Abstract
Acid sphingomyelinase occupies a prominent position in sphingolipid catabolism, catalyzing the hydrolysis of sphingomyelin to ceramide and phosphorylcholine. Enzymatic dysfunction of acid sphingomyelinase results in Niemann-Pick disease, a lysosomal storage disorder characterized at the cellular level by accumulation of sphingomyelin within the endo-lysosomal compartment. Over the past decade interest in the role of acid sphingomyelinase has moved beyond its ‘housekeeping’ function in constitutive turnover of sphingomyelin in the lysosome to include study of regulated ceramide generation. Ceramide functions as a bioactive sphingolipid with pleiotropic signaling properties, and has been implicated in diverse cellular processes of physiologic and pathophysiologic importance. Though many cellular enzymes have the capacity to generate ceramide, there is growing appreciation that ‘all ceramides are not created equal.’ Ceramides likely exert distinct effects in different cellular/subcellular compartments by virtue of access to other sphingolipid enzymes (e.g. ceramidases), effector molecules (e.g. ceramide-activated protein phosphatases), and neighboring lipids and proteins (e.g. cholesterol, ion channels). One of the unique features of acid sphingomyelinase is that it has been implicated in the hydrolysis of sphingomyelin in three different settings – the endo-lysosomal compartment, the outer leaflet of the plasma membrane, and lipoproteins. How a single gene product has the capacity to function in these diverse settings, and the subsequent impact on downstream ceramide-mediated biology is the subject of this review.
Keywords: acid sphingomyelinase, secretory sphingomyelinase, ceramide, sphingolipid, Niemann-Pick disease
1. Introduction
Once considered inert structural components of cellular membranes, sphingolipids are increasingly recognized as bioactive lipids [1]. Ceramide and its metabolites have been shown to influence various cellular processes, including apoptosis, senescence, differentiation, migration, angiogenesis, proliferation, infection, protein trafficking, autophagy, and inflammation [1–5]. However, the molecular mechanism(s) whereby ceramide exerts its effects on cellular processes has remained somewhat of a mystery.
Enzymes of sphingolipid metabolism determine cellular levels of ceramide, and thus understanding the regulation of these enzymes provides crucial insight into the cellular mechanisms underlying ceramide generation, accumulation, and action. Ceramide can be generated de novo, beginning with the condensation of serine and palmitoyl-CoA [6], from the hydrolysis of complex sphingolipids, such as sphingomyelin (SM), or by the recently characterized ceramide salvage pathway (see Figure 1) [7]. Hydrolysis of SM is carried out by sphingomyelinases (SMases), which cleave the phosphodiester bond of SM releasing the phosphorylcholine head group to generate ceramide (see Figure 2). SMases are characterized by pH optima for enzymatic activity, into alkaline, neutral, and acidic SMases, and further sub classified based on requirement for divalent cations [8, 9]. Of the four putative SMases (sphingomyelin phosphodiesterase, SMPD 1–4) that have been identified and cloned, acid sphingomyelinase (aSMase, SMPD1) and Mg2+-dependent neutral sphingomyelinase 2 (nSMase2, SMPD3) are the most commonly studied enzymes in regulated SM hydrolysis in response to a range of stress stimuli [9].
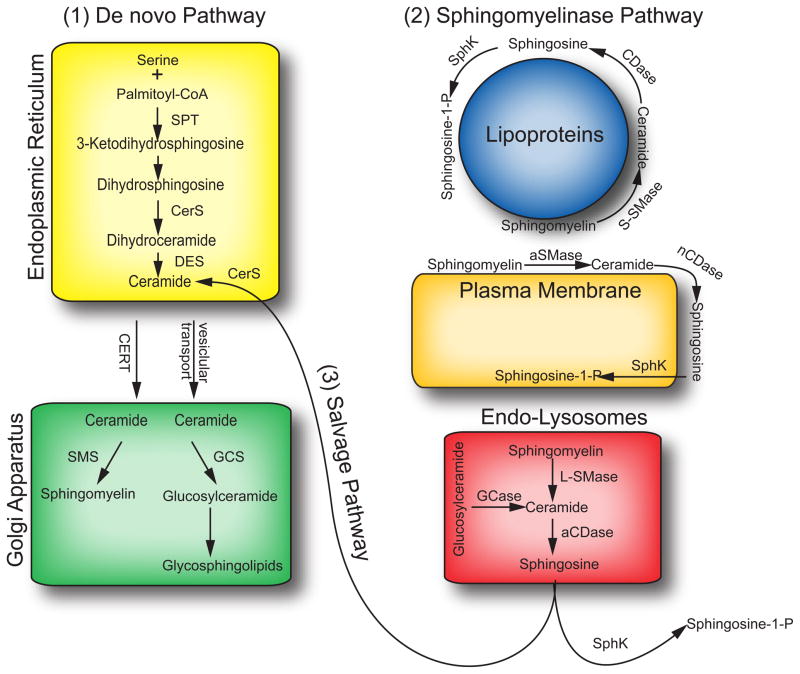
Figure 1. Sphingolipid metabolism.
[1]De novo ceramide synthesis. Within the endoplasmic reticulum, serine and palmitoyl-CoA are condensed by serine palmitoyltransferase (SPT) to form in the first step of the de novo sphingolipid synthesis. Subsequently, 3-ketosphinganine is metabolized to dihydrosphingosine. Dihydrosphingosine is acylated by (dihydro)ceramide synthase (CerS) producing dihydroceramide, which is then converted to ceramide by dihydroceramide desaturase (DES). Ceramide is then trafficked to the Golgi apparatus via vesicular transport or via the ceramide transfer protein (CERT) where it is the substrate for the synthesis of more complex sphingolipids, such as sphingomyelin and glycosphingolipids. [2] Sphingomyelinase pathway. The breakdown of sphingomyelin by aSMase within the endo-lysosomal system, at the outer leaflet of the plasma membrane, and in association with lipoproteins. Ceramide generated from the breakdown of SM can be deacylated by acid or neutral ceramidases (aCDase, nCDase) to yield free sphingosine. Sphingosine can be the substrate for either CerS, via the [3] ceramide salvage pathway or sphingosine kinases (SphK) to form ceramide and S1P, respectively. For simplicity, neutral and alkaline sphingomyelinases are not depicted in this diagram.
Abbreviations: SPT – serine palmitoyltransferase; CerS – (dihydro)ceramide synthase; DES – dihydroceramide desaturase; CERT – ceramide transfer protein; SMS – sphingomyelin synthase; GCS – glucosylceramide synthase; aSMase – acid sphingomyelinase; S-SMase – secretory sphingomyelinase; L-SMase – lysosomal sphingomyelinase; GCase – glucosylceramidase; aCDase – acid ceramidase; nCDase – neutral ceramidase; SphK – sphingosine kinase.
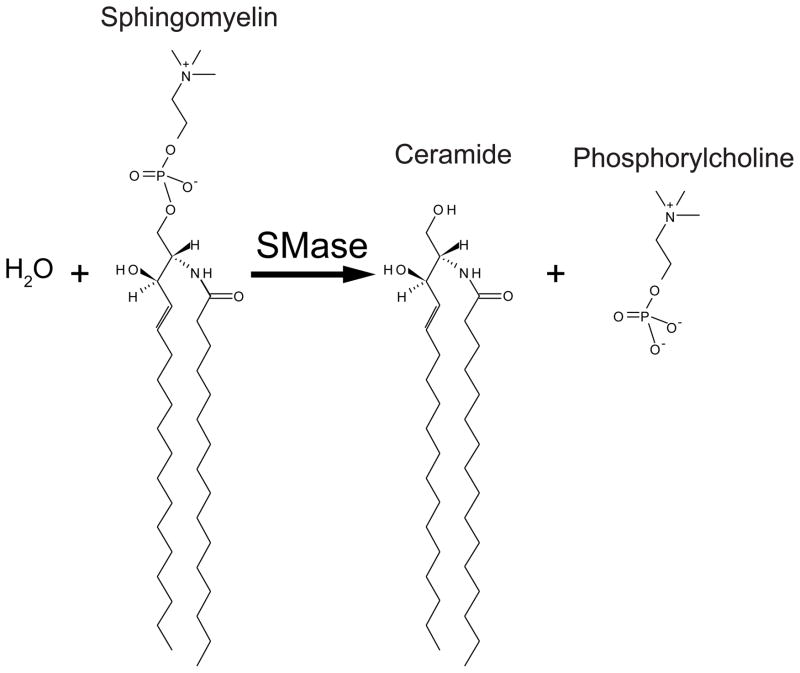
Figure 2.
Sphingomyelinase reaction Sphingomyelin is hydrolyzed by acid sphingomyelinase to form ceramide and phosphorylcholine. Shown is the conversion of 2S, 3R, 4E-C16-sphingomyelin to 2S, 3R, 4E-C16-ceramide.
Deficiency of aSMase results in Niemann-Pick disease types A and B (NPD) (OMIM; NPD-A: 257200, NPD-B: 607616) [10]. Patients with NPD-A develop severe neurological and visceral pathology and seldom live beyond three years of age [11], while patients with NPD-B typically live into adolescence or early adulthood and characteristically do not manifest neurological signs or symptoms [12]. Following the landmark discovery that cells and tissues derived from mouse models of NPD (i.e. aSMase knockout mice) displayed marked protection from potent inducers of cell death [13, 14], and coincident with mounting evidence supporting a role of sphingolipids in signal transduction [15], several groups began investigating the role and regulation of aSMase in ceramide-mediated cellular signaling. Subsequently, aSMase has been demonstrated to play a central role in the cellular response to a variety of cellular stresses, including environmental insults, infection with various pathogens, ligation of death receptors, and chemotherapeutic drugs [3, 16].
Despite growing interest in aSMase/ceramide signaling, an incomplete understanding of the molecular mechanism(s) of aSMase regulation and its precise role in ceramide-mediated biological processes has led to many contradictory findings [17, 18]. One possible reason for these discrepancies is that the gene that encodes aSMase gives rise to two unique enzymes [19]. Via differential trafficking of a common protein precursor, the aSMase gene gives rise to the often-studied lysosomal sphingomyelinase (L-SMase), as well as a secretory sphingomyelinase (S-SMase) that is released extracellularly [20]. The existence of two forms of aSMase suggests that they may serve distinct metabolic roles and that the biological impact of hydrolysis of sphingomyelin to ceramide may differ depending on its compartmentalization. Of the several hundred studies reporting regulated activation of aSMase, only a handful have addressed the regulation of both S-SMase and L-SMase, highlighting the need for more focused investigation into the individual contribution of S-SMase and L-SMase into biological processes previously ascribed generically to ‘aSMase.’ In the sections that follow, we will review the regulation, metabolic function, and biological role of aSMase with emphasis on the reported (and inferred) contributions of S-SMase and L-SMase in the context of the natural history of the aSMase molecule.
2. Acid Sphingomyelinase – One Gene, Two Enzymes, Multiple Regulatory Mechanisms
The ability of aSMase to perform its metabolic function is subject to multiple levels of cellular regulation, requiring successful transcription, translation, post-translational modification, as well as proper trafficking to give rise to a mature, functional enzyme. In addition to proper synthesis and trafficking, the enzymatic activity of aSMase is influenced by lipids, cations, pH, redox, and other proteins. Here we will detail the life history of a single aSMase molecule from DNA to mature enzyme, and the steps at which the cell regulates aSMase to impact SM and Cer levels.
