Abstract
Several studies have demonstrated that polyphenolics from pomegranate (Punica granatum L.) are potent inhibitors of cancer cell proliferation and induce apoptosis, cell cycle arrest, and also decrease inflammation in vitro and vivo. There is growing evidence that botanicals exert their cytotoxic and anti-inflammatory activities, at least in part, by decreasing specificity protein (Sp) transcription factors. These are overexpressed in breast-tumors and regulate genes important for cancer cell survival and inflammation such as the p65 unit of NF-κB. Moreover, previous studies have shown that Pg extracts decrease inflammation in lung cancer cell lines by inhibiting phosphatidylinositol 3,4,5-trisphosphate (PI3K)-dependent phosphorylation of AKT in vitro and inhibiting the activation of NF-kB in vivo. The objective of this study was to investigate the roles of miR-27a-ZBTB10-Sp and miR-155-SHIP-1-PI3K on the anti-inflammatory and cytotoxic activity of pomegranate extract.
Pg extract (2.5–50 µg/ml) inhibited growth of BT-474 and MDA-MB-231 cells but not the non-cancer MCF-10F and MCF-12F cells. Pg extract significantly decreased Sp1, Sp3, and Sp4 as well as miR-27a in BT474 and MDA-MB-231 cells and increased expression of the transcriptional repressor ZBTB10. A significant decrease in Sp proteins and Sp-regulated genes was also observed. Pg extract also induced SHIP-1 expression and this was accompanied by downregulation of miRNA-155 and inhibition of PI3K-dependent phosphorylation of AKT. Similar results were observed in tumors from nude mice bearing BT474 cells as xenografts and treated with Pg extract. The effects of antagomirs and knockdown of SHIP-1 by RNA interference confirmed that the anti-inflammatory and cytotoxic effects of Pg extract were partly due to the disruption of both miR-27a-ZBTB10 and miR-155-SHIP-1.
In summary the anticancer activities of Pg extract in breast cancer cells were due in part to targeting microRNAs155 and 27a. Both pathways play an important role in the proliferative/inflammatory phenotype exhibited by these cell lines
Keywords: Breast Cancer, Polyphenolics, Pomegranate, Xenografts, Inflammation, Cytotoxicity
Introduction
Polyphenols from fruits, vegetables and spices have demonstrated anti-inflammatory and anticarcinogenic activities in vitro and vivo [1–11]. This includes several reports on the cytotoxic activities of polyphenols in the prevention and treatment of breast and other cancer cell lines [2, 7, 11–17]. Pomegranate (Punica granatum L.) is rich in polyphenols. The predominant and therapeutically relevant compounds are ellagic acid, ellagitannins, flavonoids, and 3-glucosides/3,5-diglucosides of the anthocyanins delphinidin, cyanidin, and pelargonidin [18], that exert antioxidant, anti-inflammatory, and anticarcinogenic activities in vitro and vivo [19–21]. Polyphenolics from pomegranate juice and peels inhibited aromatase activity relevant to the prevention of breast cancer [2, 22], exhibited cytotoxic activities in hepatocellular carcinomas in rats [23], and suppressed chemical-induced colon cancer in rats [10, 24]. The inhibition of NF-κB and other inflammatory markers by pomegranate polyphenolics have been reported for breast [2, 5], lung [3, 4] and prostate cancer cell lines [11, 25, 26].
In previous studies, we have demonstrated that the cytotoxicity of several botanicals is due, in part, to downregulation of specificity protein (Sp) transcription factors (Sp1, Sp3, Sp4). These transcription factors are widely overexpressed in tumors and regulate genes required for cell proliferation, survival and angiogenesis [27–33]. Sp-1 is also involved in the regulation of NF-κB through both a GC-rich binding site in the promotor region of the NF-κB p65 subunit and agents such as curcumin that downregulate Sp transcription factors also decreased NF-κB [6].The high expression of Sp1, Sp3 and Sp4 in breast and other cancer cell lines and the effects of botanical drugs on downregulation of Sp transcription factors is due to several pathways, including both proteasome-dependent and independent mechanisms [6, 34]. In breast cancer cells, the high expression of Sp is due, to suppression of ZBTB10, an Sp-repressor by miR-27a. It has been shown that several anticancer agents act through downregulation of miR-27a which is accompanied by induction of ZBTB10 and downregulation of Sp proteins [6, 30–33, 35] and our preliminary screening of miRNAs indicated that miR-27a expression was significantly decreased by pomegranate extract.
Previous studies on the anti-inflammatory properties of pomegranate indicate that pomegranate extract decreased NF-κB [36] [37]. Our preliminary screening of inflammation-involved microRNAs showed that pomegranate extract decreased the expression of miR-155 and this was accompanied by induction of the miR-155-regulated inositol 5'-phosphatase SHIP-1. This phosphatase is a crucial regulator of phosphatidylinositol-3,4,5-trisphosphate (PI3K), a second messenger in the activation of AKT and nuclear translocation of NF-κB [38] [39]. Previous studies reported that Pg extracts decreased inflammation and repressed lung-tumor growth in mice by inhibiting PI3K-dependent phosphorylation of AKT and decreasing the activation of NF-κB in vivo [3, 4]. Moreover, the polyphenols resveratrol and quercetin decreased miRNA-155 and inhibited NF-κB-involved inflammation in a preclinical murine model and in vitro cell study [40–43]. This suggests that pomegranate polyphenolics may act through miR-155, SHIP-1 and AKT associated pathway [44].
