Abstract
Previously, we identified a novel gene, pmgA, as an essential factor to support photomixotrophic growth of Synechocystis species PCC 6803 and reported that a strain in which pmgA was deleted grew better than the wild type under photoautotrophic conditions. To gain insight into the role of pmgA, we investigated the mutant phenotype of pmgA in detail. When low-light-grown (20 μE m−2 s−1) cells were transferred to high light (HL [200μE m−2 s−1]), pmgA mutants failed to respond in the manner typically associated with Synechocystis. Specifically, mutants lost their ability to suppress accumulation of chlorophyll and photosystem I and, consequently, could not modulate photosystem stoichiometry. These phenotypes seem to result in enhanced rates of photosynthesis and growth during short-term exposure to HL. Moreover, mixed-culture experiments clearly demonstrated that loss of pmgA function was selected against during longer-term exposure to HL, suggesting that pmgA is involved in acquisition of resistance to HL stress. Finally, early induction of pmgA expression detected by reverse transcriptase-PCR upon the shift to HL led us to conclude that pmgA is the first gene identified, to our knowledge, as a specific regulatory factor for HL acclimation.
Acclimation to light regimes is one of the most important and complex responses of photosynthetic organisms to varying environmental conditions. Under different growth light cyanobacteria and plants regulate antenna pigment complexes, photochemical reaction centers, and enzymes for CO2 fixation to optimize utilization of light energy (for review, see Anderson, 1986; Melis, 1991; Anderson et al., 1995). Under light-limiting conditions, antenna pigments are selectively accumulated to collect light energy efficiently. It is well known that cyanobacteria increase their antenna size by elongation of the phycobilisome rods and by an increase in the number of phycobilisomes per unit area of thylakoid membrane upon the shift to LL (Knanna et al., 1983; Lönneborg et al., 1985). Higher plants and green algae having chlorophyll b as an antennae pigment show a marked decline in chlorophyll a to b ratio upon the shift to LL, reflecting accumulation of the light-harvesting chlorophyll a/b complex (Leong and Anderson, 1984). However, under light-saturating conditions these organisms reduce their antenna size. Moreover, Rubisco, the rate-limiting enzyme for CO2 fixation, is accumulated to balance with high photochemical activities (Björkman, 1981). Expression of enzymes such as catalase and superoxide dismutase is also enhanced to scavenge reactive oxygen species, which are generated by excess light energy (Foyer et al., 1994). The amount of PSII relative to that of PSI, the photosystem stoichiometry, is another target for the regulation in response to light intensity, since photosynthetic electron transport to generate NADPH and ATP is driven by coordination of the two photosystems with distinct antenna size (Melis et al., 1985; Fujita et al., 1987, 1994). In general, the antenna size of PSII is variable, whereas that of PSI is unchanged under various light conditions. Under LL the photosystem stoichiometry is optimized based on their antenna sizes, whereas it must be kept near unity irrespective of the antenna size under HL. Thus, organisms must balance the electron flow between the two photosystems by modulating both antenna complexes and photosystem stoichiometry at different light intensities.
Although a number of reports have provided information on the physiological and biochemical characterization of various light acclimation, very little is known about molecular mechanisms for sensing light conditions or for modulating expression and/or assembly of the photosynthetic apparatus (Allen, 1995; Anderson et al., 1995). One exception is the complementary chromatic adaptation, which has been studied extensively in a cyanobacterium having inducible genes for phycocyanin and phycoerythrin. Several genes, including a possible photoreceptor and signal transduction components, have been identified based on the characterization of mutant phenotypes (Chiang et al., 1992; Grossman et al., 1994; Kehoe and Grossman, 1996). Since many light-acclimation responses are supposed to be common to both cyanobacteria and plants, cyanobacteria seem to be better models for molecular studies of light acclimation in photosynthetic organisms.
Here we report that modulation of photosystem stoichiometry, one of the responses typically observed upon the shift to HL, is specifically supported by a novel gene, pmgA, in the cyanobacterium Synechocystis sp. PCC 6803. The gene was initially identified as an essential factor required to support photomixotrophic growth with both light and Glc. However, its mutants could grow better than the wild type under photoautotrophic conditions (Hihara and Ikeuchi, 1997). Surprisingly, the mutant, which appeared spontaneously in a wild-type culture under photoautotrophic conditions, completely took over the culture for a year or so in our laboratory. Further characterization of pmgA mutants in this communication revealed its specific role in light acclimation. We also provide evidence for the physiological role of modulation of photosystem stoichiometry as a HL response.
MATERIALS AND METHODS
Strains and Culture Conditions
A Glc-tolerant wild-type strain of Synechocystis sp. PCC 6803 (WS) and mutants (WL strain with 1 base replacement in pmgA and a disruptant with spectinomycin-resistance cassette: WL and pmgA::SpR) (Hihara and Ikeuchi, 1997) were grown at 32°C in BG-11 medium with 20 mm Hepes-NaOH (pH 7.0) under continuous illumination provided by fluorescent lamps. Liquid cultures were bubbled with air containing 1.0% (v/v) CO2. The pmgA-disrupted mutant was usually maintained with 20 μg/mL spectinomycin. PPFD was measured by a quantum sensor (model LI-250, Li-Cor, Lincoln, NE). Cell density was estimated as A730 with a spectrophotometer (model UV-160A, Shimadzu, Kyoto, Japan).
In all of the experiments, fresh media were inoculated at a cell density of A730 = 0.05 with precultures grown to late log phase (A730 = 0.8–0.9) under LL and then transferred to HL or LL. Unless otherwise stated, cells were grown in volumes of 50 mL with test tubes (3 cm in diameter). A larger volume of culture was provided in 500- or 1000-mL flat vessels illuminated with the same HL. The fixed culture conditions were important for HL, since the self-shading started to cancel HL at the later stage of the batch culture.
Absorption and 77 K Fluorescence Emission Spectra
In vivo absorption spectra of whole cells of the wild type and mutants suspended in BG-11 medium were measured at room temperature using a spectrophotometer (model U3500, Hitachi, Tokyo, Japan) with an end-on photomultiplier. Chlorophyll and phycocyanin were calculated using the equations of Arnon et al. (1974). Low-temperature fluorescence emission spectra at 77 K were recorded using a custom-made apparatus (Sonoike and Terashima, 1994). Cells containing 100 μg chlorophyll/mL in BG-11 medium were placed in a sample holder. Pigments were excited with light 400 to 600 nm in wavelength produced by passing white light from a 100-W halogen lamp through a filter (CS4–96, Corning Inc., Corning, NY). Before measurement, cells were dark adapted (>10 min) at room temperature to eliminate possible effects of the state transition.
Measurement of Rates of Electron-Transfer Reactions
Oxygen evolution and consumption of cells were measured in BG-11 medium with a Clark-type electrode at a chlorophyll concentration of 2.5 μg/mL. The medium was continuously stirred at 25°C and illuminated with saturating actinic light (4000 μE m−2 s−1). Whole-cell photosynthetic activity or PSII-mediated electron transfer activity was measured as oxygen evolution supported by 2 mm NaHCO3 or 2 mm 2,6-DCBQ, respectively. PSI-mediated electron transfer activity was measured as oxygen consumption in the presence of 1 mm ascorbic acid, 5 mm DAD, 2 mm MV, 20 μm DCMU, and 1 mm KCN.