2.1. Acid Sphingomyelinase: The Gene (SMPD1)
As aSMase was the first cloned sphingomyelin phosphodiesterase, it was designated SMPD1 (sphingomyelin phosphodiesterase 1, OMIM: 607608). The full-length SMPD1 gene maps to chromosome 11p15.1–11p15.4, spanning ~6 kilobases, consisting of six exons, and five introns [21, 22]. A single mRNA gives rise to at least three different splice variants, although only the type 1 transcript gives rise to a functional enzyme [23]. The full-length aSMase cDNA for the type 1 transcript has an open-reading frame of 1890 bp, giving rise to a polypeptide of 629 amino acids [23, 24]. Murine aSMase shares 82% identity with human aSMase and maps to chromosome 7 [25]. Interestingly, C. elegans possesses two separate genes that each encode functional aSMase enzymes (asm-1 and asm-2) which exhibit distinct temporal expression profiles corresponding to different developmental stages [26].
The promoter region of human aSMase was determined following isolation of a genomic clone of the gene encoding aSMase [22]. Analysis of the upstream region revealed GC-rich regions and several potential promoter elements, including consensus binding sites for transcription factors such as Sp1 and AP-1 [22]. Transcriptional upregulation of aSMase has since been reported in a variety of settings, including drug-induced differentiation of leukemia cells [27], differentiation of monocytes to macrophages [28], butyrate treatment [29], in senescent fibroblasts from patients with the premature aging disorder Werner syndrome [30], and in genetic and diet-induced mouse models of obesity [31, 32].
SMPD1 is also subject to epigenetic regulation through methylation [33]. A role for genetic imprinting was initially suggested when a patient with Beckwith-Wiedemann syndrome (BWS) presented with signs and symptoms consistent with NPD and was found to exhibit only 35% aSMase activity in isolated skin fibroblasts [34]. BWS is a genetic condition resulting from uniparental disomy in which a child receives two copies of a gene from one parent, and none from the other parent. Because SMPD1 is paternally imprinted (i.e. expressed from the maternal allele), if a child receives two copies of the short arm of chromosome 11 from the father, then expression of all genes in this region will be diminished [35]. Thus, residual aSMase activity from the patient was likely due to expression of aSMase from the non-methylated maternal allele. It has been suggested that genetic imprinting at the SMPD1 locus may contribute to some of the heterogeneity of disease seen in NPD type B patients, which may in fact be heterozygous for functional aSMase [33]. The role of epigenetic regulation of aSMase in other human diseases is not currently known; however, a recent study highlighted abnormalities in chromosome 11p15 in the development of Wilms tumor [36]. Interestingly, children with BWS are at increased risk of developing Wilms tumor [37]. Given the putative role of Cer as a tumor suppressor lipid [5], epigenetic regulation of SMPD1 expression may serve as a mechanism for reducing the levels of ceramide, and thus promoting tumor growth.
2.2. Acid Sphingomyelinase: Post-Translational Modification and Subcellular Trafficking
Polypeptide Synthesis in the ER
Similar to other enzymes of the endo-lysosomal system, aSMase has a NH2-terminal ER signal sequence that is sufficient for co-translational introduction of the nascent precursor polypeptide into the endoplasmic reticulum (see Figure 3). Within the ER, the aSMase precursor begins a process of maturation common to all proteins of the secretory pathway. Expression of a human aSMase deletion mutant lacking a signal peptide results in production of a non-glycosylated, cytosolic protein that lacks enzyme activity in cellular extracts, and is not secreted [38]. Of note, the signal peptide is a reported polymorphic region within the aSMase protein [39], and while the biologic relevance of this polymorphism is currently not known, given its localization within the signal peptide, it is unlikely that this region influences catalytic activity per se.
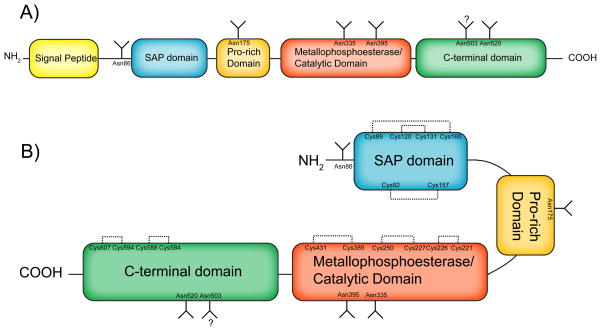
Figure 3. Primary structure of acid sphingomyelinase.
A) Primary structure of aSMase polypeptide. Domain structure and N-glycosylation sites are shown. B) Stylized conformation of mature aSMase. Domain structure, N-glycosylation sites, and disulfide bridges are shown. Emphasized are the hypothesized roles of the proline-rich domain as a hinge region and the SAP region is an intramolecular activating domain. Signal peptide (amino acids 1-46), SAP domain (89–165), Pro-rich domain (166–198), metallophoesterase/catalytic domain (199–461), C-terminal domain (462–629). N-glycosylation sites (Asn86, Asn175, Asn335, Asn395, Asn503, Asn520). Disulfide bridges (Cys89-Cys165, Cys92-Cys157, Cys120-Cys131, Cys221-Cys226, Cys227-Cys250, Cys385-Cys431, Cys584-Cys588, Cys594-Cys607).
Sulfhydryl Modifications
Unlike its Mg-dependent neutral SMase counterpart, aSMase is highly sensitive to reducing agents [40]. The basis for this inactivation likely involves reduction of aSMase’s eight intramolecular disulfide bonds [41]. These disulfide bridges are formed by protein disulfide isomerases within the oxidizing lumen of the ER. Interestingly, the type 2 and type 3 aSMase splice variants that encode enzymatically inactive proteins both lack cysteine residues in the putative catalytic domain which may explain the absence of activity for the protein products of these splice variants. Interestingly, only sixteen of aSMase’s seventeen cysteine residues are involved in disulfide bond formation. Deletion or oxidation of the unbridged, carboxyterminal Cys629 resulted in a 4–5-fold increase in aSMase activity [42]. It should be noted that the relevance of C-terminal modification in the context of the maturation of S-SMase and L-SMase is currently not known. However, given that C-terminal processing has been reported for other lysosomal enzymes (e.g. acid β-glucosidase [43], cathepsin D [44]), C-terminal modification of Cys629 may serve as a cellular mechanism of aSMase maturation/activation.
N-Glycosylation
There are six predicted N-glycosylation sites in the aSMase polypeptide chain, of which five are utilized (Asn86, Asn175, Asn335, Asn395, and Asn520 – not Asn503) [45]. Mutation of Asn520 to glutamine resulted in impaired secretion of S-SMase in addition to complete loss of L-SMase activity, presumably resulting from defective trafficking [45]. The modification of putative N-glycosylation sites was further validated by mass spectrometric analysis of both purified placental aSMase and rh-aSMase (S-SMase) [41]. Of note, murine aSMase [46] and recombinant human aSMase from Sf21 insect cells are glycosylated on all six predicted glycosylation sites [47, 48].
In addition to mediating proper folding and trafficking, glycosylation of aSMase is believed to serve a protective role to prevent destruction in the harsh environment of the lysosome [46]. Glycosylation may also be important for maintaining the mature enzyme in an active conformation, as in vitro glycosidase treatment results in loss of activity [47]. Lastly, differences in the oligosaccharide processing of N-glycans distinguishes L-SMase from S-SMase. Differential sensitivity of S-SMase and L-SMase to glycosidases is considered proof of the alternative trafficking model of aSMase by virtue of further oligosaccharide processing of N-glycans in different regions of the Golgi, as L-SMase possesses a high mannose N-glycan composition, while S-SMase exhibits a complex type pattern [49, 50].
Mannose-6-Phosphorylation and Lysosomal Trafficking
The primary mechanism of targeting acid hydrolases to the lysosome is the mannose-6-phosphate (M6P) receptor system [51]. Evidence for M6P-based trafficking of L-SMase to the lysosome was provided using fibroblasts from patients with I-cell disease, a disease in which lysosomal enzymes are missorted to the secretory pathway due to mutations in enzymes of the M6P machinery (e.g. N-acetyl glucosamine phosphotransferase, NAGPT) [52, 53]. In turn, these enzymes are trafficked through the default Golgi secretory pathway and released extracellularly. In I-cell fibroblasts, more than 70% of the aSMase precursor was secreted (and is activated by zinc [54]), and the portion that remained intracellularly was insensitive to endoglycosidase H, indicating the presence of a complex type glycosylation pattern [50]. Other mechanisms of trafficking L-SMase to the lysosome have been reported, such as sortilin-mediated transport which is utilized for the different saposins [55]. However, the M6PR-mediated pathway appears to be the more dominant of the two pathways.
Regulated secretion of S-SMase was first reported in cultured endothelial cells in response to inflammatory cytokines [56]. Despite mounting evidence for regulation of S-SMase secretion, a mechanistic understanding of the regulated trafficking of aSMase is lacking. One theory, first submitted by Marathe et al. [56], is that the M6P machinery itself may be regulated. Their data demonstrated that enhanced S-SMase secretion occurred with concomitant diminution of L-SMase activity, possibly reflecting regulated trafficking of the common aSMase precursor. Takahashi and colleagues investigated this hypothesis indirectly by employing a screening mutagenesis approach to identify lysine residues critical for trafficking of the aSMase precursor [54]. Mutagenesis studies have revealed that the three-dimensional conformation of specific lysine residues mediates the interaction of the protein substrate with NAGPT [57]. Mutation of lysine 93 to alanine resulted in diminished activity in cellular extracts with enhanced activity of S-SMase in the culture medium, suggesting that this residue may serve as one of the crucial determinants for the proper interaction with NAGPT. Thus, loss of Lys93 results in decreased interaction with the enzyme, decreased addition of M6P to the precursor, diminished L-SMase activity, and elevated S-SMase activity in the media. It has yet to be determined if enhanced secretion results from post-translational modifications to the aSMase precursor itself or the M6P machinery as a whole.
Proteolytic Processing
Soon after entry into the ER, a signal peptidase removes the N-terminal ER signal sequence (amino acids 1-46) and likely a few additional residues as rh-aSMase is reported to consist of 570 amino acids (i.e. loses amino acids 1-59). However, although the N-terminus of S-SMase begins with His60, that of L-SMase begins with Gly66 suggesting additional processing of L-SMase [49]. Moreover, N-terminal sequencing of L-SMase purified from human placenta indicated that the Gly83 was the first amino acid which may indicate further N-terminal processing in the endo-lysosomal compartment [48].
Evidence for multiple proteolytic processing steps has come from the Sandhoff group using pulse-chase labeling experiments to track the maturation of aSMase over time [50]. Processing of the initial 75 kDa prepro form of aSMase to the 72 kDa proform occurred within 2–3 hours of chase whereas formation of the mature 70 kDa form was evident after 4–8 hr. Maturation of the prepro form (75 kDa) to the 72 and 70 kDa forms was inhibited by brefeldin A, suggesting a requirement for processing in the acidic compartment. Lastly, the 70 kDa form is lost upon treatment with desipramine suggesting that this form represents the mature enzyme [58].
Phosphorylation
L-SMase is a reported phosphoprotein by virtue of incorporation of phosphate into oligosaccharide structures (e.g. mannose-6-phosphate) [50]. Given that in vitro deglycosylation removed only 70% of the phosphate label, aSMase may also be phosphorylated on the polypeptide backbone [45]. Recently, Zeidan et al. published a series of studies reporting that phosphorylation of aSMase on serine 508 is requisite for activation and relocalization of aSMase to the plasma membrane [59–61]. There are several aspects of this mechanism that require further explanation, including the phosphorylation of the luminal aSMase by the cytosolic protein kinase C δ (PKCδ). Confirmatory studies by Lee et al. failed to demonstrate phosphorylation of L-SMase in response to phorbol ester and other agonists [62]; however, the phosphorylation of S-SMase was not investigated nor was the presence of PKCδ, which appears to be the critical PKC isoform required for activation of aSMase.