Hence, the objective of this study was to investigate the role of miR27a-ZBTB10 and miR-155-SHIP-1 in mediating the anti-inflammatory and cytotoxic effects of pomegranate polyphenolics.
Materials and methods
Botanical extracts
Stiebs Pomegranate Products (Madera, CA) provided the pomegranate juice concentrate (Pg) utilized in this study from the 2009 California crop (Sample # 0622-33808). Pg was stored at 4°C upon receipt and isolated within 48 h. Polyphenols from Pg were diluted with water to facilitate loading and partitioning from a Sep Pack Vac 20 g C18 cartridge (Waters Corp. Milford, MA) previously conditioned with 100% methanol containing 0.01% HCl. Compounds were eluted with 100% methanol and solvent removed by rotary evaporation (Büchi Labortechnik AG, Flawil, Switzerland) at 40°C. Residual water was removed on a speedvac system (Savant, Thermo Scientific Inc, Pittsburgh, PA). The dried extract stored at −80°C prior to weighing and dissolution in dimethyl sulfoxide (DMSO) for cell culture and analytical procedures.
HPLC-PDA Analysis
The pomegranate polyphenolics were analyzed in negative ESI-MS and quantified against a standard of punicalagins A and B and cyanidin-3-gluoside. Separations were made on a SunFireTM C18 column (Supelco Inc. Bellefonte, PA) (250 × 4.8 mm, 5 µm) at room temperature. A mobile phase of water/acetic acid was run (98:2) in Phase A and acetonitrile/water/acetic acid was run (68:30:2) in Phase B. A gradient program at 0.4 mL/min initially ran Phase B at 0%, from 0 to 5% Phase B in 1 min, from 5 to 30% Phase B in 15 min, from 30 to 65% Phase B in 40 min, and 65 to 95% Phase B in 50 min before returning to initial conditions. Detection was at 280 and 520 nm for ellagitannins and anthocyanins, respectively. Compounds were tentatively identified based on mass spectrometric analysis. This was performed on a Thermo Finnigan LCQ Deca XP Max ion trap mass spectrometer with an electrospray ionization probe in negative ion mode under the following conditions: sheath gas (N2), 60 units/min; auxiliary gas (N2), 5 units/min; spray voltage, 3.3 kV; capillary temperature, 250°C; capillary voltage, 1.5 V; tube lens offset, 0 V. Standard compounds for the identification and quantitative analysis of ellagitannins and anthocyanins were obtained from Acros Organics (Morris Plains, NJ.) and ChromaDex (Irvine, CA), respectively.
Reagents
Antibodies against cleaved caspase-3, poly-(ADP-ribose)-polymerase (PARP), NF-κB (p65), phosphorylated NF-κB (p65) as well as SHIP-1 were purchased from Cell Signaling Technology (Beverly, MA). All other antibodies and SHIP-1 small interfering RNA (SiRNA) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Reporter lysis buffer and luciferase reagent for luciferase studies were obtained from Promega (Madison, WI). Invitrogen (Grand Island, NY) supplied LipofectAMINE 2000 reagent. Western lighting chemiluminescence reagent was purchased from Perkin-Elmer Life Sciences (Waltham, MA). Primers for Sp1, VEGF, VEGFR-1, survivin, ZBTB10, SHIP-1 were purchased from Integrated DNA Technologies (San Diego, CA). Primers for Sp3 and Sp4 were obtained from Qiagen; antagomers of miR-27a (inhibitor) and miR155 (inhibitor), as well as scrambled miRNA were from Dharmacon, Inc. (Lafayette, CO). mirVana TM extraction kit, reverse transcription (RT) and real-time PCR amplification kits were purchased from Applied Biosciences (Foster City, CA). Sp1, Sp3 and NF-κB promoter constructs were kindly provided by Dr. Yanan Tian (Texas A&M University).
Cell culture
Human mammary carcinoma cell lines BT474 and MDA-MB-231 as well as non-cancer breast fibroblasts MCF-10F and MCF-12F, were obtained from ATCC and maintained according to the suppliers guidelines (American Type Tissue Collection (ATCC, Manassas, VA).
Cell proliferation
The cell proliferation was assessed with an electronic cell counter at 48 h (Z1™ Series, Beckman Coulter, Inc, Fullerton, CA), as previously described, [31] and are presented as net growth.
Real-Time PCR analysis of mRNA and miRNA
BT474 and MDA-MB-231 cells were seeded (3 × 105 cells onto a 6-well plate) and incubated for 24h to allow cell attachment. Cells were treated with Pg extract and after 24 h mRNA was extracted for gene expression analysis as previously described [31]. RNA-quality and quantity was assessed using the NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Reverse transcription (Invitrogen Corp., Grand Island, NY) and qRT-PCR were carried out as previously described [31] on an ABI Prism 7900 Sequence Detection System (Applied Biosystems Inc, Foster City, CA). Primers for TBP, ZBTB10, Sp1, Survivin, VEGF and VEGFR-1 have been previously described [31]. The primer-sequences for SHIP-1were F:5’-GTG-GAG-AGA-TCT-GGC-CTC-AG-3’ and R:5’-GGG-AGC-AAC-AGC-AAA-GAC-TC-3’, for VEGF, the sequence were F:5’-AAG GAG GAG GGC AGA ATC AT-3’, and R:5’-ATC TGC ATG GTG ATG TTG GA-3’. Integrated DNA Technologies, Inc. (San Diego, CA). Primers were designed using Primer Express software (Applied Biosystems, Foster City, Ca), homology-searched by an NCBI BLAST and examined by dissociation curve analysis. Primers for Sp3 and Sp4 were purchased from Qiagen. microRNA was extracted, reverse transcribed, and qRT-PCR reaction was performed as previously performed [30, 31].