Determination of Photosystems
Thylakoid membranes used for measurements of PSII and PSI were isolated from cells grown in 1000 mL of culture volume. Cells suspended in HN buffer (5 mm Hepes-NaOH and 10 mm NaCl, pH 7.5) were broken with a Mini-Bead Beater (Biospec, Bartlesville, OK) with zircon beads (100 μm in diameter, Biospec) for three pulses of 50 s each with 2-min cooling intervals at 0°C. After brief centrifugation to remove the beads, cell debris and thylakoid membranes were collected at 45,000g for 20 min with a rotor (RP80AT, Hitachi). Pellets were resuspended in HN buffer and sonicated for three pulses of 10 s each with 10-s cooling intervals at 0°C to liberate thylakoid membranes from cell debris. After centrifugation at 2,500g for 5 min with a RT15A8 rotor (Hitachi), thylakoid-containing supernatants were used to determine P700 and Cyt b559.
PSII content was estimated as one-half molar of Cyt b559, as described in Fujita and Murakami (1987). Cyt b559 was determined from the difference spectrum (520–600 nm) between ascorbate- and hydroquinone-reduced conditions using a U3500 spectrophotometer. The chlorophyll concentration of thylakoid membranes was 80 μg/mL, and a difference absorption coefficient of 21 mm−1 cm−1 (Garewall and Wasserman, 1974) was used.
PSI content was estimated as photoactive P700 content upon illumination by continuous light. Absorbance changes at 703 nm were measured with a spectrophotometer (model 356, Hitachi) (Terashima et al., 1994). The reaction mixture contained thylakoid membranes at a chlorophyll concentration of 3 μg/mL in 50 mm Tris-HCl (pH 7.5), 10 mm sodium ascorbate, 30 μm TMPD, 10 μm MV, and 0.05% dodecylmaltoside. The reduced-minus-oxidized differential absorption coefficient of P700 is known to vary with species (Hiyama and Ke, 1972; Sonoike and Katoh, 1990) and with the preparation used (Sonoike and Katoh, 1988, 1989). Thus, we determined the absorption coefficient of P700 in the thylakoid membranes from Synechocystis sp. PCC 6803 by measuring oxidation of TMPD coupled with the reduction of flash-oxidized P700, basically as described by Hiyama and Ke (1972). Flash-induced absorbance changes on a millisecond time scale were measured with a single-beam spectrophotometer (model RA-401, Otsuka Electronics, Osaka, Japan) under aerobic conditions.
Absorption changes were measured at 703 nm for P700 and 575 nm for TMPD, and the absorption coefficient of oxidized TMPD at this wavelength was assumed to be 10.7 mm−1 cm−1 (Hiyama and Ke, 1972). Saturating xenon flashes (half-duration time of 5 μs) that passed through two band-pass filters (CS 4–96 and CS 7–59, Corning) and a dichroic filter (DF-B, Japan Vacuum Optics, Gotenba, Japan) were fired at 0.1 Hz, and signals were recorded with a photomultiplier (R374, Hamamatsu Photonics, Shizuoka, Japan) blocked with two cutoff filters (R-69, Toshiba, Tokyo, Japan) for P700 or with an orange cutoff filter (O-57, Toshiba) and a dichroic filter (DF-C, Japan Vacuum Optics) for TMPD. The reaction mixture contained thylakoid membranes equivalent to 3 μg/mL chlorophyll, 0.8 mm TMPD, 10 μm DCMU, 1 mm KCN, 0.05% dodecylmaltoside, and 50 mm Tris/HCl (pH 7.5). The absorption coefficient of P700 in thylakoid membranes from Synechocystis sp. PCC 6803 in the presence of dodecylmaltoside was determined as 71 ± 3 mm−1 cm−1. This value was significantly greater than that determined for Synechococcus elongatus in similar conditions (Sonoike and Katoh, 1988), but close to the value for the PSI preparation from Triton-solubilized thylakoid membranes from Anabaena variabilis (Hiyama and Ke, 1972).
Immunoblot Analysis
Whole-cell extracts before removal of cell debris, as described above, were treated with LDS and subjected to SDS-PAGE. For detection of D2, PsbO, and Rubisco proteins, the extracts were solubilized with 1% LDS, 60 mm DTT, and 60 mm Tris-HCl (pH 8.0) for 10 min at room temperature, whereas for PsaA/B proteins, they were solubilized with 5% LDS, 60 mm DTT, and 60 mm Tris-HCl (pH 8.0) for 2 h at room temperature to achieve complete denaturation. SDS gel electrophoresis was done by the procedure of Laemmli (1970) with a gel containing 12.5% acrylamide and 6 m urea for D2, PsbO, and Rubisco or a gradient gel of 16% to 22% acrylamide with 7.5 m urea for PsaA/B. Samples corresponding to 0.32 × 107, 0.48 × 107, 1.92 × 107, and 1.15 × 107 cells were loaded for the detection of D2, PsbO, and Rubisco and PsaA/B, respectively. Proteins were electroblotted onto PVDF membranes (Immobilon-P; Millipore). The antiserum against PsaA/B from S. elongatus was kindly provided by Dr. I. Enami (Science University of Tokyo). The antiserum against Rubisco from spinach was a generous gift from Dr. K. Okada (Tokyo University of Pharmacy and Lifescience). The antisera against D2 and PsbO were from spinach (Ikeuchi and Inoue, 1987). Reaction with antisera and immunodetection by alkaline phosphatase or peroxidase were performed according to the manufacturer's instructions (Bio-Rad).
Direct Sequencing Analysis
Direct sequencing analysis of pmgA was performed as described previously (Hihara and Ikeuchi, 1997).
Preparation of Total RNA
Cells were collected by brief centrifugation at 4°C and stored in liquid N2. The frozen cells were thawed with 20 mm EDTA and 50 mm Tris-HCl (pH 8.0) and immediately treated with phenol at 75°C for 10 min. Cells were further treated with 0.8% (w/v) SDS at 75°C for 10 min with shaking, and then extracted once with phenol/chloroform and twice with chloroform. After precipitation with ethanol, RNA was solubilized in 8 m guanidine-HCl, 0.1 m sodium acetate, pH 5.2, 5 mm DTT, and 0.5% sodium lauryl sarcosinate and precipitated with ethanol. Residual DNA in the RNA preparation was removed by digestion with DNase I at 25°C for 2 h. After ethanol precipitation, the amount of RNA was determined by UV absorption at 260 nm.
RT-PCR
First-strand cDNA was synthesized using 1 μg of total RNA with a RT-PCR High Kit (Toyobo, Osaka, Japan) in a final volume of 20 μL, according to the manufacturer's instructions. The amount of cDNA used as a template was experimentally determined for each set of primers to achieve proportional production of the PCR product (the cDNA equivalent to RNA of 0.1 μg and 5 pg was used for amplification of pmgA and rnpB, respectively). The oligonucleotide primers 5′-TGTAAAACGACGGCCA-GTCAGCACATTCAGGCCTCC-3′ and 5′-CAGGAAAC-AGCTATGACCGCTTAATTTTCTTGCTGA-3′ were used for amplification of a 565-bp fragment of pmgA and 5′-AGTTAGGGAGGGAGTTGC-3′ and 5′-TAAGCCGGGTTCTGTTCC-3′ were used for amplification of a 417-bp fragment of the constitutive RNase P gene, rnpB, as a positive control (Frías et al., 1994). After a first denaturation step of 3 min at 93°C, 30 PCR cycles were performed (93°C for 30 s, 57°C for 2 min, and 72°C for 2 min) followed by a final extension step of 10 min at 72°C. As a negative control for RT-PCR, 1 μg of RNA without the RT reaction was subjected to PCR amplification of rnpB.