2.3. Acid Sphingomyelinase: Enzymology
Once mature S-SMase and L-SMase reach their respective cellular (and extracellular) destinations, they are still at the mercy of various regulatory factors that influence substrate affinity (Km) or catalytic velocity (Vmax). The enzymologic features of aSMase have been characterized using various sources of enzyme, including human brain [63], human placenta [64, 65], and urine [66]. More recently, use of recombinant human aSMase has supplanted purified endogenous aSMase for enzymologic studies [47, 67]. In the section that follows, the many biochemical factors capable of influencing both L-SMase and S-SMase activity will be discussed.
Effect of pH
Using aSMase purified from human placenta (L-SMase), Callahan et al. first reported that the requirement for acid pH for proper function actually involved mediating substrate binding (Km), not catalytic velocity (Vmax) [65]. While L-SMase seems well suited to operate at the low pH of the endolysosomal compartment, S-SMase is proposed to participate in SM hydrolysis at the neutral pH of the extracellular milieu or in association with lipoproteins in serum. Schissel et al. demonstrated that S-SMase was capable of hydrolyzing SM associated with atherogenic lipoproteins at neutral pH [68]. The precise molecular mechanism whereby enzyme activity is promoted by atherogenic lipoproteins remains unknown.
Cation Requirement
Both S-SMase and L-SMase are zinc metalloenzymes although L-SMase encounters and tightly binds Zn during its maturation and thus retains activity in the presence of mild Zn-chelators. Importantly, although S-SMase was characterized as a Zn-dependent enzyme nearly twenty years ago [69], the precise Zn-coordinating residues and the influence of Zn on enzyme function have yet to be experimentally defined. The role of other cations, especially under different experimental conditions (e.g. neutral pH) has not been investigated extensively although copper has been reported to exert a strong activating effect on some aSMase preparations [42, 47]. Given that L-SMase is reported to acquire zinc during its trafficking/maturation, the availability of zinc or zinc-regulating factors may prove to be important mediators of cellular L-SMase activity. For S-SMase, the requirement for cation for enzyme activity is less well understood.
Saposin-Like Domain
The primary structure of the N-terminus of aSMase predicts a saposin-like (SAP) domain extending from amino acid 89-165 immediately following the signal peptide, [70]. SAPs (sphingolipid activator proteins) are non-enzymatic glycoproteins found predominantly in the acidic compartment and function to facilitate degradation of various sphingolipids via mobilizing lipids from membranes, thus exposing substrates to the active site of the respective enzyme [71]. Biochemical characterization of SAP function has shown that mimicking lysosomal conditions (e.g. acidic pH, low cholesterol content, and increased levels of anionic lipids) promotes substrate binding and lipid extraction [72]. As the SAP domain is separated from the putative catalytic domain by a proline-rich domain, it has been postulated that the SAP domain within aSMase functions as an intramolecular activating region for SM hydrolysis with the proline-rich domain serving as a hinge region (see Figure 3). The role of the intramolecular SAP region was reviewed and investigated by Ferlinz et al. in an effort to explain contradictory findings regarding the requirement for different SAPs [73]. Mutation of prosaposin in humans, with resultant loss of all SAPs, does not result in SM accumulation [74, 75], suggesting that the SAP-like region is functional in vivo. Kolzer et al. mutated various conserved amino acids within the SAP domain of aSMase and assessed both micellar and liposomal aSMase activity, with and without exogenous SAP [76]. As predicted, mutations in the conserved residues resulted in impaired liposomal aSMase activity due to impaired lipid-extraction function, which could be rescued by exogenous SAP protein. However, all but one of these mutants exhibited decreased activity in a micellar system that could not be restored with exogenous SAP, suggesting the internal SAP domain may have other functions besides lipid extraction.
Lipid Activators and Inhibitors
Given that the substrate for aSMase resides in biologic membranes, and that aSMase is a soluble enzyme, it seems likely that interaction of aSMase with neighboring lipids could serve as a mechanism to regulate enzyme function. Preparation of SM-containing liposomes with various lipids found to be enriched in the lysosome revealed that bismonoacylglycerophosphate (BMP or lysobisphosphatidic acid, LBPA) and phosphatidylinositol (PI) enhance hydrolysis of SM by rh-aSMase [77]. Whereas PI serves as an activating lipid for aSMase activity, various phosphorylated derivatives of PI (e.g. PI-3,5-P2, and PI-3,4,5-P3) have been demonstrated to function as in vitro inhibitors [78, 79]. An interesting feature of the various phosphoinositides is relative compartmentalization of specific PI derivatives [80]. Thus, the presence (or absence) of various lipid activators/inhibitors may function to limit aSMase function in certain compartments (e.g. PI-3,4,5-P3 at the plasma membrane) while promoting its action in others (e.g. increased BMP in the lysosome). It is also worth mentioning that while S-SMase and L-SMase will encounter distinct lipidic environments, the effects of differences in lipid binding on activation/inhibition have yet to be described.
Niemann-Pick diease type C and Cholesterol
Unlike NPD types A and B, which result from mutations in SMPD1, Niemann-Pick disease type C results from mutations in NPC1 and NPC2. NPC1 and NPC2 encode proteins involved in cholesterol trafficking in the endo-lysosomal compartment, and mutation of either of these proteins results in massive accumulation of unesterified cholesterol in perinuclear lysosomes (NPC-1, OMIM: 257220; NPC-2, OMIM: 607625) [81]. Despite the absence of genetic aberrations in SMPD1, fibroblasts from NPD-C patients exhibit marked inhibition of aSMase activity in cellular extracts [82, 83]. Loss of aSMase activity occurs via post-translational mechanisms and is a direct result of accumulation of cholesterol in the endo-lysosomal compartment [82]. While mature lysosomes are enriched in anionic lipids, such as BMP, the levels cholesterol are low, and this is attributed to depletion of cholesterol during lysosomal maturation [84]. The accumulation of cholesterol in perinuclear lysosomes in NPD-C appears to originate from lipoproteins since removal of the lipoprotein fraction from culture media corrected aSMase deficiency in NPD-C fibroblasts [85]. Whether the functional loss of aSMase activity is a result of altered trafficking (e.g. cholesterol influences trafficking of aSMase precursor), or via direct action on the enzyme is unclear. However, certain cholesterol derivatives (e.g. 7-ketocholesterol) have been shown to inhibit L-SMase activity in a cell-free system [86]. This finding may explain the influence of exogenous LDL or cholesterol species on aSMase activity and subsequent biology.
3. Metabolic and Biological Role of Secretory and Lysosomal Sphingomyelinase
Given that the majority of studies on the role of aSMase in the (patho)physiology of human disease rely on loss-of-function studies, a distinction between the metabolic and biologic roles of S-SMase and L-SMase has yet to be made. However, several inferences regarding proposed roles of S-SMase (and L-SMase) can be made on the basis of the experimental evidence collected from various studies. In this section, we will review the existing evidence regarding regulation of S-SMase and L-SMase, as well as their purported roles.
3.1. Lysosomal Acid Sphingomyelinase
While the prevailing model of aSMase action upon induction of cellular stress highlights relocalization to the PM as a crucial event, there remains the question of the regulated role of lysosomal aSMase in Cer signaling. The targets of L-SMase-derived ceramide are poorly defined although activation of aSMase in certain contexts has been associated with activation of classical apoptotic signaling, such as Bax activation [87, 88], mitochondrial injury [59], and cathepsin D release into the cytosol [89]. However, several groups have suggested that lysosomal Cer is not associated with apoptosis [90, 91]. Additionally, the likelihood of lysosomal Cer serving as a signaling molecule has been called into question as it cannot escape the lysosome [92].
Despite these reports, a signaling role for L-SMase cannot be excluded as several lysosomotropic inhibitors of aSMase have been shown to disrupt putative Cer-signaling events [93], including apoptotic signaling [94–97]. Several cationic amphiphilic drugs have been shown to induce irreversible loss of L-SMase by promoting proteolysis of the mature enzyme [58, 98–101], and unlike agents which induce alkalinization of lysosomes (e.g. ammonium chloride), desipramine and related compounds do not induce massive lysosomal disruption but rather induce proteolysis of the mature L-SMase enzyme [58, 98]. It should be emphasized that these compounds do not function as direct in vitro inhibitors [100], and L-SMase proteolysis is unlikely a selective process. For example, desipramine has recently been shown to induce proteolysis of mature acid ceramidase (aCDase) [102, 103]. This finding is of particular importance given the possible role of L-SMase in the ceramide salvage pathway [87]. L-SMase may act upstream in the ceramide salvage pathway, which employs an initial generation of Cer followed by processing by CDase to generate Sph which can escape the lysosome, which can then be reacylated to Cer by ceramide synthases (CerS) in different compartments [7]. Desipramine disrupts two components of the pathway – aSMase and aCDase – and thus the action of the compound may be to limit production of Cer by CerS [61, 87] or via inhibition of intracellular S1P by depletion of Sph [102].
While the metabolic role and biological function of L-SMase remain unresolved, dysfunction or absence of L-SMase results in cellular dysfunction and subsequent systemic disease, as exemplified in NPD. Dhami et al. provided in vivo evidence that even minimal restoration of L-SMase function was capable of alleviating neurological deficits in mouse models of NPD-A [104]. Interestingly, although visceral SM accumulation and pathology remained, mice expressing a ‘L-SMase only’ chimeric-aSMase transgene (aSMase fused to lysosome targeting sequence of LAMP1) did not exhibit ataxia or tremor, and exhibited normal growth curves. This finding suggested that residual L-SMase activity might explain the lack of neurological involvement in non-neuronopathic type B NPD.
3.2. Acid Sphingomyelinase at the Cell Surface, Lipid Rafts and Apoptosis
Acid Sphingomyelinase and Apoptosis
The strongest evidence for a prominent role of aSMase in the cellular stress response comes from genetic models of aSMase deficiency (e.g. aSMase knockout mice). In recent years, aSMase-null mice have been shown to be protected against a variety of stress stimuli, including Fas ligand [105], lipopolysaccharide (LPS) [13], ionizing radiation [14, 106], photocytotoxicity [107], ischemia/reperfusion injury [93], cisplatin [108], and tumor necrosis factor-α (TNF-α) [109, 110] owing to impaired ceramide generation. Protection from injury in these studies was attributed to decreased cell death, predominantly in the endothelium. Similar findings have been reported in various cell culture models, showing that aSMase activation and subsequent ceramide are critical to the cellular stress response. These and related studies have established a prominent role for a product of the aSMase gene in cellular stress signaling. However, not all tissues/cells are equally protected from these insults raising questions as to the precise involvement of aSMase, and ceramide, in these processes [105, 111]. Furthermore, other groups have shown that aSMase is dispensable for apoptosis in response to many of these same stimuli [91], raising questions as the precise involvement of aSMase.
While some of these discrepancies may be ascribed to inherent differences in model systems [17], cell types, etc., another possible explanation is that genetic models of aSMase deficiency naturally lack both S-SMase and L-SMase. Following up on studies by Garcia-Barros et al. [112, 113], Smith and Schuchman investigated the specific role of S-SMase in the anti-tumor effects of ionizing radiation on tumor microvasculature [114]. Acidification of culture media to accommodate the pH optima of S-SMase (and mimic the tumor microenvironment) and administration of rh-aSMase (a form of S-SMase) sensitized cancer cells to IR-induced cell death. Additionally, xenografts of B16 melanoma cell overexpressing aSMase were markedly sensitized to IR and exhibited noticeably diminished recruitment of small blood vessels, once again highlighting a connection between the endothelium and S-SMase.