Transfection with antagomers of miR-155 and miR-27a (inhibitors), miR-27a mimic, or small interfering RNA (siRNA) against SHIP-1 gene
Cells seeded (1×105) into 12-well plates were incubated for 24h to allow cell attachment. Transfections with 10nM siRNA against SHIP-1 gene Santa Cruz Biotechnology Inc. (Santa Cruz, CA) and antagomers (inhibitors) of 20 nM, 40 nM and 80 nM miR-27a and miR-155, and also mimic of 20 nM miR-27a (Dharmacon, Lafay ette, CO) were performed as previously described [6, 31]
Reporter gene transfection and luciferase assays
Cells were transfected with constructs as previously described [6, 9, 31].
Western blotting
Cells (4 × 105) were seeded in 6-well plates and incubated 24 h to allow cell attachment. They were also treated with Pg extract (0–10µg/mL) for 24 h. Cells were harvested and prepared for Western Blotting as previously performed [30, 31]. Proteins were visualized with a Kodak Molecular Imaging System (Carestream Health, Rochester, NY).
Xenograft study
Female athymic BALB/c nude mice (age 3–4 weeks) from Harlan laboratory (Houston, TX) were implanted with BT474 cells (2×106 cells) in matrigel (BD Bioscience San Jose, CA) s.c into the flank [31]. After 10 days mice were treated with either 100 µl vehicle (1% DMSO in corn oil) by oral gavage, or 0.8mg gallic acid equivalent (GAE)/kg/day of Pg extract/d for 35 days after approval by the Institutional Animal Care and Use Committee at Texas A&M University (College Station, TX). Final body and tumor weights were determined. Tissues were flash-frozen in liquid nitrogen and stored at −80°C for mRNA and protein analysis. 4-AM-thick paraffin-embedded tumor sections were cut and stained with Hematoxylin and Eosin (H&E) for bright field microscopy.
Statistical analysis
Quantitative data represent mean values with the respective standard deviation (SD) or standard error of the mean (SE) corresponding to 3 or more replicates. Data were analyzed by one-way analysis of variance (ANOVA) using SAS version 9.3 (SAS institute Inc., Cary, NC). Tukey’s Post Hoc multiple comparisons were used (p<0.05) to establish significant statistical difference.
Results
Polyphenolic Composition of Pomegranate Extract Determined by HPLC-MS
The polyphenolic profile of pomegranate varities was previously reported as being rich in ellagitannins and anthocyanins [19, 20, 25, 45]. The primary ellagitannin in pomegranate juice is punicalagin [45], that can be converted into free ellagic acid upon hydrolysis. In this study, a polyphenolic extract was prepared from a pomegranate juice extract (Pg) that was kindly provided by Stiebs LLC (Kirkland, WA). The chromatographic profiles (Fig. 1a and 1b) show the ellagitannins punicalagin A (peak 1), punicalagin B (peak 2), anthocyanins delphinidin 3-glucoside (peak 3) and cyanidin-3-glucoside (peak 4). Low concentrations of ellagic acid glucoside (peak 5) and free ellagic acid (peak 6) were also detected.
Fig. 1.
Representative chromatogram of polyphenolic compounds in an extract of pomegranate juice concentrate (a) Ellagitannins at 280 nm, tentative peak assignments: 1, Punicalagin A; 2, Punicalagin B; 5, Ellagic acid glucoside and 6, Ellagic acid; (b) Anthocyanins at 520 nm, tentative peak assignments: 3, Delphinidin-3-glucoside; 4, Cyanidin-3-glucoside.
Anti-proliferative and Pro-apoptotic Activities of Pomegranate Extract
The cytotoxic activities of Pg were investigated in human BT474 and MDA-MB-231 breast cancer cell-lines and non-cancer breast fibroblasts MCF-10F and MCF-12F (Fig. 2a). In both cancer cell lines, there was a concentration-dependent decrease in cell viability after treatment with Pg (2.5–25 µg/ml) after 48 h. In contrast, no significant cytotoxic effects were observed in non-transformed cells treated under the same conditions (Fig. 2a). Similar results were observed after 72 h (data not shown). Pg-induced cytotoxicity was accompanied by activation of capase-3, a primary apoptosis-executing enzyme, and the cleaved product of the substrate Poly (ADP-ribose)-polymerase1 (PARP) [46]. Full lengths PARP protein was not decreased by Pg whereas the highest concentration of Pg decreased full length Caspase-3 (Fig. 2b).
Fig. 2.
Pomegranate extract (Pg) inhibited proliferation and induced apoptosis in cancer cells. (a) Cells were seeded and treated with DMSO (vehicle control) or different concentrations of Pg (2.5–10 µg/ml treatement) for 48 h. (b) Western blot analysis of apoptosis-associated proteins (Full length capase-3 and cleaved capase-3; full length PARP and cleaved PARP). BT474 cells were treated with DMSO (control vehicle) or different concentrations of Pg extract 2.5–10 µg/ml for 24 h, and whole-cell lysates were analyzed by western blot analysis. All experiments were performed as described in the material and method section and were performed at least three times, and result were expressed as mean ± SE.* indicates significant changes at p<0.05.