RESULTS
Absorption Spectra and Content of Photosynthetic Pigment
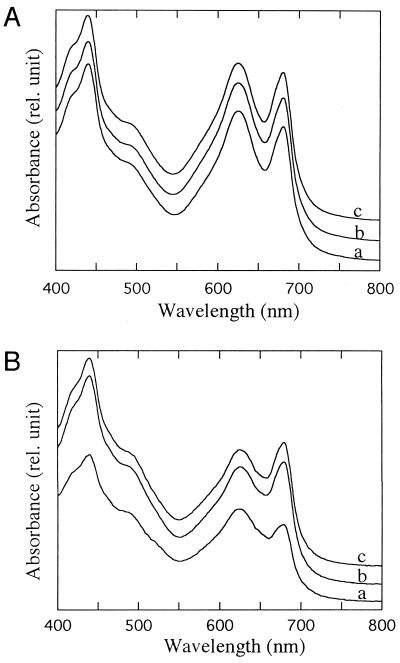
We isolated a mutant clone with a larger colony size (WL strain) compared with smaller colonies of the wild type (WS strain) under HL and identified a point mutation in a novel gene, pmgA, responsible for the change of colony size (Hihara and Ikeuchi, 1997). Here we observed that the WL strain and pmgA-disruptant (pmgA::SpR) cells in the liquid culture showed enhanced pigmentation and slightly higher cell density relative to wild-type cells under HL, as shown in Figure 1. In HL, doubling time of the WL strain (5.39 ± 0.25 h) and the pmgA-disruptant strain (5.41 ± 0.28 h) was significantly shorter than that of the wild type (5.82 ± 0.14 h). Figure 2 shows absorption spectra of cells grown in liquid culture under HL or LL. Absorption spectra were significantly different between the wild type and pmgA mutants under HL: the peak of chlorophyll absorption at 678 nm was higher than the peak of phycocyanin absorption at 628 nm in the mutants, whereas it was lower in the wild type (Fig. 2B). However, there was no difference in the relative peak heights between the wild type and mutants when grown under LL (Fig. 2A). Compared with cells grown under HL, the content of both pigments increased relative to cell density and to carotenoid absorption (approximately 495 nm) in both cell types under LL. The difference in pigmentation was barely discernible for colonies on agar plates under similar HL conditions (Hihara and Ikeuchi, 1997) or for liquid cultures under LL conditions (data not shown).
Figure 1.
Liquid culture of wild type (WS) and pmgA mutants (WL and the disruptant pmgA::SpR) at log phase (20 h after inoculation) under HL. A730 of WS, WL, and pmgA-disruptant was 0.56, 0.73, and 0.66, respectively.
Figure 2.
Absorption spectra of cells grown under different light intensities. A, Absorption spectra of LL-grown cells. B, Absorption spectra of cells 18 h after shift to HL. The spectra of wild type (a), WL (b), and pmgA-disruptant cells (c) are normalized at A730. rel., Relative.
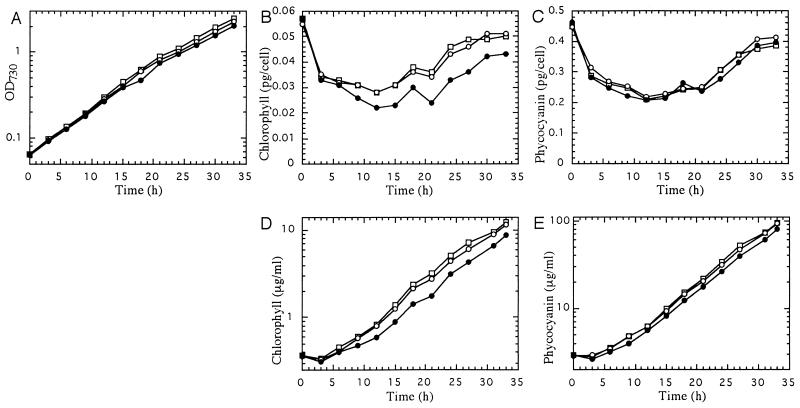
Figure 3 shows time-course changes in cell density and pigment abundance in the small batch culture defined in Methods. Data at time 0, representing LL-grown cells, showed no significant differences between the wild type and pmgA mutants. Upon transfer to HL, the content of both chlorophyll (Fig. 3B) and phycocyanin (Fig. 3C) on a per-cell basis showed changes with three different phases: (a) drastically reduced to about two-thirds within 3 h, (b) further decreased but at a lesser rate until 12 h, and (c) gradually recovered, although cells continued to grow logarithmically (Fig. 3A). Notably, the chlorophyll content was significantly greater in pmgA mutants than in the wild type after 9 h, whereas the phycocyanin content was not much different between the strains throughout the batch culture. The cellular content of both pigments almost recovered to the initial level after 30 h due to the self-shading effect at high cell density. Taking into account that cells were dividing logarithmically, accumulation of pigments, expressed per milliliter of culture volume, is shown on a log scale (Fig. 3, D and E). Accumulation of both pigments stopped during the initial 3 h (phase 1). The pigment accumulation restarted at a low rate from 3 to 12 h (phase 2) and accelerated after 12 h (phase 3). Clearly, the pmgA mutants differed from the wild type in their chlorophyll accumulation during phase 2 (Fig. 3D), leading to a higher cellular chlorophyll content in phase 3 (Fig. 3B). However, accumulation of phycocyanin did not differ between the wild type and the mutants (Fig. 3E), although its time course was similar to that of chlorophyll. In short, loss of pmgA function seems to abolish the specific retardation of chlorophyll synthesis at phase 2 (3–12 h) in response to HL. However, the initial suppression of chlorophyll synthesis, the recovery of chlorophyll synthesis during phase 3, and phycocyanin synthesis do not seem to be affected by the pmgA mutation.
Figure 3.
Growth curve and changes in the pigment content in the course of a batch culture under HL. A, Cell density. B, Chlorophyll content expressed on a per-cell basis. C, Phycocyanin content expressed on a per-cell basis. D, Chlorophyll accumulation expressed per milliliter of culture volume. E, Phycocyanin accumulation expressed per milliliter of culture volume. At time 0, the batch culture was inoculated with LL-grown cells. •, WS; ○, WL; □, pmgA::SpR.