While several groups had reported connections between endothelial viability and aSMase, the study by Smith and Schuchman is the first to address the role of S-SMase directly. For example, elevations in endogenous S-SMase have been reported in patients undergoing radiation treatment [115], and aSMase −/− mice have been shown to be protected from IR induced microvascular cell death in multiple tissues. Similarly, S-SMase is elevated in sepsis [116], LPS induces S-SMase secretion in mice [117], and aSMase −/− mice are protected from LPS induced endothelial apoptosis [13]. Thus, endothelial cells represent not only a rich source of S-SMase [56], but perhaps a sensitive target tissue to study the effects of ceramide-mediated apoptosis.
Biophysical and biological aspects of ceramide-enriched microdomains
Generation of ceramide at the plasma membrane has been shown to influence membrane dynamics [118, 119]. Increased Cer alters the biophysical properties of membrane bilayers (e.g. gel-fluid transition temperature), which is most often detected experimentally by detergent resistance. While SM is soluble in cold Triton X-100, as little as 5 mol % Cer is sufficient to induce detergent resistance [120]. Rapid generation of Cer from SM hydrolysis results in a relatively slow reorganization of membranes with resultant microdomain formation [121]. These Cer-enriched microdomains have been shown to exclude cholesterol from model membranes [122, 123], alter membrane fluidity [124], change membrane curvature [125], and induce transbilayer flip-flop [126, 127], although the biological consequences of these biophysical features of Cer remain incompletely understood.
One potential biological consequence of Cer generation at the PM and function related to changes in membrane dynamics involves lipid rafts. Hydrolysis of SM to Cer results in formation of lipid microdomains which have the capacity to fuse together to form larger platforms [121, 128, 129]. Acute activation and relocalization of aSMase to membrane rafts has been demonstrated in response to multiple stimuli, including CD95 activation [128, 130, 131], pathogens [132–134], and UV irradiation [135], although rafts were defined primarily by microscopy using raft markers such as cholera toxin (which binds GM1 ganglioside), or by sensitivity to cholesterol-depleting agents. While these rafts have been implicated in promoting clustering of membrane receptors, which has been suggested to organize signaling molecules and amplify membrane signals, the precise relationship between aSMase, SM hydrolysis to Cer at the PM, and lipid rafts remains unresolved.
3.3. Secretory Sphingomyelinase and Cardiovascular Disease
S-SMase and Atherosclerosis
Given that the earliest report of extracellular sphingomyelinase activity utilized serum as a source of enzyme [69], it is not surprising that there has been intense study into the role of serum sphingomyelinase in cardiovascular pathophysiology. One of the best-characterized roles for S-SMase is in the subendothelial retention of aggregated low-density lipoproteins (LDL), an early atherogenic event in the arterial wall (reviewed in [136]). Action of S-SMase on lipoprotein-bound SM yields Cer-enriched LDL, which is prone to aggregation. LDL aggregation in turn promotes subendothelial retention of these atherogenic lipoproteins that are engulfed by arterial wall macrophages, which over time become macrophage foam cells. These foam cells promote atherosclerosis via multiple mechanisms [137, 138].
Several lines of evidence support a positive role for S-SMase in the subendothelial retention of atherogenic lipoproteins. First, treatment of native LDL particles with S-SMase or bacterial SMase promotes LDL aggregation and foam cell formation [68, 139, 140]. Second, Cer-enriched, aggregated LDL are evident in human (and animal model) atherosclerotic lesions [141]. Third, S-SMase is the only known extracellular SMase which would have access to SM associated with LDL [136]. Fourth, endothelial cells are rich sources of S-SMase [56]. Lastly, S-SMase interacts with components of the extracellular matrix (e.g. collagen) which are found in the subendothelium [142].
Most recently, Devlin et al. showed that aSMase-null mice, when crossed with atherosclerosis-prone apoE −/− or LDL-R −/− mice, were protected from development of atherosclerotic lesions [143]. Mice lacking aSMase had no change in serum cholesterol or circulating LDL relative to control mice in the apoE −/− or LDL-R −/− background, but showed decreased focal atheroma formation (40–50% reduction). The basis for diminished atheroma formation may in fact be a result of diminished LDL retention in the subendothelium, as early foam cell lesions were reduced by over 80% in aSMase-null mice in both the apoE −/− and LDL-R −/− backgrounds.
From a biochemical standpoint, one of the most fascinating aspects of the S-SMase/atherosclerosis story is the capacity of S-SMase to function outside its pH optimum. S-SMase was shown to hydrolyze atherogenic, but not native, lipoprotein-associated SM at neutral pH [68]. Interestingly, one of the features of these ‘atherogenic’ lipoproteins was an increased sphingomyelin:phosphatidylcholine ratio (SM:PC). In support of these data, elevated levels of SM in lipoproteins have been shown to correlate with atherosclerotic progression [144], and reduction in SM has shown to serve a protective role [145–147]. The atherogenic lipoproteins in apoE −/− mice (derivatives of chylomicrons) are similarly enriched in SM (4-fold higher than wild-type) and are excellent substrates for S-SMase [148].
An intriguing conundrum arising from the study by Devlin et al. is the apparent contradiction with the known predisposition of NPD patients to atherogenic lipoprotein profiles and coronary artery disease [149–151]. Thus, the protective role of aSMase deficiency in mouse models of atherogenesis ought to be interpreted with caution. Future studies aimed at determining the precise contribution of S-SMase in the absence of defective housekeeping function of L-SMase will permit greater understanding of the role of S-SMase in physiologic and pathophysiologic lipoprotein metabolism.
Serum S-SMase, Diabetes and Heart Disease
While basolateral secretion of S-SMase from endothelial cells is thought to mediate the subendothelial retention of atherogenic lipoproteins and subsequent plaque formation, apical secretion by endothelial cells is believed to account for the elevated S-SMase activity in serum that has been reported in patients with inflammatory conditions (see below), type II diabetes [152] and chronic heart failure [153]. In addition to development of microvascular disease, patients with diabetes mellitus type II (DMII) develop macrovascular disease, including atherosclerosis. Although the elevated serum Zn-dependent S-SMase present in type II diabetics has not been causally linked to increased risk of atherosclerosis, elevated Cer levels have been demonstrated in skeletal muscle of patients with DMII [154, 155], and ceramide is suggested to contribute to insulin resistance via multiple mechanisms (reviewed in [156]). The role of inflammatory mediators in the regulation of S-SMase secretion in the context of insulin resistance is not known; however, patients with DMII are known to have chronic low level inflammation [157]. It is conceivable that inflammation-induced S-SMase may contribute to insulin resistance via generation of ceramide.
Similar to patients with DMII, blood levels of inflammatory cytokines are elevated in patients with chronic heart failure [158]. Elevated serum S-SMase activity in patients with chronic heart failure correlates with severity of disease, with highest levels seen in patients with cachectic heart failure. Cardiac cachexia is characterized by elevated serum TNF-α levels, severe muscle wasting, and portends a poor prognosis. Similar to the report of elevated S-SMase activity in DMII patients, the report by Doehner et al. does not provide evidence for the mechanism of regulation of S-SMase secretion nor the biological consequence of elevated S-SMase activity in the context of cachectic heart failure.
S-SMase and Platelet Function
Platelets represent another source of S-SMase that is relevant to cardiovascular physiology. Sphingolipids are regulated by inducers of platelet activation [159], and in turn influence various aspects of platelet function [160–164]. Platelets are devoid of neutral SMase activity but possess acid SMase activity both in platelet homogenates and in the supernatant following platelet activation [165]. In response to thrombin-induced platelet activation, S-SMase is released extracellularly [165, 166]. This elevation in S-SMase occurs with concomitant loss of intracellular L-SMase (consistent with the alternative trafficking model), and was recapitulated by phorbol ester and calcium ionophore, suggesting a role for protein kinase C (PKC) and intracellular calcium. Interestingly, following thrombin activation, levels of Cer are not significantly elevated [167], while Sph levels are increased, suggesting high endogenous CDase activity [165]. Using both bacterial SMase and S-SMase, Tani et al. implicated SM-derived Cer as a source of platelet S1P [168]. The precise biological role of S-SMase either derived from platelets, or acting on platelets, remains ill-defined, but increased ceramide has been shown to induce marked changes in energy metabolism in resting platelets [169] and translocation of signaling enzymes [170].
S-SMase and Anemia
Eryptosis, or red blood cell programmed cell death, serves to clear both aged and damaged erythrocytes [171]. Hallmarks of eryptosis include increased cytosolic calcium, decreased erythrocyte volume, and externalization of phosphatidylserine (PS). Studies using various inducers of eryptosis, including PAF [172], Aβ peptide [173], sepsis [174], and exogenous zinc, have implicated aSMase in Cer formation. However, given that erythrocytes lack organelles, including lysosomes, the aSMase probably originates in other cells.
Lang et al. recently described a role for aSMase in anemia associated with Wilson disease, a hereditary condition characterized by pathologic accumulation of copper [96]. In this study, copper intoxication was shown to activate both S-SMase and L-SMase in leukocytes [96]. Interestingly, incubating control erythrocytes with culture medium from leukocytes treated with copper induced ceramide formation and PS-externalization, suggesting that leukocytes may be the source of soluble acid SMase detected in this study. Thus, S-SMase may serve as a paracrine signaling molecule originating in copper-stimulated leukocytes and acting on neighboring erythrocytes to generate Cer, induce eryptosis, and subsequent anemia associated with Wilson disease.
3.4. Secretory Sphingomyelinase, Infection and Inflammation
Secretory Sphingomyelinase & Acute and Chronic Inflammation
The first report of regulated aSMase trafficking demonstrated upregulation of S-SMase in response to inflammatory cytokines in cultured endothelial cells [56]. Increased S-SMase activity in an inflammatory setting was also demonstrated in a mouse model of acute systemic inflammation induced by injection of LPS, IL-1β and TNF-α [13, 117]. In support of the LPS data, Salmonella typhi and Escherichia coli specifically and acutely induce upregulation of S-SMase by macrophages, suggesting that S-SMase upregulation by inflammatory mediators may involve mechanisms shared by the innate immune response [175]. Elevated serum S-SMase activity has been reported not only in patients with type II diabetes [152] and cachectic heart failure [153], but also sepsis [116], hypercytokinemia (in hemophagocytic lymphohistiocytosis) [176], and in response to spatially fractionated ionizing radiation therapy in cancer patients [115], providing further evidence of physiologic and pharmacologic regulation of S-SMase.
The precise mechanism underlying enhanced secretion of S-SMase remains unknown, although the alternative trafficking model suggests that a given aSMase precursor has a minimum of 2 fates – secretion or lysosomal targeting. In endothelial cells, increased release of S-SMase into the culture medium in response to interleukin-1β (IL-1β) occurred with concomitant diminution of L-SMase activity, consistent with the alternative trafficking model of aSMase [56]. If the mechanism of regulation were common to all lysosomal enzymes (e.g. ↓ M6P → ↓ lysosomal shuttling), then one would expect a comparable increase in other acid hydrolases in the culture medium. Although this has yet to be defined using cell culture studies, urine from patients with peritonitis displayed a > 200-fold increase in aSMase activity relative to control patients with only modest elevations in other lysosomal hydrolases [177]. Thus, the mechanism of increased secretion of S-SMase may not rely on such a global trafficking switch that would affect all M6P-trafficked enzymes equally.
