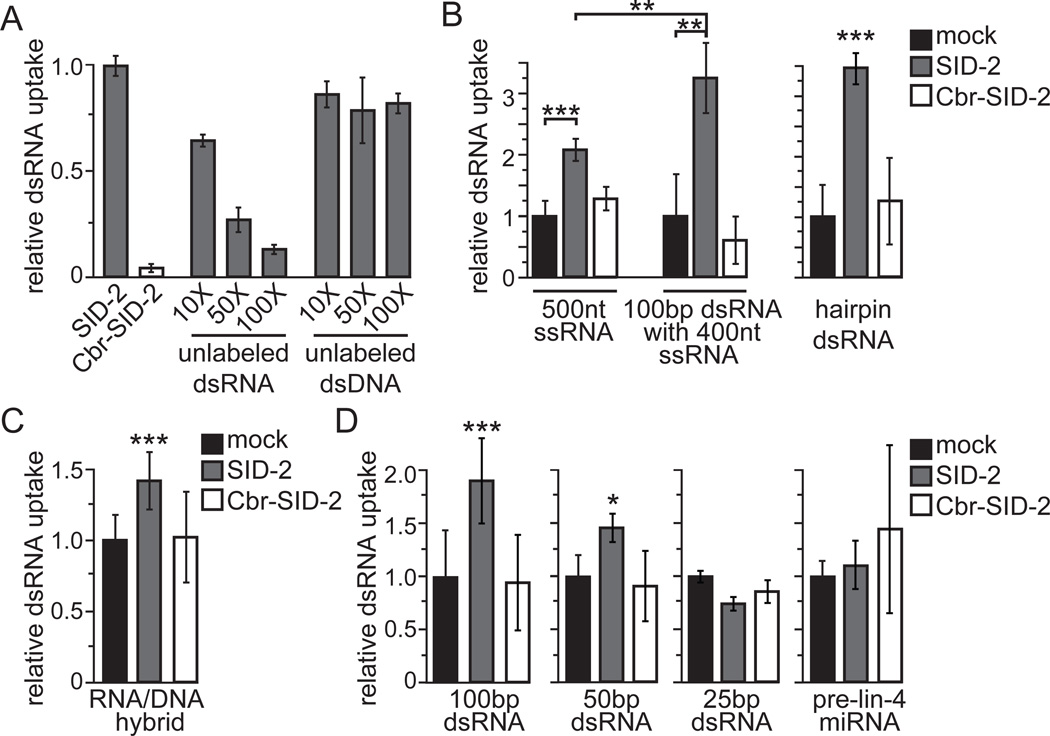
To determine whether SID-2 functions as a general scavenger receptor for any nucleic acid or is specific for dsRNA, we asked whether the uptake of 32P-labeled dsRNA is blocked when SID-2::GFP-expressing cells are incubated in an excess of unlabeled DNA or RNA of the same length and sequence. As expected, the addition of excess unlabeled dsRNA reduced the import of 32P-labeled dsRNA in a dose-dependent manner (Figure 3A). Somewhat surprisingly, the addition of even 100-fold excess of unlabeled dsRNA did not completely inhibit the internalization of 32P-labeled dsRNA, indicating that the SID-2-dependent uptake capacity of these assays is far from saturated. In contrast, unlabeled dsDNA had only a moderate effect on 32P-labeled dsRNA uptake (Figure 3A). Moreover, the addition of 10, 50, or 100-fold excess dsDNA all decreased dsRNA internalization by approximately 20% which suggests that this is a non-specific effect.
Figure 3.
SID-2-mediated transport is selective for long dsRNA. (A) The addition of unlabeled dsRNA competitor blocks the uptake of 32P-labeled dsRNA into cells expressing SID-2 while unlabeled dsDNA has only a moderate effect. Results shown are an average of 5 biological replicates. (B–D) Internalization of 32P-labeled substrates into SID-2-expressing, Cbr-SID-2-expressing, or mock-transfected S2 cells. Results shown are an average of 9 (100bp, 25bp SID-2 and Cbr-SID-2), 3 (25bp mock), or 6 (remaining conditions) biological replicates. Cells expressing Cbr-SID-2 were indistinguishable from mock-transfected cells for all tested substrates. Error bars represent 1 standard deviation. Starred conditions accumulated at least 1.4-fold more substrate than mock-transfected cells with *, p < 0.05; **, p < 0.005; ***, p < 5 × 10−4. p values determined with a two-tailed paired t test.
Since SID-2 is selective for dsRNA over dsDNA, we next asked whether SID-2 preferentially transports double-stranded substrates. We exposed cells to 500 nucleotide (nt) 32P-labeled single-stranded RNA (ssRNA) and found that SID-2-expressing cells were able to internalize more ssRNA than mock-transfected cells (Figure 3B). A caveat of this experiment, however, is that ssRNA containing 500nt is likely to fold back onto itself to form short stretches of dsRNA. Therefore, to directly test whether SID-2 differentially transports ss or dsRNA, we annealed an unlabeled 100nt complementary ssRNA to this 500nt 32P-labeled ssRNA to generate a 100bp dsRNA with a 400nt ssRNA tail. As a negative control, we similarly tested an unlabeled 100nt non-complementary ssRNA. We determined that the addition of the non-complementary ssRNA sequence did not affect the internalization of the 32P-labeled substrate (p > 0.1; data not shown), while addition of a complementary 100nt ssRNA enhanced the uptake of 500nt 32P-labeled ssRNA (Figure 3B). These results indicate that even a relatively small dsRNA region facilitates SID-2-dependent transport. In addition, these results support the notion that the observed uptake of ssRNA is likely due to internal dsRNA structures.