Chlorophyll-Fluorescence Spectra and Content of Photosystems
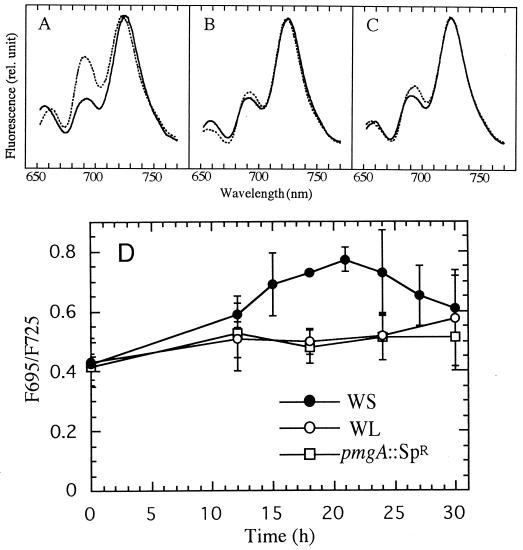
Differences in chlorophyll content are assumed to reflect changes in photosystems of cyanobacteria, since they have no apparent chlorophyll-binding antenna proteins. It is widely accepted that chlorophylls of PSII emit fluorescence around 685 and 695 nm, whereas chlorophylls of PSI emit at 720 to 730 nm at 77 K (Murata et al., 1966). Thus, as shown in Figure 4, we investigated the chlorophyll-fluorescence emission spectra of cells at 77 K to determine whether photosystem stoichiometry is different between the wild type and pmgA mutants. When the spectra were normalized at the peaks of PSI fluorescence, it was notable that the peak at 695 nm originating from PSII was about 1.5-fold higher in wild-type cells grown under HL than under LL (Fig. 4A). However, the 695-nm peak was virtually unchanged in pmgA mutants (Fig. 4, B and C). This strongly indicates that the ratio of PSII to PSI increased in the wild type in response to HL, whereas it remained unchanged in the mutants. Since the fluorescence ratio (F695/F725) is a good index of the ratio of PSII to PSI, the ratio was plotted in the course of the same experimental conditions as in Figure 3. Clearly, the ratio gradually increased in the wild type 12 h after the shift to HL, reaching the maximum (about 1.5-fold of the initial) at around 18 to 24 h, whereas the ratio changed little in pmgA mutants (Fig. 4D). These differences in the time-course change of the F695/F725 ratio between the wild type and pmgA mutants appear to reflect the difference in chlorophyll accumulation (Fig. 3D). A decline of the ratio after 20 h in the wild type is possibly due to the self-shading effect in the large vessel.
Figure 4.
Low-temperature (77 K) fluorescence emission spectra of cells. A, Fluorescence emission spectra of WS (wild type) at 77 K. B, Fluorescence emission spectra of WL at 77 K. C, Fluorescence emission spectra of pmgA-disruptant at 77 K. Spectra of LL-grown cells (solid line) and cells 20 h after a shift to HL (dashed line) were normalized at the 725-nm peak of PSI. D, Time course of the change in the ratio of F695/F725 under HL. Conditions for the culture were the same as in Figure 3. Data are the means ± se for at least three separate experiments. rel., Relative.
To confirm the results of fluorescence measurement, we determined the photosystem content of thylakoid membranes isolated from LL-and HL-grown cells by measuring Cyt b559 and P700. Cyt b559 has been known to be tightly associated with the PSII reaction center in thylakoid membranes at a molar ratio of 2:1 (Whitmarsh and Ort, 1984), whereas P700, a photoactive pigment of PSI reaction center, was used to determine PSI content (Hiyama and Ke, 1972). Table I shows that the PSII content on a per-cell basis decreased to about 74% in the wild type during the first 13 h after the shift to HL conditions, whereas the PSI content markedly decreased to about 40% of the initial value. As a result, the ratio of PSII to PSI increased from 0.48 for LL-grown cells to 0.81 for HL-grown cells. Since cells grew logarithmically during this period, the accumulation of PSI and PSII may have been transiently suppressed and recovered as chlorophyll synthesis, as shown in Figure 3D. In pmgA mutants, content of both photosystems similarly decreased under HL but the marked suppression of PSI accumulation did not take place. As a result, the photosystem stoichiometry remained unchanged in the mutants under HL. Wild-type cells showed a slight recovery of their photosystem content and stoichiometry after 22 h. Consistently, the low-temperature fluorescence ratio (F695/F725) of the wild-type thylakoids was at the maximum level at 13 h and slightly lower at 22 h, whereas the ratio of the mutant thylakoids was not much changed (data not shown). These fluorescence changes seemed to occur slightly earlier than those observed in Figure 4D. This may be due to higher self-shading in the larger culture volume required for the measurement of Cyt b559.
Table I.
Content of photosystems in the wild type and pmgA mutantsa
| Strain | Timeb | PSII | PSI | PSII/PSI |
|---|---|---|---|---|
| h | 10−19 mol/cell | |||
| WS (wild type) | 0 | 2.01 ± 0.19 | 4.21 ± 0.13 | 0.48 ± 0.06 |
| 13 | 1.48 ± 0.23 | 1.68 ± 0.11 | 0.81 ± 0.08 | |
| 22 | 1.55 ± 0.16 | 2.13 ± 0.19 | 0.73 ± 0.07 | |
| WL | 0 | 2.17 ± 0.38 | 4.27 ± 0.08 | 0.51 ± 0.08 |
| 13 | 1.57 ± 0.05 | 2.67 ± 0.24 | 0.60 ± 0.07 | |
| 22 | 1.85 ± 0.04 | 3.49 ± 0.32 | 0.53 ± 0.06 | |
| pmgA::SpR | 0 | 2.10 ± 0.32 | 4.16 ± 0.21 | 0.50 ± 0.07 |
| 13 | 1.47 ± 0.09 | 2.51 ± 0.18 | 0.59 ± 0.04 | |
| 22 | 1.79 ± 0.08 | 4.31 ± 0.52 | 0.42 ± 0.04 | |
Number of PSI and PSII on a per-cell basis were calculated from P700 and one-half of Cyt b559, respectively. Data are the means ± se for at least three separate experiments.
The culture was inoculated with LL-grown cells and transferred to HL at time 0. Conditions for the culture were the same as in Figure 3, except for a larger culture volume in this experiment.
From the data in Table I and the phycocyanin content of the samples, we also calculated the ratio of phycocyanin to PSII, which represents the antenna size of PSII. It dropped to about 80% not only in the wild type but also in pmgA mutants during the first 13 h after the shift to HL conditions (data not shown). Thus, we conclude that pmgA is essential for the modulation of the PSII-to-PSI ratio as acclimation to HL but not for the adjustment of antenna size of PSII.
Electron-Transfer Activities under Different Light Intensities
We pursued the relationship between the two phenotypic features of pmgA mutants, the lack of modulation of the photosystem stoichiometry and the enhanced growth under photoautotrophic conditions, by measuring photosynthetic activities (Table II). Under LL, there were no significant differences in activities of whole-cell photosynthesis (from water to CO2), PSII (from water to 2,6-DCBQ), and PSI (from reduced DAD to MV) between the wild type and pmgA mutants. This is consistent with our previous observation that the growth rate of pmgA mutants was comparable to that of the wild type under LL (Hihara and Ikeuchi, 1997). When cells were grown under HL for 18 h, the whole photosynthetic activity of the mutants was much greater than that of the wild type. This seems to account partly for the fact that pmgA mutants grew slightly better than the wild type under HL (Fig. 3A). The PSI activity was higher in pmgA mutants than in the wild type. This is because the wild type markedly decreased PSI activity as well as PSI content in response to HL, whereas pmgA mutants did not. The slight discrepancy was observed between P700 content and PSI activity. Since DAD indirectly as well as directly donates electrons to P700, the PSI activity may also be affected by other unknown conditions (Izawa, 1980). The PSII activity was also higher in pmgA mutants than in the wild type under HL. This was due to a significant decrease in the ratio of PSII activity to PSII content only in wild-type cells transferred to HL. A possible reason for the HL effect will be addressed in Discussion.
Table II.