Modulation of Sp transcription factors and Sp-regulated genes and disruption of miR-27a:ZBTB10
Treatment of BT474 breast cancer cells with Pg (2.5–10 µg/ml) decreased expression of Sp1, Sp3, and Sp4 mRNA (Fig. 3a) and protein (Fig. 3b). Pg also decreased luciferase-activity in BT474 cells transfected with a plasmid construct containing the GC-rich regions from the Sp1 and Sp3 promoters [31] (Fig. 3c). Additionally, Pg induced expression of the Sp-repressor ZBTB10 (Fig. 3d) and decreased VEGF, VEGFR-1 and survivin m RNA and protein (Fig. 3e) and (Fig. 3f). [31].
Fig. 3.
Effects of Pg on Sp1, Sp3, Sp4 and Sp-regulated genes in BT474 cells. Pg extract decreased Sp1, Sp3 and Sp4, and Sp-regulated genes and induced apoptosis. Cells were treated with solvent DMSO (control vehicle) or different concentration of Pg (2.5–10 µg/ml) for 24h. (a) Expression of mRNA and (b) protein of Sp1, Sp3 and Sp4. (c) Luciferase activity of Sp1 and Sp3 promoter constructs transfected into BT474 cells. Data represent ratios of luciferase/β-gal activity in transfected cells treated with DMSO (control) or Pg (2.5–10µg/ml) for 24h. (d) mRNA levels of ZBTB10 of cells treated with DMSO (control) or Pg (2.5–10µg /ml) for 24h. (e) Survivin, VEGF and VEGFR-1 mRNA and (f) protein levels of cells treated with DMSO (control) or Pg (2.5–10µg /ml) for 24h. (g) miR-27a and (h) miR-27a in different breast cancer and non40 cancer breast fibroblasts (i) Effects of Pg with and without the antagomir (Ant.) for miR-27a on the expression of miR-27a, (j) on the expression of ZBTB10 mRNA and (k) Effects of Pg with and without the mimic for miR-27a on the expression of miR-27a. All experiments were performed as described in the material and method section and were performed at least three times, and result were expressed as mean ± SE.* indicates significant changes at p<0.05.
The basal expression of miR27a was higher in BT474 and MDA-MB-231 breast cancer cells compared to the non-cancer breast fibroblasts (Fig. 3g). Pg (2.5–10 µg/ml) significantly decreased miR-27a expression only in the cancer cell lines in a concentration-dependent manner, in the non-cancer fibroblasts minimal effects on miR-27a were observed (Fig. 3h). When BT474 cells were transfected with the antagomir (Ant.) of miR-27a, miR-27a was decreased and ZBTB10 mRNA was induced and this was comparable to the effects of Pg extract (Fig. 3d and 3h) Both the miR-27a antagomir and Pg extract alone and in combination decrease miR-27a (Fig. 3i) and induce ZBTB10 (Fig. 3j). Transfected of BT474 cells with miR27a mimic increased miR-27a level and this was partially reversed by the treatment with Pg, indicating that Pg directly or indirectly targets miR27a (Fig. 3k). We also observed the Pg extract decreased Sp1, NF-κB (p65) and VEGF protein (Fig. 4a) and mRNA (Fig. 4b) expression in MDA-MB-231 cells and this was accompanied by induction of ZBTB10 mRNA (Fig. 4c) and this is similar to the effects of Pg extract in BT474 cells.
Fig. 4.
Effects of Pg on Sp1 and Sp-regulated genes in MDA-MB-231 cells. Pg extract decreased Sp1, and Sp-regulated genes and induced apoptosis. Cells were treated with solvent DMSO (control vehicle) or different concentration of Pg (2.5–10 µg/ml) for 24h. (a) Expression of protein and (b)mRNA of Sp1, NF-κB and VEGF (c) mRNA levels of ZBTB10 in cells treated with DMSO (control) or Pg (2.5–10µg /ml) for 24h. All experiments were performed as described in the material and method section and were performed at least three times, and result were expressed as mean ± SE.* indicates significant changes at p<0.05.