We next asked whether SID-2 can transport dsRNAs that contain an internal ssRNA region. We found that SID-2-expressing cells took up more of a 32P-labeled hairpin substrate containing 1.4kb dsRNA and a 497nt ssRNA loop than mock-transfected cells, demonstrating that this internal ssRNA region did not prevent SID-2-dependent transport (Figure 3B). To further probe the nucleic acid specificity of SID-2, we tested whether SID-2 can transport a double-stranded nucleic acid formed from a 500nt 32P-labeled ssRNA bound to a complementary strand of unlabeled 500nt ssDNA (RNA/DNA hybrid). Unlike for dsRNA, SID-2-expressing cells internalized only slightly more RNA/DNA hybrid than control cells (Figure 3C), indicating that two RNA strands are required for efficient SID-2 transport. Taken together, SID-2 transports dsRNA more efficiently than ssRNA or RNA/DNA hybrid and its function is not inhibited by internal or terminal stretches of ssRNA.
SID-2 transports 50bp dsRNA but not 25bp dsRNA or pre-miRNA
Since long dsRNAs and hairpin RNAs are cleaved into short RNAs of ~22bp during RNAi processing, we asked whether these short RNAs are also SID-2 substrates. We initially investigated whether SID-2 transport is length-dependent by exposing cells to 32P-labeled 100bp, 50bp, or 25bp dsRNA. We determined that cells expressing SID-2 imported more 100bp and 50bp dsRNA than mock-transfected cells but uptake of 32P-labeled 25bp dsRNA was indistinguishable from control cells (Figure 3D). We also directly tested whether SID-2 can transport pre-microRNA (pre-miRNA), a ~60nt RNA that forms an imperfectly complementary 22bp hairpin. Similar to the 25bp dsRNA, SID-2-expressing cells did not internalize more pre-lin-4 miRNA than mock-transfected cells (Figure 3D). Therefore, SID-2-mediated transport, and presumably environmental RNAi, requires a minimum length greater than 25bp and does not import pre-miRNA molecules.
The SID-2 extracellular domain contains dsRNA-transport activity
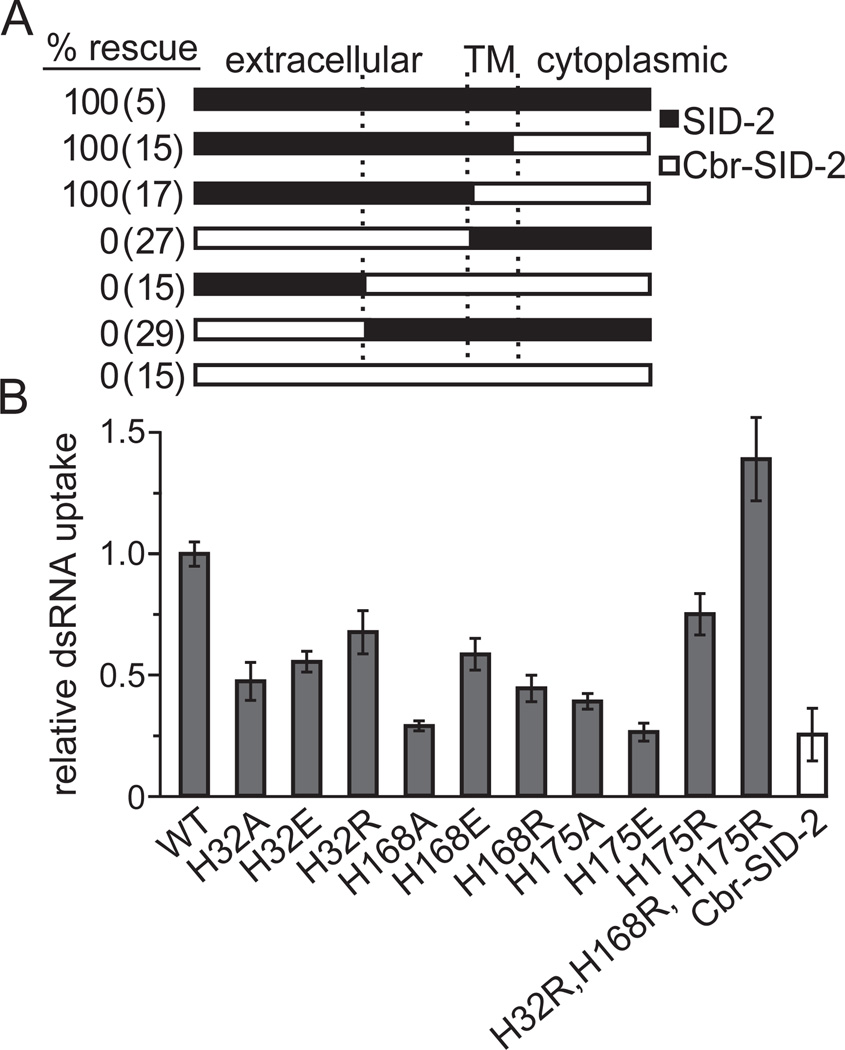
To identify domains required to recognize and/or mediate the import of long dsRNA, we mapped the region(s) that enables SID-2 transport activity by testing a variety of C. elegans/C. briggsae hybrid SID-2 proteins for their ability to rescue the environmental RNAi defect in sid-2(gk505) deletion worms. To confirm that hybrid proteins were properly expressed and localized, we fused GFP to the cytoplasmic C-terminus of each construct. All constructs produced GFP signal that localized to the intestinal apical membrane, indicating production of full-length products (Figure S2A). We found that a construct containing the SID-2 extracellular domain and Cbr-SID-2 membrane and cytoplasmic regions rescued environmental RNAi in sid-2(gk505) animals. In contrast, a fusion construct containing the Cbr-SID-2 extracellular domain fused to SID-2 membrane and cytoplasmic regions failed to rescue sid-2(gk505) (Figure 4A). These results indicate that the difference between SID-2 and Cbr-SID-2 is localized to the extracellular domain (Figure 4A). We attempted to further delineate the functional domain by swapping the first or second half of the SID-2 extracellular domain with Cbr-SID-2. However, neither construct rescued sid-2(gk505), indicating that multiple regions of the SID-2 extracellular domain are required for environmental RNAi (Figure 4A). Because the SID-2 extracellular domain is poorly conserved among the sequenced SID-2 homologs (Figure S2B), none of which enable environmental RNAi, the smaller domains or residues enabling environmental RNAi cannot be determined by simple sequence analysis.