Photosynthetic activities of cells
| Reaction | Activities
|
||
|---|---|---|---|
| WS (wild type) | WL | pmgA::SpR | |
| μmol O2 evolved 109 cells−1 h−1 | |||
| LL | |||
| H2O to HCO−3 | 6.60 ± 0.51 | 6.49 ± 0.50 | 6.53 ± 0.77 |
| PSII (water to 2,6-DCBQ) | 21.72 ± 0.30 | 22.73 ± 0.51 | 22.27 ± 0.69 |
| PSI (DAD/ascorbic acid to MV) | −19.59 ± 3.31 | −13.41 ± 2.82 | −14.43 ± 2.89 |
| HLa | |||
| H2O to HCO−3 | 8.87 ± 0.16 | 12.41 ± 1.06 | 11.35 ± 0.55 |
| PSII (water to 2,6-DCBQ) | 9.24 ± 0.33 | 13.56 ± 1.41 | 12.67 ± 0.83 |
| PSI (DAD/ascorbic acid to MV) | −6.02 ± 0.74 | −11.24 ± 3.53 | −11.41 ± 2.04 |
All values represent the means ± se for at least three separate experiments.
Activities of cells 18 h after shift to HL.
It is also of interest that the ratio of the whole photosynthetic activity to PSII or PSI activity was much higher in HL-grown than in LL-grown cells, regardless of functionality of pmgA. This suggests that the enzyme(s) for carbon assimilation were up-regulated under HL, in contrast to the down-regulation of chlorophyll content. Both regulations are typical responses of photosynthetic organisms to HL (Björkman, 1981; Raps et al., 1983).
Western Analysis
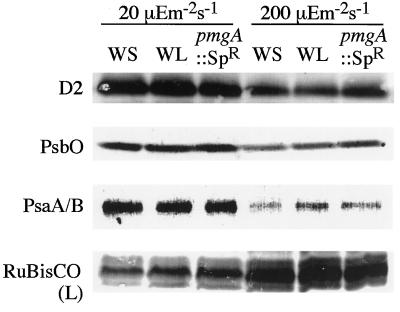
To confirm the measurements of photosystem content and activities, photosystem proteins and Rubisco were probed with specific antibodies as shown in Figure 5. Clearly, the cellular content of the D2 protein of PSII reaction centers, the PsbO protein of PSII oxygen-evolving complexes, and the PsaA/B proteins of PSI reaction centers were down-regulated under HL, whereas the content of the Rubisco large subunit was up-regulated. These differences in accumulation of photosystems and Rubisco proteins seem to coincide with the data on photosynthetic activity. It is interesting that only PsaA/B proteins under HL seemed to be down-regulated in the wild type and not in pmgA mutants. Although the difference in PsaA/B content was not clear, possibly due to inaccuracy of the method, the results seem to support the idea that pmgA is involved in the modulation of photosystem stoichiometry by regulating the accumulation of PSI under HL.
Figure 5.
Immunoblotting of polypeptides of the wild type (WS) and pmgA mutants (WL and the disruptant pmgA::SpR). Total cell extracts of LL-grown cells or cells 18 h after a shift to HL were separated by SDS-PAGE, electrotransferred, and challenged with anti-D2, anti-PsbO, anti-PsaA/B, or anti-Rubisco (RuBisCO). Proteins extracted from the same number of cells were probed with each antibody (see Methods).
Mixed-Culture Experiments at Various Light Intensities
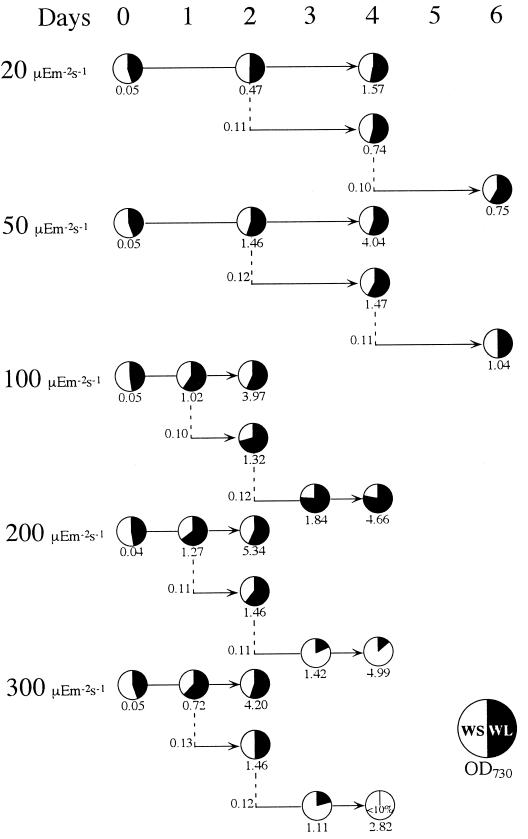
We have shown that the higher chlorophyll content and the lower PSII-to-PSI ratio in the pmgA mutants were apparently linked to the higher photosynthetic activities per cell and the higher growth rates than the wild type under HL. Next, we explored the physiological significance of the pmgA-mediated regulation mechanism, which had been acquired in wild-type Synechocystis in evolution. We attempted to answer this question by examining growth rates of a point mutant of pmgA (WL) and the wild type in mixed-culture experiments as shown in Figure 6. Relative growth was estimated by direct sequencing of the pmgA gene in genomic DNA extracted from the mixed cultures, which consisted of several consecutive batch cultures. Direct sequencing provided information about the population of the two strains at the time of sampling irrespective of the extent of photodamage. Examination by direct sequencing seemed to be more reliable than subsequent growth tests, especially for damaged cells. Consistent with our previous observation on agar plates (Hihara and Ikeuchi, 1997), the mutant and the wild type grew similarly under LL or medium light (50 μE m−2 s−1). Under higher-light conditions (100 μE m−2 s−1), the mutant became dominant in the second and third culture. We also confirmed that the mutant became dominant in the second culture at 200 and 400 μE m−2 s−1, when the culture was transferred every 2 d (data not shown), as shown in our previous report (Hihara and Ikeuchi, 1997). However, the WL mutant suddenly disappeared from the mixed culture under similar conditions when transferred every 24 h (Fig. 6, 200–300 μE m−2 s−1). The latter culture regime kept cells at a relatively low density so that they would receive more HL stress due to reduced self-shading. Therefore, disappearance of the pmgA mutant indicates that the pmgA mutation responsible for higher photosynthetic activities (Table II) is fatal under prolonged conditions of HL stress, suggesting that alteration in the PSII-to-PSI ratio has been selected during evolution as an adaptation to HL in Synechocystis.
Figure 6.
Effects of light intensities for mixed-liquid culture on the ratio of the WL to WS genotype as determined by direct sequencing. The ratio is expressed as a pie chart, whereas cell density is shown as A730 under each pie chart. A horizontal line indicates each batch culture, and a dotted line indicates the inoculation of the following batch culture.