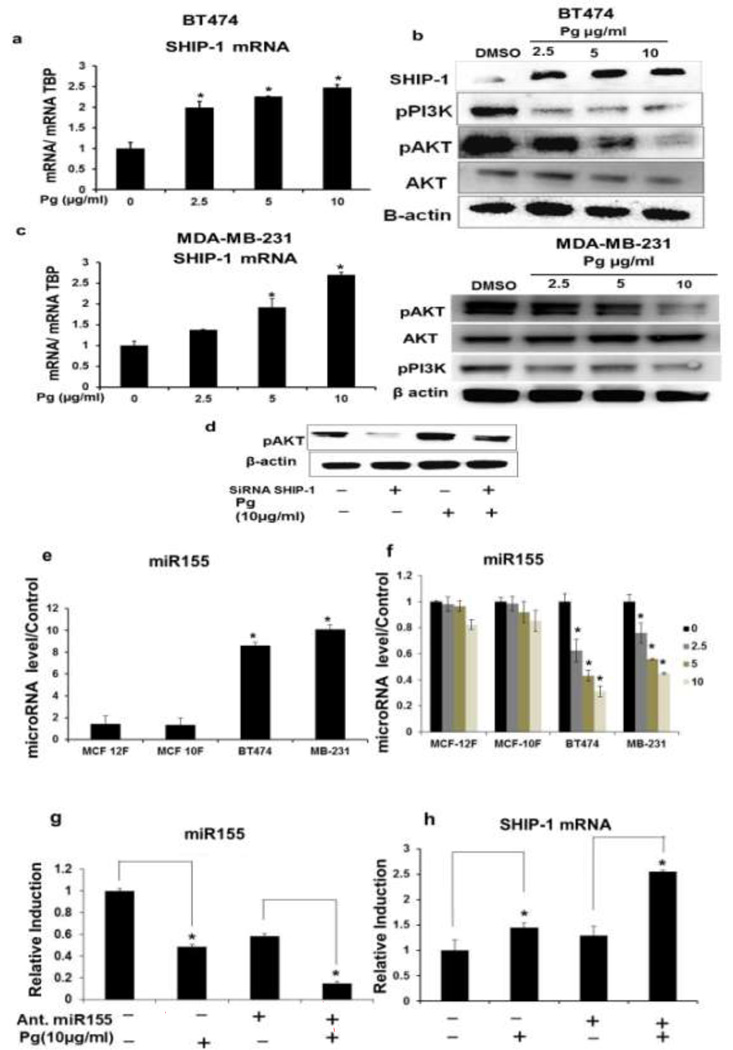
Pg-induced Modulation of SHIP-1, PI3K and AKT and Involvement of SHIP-1 and miR-155 in the Modulation of AKT and NF-κB
Pg also induced the upregulation of SHIP-1 mRNA and protein in BT474 (Fig. 5a, 5b) and this was accompanied by a decrease in pPI3K and pAKT while but not AKT (total protein) (Fig. 5b). Pg extract also caused similar effects on SHIP-1, pAKT, pPI3K and AKT in MDA-MB-231 breast cancer cells (Fig. 5c). To better understand the effects of Pg on SHIP-1, PI3K and AKT, BT474 cells were transfected with the siRNA of SHIP-1. SiRNA SHIP-1 increased pAKT protein levels. In contrast, Pg decreased pAKT protein expression (Fig. 5b) and these effects were partially reversed by siRNA SHIP-1 (Fig. 5d). MiR-155 is overexpressed in several breast cancer cell lines [47–50]. It was previously reported that polyphenolic extracts decrease miR-155 [40, 41, 51]. Basal expression of miRNA-155 was elevated in BT474 and MDA-MB-231 cells compared to the non-cancer cell lines (Fig. 5e). Pg (2.5–10 µg/ml) significantly decreased miR-155 (Fig. 5f) in the cancer cell lines (compared MCF-10F and MCF-12F) and this correlates with the upregulation of SHIP-1 mRNA levels in BT474 and MDA-MB-231 cells (Fig. 5a and 5c). Transfection of BT474 cells with miR-155 antagomir of treatment with 10 µg/ml Pg alone or in combination decreased miR-155 expression (Fig. 5g) and increased SHIP-1 mRNA level (Fig. 5h). Further confirming that Pg extract disrupts miR155: SHIP-1 interaction in BT474 cells.
Fig. 5.
Effects of Pg on AKT-associated kinase pathways in BT474 and MDA-MB-231 cells. (a) Effects of Pg on SHIP-1 mRNA levels and (b) SHIP-1, pP13K and pAKT protein expression in BT474 cells. Cells were treated with solvent DMSO (control) or different concentration of Pg (2.5–10 µg/ml) for 24 h (c) SHIP-1 mRNA levels and protein expression of SHIP-1, pP13K and pAKT in MDA-MB-231 cells. Cells were treated with solvent DMSO (control) or different concentration of Pg (2.5–10 µg/ml) for 24 h (d) Effect of SiRNA SHIP-1 with and without Pg on pAKT protein (e) Basal levels of miR-155 in different breast cancer and non-cancer breast fibroblasts. (f) Effects of Pg on miR-155 levels in different breast cancer and non-cancer breast fibroblasts. (g) Effects of Pg with and without Ant. miR155 in BT474 cells on the expression of miR155 and (H) SHIP-1 mRNA levels. All experiments were performed as described in the material and method section and were performed at least three times, and result were expressed as mean ± SE.* indicates significant changes at p<0.05.
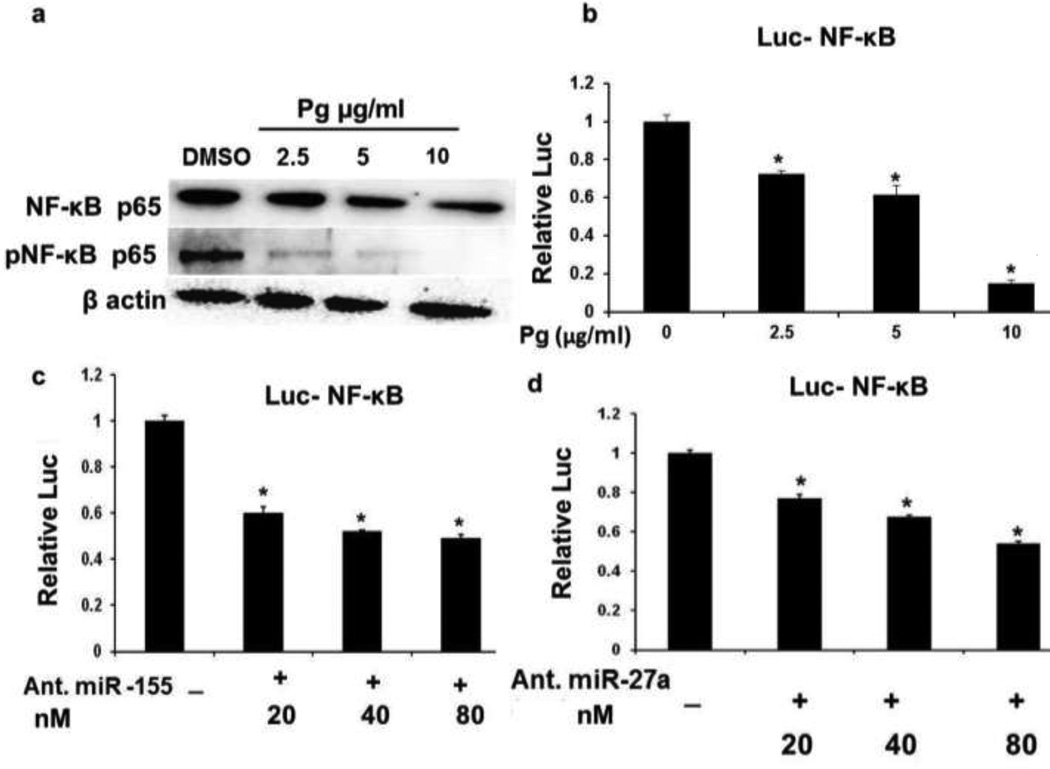
Effects of Pg on NF-κB
Pg significantly inhibited the constitutive expression and phosphorylation of NF-κB p65 in BT474 cells (Fig. 6a) and in MDA-MB-231 cells was also decreased by Pg (Fig. 4a) Furthermore, Pg extract decreased luciferase activity in BT474 cells transfected with pNF-κB-Luc (Fig. 6b) and similar results were observed in cell co transfected with miR-155 (Fig. 6c) or miR-27a (Fig 6d) antagomirs.. These results suggest that both microRNAs are involved in Pg-induced repression of NF-κB.