Figure 4.
Domain swaps identify a critical role for the SID-2 extracellular domain in environmental RNAi. (A) sid-2(gk505) deletion worms expressing SID-2, Cbr-SID-2, or hybrids of the C. elegans/C. briggsae SID-2 proteins were scored as rescued if exposure to ingested pal-1 dsRNA caused >95% embryonic lethality. Only proteins containing the extracellular SID-2 domain could rescue the environmental RNAi defect. (B) S2 cells expressing mutagenized SID-2 constructs internalize less 32P-labeled dsRNA than the wild type (WT) SID-2 control. An exception to this is the triple histidine-to-arginine mutant, which internalizes more 32P-labeled dsRNA than WT. Results shown are an average of 5 biological replicates. Error bars represent 1 standard deviation. See also Figure S2.
Extracellular SID-2 histidines mediate dsRNA uptake and environmental RNAi
While the extracellular domain does not contain any recognized functional motifs, it is predicted to localize to the intestinal lumenal space (Winston et al., 2007) where it presumably encounters and interacts with ingested dsRNAs. Since RNA is negatively-charged, we tested whether positively-charged extracellular residues are necessary for transport. Specifically, we targeted histidine residues because the imidazole groups they contain are only protonated in the acidic conditions required for SID-2 function. Moreover, histidines are essential for the function of Toll-like receptor 3, a mammalian membrane protein which, like SID-2, shows pH-dependent dsRNA interactions - only binding dsRNA at acidic pH (Fukuda et al., 2008; Liu et al., 2008). A crystal structure of dsRNA bound to the Toll-like receptor 3 ectodomain shows multiple histidines in direct contact with phosphate groups in the RNA backbone (Liu et al., 2008).
SID-2 contains three extracellular histidines (His32, His168, and His175) at least one of which is present in each extracellular portion tested in the domain swap experiments. Additionally, none of these histidines are conserved in Cbr-SID-2 (Figure S2B). To determine whether these residues are necessary for dsRNA transport, we replaced each histidine with a neutral alanine or negatively-charged glutamic acid. Western blot analysis and fluorescence from a C-terminal GFP fusion again confirmed that all constructs produced full-length proteins, expressed at similar levels, and localized to the proper membrane (Figures S2C and data not shown). When expressed in S2 cells, all mutagenized SID-2 proteins showed reduced or eliminated dsRNA transport as compared to wild-type SID-2 (Figure 4B). Since disrupting the charge of each extracellular histidine reduced dsRNA transport, we next asked whether positively-charged arginine can restore SID-2 function or if the histidines are specifically required. While individual arginine substitutions retained more function in S2 cells than alanine mutants, they did not recover wild-type transport levels. Taken together, the transport defects of these mutants show that each extracellular histidine is necessary for efficient dsRNA transport. Surprisingly, S2 cells expressing a SID-2 protein with arginine substitutions for all three histidines internalized more dsRNA than when expressing wild-type SID-2 (p < 0.05; Figure 4B), however this uptake remained pH dependent (data not shown).
To confirm that extracellular histidines are similarly required for SID-2 function in C. elegans, we expressed a subset of the histidine mutagenesis constructs in sid-2(gk505) deletion worms and tested for rescue of the environmental RNAi defect. Expression of SID-2H168A, a mutant with no detectable dsRNA uptake in S2 cells, only partially rescued environmental RNAi targeting genes expressed in the hypodermis (bli-1) or germline (pal-1). In contrast, all other tested constructs fully rescued sid-2(gk505) (Table 1). However, significant differences between the dsRNA uptake assays in S2 cells and the environmental RNAi rescue experiments may mask reduced dsRNA transport. Most notably, the sid-2 transgenes are overexpressed and the animals are exposed to dsRNA for multiple days. Consequently, the detection of only partial environmental RNAi rescue by SID-2H168A strongly implicates this extracellular histidine in uptake of ingested dsRNA.
Table 1.