RT-PCR of pmgA mRNA
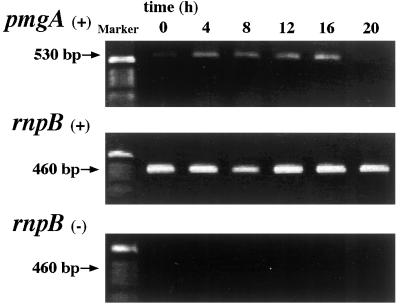
Although we have shown that pmgA is essential for modulation of photosystem stoichiometry, which molecular processes are mediated by pmgA is still unknown. To learn more about the role of pmgA, we investigated the expression of pmgA in the wild type by RT-PCR as shown in Figure 7. In contrast to relatively constant amplification of the constitutive RNase P gene (rnpB) (Frías et al., 1994), production of a pmgA fragment was largely dependent on the light conditions. When cells were grown under LL, cDNA for pmgA was barely detectable, as can be seen at time 0. After 4 h of HL, cDNA for pmgA increased severalfold and was then maintained at the high level for up to 16 h. A decrease in the cDNA level was observed at 20 h, possibly due to the self-shading effect. It was also interesting that the amount of cDNA sufficient for PCR amplification was more than 10,000 times higher for pmgA than for rnpB, suggesting that expression of pmgA is very low even under inducible conditions. We confirmed almost no DNA in the RNA preparation before the RT reactions, as shown in the negative control experiments. These suggest that the pmgA gene becomes active upon exposure to HL to acclimate to conditions of HL stress.
Figure 7.
Expression of pmgA in the wild type revealed by RT-PCR. The top and middle panels show PCR with primers specific to pmgA and rnpB, respectively. (+) indicates use of reverse-transcribed RNA from wild-type cells at HL as a template of PCR. The bottom panel shows PCR with primers specific to rnpB. (−) indicates use of RNA before reverse-transcription as a template. The culture for RNA isolation was inoculated with LL-grown cells and transferred to HL at time 0.
DISCUSSION
We have demonstrated that functional pmgA is specifically involved in acclimation to HL by modulating photosystem stoichiometry and, partly, chlorophyll synthesis. In our experimental conditions with Synechocystis sp. PCC 6803, we observed many changes due to HL acclimation: (a) decrease of cellular pigment content; (b) decrease of photochemical activities (on a per-cell basis); (c) decrease of antenna size of PSII; (d) increase of the PSII to PSI ratios; (e) increase of Rubisco; and (f) increase of maximum photosynthetic rate. These responses, possibly resulting from HL-induced modulation of accumulation of pigments and proteins for the photosynthetic apparatus, have been widely recognized in cyanobacteria, algae, and higher plants (Kawamura et al., 1979; Vierling and Alberte, 1980; Björkman, 1981; Raps et al., 1983; Zevenboom and Mur, 1984; Anderson et al., 1988). Our mutant analysis revealed that pmgA is specifically responsible for the slow recovery of chlorophyll accumulation during phase 2 after the shift from LL to HL (Fig. 3D) and for an increase in the PSII-to-PSI ratio (Fig. 4; Table I). Importantly, pmgA is not directly involved in other responses. To our knowledge, this is the first gene to be identified as a regulatory factor for HL acclimation. Since modulation of photosystem stoichiometry under different growth irradiances has been well documented in various cyanobacteria, algae, and higher plants (Kawamura et al., 1979; Falkowski et al., 1981; Leong and Anderson, 1984; Neale and Melis, 1986; Wild et al., 1986; Anderson et al., 1988; Smith and Melis, 1988; Murakami and Fujita, 1991; Yokoyama et al., 1991), it would be interesting to survey other cyanobacteria and/or plants for a pmgA homolog.
The phenotype causing photoautotrophic growth of pmgA mutants on agar plates to be much better than the wild type under almost all light intensities (Hihara and Ikeuchi, 1997) was totally unexpected. In this report we showed that the mutants grew significantly faster than the wild type in liquid medium when grown separately (Fig. 3A) or in mixed culture (Fig. 6). The higher growth rate of the mutants seems to be accounted for by their higher whole-cell photosynthetic activity (Table II). The higher photosynthetic activity of the mutants as compared with the wild type seems to reflect their higher activity of PSI, which is consistent with the results of P700 measurements (Table I) and immunoblotting of PSI reaction center PsaA/B proteins (Fig. 5). However,the PSII activity of the mutants was also higher than the wild type (Table II), although the cellular content of Cyt b559 and reaction center D2 protein were not much different between the mutant and wild-type cells (Table I; Fig. 5). This could be explained by the difference in sensitivity to PSII photoinhibition under HL. Since photoinhibition of PSII is caused by accumulation of reduced quinone at the primary acceptor QA site (Aro et al., 1993), the higher activity of PSI in the mutants is supposed to extract more electrons from PSII, possibly resulting in less photoinhibition of PSII, namely higher PSII activity. Thus, it can be concluded that the higher photosynthetic activity of the mutants resulting from enhanced accumulation of PSI due to loss of pmgA function is responsible for the mutant's ability to grow faster than the wild type under HL conditions.
However, our results with mixed-culture experiments under extended HL stress (Fig. 6) demonstrated that the pmgA mutant, with its higher PSI content, was more sensitive to prolonged stress than the wild type. One explanation for this apparent discrepancy is increased accumulation of reactive oxygen species in the cells of the pmgA mutant under HL conditions. The increase of PSI content mitigates photoinhibition of PSII, and results in the higher activity of whole-electron transport. The increase of PSI content also results in the accumulation of electrons on the reducing side of PSI rather than on the QA site of PSII. Both the increase in the electron-transfer rate and the accumulation of electrons on the reducing side of PSI are conditions that stimulate the production of reactive oxygen species. Thus, the increased PSI content in the mutants may result in much more production of reactive species of oxygen, which might account for the loss of viability of the mutants under the prolonged stress of HL. It is widely accepted that electrons generated from PSI react with oxygen to produce the superoxide anions, which are mainly scavenged by superoxide dismutase and ascorbate peroxidase (Asada, 1992; Herbert et al., 1992). It was recently demonstrated that irreversible photoinhibition of PSI occurs at its acceptor side in chilling-sensitive plants under chilling stress, possibly due to a loss of protection against the reactive species of oxygen (Sonoike and Terashima, 1994; Sonoike, 1996). The protection against reactive oxygen species is particularly important under HL stress (Foyer et al., 1994).
The physiological significance of the HL response that adjusts photosystem stoichiometry has not been fully established, although the adjustment is widespread in photosynthetic organisms. For example, it was simply stated in a review (Anderson et al., 1995) that adaptation of the photosystem stoichiometry serves to regulate the distribution of excitation energy between the photosystems and correct any imbalances. This is in contrast to many other responses of HL acclimation, such as the reduction in pigments and antenna size and the increase in CO2 fixation activity, which can be easily recognized as avoiding photoinhibition (Björkman, 1981; Anderson et al., 1988). Here we proposed another explanation for the adjustment of photosystem stoichiometry under HL: the decrease of PSI content makes cells resistant to HL stress by reducing the production of reactive oxygen species, which is otherwise lethal under prolonged HL stress. In conclusion, relative decrease of PSI content under HL conditions mediated by pmgA in Synechocystis can be a physiological response to the HL stress. pmgA mutants lack this response, resulting in the production of reactive oxygen species. It would be interesting to attempt to detect generation of the reactive oxygen species under HL in pmgA mutants.
pmgA mutants have another interesting phenotypic character: They are unable to grow on agar plates under photomixotrophic conditions with 5 mm Glc even under medium light (50 μE m−2 s−1) (Hihara and Ikeuchi, 1997). On the other hand, they can grow in liquid under the same photomixotrophic conditions, although their growth is significantly slower than the wild type, as demonstrated by mixed culture (Hihara and Ikeuchi, 1997). Here we observed that wild-type cells grown in liquid under photomixotrophic conditions showed a much reduced chlorophyll content on a per-cell basis and a higher ratio of PSII to PSI than those under photoautotrophic conditions. However, pmgA mutants did not show any change in the photosystem stoichiometry under the same photomixotrophic conditions (data not shown). Since these changes were almost the same as those of HL-grown cells, the addition of Glc is supposed to intensify the light stress. This interpretation may be reasonable, since Glc provided cells with NADPH via the oxidative pentose phosphate cycle (Pelroy et al., 1972), which presumably makes the acceptor side of PSI more reductive (like the HL treatment). Thus, the phenotype of pmgA mutants unable to grow under the photomixotrophic conditions could be also explained by the inability to reduce the PSI content.