Acid SMase, Ceramide, and the Host Response to Infection
There are numerous reports suggesting a prominent role for aSMase in the response to various pathogens [178, 179]. In response to infection, aSMase has been shown to relocalize to the outer leaflet of the PM, generating Cer, which in turn promotes microdomain formation [132]. While the specific mechanism of Cer-mediated pathogen clearance has yet to be elucidated, Cer has been suggested to promote fusion of different membrane-bound compartments [180] and the maturation of the phago-lysosome [181, 182]. For a comprehensive discussion of these studies, readers are referred to several reviews [16, 180, 183, 184].
One of the most intensely studied pathogens linked to aSMase activation is Pseudomonas aeruginosa (PA). Mice deficient in aSMase were unable to clear the infection due to impaired Cer generation, which resulted in hyperinflammatory response, sepsis and death [132]. Given that patients with cystic fibrosis (CF) are predisposed to pulmonary infections with PA, there has been increasing interest in manipulating aSMase to improve the course of CF by alleviating PA-mediated disease [184]. However, a recent study by the same group demonstrated that aSMase-derived Cer promoted inflammation, lung epithelial cell death, and increased susceptibility in a mouse model of cystic fibrosis [185]. The authors also claimed that the aSMase inhibitor amitryptiline may serve as a useful therapeutic agent in the treatment of PA-infection in CF patients. However, it should be noted that the role of S-SMase in response to PA infection, or in the context of impaired function of cystic fibrosis transmembrane conductance regulator (CFTR) has yet to investigated. Given that exogenous, bacterial SMase has been shown to influence CFTRchloride channel function via hydrolysis of SM at the plasma membrane [186, 187], it is likely that aSMase is involved in the pathophysiology of CF at multiple levels. Additionally, aSMase likely serves distinct roles in different cell types as aSMase was recently shown to enhance PA-mediated apoptosis of macrophages [188]. Finally, studies of NPD-type C cells have shown some similarities with CFTR −/− cells in terms of cholesterol storage and hyperinflammatory responses [189]. The intersection of cholesterol, aSMase trafficking, and CFTR function as it relates to PA function is not yet known, but future studies taking into account both S-SMase and L-SMase function will provide more concrete evidence of regarding the role of aSMase at the PM versus the endo-lysosomal compartment.
4. Unanswered Questions & Future Directions
Despite the multitude of studies implicating the aSMase/Cer pathway in important cellular processes, many important questions regarding the roles and regulation of aSMase remain unanswered. The molecular mechanism(s) of aSMase regulation, the metabolic impact of S-SMase vs. L-SMase, and the subsequent biological consequence of Cer formation in distinct regions of the cell are at present unresolved. Two essential features of aSMase regulation that remain unknown or incompletely defined include (1) the cellular pathways involved in regulating aSMase trafficking, localization, and activity, and (2) the molecular mechanism of aSMase (e.g. S-SMase) action on sphingomyelin in the outer leaflet of the plasma membrane. While the second component addresses the biochemical characteristics of both S-SMase and L-SMase and will require side-by-side comparison of both enzymes, the first component is a molecular cell biology question necessitating focused investigation into the cellular regulation of aSMase trafficking.
Understanding the cellular and biochemical regulation of aSMase function will be essential for the assessment of the metabolic impact of S-SMase and L-SMase. Both S-SMase and L-SMase hydrolyze SM to Cer, but act on different pools of SM. One of the most glaring unanswered questions is the impact of compartmentalization of Cer generation. The precise role of Cer in biological processes remains to be well defined, but likely involves the intersection of classical signaling paradigms with the unique membrane-modifying properties of Cer [190]. One of the impediments to progress has been the paucity of tools to address the subcellular localization of specific Cer molecules. While sphingolipidomic analysis has been advanced by the use of mass spectrometry to analyze the cellular complement of sphingolipid species and subspecies, a comparable advance to assess compartmentalization and topology has yet to be realized. Greater capacity to both understand and manipulate the cellular Cer profile in a compartment-specific fashion will allow researchers to ask more focused question about the biological role of Cer and Cer-producing enzymes/pathways.
5. Conclusions
S-SMase and L-SMase are distinct enzymatic entities that arise through alternative trafficking of a common aSMase protein precursor. While several studies have reported regulation of L-SMase (e.g. increased specific activity, translocation), far fewer studies have addressed the role and regulation of S-SMase. Additionally, the most robust findings suggesting a central role for aSMase in cellular stress signaling involves loss-of-function studies that do not discriminate between the contributions of S-SMase and L-SMase. While the use of RNAi, knockout mice, and NPD patient tissues have been instrumental in advancing the field of aSMase research to its current standing, new tools are needed to address the remaining unanswered questions. Advancing our understanding of the molecular mechanisms underlying the regulation, activity, and action of both S-SMase and L-SMase may permit the development of more specific strategies to modulate Cer levels in distinct regions of the cell to alter the course of human disease.
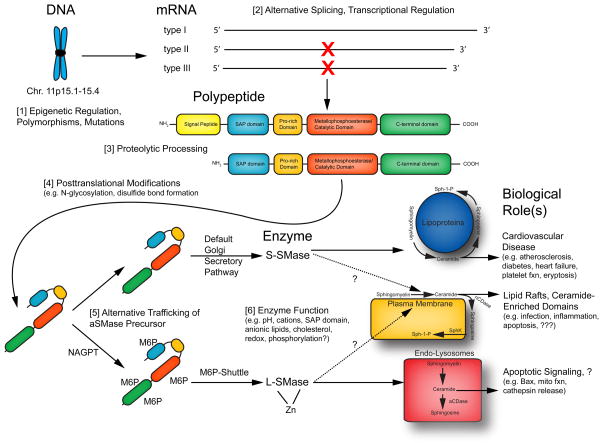
Figure 4.
Summary - The life history of acid sphingomyelinaose.
Acknowledgments
This work was funded in part by AHA 081509E (R.W.J.), GM08716 (MSTP ~ R.W.J.) and CA 97132 (Y.A.H.).
Special thanks to Leah Siskind, Nana Bartke, and Christopher J. Clarke for reviewing this manuscript. Additional thanks to Thomas Mullen for his assistance in preparing the graphics and figures.
Abbreviations
- aSMase
acid sphingomyelinase
- SMPD1
sphingomyelin phosphodiesterase 1
- S-SMase
secretory sphingomyelinase
- L-SMase
lysosomal sphingomyelinase
- Cer
ceramide
- SM
sphingomyelin
- NPD
Niemann-Pick disease
- rh-aSMase
recombinant human aSMase
References
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 2.Carpinteiro A, et al. Ceramide-induced cell death in malignant cells. Cancer Lett. 2008;264(1):1–10. doi: 10.1016/j.canlet.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R11–26. doi: 10.1152/ajpregu.00416.2005. [DOI] [PubMed] [Google Scholar]
- 4.Pettus BJ, Chalfant CE, Hannun YA. Sphingolipids in inflammation: roles and implications. Curr Mol Med. 2004;4(4):405–18. doi: 10.2174/1566524043360573. [DOI] [PubMed] [Google Scholar]
- 5.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4(8):604–16. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 6.Perry DK. Serine palmitoyltransferase: role in apoptotic de novo ceramide synthesis and other stress responses. Biochim Biophys Acta. 2002;1585(2–3):146–52. doi: 10.1016/s1388-1981(02)00335-9. [DOI] [PubMed] [Google Scholar]
- 7.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20(6):1010–8. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goni FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 2002;531(1):38–46. doi: 10.1016/s0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- 9.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82(1):27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 10.Schuchman EH. The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann-Pick disease. J Inherit Metab Dis. 2007;30(5):654–63. doi: 10.1007/s10545-007-0632-9. [DOI] [PubMed] [Google Scholar]
- 11.McGovern MM, et al. Natural history of Type A Niemann-Pick disease: possible endpoints for therapeutic trials. Neurology. 2006;66(2):228–32. doi: 10.1212/01.wnl.0000194208.08904.0c. [DOI] [PubMed] [Google Scholar]
- 12.McGovern MM, et al. A prospective, cross-sectional survey study of the natural history of Niemann-Pick disease type B. Pediatrics. 2008;122(2):e341–9. doi: 10.1542/peds.2007-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haimovitz-Friedman A, et al. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med. 1997;186(11):1831–41. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santana P, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86(2):189–99. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 15.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274(5294):1855–9. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 16.Smith EL, Schuchman EH. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J. 2008 doi: 10.1096/fj.08-108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozano J, et al. Niemann-Pick Disease versus acid sphingomyelinase deficiency. Cell Death Differ. 2001;8(1):100–3. doi: 10.1038/sj.cdd.4400775. [DOI] [PubMed] [Google Scholar]
- 18.Nix M, Stoffel W. Perturbation of membrane microdomains reduces mitogenic signaling and increases susceptibility to apoptosis after T cell receptor stimulation. Cell Death Differ. 2000;7(5):413–24. doi: 10.1038/sj.cdd.4400666. [DOI] [PubMed] [Google Scholar]
- 19.Tardy C, et al. Lysosomes and lysosomal proteins in cancer cell death (new players of an old struggle) Biochim Biophys Acta. 2006;1765(2):101–25. doi: 10.1016/j.bbcan.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Schissel SL, et al. Zn2+-stimulated sphingomyelinase is secreted by many cell types and is a product of the acid sphingomyelinase gene. J Biol Chem. 1996;271(31):18431–6. doi: 10.1074/jbc.271.31.18431. [DOI] [PubMed] [Google Scholar]
- 21.da Veiga Pereira L, et al. Regional assignment of the human acid sphingomyelinase gene (SMPD1) by PCR analysis of somatic cell hybrids and in situ hybridization to 11p15.1----p15.4. Genomics. 1991;9(2):229–34. doi: 10.1016/0888-7543(91)90246-b. [DOI] [PubMed] [Google Scholar]
- 22.Schuchman EH, et al. Structural organization and complete nucleotide sequence of the gene encoding human acid sphingomyelinase (SMPD1) Genomics. 1992;12(2):197–205. doi: 10.1016/0888-7543(92)90366-z. [DOI] [PubMed] [Google Scholar]
- 23.Schuchman EH, et al. Human acid sphingomyelinase. Isolation, nucleotide sequence and expression of the full-length and alternatively spliced cDNAs. J Biol Chem. 1991;266(13):8531–9. [PubMed] [Google Scholar]
- 24.Quintern LE, et al. Isolation of cDNA clones encoding human acid sphingomyelinase: occurrence of alternatively processed transcripts. Embo J. 1989;8(9):2469–73. doi: 10.1002/j.1460-2075.1989.tb08382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newrzella D, Stoffel W. Molecular cloning of the acid sphingomyelinase of the mouse and the organization and complete nucleotide sequence of the gene. Biol Chem Hoppe Seyler. 1992;373(12):1233–8. doi: 10.1515/bchm3.1992.373.2.1233. [DOI] [PubMed] [Google Scholar]