SID-2 constructs with mutant histidines can rescue environmental RNAi when expressed in sid-2(gk505) deletion worms as determined by feeding transgenic worms dsRNA targeting genes in the hypodermis (bli-1) or the germline (pal-1).
| % adults responding to dsRNA targeting | ||
|---|---|---|
| sid-2(gk505) expressing | hypodermis: bli-1 |
germline: pal-1 |
| no construct | 0 (51) | 0 (31) |
| SID-2 | 91 (22) | 95 (22) |
| SID-2 H32A | 80 (30) | 88 (32) |
| SID-2 H168A | 32 (31) | 61 (46) |
| SID-2 H175A | 92 (26) | 100 (25) |
| SID-2 H32R, H168R, H175R | 95 (21) | 96 (22) |
SID-2-dependent dsRNA uptake is a slow, energy-dependent process
While both SID-2 and SID-1 are required for environmental RNAi in C. elegans and both are sufficient to increase the uptake of extracellular dsRNA into S2 cells, the differing effect of extracellular pH suggests that these proteins transport dsRNA by distinct mechanisms. We have previously shown that SID-1-dependent dsRNA uptake is extremely rapid, plateauing within one minute (Shih et al., 2009), and is largely energy independent (Feinberg and Hunter, 2003). In contrast, when we conducted a time course analysis, we found that the concentration of intracellular 32P-labeled dsRNA increased slowly in cells expressing SID-2; at 10 minutes cells contained less than 2% of the dsRNA internalized by 200 minutes. Even with extended incubation times, the cells did not become saturated but continued to internalize dsRNA at a roughly constant rate (Figure 5A). Furthermore, we found that this steady uptake of dsRNA was abolished by pre-treatment with the F1-ATPase inhibitor oligomycin, indicating that dsRNA uptake by SID-2-expressing S2 cells is energy dependent (Figure 5B). Importantly, oligomycin did not prevent SID-1-dependent uptake (Figure 5C), which demonstrates that oligomycin-treated cells are viable and capable of importing dsRNAs. These results confirm that SID-2 is mechanistically distinct from the dsRNA-channel SID-1 and potentially functions as a receptor or pump to internalize dsRNA.
Figure 5.
Vesicle trafficking is required for SID-2 to take up dsRNA. (A) In S2 cells, SID-2-dependent uptake of 32P-labeled dsRNA is a continuous process. Results shown are an average of 8 (100 min) or 5 (remaining conditions) biological replicates. (B) SID-2-dependent uptake of 32P-labeled dsRNA is strongly reduced by exposing the S2 cells to oligomycin (inhibits ATP synthase), latrunculin A (latA; inhibits actin polymerization), and bafilomycin A1 (bafA; inhibits vesicle maturation); SID-2-expressing cells exposed to these inhibitors internalize less than 2-fold more dsRNA than relevant control cells. Results shown are an average of 5 (SID-2) or 4 (Cbr-SID-2) biological replicates. (C) Endocytosis inhibitors do not prevent SID-1-dependent internalization of 32P-labeled dsRNA; SID-1-expressing cells exposed to these inhibitors internalize between 7.5 and 37-fold more dsRNA than relevant control cells. Results shown are an average of 5 biological replicates. (D) LatA inhibits cytoplasmic SID-2::GFP structures and reduces the internalization of cy5-labeled dsRNA (7/50 cells contain at least 3 points of co-localization between cy5-dsRNA and SID-2::GFP). Scale bar, 10 µm. *, p < 0.05; **, p < 0.005; ***, p < 5 × 10−5. Error bars represent 1 standard deviation. p values determined with a two-tailed paired t test.
Endocytosis is important for SID-2-dependent dsRNA uptake in S2 cells
The kinetics of SID-2-dependent dsRNA uptake (Figure 5A) combined with our observation that SID-2 and internalized dsRNA may co-localize in punctate cytoplasmic structures approximating the size and shape of vesicles (Figure 2A) suggests that SID-2 imports dsRNA via endocytosis. To test this hypothesis more directly, we used the pharmacological inhibitors latrunculin A (latA) and bafilomycin-A1 (bafA) to disrupt different aspects of endocytosis; latA disrupts actin polymerization, which is required for vesicle formation (Coué et al., 1987; Yarar et al., 2005), while bafA prevents acidification-dependent vesicle turnover, which is required for endosome maturation (Hurtado-Lorenzo et al., 2006). Treatment with either drug strongly inhibited SID-2-dependent 32P-labeled dsRNA internalization (Figure 5B). We additionally found that incubating cells in latA severely reduced the number of vesicle-like SID-2::GFP structures and limited the amount of internalized cy-5-labeled dsRNA (Figure 5D). In contrast, SID-1-expressing cells similarly treated with either latA or bafA retained substantial dsRNA transport capacity as compared to controls, which again demonstrates that these treatments have only a minor effect on S2 cell integrity or viability (Figure 5C). Thus, SID-2 dependent dsRNA uptake is uniquely sensitive to treatments that disrupt endocytosis. RNAi targeting Drosophila endocytosis proteins clathrin and rab-5 caused severe effects on cell viability (not shown), precluding analysis of their effects on SID-2-mediated dsRNA uptake.