How does pmgA work on the accumulation of chlorophyll and photosystem stoichiometry? Our data on photosystem content suggest that photosystem stoichiometry was mainly modulated by accumulation of PSI. Studies on light acclimation in cyanobacteria demonstrated that cellular PSI content is more variable than PSII during the adjustment of photosystem stoichiometry (Kawamura et al., 1979; Murakami and Fujita, 1991). However, it remains to be determined whether chlorophyll accumulation or PSI accumulation is the primary target of the pmgA-mediated acclimation to HL. The retardation of chlorophyll accumulation (Fig. 3D) seemed to precede the increase of the F695/F725 ratio (Fig. 4D). However, it would be rather difficult to imagine a mechanism whereby chlorophyll biosynthesis preferentially regulates assembly of the PSI complex. It should be noted that mRNA levels of pmgA were elevated severalfold for the initial 4 h after HL was begun (Fig. 7). This observation, coupled with the fact that the level of pmgA is very low even after HL induction, suggests that the pmgA product is involved in an early signaling process of the HL acclimation.
So far, two genes have been documented to be specifically involved in accumulation of PSI complexes but not PSII (Wilde et al., 1995; Bartsevich and Pakrasi, 1997; Boudreau et al., 1997). Disruption of a chloroplast open reading frame, ycf4, and a Synechocystis homolog, orf184, induced a significant decline in PSI content per unit of chlorophyll. As a result, the PSII-to-PSI ratio was elevated about 3-fold in the mutant compared with wild-type Synechocystis (Wilde et al., 1995), whereas there was almost no accumulation of the PSI complex in the C. reinhardtii mutant (Boudreau et al., 1997). A second gene, btpA, seems to regulate a posttranscriptional process that affects biogenesis of the PSI complex in Synechocystis (Bartsevich and Pakrasi, 1997). A disruption mutant of btpA had only 10% to 15% of PSI reaction center proteins compared with the wild type, whereas the PSII content remained unaffected. These different genes may regulate accumulation of PSI at a step of translation, assembly, or turnover of the PSI complex, although no relevant data were presented that indicated their involvement in physiological adjustment of photosystem stoichiometry under varying environment conditions. To our knowledge, pmgA is the first gene shown to be involved in the regulation of the PSII-to-PSI ratio under physiological conditions.
ACKNOWLEDGMENTS
We thank Dr. Gerry Plumley for critical reading of the manuscript, Dr. Akio Murakami for advice on the measurement of Cyt b559, Dr. Arthur Grossman for advice on preparation of total RNA, and Ms. Ayako Kamei for her help with the experiments. We are also grateful to Drs. Isao Enami and Katsuhiko Okada for providing antibodies.
Abbreviations:
- 2,6-DCBQ
2,6-dichlorobenzoquinone
- DAD
diaminodurene
- HL
high light (in this study, 200 μE m−2 s−1)
- LDS
lithium dodecyl sulfate
- LL
low light (in this study, 20 μE m−2 s−1)
- MV
methyl viologen
- RT-PCR
reverse transcriptase-PCR
- TMPD
N,N,N′,N′-tetramethyl-p-phenylenediamine
Footnotes
This work was supported by a Research Fellowship for Young Scientists from the Japan Society of the Promotion of Science (to Y.H.); by grants-in-aid for encouragement of young scientists (no. 09740590 to K.S.), for scientific research on priority areas (no. 07251204 to M.I.), and for scientific research C (no. 08836002 to M.I.) from the Ministry of Education, Science, and Culture, Japan; and by a grant for Scientific Research from the Human Frontier Science program (to M.I.).
LITERATURE CITED
- Allen JF. Thylakoid protein phosphorylation, state 1-state 2 transitions, and photosystem stoichiometry adjustment: redox control at multiple levels of gene expression. Physiol Plant. 1995;93:196–205. [Google Scholar]
- Anderson JM. Photoregulation of the composition, function, and structure of thylakoid membranes. Annu Rev Plant Physiol. 1986;37:93–136. [Google Scholar]
- Anderson JM, Chow WS, Goodchild DJ. Thylakoid membrane organization in sun/shade acclimation. Aust J Plant Physiol. 1988;15:11–26. [Google Scholar]
- Anderson JM, Chow WS, Park Y-I. The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res. 1995;46:129–139. doi: 10.1007/BF00020423. [DOI] [PubMed] [Google Scholar]
- Arnon DI, McSwain BD, Tsujimoto HY, Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974;357:231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Asada K. Ascorbate peroxidase: a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant. 1992;85:235–241. [Google Scholar]
- Bartsevich VV, Pakrasi HB. Molecular identification of a novel protein that regulates biogenesis of photosystem I, a membrane protein complex. J Biol Chem. 1997;272:6382–6387. doi: 10.1074/jbc.272.10.6382. [DOI] [PubMed] [Google Scholar]
- Björkman O. Responses to different quantum flux densities. In: Lange OLL, Nobel PS, Osmond CB, Ziegler H, editors. Physiological Plant Ecology I. Responses to the Physical Environment. Encyclopedia of Plant Physiology, New Series 12A. Berlin: Springer-Verlag; 1981. pp. 57–107. [Google Scholar]
- Boudreau E, Takahashi Y, Lemieux C, Turmel M, Rochaix J-D. The chloroplast ycf3 and ycf4 open reading frames of Chlamydomonas reinhardtii are required for the accumulation of the photosystem I complex. EMBO J. 1997;16:6095–6104. doi: 10.1093/emboj/16.20.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang GG, Schaefer MR, Grossman AR. Complementation of a red-light-indifferent cyanobacterial mutant. Proc Natl Acad Sci USA. 1992;89:9415–9419. doi: 10.1073/pnas.89.20.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski PG, Owens TG, Ley AC, Mauzerall DC. Effect of growth irradiance levels on the ratio of reaction centers in two species of marine phytoplankton. Plant Physiol. 1981;68:969–973. doi: 10.1104/pp.68.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Frías JE, Flores E, Herrero A. Requirement of the regulatory protein NtcA for the expression of nitrogen assimilation and heterocyst development genes in the cyanobacterium Anabaena sp. PCC 7120. Mol Microbiol. 1994;14:823–832. doi: 10.1111/j.1365-2958.1994.tb01318.x. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Murakami A. Regulation of electron transport composition in cyanobacterial photosynthetic system: stoichiometry among photosystem I and II complexes and their light-harvesting antennae and cytochrome b6/f complex. Plant Cell Physiol. 1987;28:1547–1553. [Google Scholar]
- Fujita Y, Murakami A, Aizawa K, Ohki K. Short-term and long-term adaptation of the photosynthetic apparatus: homeostatic properties of thylakoids. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 677–692. [Google Scholar]
- Fujita Y, Murakami A, Ohki K. Regulation of photosystem composition in the cyanobacterial photosynthetic system: the regulation occurs in response to the redox state of the electron pool located between the two photosystems. Plant Cell Physiol. 1987;28:283–292. [Google Scholar]