- 26.Lin X, Hengartner MO, Kolesnick R. Caenorhabditis elegans contains two distinct acid sphingomyelinases. J Biol Chem. 1998;273(23):14374–9. doi: 10.1074/jbc.273.23.14374. [DOI] [PubMed] [Google Scholar]
- 27.Murate T, et al. Up-regulation of acid sphingomyelinase during retinoic acid-induced myeloid differentiation of NB4, a human acute promyelocytic leukemia cell line. J Biol Chem. 2002;277(12):9936–43. doi: 10.1074/jbc.M111594200. [DOI] [PubMed] [Google Scholar]
- 28.Langmann T, et al. Transcription factors Sp1 and AP-2 mediate induction of acid sphingomyelinase during monocytic differentiation. J Lipid Res. 1999;40(5):870–80. [PubMed] [Google Scholar]
- 29.Wu J, et al. Acid sphingomyelinase is induced by butyrate but does not initiate the anticancer effect of butyrate in HT29 and HepG2 cells. J Lipid Res. 2005;46(9):1944–52. doi: 10.1194/jlr.M500118-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Lecka-Czernik B, et al. Identification of gene sequences overexpressed in senescent and Werner syndrome human fibroblasts. Exp Gerontol. 1996;31(1–2):159–74. doi: 10.1016/0531-5565(95)02014-4. [DOI] [PubMed] [Google Scholar]
- 31.Shah C, et al. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem. 2008;283(20):13538–48. doi: 10.1074/jbc.M709950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samad F, et al. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55(9):2579–87. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- 33.Simonaro CM, et al. Imprinting at the SMPD1 locus: implications for acid sphingomyelinase-deficient Niemann-Pick disease. Am J Hum Genet. 2006;78(5):865–70. doi: 10.1086/503750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rethy LA, et al. Acid sphingomyelinase deficiency in Beckwith Wiedemann syndrome. Pathol Oncol Res. 2000;6(4):295–7. doi: 10.1007/BF03187335. [DOI] [PubMed] [Google Scholar]
- 35.Elliott M, Maher ER. Beckwith-Wiedemann syndrome. J Med Genet. 1994;31(7):560–4. doi: 10.1136/jmg.31.7.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott RH, et al. Constitutional 11p15 abnormalities, including heritable imprinting center mutations, cause nonsyndromic Wilms tumor. Nat Genet. 2008 doi: 10.1038/ng.243. [DOI] [PubMed] [Google Scholar]
- 37.Scott RH, et al. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet. 2006;43(9):705–15. doi: 10.1136/jmg.2006.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferlinz K, et al. Occurrence of two molecular forms of human acid sphingomyelinase. Biochem J. 1994;301(Pt 3):855–62. doi: 10.1042/bj3010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan Q, Schuchman EH. A novel polymorphism in the human acid sphingomyelinase gene due to size variation of the signal peptide region. Biochim Biophys Acta. 1995;1270(2–3):207–10. doi: 10.1016/0925-4439(95)00050-e. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Hannun YA. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272(26):16281–7. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- 41.Lansmann S, et al. Human acid sphingomyelinase. Eur J Biochem. 2003;270(6):1076–88. doi: 10.1046/j.1432-1033.2003.03435.x. [DOI] [PubMed] [Google Scholar]
- 42.Qiu H, et al. Activation of human acid sphingomyelinase through modification or deletion of C-terminal cysteine. J Biol Chem. 2003;278(35):32744–52. doi: 10.1074/jbc.M303022200. [DOI] [PubMed] [Google Scholar]
- 43.Wisselaar HA, et al. Structural and functional changes of lysosomal acid alpha-glucosidase during intracellular transport and maturation. J Biol Chem. 1993;268(3):2223–31. [PubMed] [Google Scholar]
- 44.Yonezawa S, et al. Structures at the proteolytic processing region of cathepsin D. J Biol Chem. 1988;263(31):16504–11. [PubMed] [Google Scholar]
- 45.Ferlinz K, et al. Functional characterization of the N-glycosylation sites of human acid sphingomyelinase by site-directed mutagenesis. Eur J Biochem. 1997;243(1–2):511–7. doi: 10.1111/j.1432-1033.1997.511_1a.x. [DOI] [PubMed] [Google Scholar]
- 46.Newrzella D, Stoffel W. Functional analysis of the glycosylation of murine acid sphingomyelinase. J Biol Chem. 1996;271(50):32089–95. doi: 10.1074/jbc.271.50.32089. [DOI] [PubMed] [Google Scholar]
- 47.Bartelsen O, et al. Expression of recombinant human acid sphingomyelinase in insect Sf21 cells: purification, processing and enzymatic characterization. J Biotechnol. 1998;63(1):29–40. doi: 10.1016/s0168-1656(98)00070-4. [DOI] [PubMed] [Google Scholar]
- 48.Lansmann S, et al. Purification of acid sphingomyelinase from human placenta: characterization and N-terminal sequence. FEBS Lett. 1996;399(3):227–31. doi: 10.1016/s0014-5793(96)01331-2. [DOI] [PubMed] [Google Scholar]
- 49.Schissel SL, et al. The cellular trafficking and zinc dependence of secretory and lysosomal sphingomyelinase, two products of the acid sphingomyelinase gene. J Biol Chem. 1998;273(29):18250–9. doi: 10.1074/jbc.273.29.18250. [DOI] [PubMed] [Google Scholar]
- 50.Hurwitz R, et al. Processing of human acid sphingomyelinase in normal and I-cell fibroblasts. J Biol Chem. 1994;269(7):5440–5. [PubMed] [Google Scholar]
- 51.Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987;1(6):462–8. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- 52.Hasilik A, Waheed A, von Figura K. Enzymatic phosphorylation of lysosomal enzymes in the presence of UDP-N-acetylglucosamine. Absence of the activity in I-cell fibroblasts. Biochem Biophys Res Commun. 1981;98(3):761–7. doi: 10.1016/0006-291x(81)91177-3. [DOI] [PubMed] [Google Scholar]
- 53.Hasilik A, et al. Phosphorylated oligosaccharides in lysosomal enzymes: identification of alpha-N-acetylglucosamine(1)phospho(6)mannose diester groups. Proc Natl Acad Sci U S A. 1980;77(12):7074–8. doi: 10.1073/pnas.77.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi I, et al. Acid sphingomyelinase: relation of 93lysine residue on the ratio of intracellular to secreted enzyme activity. Tohoku J Exp Med. 2005;206(4):333–40. doi: 10.1620/tjem.206.333. [DOI] [PubMed] [Google Scholar]
- 55.Ni X, Morales CR. The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic. 2006;7(7):889–902. doi: 10.1111/j.1600-0854.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 56.Marathe S, et al. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J Biol Chem. 1998;273(7):4081–8. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- 57.Tikkanen R, et al. Several cooperating binding sites mediate the interaction of a lysosomal enzyme with phosphotransferase. Embo J. 1997;16(22):6684–93. doi: 10.1093/emboj/16.22.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hurwitz R, Ferlinz K, Sandhoff K. The tricyclic antidepressant desipramine causes proteolytic degradation of lysosomal sphingomyelinase in human fibroblasts. Biol Chem Hoppe Seyler. 1994;375(7):447–50. doi: 10.1515/bchm3.1994.375.7.447. [DOI] [PubMed] [Google Scholar]
- 59.Zeidan YH, et al. A novel role for protein kinase Cdelta-mediated phosphorylation of acid sphingomyelinase in UV light-induced mitochondrial injury. FASEB J. 2008;22(1):183–93. doi: 10.1096/fj.07-8967com. [DOI] [PubMed] [Google Scholar]
- 60.Zeidan YH, Jenkins RW, Hannun YA. Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. J Cell Biol. 2008;181(2):335–50. doi: 10.1083/jcb.200705060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeidan YH, Hannun YA. Activation of acid sphingomyelinase by protein kinase Cdelta-mediated phosphorylation. J Biol Chem. 2007;282(15):11549–61. doi: 10.1074/jbc.M609424200. [DOI] [PubMed] [Google Scholar]
- 62.Lee CY, et al. Carboxyl-terminal disulfide bond of acid sphingomyelinase is critical for its secretion and enzymatic function. Biochemistry. 2007;46(51):14969–78. doi: 10.1021/bi700817g. [DOI] [PubMed] [Google Scholar]
- 63.Yamanaka T, Suzuki K. Acid sphingomyelinase of human brain: purification to homogeneity. J Neurochem. 1982;38(6):1753–64. doi: 10.1111/j.1471-4159.1982.tb06659.x. [DOI] [PubMed] [Google Scholar]
- 64.Zou L, et al. Purification to homogeneity of human placental acid sphingomyelinase. Biotechnol Appl Biochem. 1989;11(2):217–25. [PubMed] [Google Scholar]
- 65.Callahan JW, et al. The active site of lysosomal sphingomyelinase: evidence for the involvement of hydrophobic and ionic groups. J Neurosci Res. 1983;10(2):151–63. doi: 10.1002/jnr.490100205. [DOI] [PubMed] [Google Scholar]
- 66.Quintern LE, et al. Acid sphingomyelinase from human urine: purification and characterization. Biochim Biophys Acta. 1987;922(3):323–36. doi: 10.1016/0005-2760(87)90055-5. [DOI] [PubMed] [Google Scholar]
- 67.He X, et al. Characterization of human acid sphingomyelinase purified from the media of overexpressing Chinese hamster ovary cells. Biochim Biophys Acta. 1999;1432(2):251–64. doi: 10.1016/s0167-4838(99)00069-2. [DOI] [PubMed] [Google Scholar]
- 68.Schissel SL, et al. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem. 1998;273(5):2738–46. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 69.Spence MW, et al. A new Zn2+-stimulated sphingomyelinase in fetal bovine serum. J Biol Chem. 1989;264(10):5358–63. [PubMed] [Google Scholar]
- 70.Ponting CP. Acid sphingomyelinase possesses a domain homologous to its activator proteins: saposins B and D. Protein Sci. 1994;3(2):359–61. doi: 10.1002/pro.5560030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu Rev Cell Dev Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- 72.Remmel N, et al. Saposin B mobilizes lipids from cholesterol-poor and bis(monoacylglycero)phosphate-rich membranes at acidic pH. Unglycosylated patient variant saposin B lacks lipid-extraction capacity. Febs J. 2007;274(13):3405–20. doi: 10.1111/j.1742-4658.2007.05873.x. [DOI] [PubMed] [Google Scholar]
- 73.Ferlinz K, et al. Stimulation of lysosomal sphingomyelin degradation by sphingolipid activator proteins. Chem Phys Lipids. 1999;102(1–2):35–43. doi: 10.1016/s0009-3084(99)00073-0. [DOI] [PubMed] [Google Scholar]
- 74.Paton BC, et al. Additional biochemical findings in a patient and fetal sibling with a genetic defect in the sphingolipid activator protein (SAP) precursor, prosaposin. Evidence for a deficiency in SAP-1 and for a normal lysosomal neuraminidase. Biochem J. 1992;285(Pt 2):481–8. doi: 10.1042/bj2850481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bradova V, et al. Prosaposin deficiency: further characterization of the sphingolipid activator protein-deficient sibs. Multiple glycolipid elevations (including lactosylceramidosis), partial enzyme deficiencies and ultrastructure of the skin in this generalized sphingolipid storage disease. Hum Genet. 1993;92(2):143–52. doi: 10.1007/BF00219682. [DOI] [PubMed] [Google Scholar]
- 76.Kolzer M, et al. Functional characterization of the postulated intramolecular sphingolipid activator protein domain of human acid sphingomyelinase. Biol Chem. 2004;385(12):1193–5. doi: 10.1515/BC.2004.154. [DOI] [PubMed] [Google Scholar]
- 77.Linke T, et al. Stimulation of acid sphingomyelinase activity by lysosomal lipids and sphingolipid activator proteins. Biol Chem. 2001;382(2):283–90. doi: 10.1515/BC.2001.035. [DOI] [PubMed] [Google Scholar]
- 78.Testai FD, et al. Acid sphingomyelinase and inhibition by phosphate ion: role of inhibition by phosphatidyl-myo-inositol 3,4,5-triphosphate in oligodendrocyte cell signaling. J Neurochem. 2004;89(3):636–44. doi: 10.1046/j.1471-4159.2004.02374.x. [DOI] [PubMed] [Google Scholar]