Fig. 6.
NF-κB expression and activity in BT474 cells. (a) Effect of Pg on NF-κB and pNF-κB protein levels and (b) luciferase activity in a plasmid containing a NF-κB binding site in the luc-promoter region. Cells were treated with solvent DMSO (control) or different concentration of Pg (2.5–10 µg/ml) for 24h. Effects of (c) miR-155 antagomers (Ant.) and (d) miR-27a antagomers (Ant.) on luciferase activity of the NF-κB promoter construct. Luciferase activity is expressed as ratio to β-gal. All experiments were performed as described in the material and method section and were performed at least three times, and result were expressed as mean ± SE.* indicates significant changes at p<0.05.
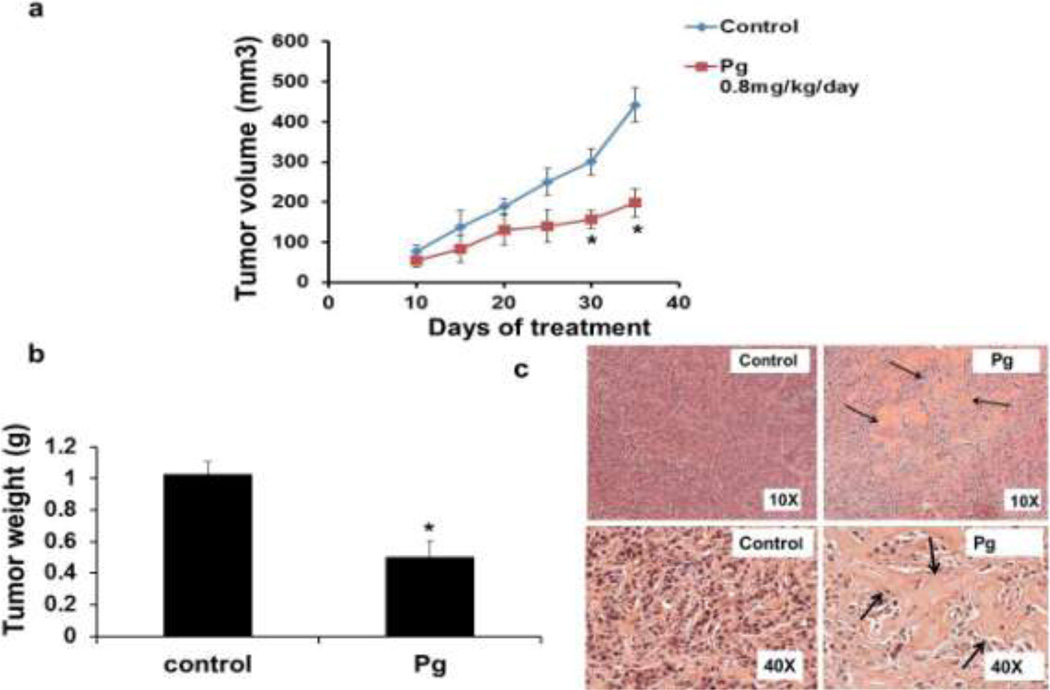
Xenograft Study in Athymic Female Nude Mice
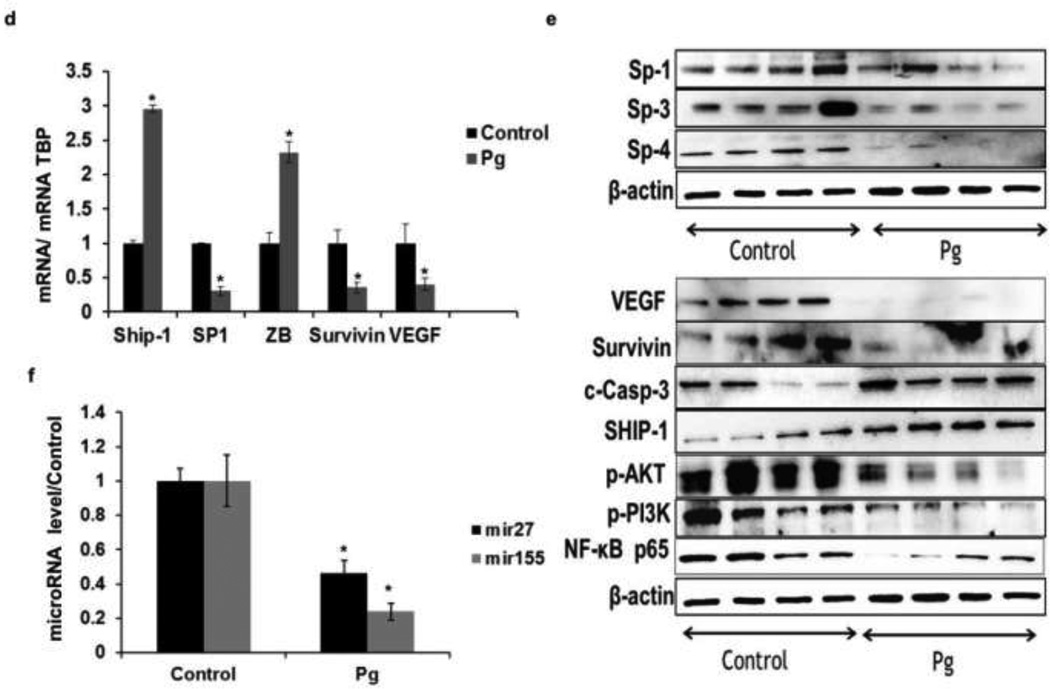
The clinical relevance of the cytotoxic activities of Pg observed in vitro was investigated in an orthotopic model of breast cancer in athymic female nude mice with BT474 cells as xenografts. Treatment with Pg (0.8mg GAE/kg/day/) significantly decreased tumor volume and weight (Fig. 7a and 7b). Histopathologic evaluations of tumors indicated that tumors of Pg-treated animals exhibited with increased apoptotic lesions compared to the control group (Fig. 7c). Moreover, Pg decreased the expression of Sp1, mRNA and Sp1, Sp3, and Sp4 protein level (Fig. 7d and 7e) and significantly decreased miR-27a (Fig. 7f) whereas ZBTB10 mRNA was upregulated (Fig. 7d). In addition, Sp-regulated genes including VEGF, survivin and NFkB p65 were also decreased at the protein and mRNA level in tumors from mice treated with Pg compared to the control tumors (Fig. 7d and Fig. 7e).In addition SHIP-1 mRNA (Fig. 7d) and protein (Fig. 7e) levels were induced in tumors. This was accompanied by a reduction of SHIP-1-regulated proteins pAKT and pPI3K in mice exposed to Pg (Fig. 7e) and miR-155 (Fig. 7f) was also decreased.
Fig. 7.