Discussion
Environmental RNAi is a remarkable process by which the contents of an organism’s surroundings can directly control the expression of any of its genes. Here we report our investigation into the activity of SID-2 in regard to the recognition and internalization of extracellular dsRNA. Our results indicate that SID-2 expressed in Drosophila S2 cells can act separately from SID-1 to enable the uptake of dsRNA. However, like SID-1, SID-2 displays remarkable specificity for dsRNA (Feinberg and Hunter, 2003; Shih et al., 2009; Shih and Hunter, 2011). We found that SID-2 efficiently internalizes 50–1,500 bp dsRNA and that long internal or terminal stretches of ssRNA did not prevent uptake. Also similar to SID-1, dsDNA and RNA/DNA heteroduplex nucleic acids are not efficiently transported by SID-2-expressing S2 cells. However, unlike SID-1, SID-2 does not transport pre-miRNAs (Shih and Hunter, 2011). These results indicate that SID-2 is selective for the transport of long RNAi triggers but not RNAi effectors. Because SID-2-dependent dsRNA import requires an acidic pH, it is likely that SID-2 directly imports these RNAi triggers from the acidic C. elegans intestinal lumen. Through structure-function analysis, we have determined that the SID-2 extracellular (lumenal) domain plays a critical role in its dsRNA-uptake activity and, more specifically, we have determined that extracellular histidines are required. These histidines are positively charged in acidic environments and so have the potential to interact with negatively-charged dsRNA. Alternatively, these histidines may be important for the structure of this SID-2 domain. Finally, we have shown that SID-2 differs from SID-1 in both pH dependency and mechanistic requirements; only SID-2-mediated transport requires endocytic processes such as vesicle trafficking.
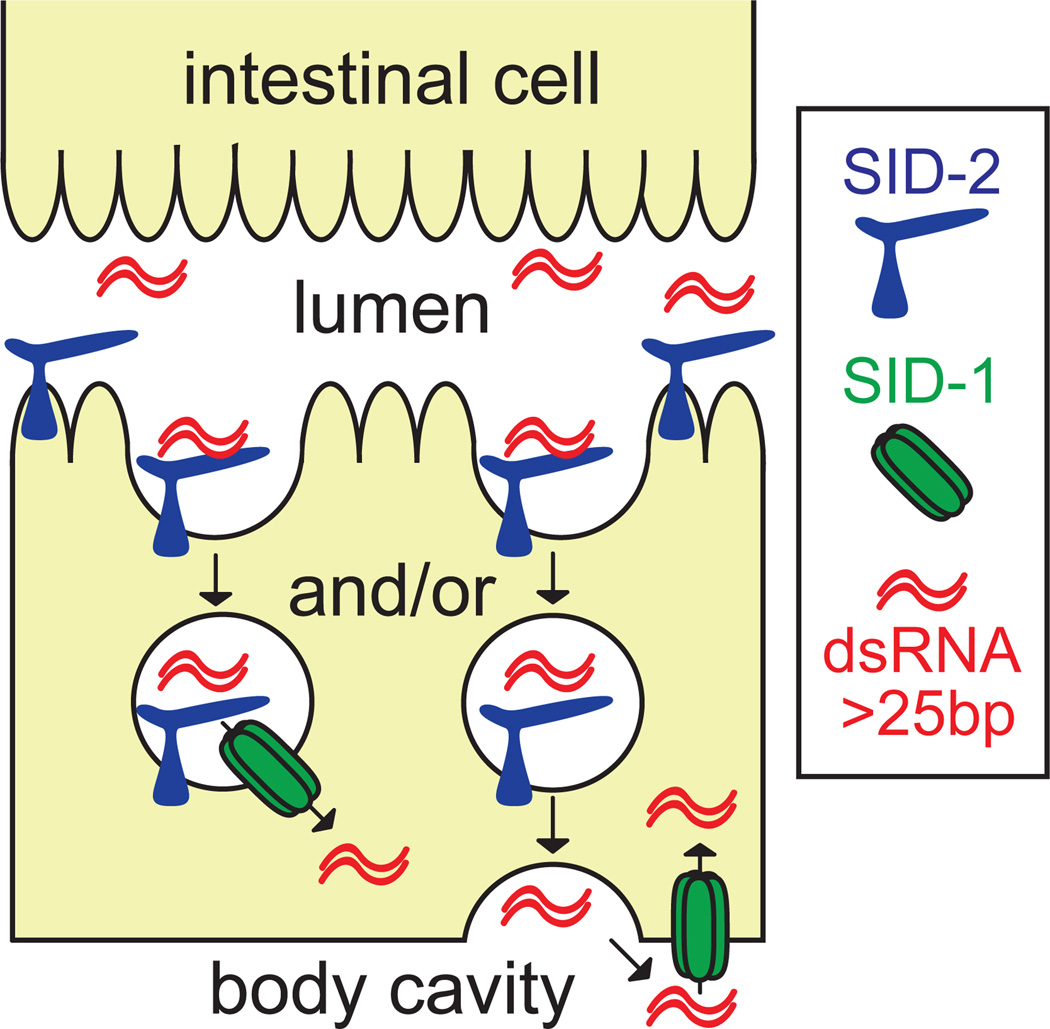
While both SID-1 and SID-2 are necessary for environmental RNAi in C. elegans, each can transport dsRNA independently of the other when expressed in S2 cells. However, as detailed above, these proteins are functionally distinct suggesting that they act separately during environmental RNAi with SID-2 mediating the initial uptake of ingested dsRNA from the lumenal space. dsRNA internalized via SID-2 is likely sequestered in cytoplasmic structures (Figure 2A), and so is inaccessible to RNAi machinery. Consequently, the probable function for SID-1 is to transport this dsRNA to the cytoplasm. This secondary transport step may be linked to SID-2-dependent import if SID-1 enables export directly from the entry vesicle (Figure 6, left). Alternatively, the encapsulated dsRNA could transit the intestinal cell and be released to the pseudocoelomic fluid from where it would then be imported into the cytoplasm by SID-1 (Figure 6, right). Analysis of SID-1 mosaic animals supports the existence of a pathway capable of transporting ingested dsRNA to the pseudocoelomic fluid independently of SID-1 activity in the intestine (Jose et al., 2009).