- 79.Kolzer M, et al. Phosphatidylinositol-3,5-Bisphosphate is a potent and selective inhibitor of acid sphingomyelinase. Biol Chem. 2003;384(9):1293–8. doi: 10.1515/BC.2003.144. [DOI] [PubMed] [Google Scholar]
- 80.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–7. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 81.Sturley SL, et al. The pathophysiology and mechanisms of NP-C disease. Biochim Biophys Acta. 2004;1685(1–3):83–7. doi: 10.1016/j.bbalip.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 82.Reagan JW, Jr, Hubbert ML, Shelness GS. Posttranslational regulation of acid sphingomyelinase in niemann-pick type C1 fibroblasts and free cholesterol-enriched chinese hamster ovary cells. J Biol Chem. 2000;275(48):38104–10. doi: 10.1074/jbc.M005296200. [DOI] [PubMed] [Google Scholar]
- 83.Pentchev PG, et al. Group C Niemann-Pick disease: faulty regulation of low-density lipoprotein uptake and cholesterol storage in cultured fibroblasts. FASEB J. 1987;1(1):40–5. doi: 10.1096/fasebj.1.1.3609608. [DOI] [PubMed] [Google Scholar]
- 84.Mobius W, et al. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4(4):222–31. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 85.Thomas GH, et al. Correction of sphingomyelinase deficiency in Niemann-Pick type C fibroblasts by removal of lipoprotein fraction from culture media. J Inherit Metab Dis. 1989;12(2):139–51. doi: 10.1007/BF01800716. [DOI] [PubMed] [Google Scholar]
- 86.Maor I, Mandel H, Aviram M. Macrophage uptake of oxidized LDL inhibits lysosomal sphingomyelinase, thus causing the accumulation of unesterified cholesterol-sphingomyelin-rich particles in the lysosomes. A possible role for 7-Ketocholesterol. Arterioscler Thromb Vasc Biol. 1995;15(9):1378–87. doi: 10.1161/01.atv.15.9.1378. [DOI] [PubMed] [Google Scholar]
- 87.Jin J, et al. Ceramide generated by sphingomyelin hydrolysis and the salvage pathway is involved in hypoxia/reoxygenation-induced Bax redistribution to mitochondria in NT-2 cells. J Biol Chem. 2008 doi: 10.1074/jbc.M801597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kashkar H, et al. Acid sphingomyelinase is indispensable for UV light-induced Bax conformational change at the mitochondrial membrane. J Biol Chem. 2005;280(21):20804–13. doi: 10.1074/jbc.M410869200. [DOI] [PubMed] [Google Scholar]
- 89.Heinrich M, et al. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11(5):550–63. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 90.Segui B, et al. Stress-induced apoptosis is not mediated by endolysosomal ceramide. FASEB J. 2000;14(1):36–47. doi: 10.1096/fasebj.14.1.36. [DOI] [PubMed] [Google Scholar]
- 91.Bezombes C, et al. Lysosomal sphingomyelinase is not solicited for apoptosis signaling. FASEB J. 2001;15(2):297–9. doi: 10.1096/fj.00-0466fje. [DOI] [PubMed] [Google Scholar]
- 92.Chatelut M, et al. Natural ceramide is unable to escape the lysosome, in contrast to a fluorescent analogue. FEBS Lett. 1998;426(1):102–6. doi: 10.1016/s0014-5793(98)00325-1. [DOI] [PubMed] [Google Scholar]
- 93.Llacuna L, et al. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology. 2006;44(3):561–72. doi: 10.1002/hep.21285. [DOI] [PubMed] [Google Scholar]
- 94.Erdreich-Epstein A, et al. Endothelial apoptosis induced by inhibition of integrins alphavbeta3 and alphavbeta5 involves ceramide metabolic pathways. Blood. 2005;105(11):4353–61. doi: 10.1182/blood-2004-08-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hundal RS, et al. Oxidized low density lipoprotein inhibits macrophage apoptosis by blocking ceramide generation, thereby maintaining protein kinase B activation and Bcl-XL levels. J Biol Chem. 2003;278(27):24399–408. doi: 10.1074/jbc.M209179200. [DOI] [PubMed] [Google Scholar]
- 96.Lang PA, et al. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med. 2007;13(2):164–70. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- 97.Osawa Y, et al. Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J Biol Chem. 2005;280(30):27879–87. doi: 10.1074/jbc.M503002200. [DOI] [PubMed] [Google Scholar]
- 98.Kolzer M, Werth N, Sandhoff K. Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic antidepressant desipramine. FEBS Lett. 2004;559(1–3):96–8. doi: 10.1016/S0014-5793(04)00033-X. [DOI] [PubMed] [Google Scholar]
- 99.Nassogne MC, et al. Cocaine induces a mixed lysosomal lipidosis in cultured fibroblasts, by inactivation of acid sphingomyelinase and inhibition of phospholipase A1. Toxicol Appl Pharmacol. 2004;194(2):101–10. doi: 10.1016/j.taap.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 100.Yoshida Y, et al. Reduction of acid sphingomyelinase activity in human fibroblasts induced by AY-9944 and other cationic amphiphilic drugs. J Biochem. 1985;98(6):1669–79. doi: 10.1093/oxfordjournals.jbchem.a135438. [DOI] [PubMed] [Google Scholar]
- 101.Masson M, et al. Calmodulin antagonist W-7 inhibits lysosomal sphingomyelinase activity in C6 glioma cells. J Neurochem. 1989;52(5):1645–7. doi: 10.1111/j.1471-4159.1989.tb09221.x. [DOI] [PubMed] [Google Scholar]
- 102.Zeidan YH, et al. Acid ceramidase but not acid sphingomyelinase is required for tumor necrosis factor-{alpha}-induced PGE2 production. J Biol Chem. 2006;281(34):24695–703. doi: 10.1074/jbc.M604713200. [DOI] [PubMed] [Google Scholar]
- 103.Elojeimy S, et al. New insights on the use of desipramine as an inhibitor for acid ceramidase. FEBS Lett. 2006;580(19):4751–6. doi: 10.1016/j.febslet.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 104.Marathe S, et al. Creation of a mouse model for non-neurological (type B) Niemann-Pick disease by stable, low level expression of lysosomal sphingomyelinase in the absence of secretory sphingomyelinase: relationship between brain intra-lysosomal enzyme activity and central nervous system function. Hum Mol Genet. 2000;9(13):1967–76. doi: 10.1093/hmg/9.13.1967. [DOI] [PubMed] [Google Scholar]
- 105.Lin T, et al. Role of acidic sphingomyelinase in Fas/CD95-mediated cell death. J Biol Chem. 2000;275(12):8657–63. doi: 10.1074/jbc.275.12.8657. [DOI] [PubMed] [Google Scholar]
- 106.Paris F, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–7. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 107.Separovic D, et al. Niemann-Pick human lymphoblasts are resistant to phthalocyanine 4-photodynamic therapy-induced apoptosis. Biochem Biophys Res Commun. 1999;258(3):506–12. doi: 10.1006/bbrc.1999.0670. [DOI] [PubMed] [Google Scholar]
- 108.Rebillard A, et al. Acid sphingomyelinase deficiency protects from cisplatin-induced gastrointestinal damage. Oncogene. 2008 doi: 10.1038/onc.2008.257. [DOI] [PubMed] [Google Scholar]
- 109.Garcia-Ruiz C, et al. Defective TNF-alpha-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J Clin Invest. 2003;111(2):197–208. doi: 10.1172/JCI16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henkes LE, et al. Acid sphingomyelinase involvement in tumor necrosis factor alpha-regulated vascular and steroid disruption during luteolysis in vivo. Proc Natl Acad Sci U S A. 2008;105(22):7670–5. doi: 10.1073/pnas.0712260105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lozano J, et al. Cell autonomous apoptosis defects in acid sphingomyelinase knockout fibroblasts. J Biol Chem. 2001;276(1):442–8. doi: 10.1074/jbc.M006353200. [DOI] [PubMed] [Google Scholar]
- 112.Garcia-Barros M, et al. Host acid sphingomyelinase regulates microvascular function not tumor immunity. Cancer Res. 2004;64(22):8285–91. doi: 10.1158/0008-5472.CAN-04-2715. [DOI] [PubMed] [Google Scholar]
- 113.Garcia-Barros M, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 114.Smith EL, Schuchman EH. Acid sphingomyelinase overexpression enhances the antineoplastic effects of irradiation in vitro and in vivo. Mol Ther. 2008;16(9):1565–71. doi: 10.1038/mt.2008.145. [DOI] [PubMed] [Google Scholar]
- 115.Sathishkumar S, et al. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther. 2005;4(9):979–86. doi: 10.4161/cbt.4.9.1915. [DOI] [PubMed] [Google Scholar]
- 116.Claus RA, et al. Role of increased sphingomyelinase activity in apoptosis and organ failure of patients with severe sepsis. FASEB J. 2005;19(12):1719–21. doi: 10.1096/fj.04-2842fje. [DOI] [PubMed] [Google Scholar]
- 117.Wong ML, et al. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci U S A. 2000;97(15):8681–6. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grassme H, Riethmuller J, Gulbins E. Biological aspects of ceramide-enriched membrane domains. Prog Lipid Res. 2007;46(3–4):161–70. doi: 10.1016/j.plipres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y, et al. Ceramide-enriched membrane domains-Structure and function. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamem.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 120.Sot J, et al. Detergent-resistant, ceramide-enriched domains in sphingomyelin/ceramide bilayers. Biophys J. 2006;90(3):903–14. doi: 10.1529/biophysj.105.067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holopainen JM, Subramanian M, Kinnunen PK. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry. 1998;37(50):17562–70. doi: 10.1021/bi980915e. [DOI] [PubMed] [Google Scholar]
- 122.Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279(11):9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 123.Staneva G, et al. The role of sphingomyelin in regulating phase coexistence in complex lipid model membranes: Competition between ceramide and cholesterol. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamem.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 124.Rebillard A, et al. Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res. 2007;67(16):7865–74. doi: 10.1158/0008-5472.CAN-07-0353. [DOI] [PubMed] [Google Scholar]
- 125.Goni FM, et al. Biophysics (and sociology) of ceramides. Biochem Soc Symp. 2005;72:177–88. doi: 10.1042/bss0720177. [DOI] [PubMed] [Google Scholar]
- 126.Ira, Johnston LJ. Sphingomyelinase generation of ceramide promotes clustering of nanoscale domains in supported bilayer membranes. Biochim Biophys Acta. 2008;1778(1):185–97. doi: 10.1016/j.bbamem.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 127.Lopez-Montero I, et al. Rapid transbilayer movement of ceramides in phospholipid vesicles and in human erythrocytes. J Biol Chem. 2005;280(27):25811–9. doi: 10.1074/jbc.M412052200. [DOI] [PubMed] [Google Scholar]
- 128.Grassme H, et al. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276(23):20589–96. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 129.Kolesnick RN, Goni FM, Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol. 2000;184(3):285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 130.Grassme H, Schwarz H, Gulbins E. Molecular mechanisms of ceramide-mediated CD95 clustering. Biochem Biophys Res Commun. 2001;284(4):1016–30. doi: 10.1006/bbrc.2001.5045. [DOI] [PubMed] [Google Scholar]
- 131.Cremesti A, et al. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276(26):23954–61. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- 132.Grassme H, et al. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9(3):322–30. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 133.Esen M, et al. Mechanisms of Staphylococcus aureus induced apoptosis of human endothelial cells. Apoptosis. 2001;6(6):431–9. doi: 10.1023/a:1012445925628. [DOI] [PubMed] [Google Scholar]