- Garewal HS, Wasserman AR. Triton X-100–4 M urea an as extraction medium for membrane proteins. I. Purification of chloroplast cytochrome b559. Biochemistry. 1974;13:4063–4071. doi: 10.1021/bi00717a001. [DOI] [PubMed] [Google Scholar]
- Grossman AR, Schaefer MR, Chiang GG, Collier JL. The responses of cyanobacteria to environmental conditions: light and nutrients. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 641–675. [Google Scholar]
- Herbert SK, Samson G, Fork DC, Laudenbach DE. Characterization of damage to photosystem I and II in a cyanobacterium lacking detectable iron superoxide dismutase activity. Proc Natl Acad Sci USA. 1992;89:8716–8720. doi: 10.1073/pnas.89.18.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hihara Y, Ikeuchi M. Mutation in a novel gene required for photomixotrophic growth leads to enhanced photoautotrophic growth of Synechocystis sp. PCC 6803. Photosynth Res. 1997;53:243–252. [Google Scholar]
- Hiyama T, Ke B. Difference spectra and extinction coefficients of P700. Biochim Biophys Acta. 1972;267:160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Inoue Y. Specific 125I labeling of D1 (herbicide-binding protein): an indication that D1 functions on both the donor and acceptor sides of photosystem II. FEBS Lett. 1987;210:71–76. [Google Scholar]
- Izawa S. Acceptors and donors for chloroplast electron transport. Methods Enzymol. 1980;69:413–433. [Google Scholar]
- Kawamura M, Mimuro M, Fujita Y. Quantitative relationship between two reaction centers in the photosynthetic system of blue-green algae. Plant Cell Physiol. 1979;20:697–705. [Google Scholar]
- Kehoe DM, Grossman AR. Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science. 1996;273:1409–1412. doi: 10.1126/science.273.5280.1409. [DOI] [PubMed] [Google Scholar]
- Knanna R, Graham JR, Myers J, Gantt E. Phycobilisome composition and possible relationship to reaction centers. Arch Biochem Biophys. 1983;224:534–542. doi: 10.1016/0003-9861(83)90241-2. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong T-Y, Anderson JM. Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll-protein complexes. Photosynth Res. 1984;5:105–115. doi: 10.1007/BF00028524. [DOI] [PubMed] [Google Scholar]
- Lönneborg A, Lind LK, Kalla SR, Gustafsson P, Öquist G. Acclimation processes in the light-harvesting system of the cyanobacterium Anacystis nidulans following a light shift from white to red light. Plant Physiol. 1985;78:110–114. doi: 10.1104/pp.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A. Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta. 1991;1058:87–106. [Google Scholar]
- Melis A, Mandori A, Glick RE, Ghirardi ML, McCauley SW, Neale PJ. The mechanism of photosynthetic membrane adaptation to environmental stress conditions: a hypothesis on the role of electron-transport capacity and of ATP/NADPH pool in the regulation of thylakoid membrane organization and function. Physiol Vég. 1985;23:757–765. [Google Scholar]
- Murakami A, Fujita Y. Regulation of photosystem stoichiometry in the photosynthetic system of the cyanophyte Synechocystis PCC 6714 in response to light-intensity. Plant Cell Physiol. 1991;32:223–230. [Google Scholar]
- Murata N, Nishimura M, Takamiya A. Fluorescence of chlorophyll in photosynthetic systems. III. Emission and action spectra of fluorescence-three emission bands of chlorophyll a and the energy transfer between two pigment systems. Biochim Biophys Acta. 1966;126:234–243. doi: 10.1016/0926-6585(66)90059-8. [DOI] [PubMed] [Google Scholar]
- Neale PJ, Melis A. Algal photosynthetic membrane complexes and the photosynthesis-irradiance curve: a comparison of light-adaptation responses in Chlamydomonas reinhardtii (Chlorophyta) J Phycol. 1986;22:531–538. [Google Scholar]
- Pelroy RA, Rippka R, Stanier RY. Metabolism of glucose by unicellular blue-green algae. Arch Microbiol. 1972;87:303–322. doi: 10.1007/BF00409131. [DOI] [PubMed] [Google Scholar]
- Raps S, Wyman K, Siegelman HW, Falkowski PG. Adaptation of the cyanobacterium Microcystis aeruginosa to light intensity. Plant Physiol. 1983;72:829–832. doi: 10.1104/pp.72.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BM, Melis A. Photochemical apparatus organization in the diatom Cylindrotheca fusiformis: photosystem stoichiometry and excitation distribution in cells grown under high and low irradiance. Plant Cell Physiol. 1988;29:761–769. [Google Scholar]
- Sonoike K. Photoinhibition of photosystem I: Its physiological significance in the chilling sensitivity of plants. Plant Cell Physiol. 1996;37:239–247. [Google Scholar]
- Sonoike K, Katoh S. Effects of sodium dodecyl sulfate and methyl viologen on the differential extinction coefficient of P-700: a band shift of chlorophyll a associated with oxidation of P-700. Biochim Biophys Acta. 1988;935:61–71. [Google Scholar]
- Sonoike K, Katoh S. Simple estimation of the differential absorption coefficient of P-700 in detergent-treated preparations. Biochim Biophys Acta. 1989;976:210–213. [Google Scholar]
- Sonoike K, Katoh S. Variation and estimation of the differential absorption coefficient of P-700 in spinach photosystem I preparations. Plant Cell Physiol. 1990;31:1079–1082. [Google Scholar]
- Sonoike K, Terashima I. Mechanism of photosystem-I photoinhibition in leaves of Cucumis sativus L. Planta. 1994;194:287–293. [Google Scholar]
- Terashima I, Funayama S, Sonoike K. The site of photoin-hibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II. Planta. 1994;193:300–306. [Google Scholar]
- Vierling E, Alberte RS. Functional organization and plasticity of the photosynthetic unit of the cyanobacterium Anacystis nidulans. Physiol Plant. 1980;50:93–98. [Google Scholar]
- Whitmarsh J, Ort DR. Stoichiometries of electron transport complexes in spinach chloroplasts. Arch Biochem Biophys. 1984;231:378–389. doi: 10.1016/0003-9861(84)90401-6. [DOI] [PubMed] [Google Scholar]
- Wild A, Höpfner M, Rühle W, Richter M. Changes in the stoichiometry of photosystem II components as an adaptive response to high-light and low-light conditions during growth. Z Naturforsch. 1986;41c:597–603. [Google Scholar]
- Wilde A, Härtel H, Hübschmann T, Hoffmann P, Shestakov SV, Börner T. Inactivation of a Synechocystis sp. strain PCC 6803 gene with homology to conserved chloroplast open reading frame 184 increases the photosystem II-to-photosystem I ratio. Plant Cell. 1995;7:649–658. doi: 10.1105/tpc.7.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama E, Murakami A, Sakurai H, Fujita Y. Effect of supra-high irradiation on the photosynthetic system of the cyanophyte Synechocystis PCC 6714. Plant Cell Physiol. 1991;32:827–834. [Google Scholar]
- Zevenboom W, Mur LR. Growth and photosynthetic response of the cyanobacterium Microcystis aeruginosa in relation to photoperiodicity and irradiance. Arch Microbiol. 1984;139:232–239. [Google Scholar]