Figure 6.
Model of SID-2 and SID-1 coordinated uptake of ingested dsRNA in C. elegans. dsRNA is internalized from the intestinal lumenal space by SID-2-mediated endocytosis. dsRNA is retained in the vesicle until directly transported into the cytoplasm by SID-1 (left) or released in the pseudocoelomic fluid for subsequent cellular import via SID-1 (right).
A dsRNA selective, multistep pathway may be a common mechanism to import extracellular dsRNA. Tissue-specific proteins like SID-2 may regularly transport dsRNA to environments that are more favorable for broadly-expressed proteins such as SID-1 to function. This hypothesis explains how C. elegans SID-1 can mediate dsRNA transport in a large and diverse range of membrane types and extracellular spaces (Winston et al., 2002) if specialized proteins, and not SID-1, mediate dsRNA interactions in each environment. Experimental evidence suggests that this model also applies to other metazoans. For example, Drosophila S2 cells import extracellular dsRNA through a dsRNA-selective endocytic process (Saleh et al., 2006; Ulvila et al., 2006) that presumably requires a secondary step to release the dsRNA to the cytoplasm. Drosophila lacks a SID-1 homolog and it is currently unknown how dsRNA escapes from the entry vesicle. In contrast to Drosophila and similar to C. elegans environmental RNAi, vertebrates may utilize the SID-1 homologs SidT1 and or SidT2 for this secondary cytoplasmic transport. Cholesterol-conjugated, lipoprotein-associated siRNA readily initiate RNAi in cultured human hepatocytes (Wolfrum et al., 2007) through a mechanism requiring two membrane transporters, SidT1 and lipoprotein endocytosis receptors. This dual requirement may be explained if the lipoprotein receptor mediates the initial internalization of siRNA in a SID-2-like process that is followed by SidT1-dependent transport across the bilayer. Based on these examples, the widespread observations of environmental RNAi in species without a SID-2 homolog could be explained by multiple different proteins that each enable the preliminary uptake of dsRNA and conserved SID-1 homologs that subsequently transport the internalized dsRNA to the cytoplasm. Similarly, if targeting ligands such as cholesterol and cell-type specific antibodies mediate an initial SID-2-like step that is capable of transporting siRNAs, this model additionally clarifies why fusing therapeutic siRNAs to these ligands increases their cellular uptake (Wolfrum et al., 2007; Zimmermann et al., 2006).
The endogenous or evolutionarily selected function of SID-2 and environmental RNAi in animals is unclear. Environmental and/or systemic RNAi may represent a novel signaling mechanism by which animals communicate information, such as an infection or other stress, which benefits the larger community. Somewhat unexpectedly, the inability of SID-2 to transport 25bp dsRNA or pre-miRNA implies that these communications signals are not the small dsRNAs produced by RNAi processing. An alternative explanation is that SID-2 substrates are derived from viruses containing dsRNA genomes or dsRNA replication intermediates. In this model, environmental RNAi could function as part of an immune response if the ingestion of damaged or non-infective viral particles triggers a protective systemic RNAi response prior to viral infection (Saleh et al., 2009). The absence of environmental RNAi in closely related species may be explained by the presence of viruses that can launch an infection starting from naked RNA. Therefore, the presence or absence of environmental RNAi potentially represents the balance of selection for or against predominant viruses in the environment. Recent data from Nuez and Félix have identified multiple new Caenorhabditis species that respond to feeding RNAi as well as many that are insensitive. Phylogenetic analysis of sensitive/insensitive species shows that environmental RNAi must have been gained or lost multiple times during evolution (Nuez and Félix, 2012). Interestingly, the ability of Cbr-SID-2 to internalize dsRNA when connected to the SID-2 extracellular domain suggests that Cbr-SID-2 is a receptor but for an unknown environmental substrate. It is also possible that the endogenous function of the SID-2 transport pathway may be unrelated to feeding RNAi and its ability to import environmental dsRNAs may only be serendipitous. Identifying the endogenous substrates of SID-2 and its homologs will help to answer these questions and shed light on whether environmental RNAi has been selected for or against during invertebrate evolution.
Experimental Procedures
All primer sequences are listed in Table S1.