Antitumorigenic activity of Pg extract in vivo. (a) In vivo studies athymic nude mice (10 per group) bearing BT474 cells as xenografts were treated with corn oil (control) or Pg extract in corn oil (0.8 mg Gallic Acid Equivalent (GAE)/kg/day) every day as described in the Materials and Methods reduced the tumor size, tumor area (b) and tumor weights (c) were determined as described in material and method section. (d) Histopathologic evaluation of tumors. Tumor from corn oil (control) and Pg extract treated mice were fixed, stained with hematoxylin and eosin (H&E) and examined histopathologically as described in the material and methods section. Pg extract decreases Sp-regulated genes and induces apoptosis in tumors. (e) Effect of Pg on SHIP-1, Sp1, survivin, VEGF and ZBTB10 mRNA levels. (f) Effect of Pg on Sp1, Sp3 and Sp4, VEGF survivin (Sp-dependent genes) and SHIP-1, pAKT, pPI3K and NF-kB protein levels. (g) In-vivo results showed miR-27a and miR-155 expression were significantly reduced in dosed (Pg treated) mice than the control. All experiments were performed as described in the material and method section and were performed at least three times, and result were expressed as mean ± SE.* indicates significant changes at p<0.05.
Discussion
This study has investigated the cytotoxic and anti-inflammatory activities of a pomegranate extract in breast cancer cells and in an animal model [2, 10, 22]. Pg decreased breast cancer cell growth, and tumor volume and activated caspase-3 (Fig. 2a, 7a and 2b). Polyphenolic pomegranate have previously been shown to induce apoptosis in cancer cell lines including breast, prostrate, liver, lung and skin cancer involving multiple pathways and inducing apoptosis involving caspase-3 [2–4, 23, 46, 52]. Previous studies have observed resistance of non-cancer cells to the effects of polyphenolics observed in this study (Fig 2a) has been previously reported with delphinidin (anthocyanin) from pomegranate extracts [2, 53, 54]. The effects of Pg extract on inhibition of tumor growth in anthymic nude mice bearing BT474 cells as xenografts (Fig. 7a) has also been observed in nude mice bearing prostrate, pancreatic and breast cancer cells [3, 6, 8, 31, 32].