- 134.Grassme H, et al. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell. 1997;91(5):605–15. doi: 10.1016/s0092-8674(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 135.Charruyer A, et al. UV-C light induces raft-associated acid sphingomyelinase and JNK activation and translocation independently on a nuclear signal. J Biol Chem. 2005;280(19):19196–204. doi: 10.1074/jbc.M412867200. [DOI] [PubMed] [Google Scholar]
- 136.Tabas I. Secretory sphingomyelinase. Chem Phys Lipids. 1999;102(1–2):123–30. doi: 10.1016/s0009-3084(99)00080-8. [DOI] [PubMed] [Google Scholar]
- 137.Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203. doi: 10.1111/j.1600-065X.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 138.Geng YJ, Libby P. Progression of atheroma: a struggle between death and procreation. Arterioscler Thromb Vasc Biol. 2002;22(9):1370–80. doi: 10.1161/01.atv.0000031341.84618.a4. [DOI] [PubMed] [Google Scholar]
- 139.Xu XX, Tabas I. Sphingomyelinase enhances low density lipoprotein uptake and ability to induce cholesteryl ester accumulation in macrophages. J Biol Chem. 1991;266(36):24849–58. [PubMed] [Google Scholar]
- 140.Marathe S, et al. Sphingomyelinase converts lipoproteins from apolipoprotein E knockout mice into potent inducers of macrophage foam cell formation. Arterioscler Thromb Vasc Biol. 2000;20(12):2607–13. doi: 10.1161/01.atv.20.12.2607. [DOI] [PubMed] [Google Scholar]
- 141.Schissel SL, et al. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest. 1996;98(6):1455–64. doi: 10.1172/JCI118934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marathe S, et al. Sphingomyelinase, an enzyme implicated in atherogenesis, is present in atherosclerotic lesions and binds to specific components of the subendothelial extracellular matrix. Arterioscler Thromb Vasc Biol. 1999;19(11):2648–58. doi: 10.1161/01.atv.19.11.2648. [DOI] [PubMed] [Google Scholar]
- 143.Devlin CM, et al. Acid Sphingomyelinase Promotes Lipoprotein Retention Within Early Atheromata and Accelerates Lesion Progression. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nelson JC, et al. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163(10):903–12. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 145.Park TS, et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2004;110(22):3465–71. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 146.Hojjati MR, et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem. 2005;280(11):10284–9. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 147.Park TS, et al. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis. 2006;189(2):264–72. doi: 10.1016/j.atherosclerosis.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 148.Jeong T, et al. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J Clin Invest. 1998;101(4):905–12. doi: 10.1172/JCI870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wasserstein MP, et al. The natural history of type B Niemann-Pick disease: results from a 10-year longitudinal study. Pediatrics. 2004;114(6):e672–7. doi: 10.1542/peds.2004-0887. [DOI] [PubMed] [Google Scholar]
- 150.McGovern MM, et al. Lipid abnormalities in children with types A and B Niemann Pick disease. J Pediatr. 2004;145(1):77–81. doi: 10.1016/j.jpeds.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 151.Lee CY, et al. Compound heterozygosity at the sphingomyelin phosphodiesterase-1 (SMPD1) gene is associated with low HDL cholesterol. Hum Genet. 2003;112(5–6):552–62. doi: 10.1007/s00439-002-0893-1. [DOI] [PubMed] [Google Scholar]
- 152.Gorska M, Baranczuk E, Dobrzyn A. Secretory Zn2+-dependent sphingomyelinase activity in the serum of patients with type 2 diabetes is elevated. Horm Metab Res. 2003;35(8):506–7. doi: 10.1055/s-2003-41810. [DOI] [PubMed] [Google Scholar]
- 153.Doehner W, et al. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J. 2007;28(7):821–8. doi: 10.1093/eurheartj/ehl541. [DOI] [PubMed] [Google Scholar]
- 154.Straczkowski M, et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50(11):2366–73. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- 155.Adams JM, 2nd, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53(1):25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 156.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 157.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14(3–4):222–31. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Levine B, et al. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323(4):236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 159.Dahm F, et al. Distribution and dynamic changes of sphingolipids in blood in response to platelet activation. J Thromb Haemost. 2006;4(12):2704–9. doi: 10.1111/j.1538-7836.2006.02241.x. [DOI] [PubMed] [Google Scholar]
- 160.Igarashi Y, Yatomi Y. Sphingosine 1-phosphate is a blood constituent released from activated platelets, possibly playing a variety of physiological and pathophysiological roles. Acta Biochim Pol. 1998;45(2):299–309. [PubMed] [Google Scholar]
- 161.Yatomi Y, et al. Sphingosine 1-phosphate induces platelet activation through an extracellular action and shares a platelet surface receptor with lysophosphatidic acid. J Biol Chem. 1997;272(8):5291–7. doi: 10.1074/jbc.272.8.5291. [DOI] [PubMed] [Google Scholar]
- 162.Yatomi Y, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997;121(5):969–73. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 163.Yatomi Y, et al. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86(1):193–202. [PubMed] [Google Scholar]
- 164.Hannun YA, Greenberg CS, Bell RM. Sphingosine inhibition of agonist-dependent secretion and activation of human platelets implies that protein kinase C is a necessary and common event of the signal transduction pathways. J Biol Chem. 1987;262(28):13620–6. [PubMed] [Google Scholar]
- 165.Simon CG, Jr, Chatterjee S, Gear AR. Sphingomyelinase activity in human platelets. Thromb Res. 1998;90(4):155–61. doi: 10.1016/s0049-3848(98)00033-4. [DOI] [PubMed] [Google Scholar]
- 166.Romiti E, et al. Characterization of sphingomyelinase activity released by thrombin-stimulated platelets. Mol Cell Biochem. 2000;205(1–2):75–81. doi: 10.1023/a:1007041329052. [DOI] [PubMed] [Google Scholar]
- 167.Simon CG, Jr, Gear AR. Sphingolipid metabolism during human platelet activation. Thromb Res. 1999;94(1):13–23. doi: 10.1016/s0049-3848(98)00186-8. [DOI] [PubMed] [Google Scholar]
- 168.Tani M, et al. Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J Lipid Res. 2005;46(11):2458–67. doi: 10.1194/jlr.M500268-JLR200. [DOI] [PubMed] [Google Scholar]
- 169.Vasta V, et al. Sphingomyelinase increases 2-deoxyglucose uptake and glucose metabolism of human platelets. Biochem Mol Biol Int. 1997;43(1):217–26. doi: 10.1080/15216549700203991. [DOI] [PubMed] [Google Scholar]
- 170.Kitatani K, et al. Acceleration by ceramide of calcium-dependent translocation of phospholipase A2 from cytosol to membranes in platelets. Arch Biochem Biophys. 2000;382(2):296–302. doi: 10.1006/abbi.2000.2028. [DOI] [PubMed] [Google Scholar]
- 171.Foller M, Huber SM, Lang F. IUBMB Life. 2008. Erythrocyte programmed cell death. [DOI] [PubMed] [Google Scholar]
- 172.Lang PA, et al. Stimulation of erythrocyte ceramide formation by platelet-activating factor. J Cell Sci. 2005;118(Pt 6):1233–43. doi: 10.1242/jcs.01730. [DOI] [PubMed] [Google Scholar]
- 173.Nicolay JP, et al. Amyloid induced suicidal erythrocyte death. Cell Physiol Biochem. 2007;19(1–4):175–84. doi: 10.1159/000099205. [DOI] [PubMed] [Google Scholar]
- 174.Kempe DS, et al. Suicidal erythrocyte death in sepsis. J Mol Med. 2007;85(3):273–81. doi: 10.1007/s00109-006-0123-8. [DOI] [PubMed] [Google Scholar]
- 175.McCollister BD, et al. Constitutive acid sphingomyelinase enhances early and late macrophage killing of Salmonella enterica serovar Typhimurium. Infect Immun. 2007;75(11):5346–52. doi: 10.1128/IAI.00689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Takahashi T, et al. Elevated sphingomyelinase and hypercytokinemia in hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol. 2002;24(5):401–4. doi: 10.1097/00043426-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 177.Quintern LE, Zenk TS, Sandhoff K. The urine from patients with peritonitis as a rich source for purifying human acid sphingomyelinase and other lysosomal enzymes. Biochim Biophys Acta. 1989;1003(2):121–4. doi: 10.1016/0005-2760(89)90244-0. [DOI] [PubMed] [Google Scholar]
- 178.Dreschers S, et al. Infections with human rhinovirus induce the formation of distinct functional membrane domains. Cell Physiol Biochem. 2007;20(1–4):241–54. doi: 10.1159/000104170. [DOI] [PubMed] [Google Scholar]
- 179.Grassme H, et al. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem. 2005;280(28):26256–62. doi: 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- 180.Utermohlen O, et al. Fusogenicity of membranes: the impact of acid sphingomyelinase on innate immune responses. Immunobiology. 2008;213(3–4):307–14. doi: 10.1016/j.imbio.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 181.Schramm M, et al. Acid sphingomyelinase is required for efficient phago-lysosomal fusion. Cell Microbiol. 2008 doi: 10.1111/j.1462-5822.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 182.Utermohlen O, et al. Severe impairment in early host defense against Listeria monocytogenes in mice deficient in acid sphingomyelinase. J Immunol. 2003;170(5):2621–8. doi: 10.4049/jimmunol.170.5.2621. [DOI] [PubMed] [Google Scholar]
- 183.Gulbins E, et al. Ceramide, membrane rafts and infections. J Mol Med. 2004;82(6):357–63. doi: 10.1007/s00109-004-0539-y. [DOI] [PubMed] [Google Scholar]
- 184.Grassme H, et al. Ceramide in bacterial infections and cystic fibrosis. Biol Chem. 2008 doi: 10.1515/BC.2008.162. [DOI] [PubMed] [Google Scholar]
- 185.Teichgraber V, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14(4):382–91. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 186.Ramu Y, Xu Y, Lu Z. Inhibition of CFTR Cl− channel function caused by enzymatic hydrolysis of sphingomyelin. Proc Natl Acad Sci U S A. 2007;104(15):6448–53. doi: 10.1073/pnas.0701354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ito Y, et al. Reduction of airway anion secretion via CFTR in sphingomyelin pathway. Biochem Biophys Res Commun. 2004;324(2):901–8. doi: 10.1016/j.bbrc.2004.09.134. [DOI] [PubMed] [Google Scholar]
- 188.Zhang Y, et al. Acid Sphingomyelinase Amplifies Redox Signaling in Pseudomonas aeruginosa-Induced Macrophage Apoptosis. J Immunol. 2008;181(6):4247–54. doi: 10.4049/jimmunol.181.6.4247. [DOI] [PubMed] [Google Scholar]
- 189.White NM, Corey DA, Kelley TJ. Mechanistic similarities between cultured cell models of cystic fibrosis and niemann-pick type C. Am J Respir Cell Mol Biol. 2004;31(5):538–43. doi: 10.1165/rcmb.2004-0117OC. [DOI] [PubMed] [Google Scholar]
- 190.van Blitterswijk WJ, et al. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J. 2003;369(Pt 2):199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]