C. elegans strains and cell culture
C. elegans strains used include VC1119 [dyf-2&sid-2(gk505)III] and HC722 [dyf-2&sid-2(gk505)III; qtIs5(SID-2::GFP)]. S2 cells were transfected with Effectene (Qiagen) 48–72 hours prior to experiments. For all SID-2::GFP experiments, fluorescence was used to confirm that approximately the same number of cells were transfected in each sample. To control for transfection variations, each condition was tested with cells from at least two separate transfections. For luciferase assays, S2 cells were transfected with a 50:1 ratio of sid:pPacPl-Pluciferase plasmids (Feinberg and Hunter, 2003). C. elegans cells were cultured as described (Shih et al., 2009).
dsRNA synthesis
Construction of nucleic acid templates can be found in Supplementary Procedures. 32P-UTP was internally incorporated into RNA as described (Shih and Hunter, 2011). cy5-UTP (Amersham) was internally incorporated into RNA synthesized using Ampliscribe T7 kit (Epicentre) and purified with P6 columns (Bio-Rad).
dsRNA uptake assays
Radiolabeled dsRNA assay was performed as described (Shih and Hunter, 2011). Briefly, 4×106 S2 cells were incubated with 32P-labeled dsRNA for 100 minutes (SID-2) or 30 minutes (SID-1), trypsin digested, PBS washed, lysed with 0.1% SDS, and assayed by scintillation counting. For inhibitor conditions, SID-2-expressing cells were pre-incubated for 30 minutes in either 5 µM oligomycin (Sigma),1 µg/mL latrunculin A (Sigma), or 100 nM bafilomycin A1 (Sigma) and then incubated with dsRNA plus inhibitor for 50 minutes. SID-1-expressing cells were pre-incubated in the inhibitors for 50 minutes and exposed to dsRNA plus inhibitor for 30 minutes. For fluorescently labeled S2 cells dsRNA assays, 1×106 S2 cells were incubated with 5 µg/mL cy5-labeled dsRNA in 100 µl Schneider’s media (Invitrogen) for 60 minutes, PBS washed, fixed in 4% paraformaldehyde for 30 minutes, and PBS washed. For fluorescently labeled dsRNA uptake assays in C. elegans, HC722 (SID-2::GFP) and VC1119 (sid-2 mutant) animals were co-incubated with 89 ng/µl cy5-labeled dsRNA for 2 hours. Live immobilized animals were imaged with a Zeiss Axiovert 200m spinning disc confocal microscope.
RNAi experiments
Worms were fed pal-1 hairpin (Baugh et al., 2005) or bli-1 dsRNA as described (Kamath et al., 2003; Winston et al., 2002) and scored as affected if < 5% viable progeny (pal-1) or a blistered cuticle were observed after 3 days (bli-1). Controls included OP50 and bacteria containing the L4440 vector with no dsRNA insert. For luciferase assays, 1 × 105 S2 cells were incubated in 10 µg/ml of 500bp luciferase dsRNA or mock-treated for 4 hours in media at the appropriate pH. Cells were washed extensively and incubated in pH 7 media without dsRNA for 24 hours. Luciferase levels were measured with Dual-Glo Luciferase Assay System (Promega) and the Tecan infinite M200 pro plate reader.
Plasmid construction and site-directed mutagenesis
Full-length SID-2 and Cbr-SID-2 were PCR amplified from pHC242 (Winston et al., 2007) (primers S1F and S1R) and mixed stage C. briggsae cDNA (primers S2F and S2R) respectively and ligated as described (Winston et al., 2007) to GFP coding and unc-54 3’UTR sequences that were PCR amplified from pPD95.75 (primers GF and GR for SID-2, primers GF and GR2 for Cbr-SID-2). The GFP fusions were cloned into the pPacPl vector at the KpnI and NotI sites (SID-2::GFP) or the KpnI site (Cbr-SID-2::GFP). SID-2 histidine mutants were generated by site-directed mutagenesis of SID-2::GFP in pPacPl using the Quikchange kit (Stratagene).
SID-2 and Cbr-SID-2 domain swap construction
SID-2 was divided into four regions, A and B (extracellular domain), C (TM domain), and D (cytoplasmic domain and GFP) and was driven by the sid-2 promoter (P). Capital letters indicate SID-2, lowercase letters indicate Cbr-SID-2. sid-2 fragments were PCR amplified from N2 genomic DNA or SID-2::GFP (Winston et al., 2007) using primers D1 and D2 (PABC), primers D1 and D3 (PAB), primers D4 and D5 (CD), primers D1 and D6 (PA), primers D7 and D5 (BCD), or primers D1 and D8 (P). Cbr-SID-2::GFP fragments were PCR amplified from Cbr-SID-2::GFP (Winston et al., 2007) using primers D9 and D5 (d), primers D10 and D5 (cd), primers D11 and D12 (pab), primers D13 and D5 (bcd), primers D14 and D15 (a), and primers D14 and D5 (abcd). Gel purified fragments (Qiagen) were PCR stitched with primers D16 and D17 or directly injected into worms for in vivo stitching. Fragments were co-injected with the pRF4 vector (25 µg/mL) into VC1119. F2 lines were selected from Rol animals with apically-localized GFP. HC722 was used for PABCD.
Supplementary Material
Highlights.
SID-2 is required to import dsRNA from the acidic C. elegans intestinal lumen
SID-2 expressed in S2 cells selectively uptakes dsRNA from acidic environments
SID-2 function requires extracellular histidine residues and vesicle trafficking
dsRNA imported by SID-2 likely becomes cytoplasmic via the dsRNA SID-1 channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baugh LR, Hill AA, Claggett JM, Hill-Harfe K, Wen JC, Slonim DK, Brown EL, Hunter CP. The homeodomain protein PAL-1 specifies a lineage-specific regulatory network in the C. elegans embryo. Development. 2005;132:1843–1854. doi: 10.1242/dev.01782. [DOI] [PubMed] [Google Scholar]
- Coué M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Letters. 1987;213:316. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Feinberg EH, Hunter CP. Transport of dsRNA into Cells by the Transmembrane Protein SID-1. Science. 2003;301:1545–1547. doi: 10.1126/science.1087117. [DOI] [PubMed] [Google Scholar]
- Fukuda L, Watanabe T, Tokisue T, Tsujita T, Nishikawa S, Hasegawa T, Seya T, Matsumoto M. Modulation of Double-stranded RNA Recognition by the N-terminal Histidine-rich Region of the Human Toll-like Receptor 3. Journal of Biological Chemistry. 2008;283:22787–22794. doi: 10.1074/jbc.M802284200. [DOI] [PubMed] [Google Scholar]
- Hunter W, Ellis J, vanEngelsdorp D, Hayes J, Westervelt D, Glick E, Williams M, Sela I, Maori E, Pettis J, et al. Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (Apis mellifera, Hymenoptera: Apidae) PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001160. e1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Lorenzo A, Skinner M, Annan JE, Futai M, Sun-Wada G-H, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, et al. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol. 2006;8:124. doi: 10.1038/ncb1348. [DOI] [PubMed] [Google Scholar]
- Huvenne H, Smagghe G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. Journal of Insect Physiology. 2010;56:227. doi: 10.1016/j.jinsphys.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Jose AM, Smith JJ, Hunter CP. Export of RNA silencing from C. elegans tissues does not require the RNA channel SID-1. Proceedings of the National Academy of Sciences. 2009;106:2283–2288. doi: 10.1073/pnas.0809760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Li F, Ding S-W. Virus Counterdefense: Diverse Strategies for Evading the RNA-Silencing Immunity. Annual Review of Microbiology. 2006;60:503. doi: 10.1146/annurev.micro.60.080805.142205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, Davies DR. Structural Basis of Toll-Like Receptor 3 Signaling with Double-Stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Yan X, Han R. Prevention of Chinese Sacbrood Virus Infection in Apis cerana using RNA Interference. Current Microbiology. 2010;61:422–428. doi: 10.1007/s00284-010-9633-2. [DOI] [PubMed] [Google Scholar]
- Maori E, Paldi N, Shafir S, Kalev H, Tsur E, Glick E, Sela I. IAPV, a bee-affecting virus associated with Colony Collapse Disorder can be silenced by dsRNA ingestion. Insect Molecular Biology. 2009;18:55–60. doi: 10.1111/j.1365-2583.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- Nuez I, Félix M-A. Evolution of Susceptibility to Ingested Double-Stranded RNAs in Caenorhabditis Nematodes. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0029811. e29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer J, Johnson D, Nehrke K. Oscillatory Transepithelial H+ Flux Regulates a Rhythmic Behavior in C. elegans. Current Biology. 2008;18:297. doi: 10.1016/j.cub.2008.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M-C, Tassetto M, van Rij RP, Goic B, Gausson V, Berry B, Jacquier C, Antoniewski C, Andino R. Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature. 2009;458:346. doi: 10.1038/nature07712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MC, van Rij RP, Hekele A, Gillis A, Foley E, O'Farrell PH, Andino R. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol. 2006;8:793–802. doi: 10.1038/ncbl439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarathi M, Simon M, Venkatesan C, Hameed A. Oral Administration of Bacterially Expressed VP28dsRNA to Protect Penaeus monodon from White Spot Syndrome Virus. Marine Biotechnology. 2008;10:242. doi: 10.1007/s10126-007-9057-6. [DOI] [PubMed] [Google Scholar]
- Shih JD, Fitzgerald MC, Sutherlin M, Hunter CP. The SID-1 double-stranded RNA transporter is not selective for dsRNA length. RNA. 2009;15:384–390. doi: 10.1261/rna.1286409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JD, Hunter CP. SID-1 is a dsRNA-selective dsRNA-gated channel. RNA. 2011;17:1057–1065. doi: 10.1261/rna.2596511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H, Grishok A, C. Mello C. REVERSE GENETICS:RNAi in C. elegans: Soaking in the Genome Sequence. Science. 1998;282:430–431. doi: 10.1126/science.282.5388.430. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Ulvila J, Parikka M, Kleino A, Sormunen R, Ezekowitz RA, Kocks C, Ramet M. Double-stranded RNA Is Internalized by Scavenger Receptor-mediated Endocytosis in Drosophila S2 Cells. J Biol Chem. 2006;281:14370–14375. doi: 10.1074/jbc.M513868200. [DOI] [PubMed] [Google Scholar]
- Whangbo JS, Hunter CP. Environmental RNA interference. Trends in Genetics. 2008;24:297. doi: 10.1016/j.tig.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans Requires the Putative Transmembrane Protein SID-1. Science. 2002;295:2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- Winston WM, Sutherlin M, Wright AJ, Feinberg EH, Hunter CP. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proceedings of the National Academy of Sciences. 2007;104:10565–10570. doi: 10.1073/pnas.0611282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotech. 2007;25:1149. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- Yarar D, Waterman-Storer CM, Schmid SL. A Dynamic Actin Cytoskeleton Functions at Multiple Stages of Clathrin-mediated Endocytosis. Mol Biol Cell. 2005;16:964–975. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann TS, Lee ACH, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.