Sp transcription factors (Sp1, Sp3, Sp4) that are overexpressed in multiple tumors and regulate genes required for cell survival and angiogenesis and it has been reported that suppression of Sp transcription factors results in growth inhibition and apoptotic cell death [6, 30, 32]. Overexpression of Sp in cancer cell lines and tumor is usually accompanied by upregulation of genes involved in cell survival, growth promotion and angiogenesis [30, 31]. In this study, Pg extract decreased Sp1, Sp3, and Sp4 mRNA (Fig. 3a) as well as protein (Fig. 3b) levels and also decreased Sp-regulated genes involved in cell proliferation, angiogenesis and inflammation, namely cyclin D1, bcl2, survivin VEGF and its receptor (VEGFR-1) and NF-κB (Fig. 3e and 3f). These results are consistent with previous reports, where secondary plant compounds, such as curcumin and a pentacyclic triterpene betulinic acid, decreased Sp1, Sp3 and Sp4 and Sp-dependent genes [6, 30–32]. We previously investigated the regulation of Sp transcription factors by microRNA-27a, that is also overexpressed in breast cancer and other cancer cell lines [30, 31, 33, 55, 56]. A specific antagomir for miR-27a (as-miR-27a) increased the expression of ZBTB10, a zinc finger protein [30, 31, 33] that in turn suppresses Sp and Sp-dependent mRNA/protein expression [30, 31]. The regulation of Sp and Sp-dependent genes through the miR27a-ZBTB10-Sp-axis by both botanical compounds and synthetic derivatives, has been shown in cancer cells and tumors derived from multiple sites [9, 31–33, 56, 57]. Pg also significantly decreased miR-27a in breast cancer cells, but not in non-transformed cell lines (Fig. 3g) and upregulated ZBTB10 mRNA expression (Fig. 3d and 4c) in BT474 and MDA-MB-231 cells. When cells were transfected with the antagomir of miR-27a, the expression of miR-27a was decreased and mRNA of ZBTB10 increased (Fig. 3i and 3j) where Pg further enhanced as-miR27a-induced effects. This indicates that miR-27a-ZBTB10-Sp is involved in the Pg-induced downregulation of NF-kB and genes involved in survival and angiogenesis. Moreover, when cells were transfected with the mimic of miR-27a, the effects of Pg, were reversed by the mimic (Fig. 3k) indicating that Pg either directly or indirectly targets miR-27a. Previous reports indicate that botanicals decreased miR-27a expression however, this has not been confirmed for a complex polyphenolic extract [31] [33, 57]. Results observed in athymic female nude mice study correspond to the in vitro studies where miR-27a was decreased and ZBTB10 was increased by the treatment of mice with Pg (Fig. 7d and Fig 7f).
Pg extracts decreased luciferase-activity in BT474 cells transfected with pNF-κB-Luc indicating a decrease of overall NF-kB activity (Fig. 6b) and a miR-27a, antagomir also decreased luciferase activity in BT474 cells transfected with pNF-κB-luc (Fig. 6d), confirming a role of this microRNA in Pg-induced suppression of NF-kB activity. In vivo, the expression of Sp transcription factors Sp1, Sp3 and Sp4 and Sp-regulated genes survivin and VEGF (Fig. 7d and 7e) were significantly decreased in Pg-treated animals and this was accompanied by a decreased expression of miR-27a (Fig. 7f). Our previous studies with betulinic acid showed a significant decrease in tumor size in a model of ER-negative breast cancer in athymic female nude mice xenografted with MDA-MB-231 cells, where also miR-27a-ZBTB10-Sp was involved in the underlying mechanism [31]. This further confirms the role of miR-27a in the cytotoxicity of Pg in this study.
Several studies show that NF-kB –activity, at least in part, is mediated through AKT, a kinase that in part is regulated through PI3K a key regulator in cell survival and cell function [3, 4]. Inositol 5'-phosphatase (SHIP-1) a 145-kDa protein that contains a Src homology 2 domain is a regulator of phosphatidylinositol 3,4,5-trisphosphate (PI3K) [58–60]. SHIP-1 has been identified as tumor suppressor in hematopoietic cancer [61–63] but the role of SHIP-1 in solid tumors has not been thoroughly investigated. In this study, Pg induced activation of NF-kB by inhibiting the phosphorylation of p65 (Fig. 6a) as well as the phosphorylation of PI3K/p85, and AKT (Ser473) (Fig. 5b, and Fig. 5c). These findings are in consistent with previous studies where it was demonstrated that pomegranate extracts decreased inflammation and repressed lung tumors in mice and also decreased PI3K-dependent phosphorylation of AKT and phosphorylation and activation of NF-κB were decreased [3, 4]. Based on these reports one of the objectives of this research was to determine whether the inhibition of NF-kB activity by Pg, was due, in part by SHIP-1-PIP3-AKT-NF-kB interactions. Pg extract increased expression of SHIP-1 protein which was accompanied by down-regulation of miRNA 155 (Fig. 5a, Fig. 5b and Fig. 5e). SHIP-1 is regulated by miR-155 via a target-binding site in the 3’ UTR region of the SHIP-1 mRNA [51]. Previously, the polyphenolics resveratrol and quercetin decreased miR155 levels in THP-1 monocytic cells (36). In this study Ant-miR-155 and Pg decreased miR-155 and increased SHIP-1 mRNA (Fig. 5g and 5h) [44, 51] and si-SHIP-1 increased the phosphorylation of AKT, while Pg (and also as-miR-155) partially reversed this effect (Fig. 5b and 5d). Ant-miR-155 also decreased the activity of NF-kB in a concentration-dependent manner in cells transfected with pNF-κB-luc (Fig. 6c) and in vivo, Pg extract decreased the expression of miR155 (Fig. 7f). Pg increased both, SHIP-1 mRNA and protein expression (Fig. 7d and 7e) where the phosphorylation of AKT and PI3K protein expression compared to tumors from control animals (Fig. 7e). Based on result of our mimic and inhibitor studies and on previous reports, we conclude both miR-27a and miR-155 are targets of Pg and their downregulation plays a significant role in the anti-inflammatory and cytotoxic efficacy of pomegranate extract and possibly other botanicals.
Conclusion
In summary, pomegranate extract exhibited cytotoxic and anti-inflammatory activities in breast cancer cells in vitro and in vivo. In addition there is evidence that these activities are significantly mediated in part through the effects of pomegranate extract n miR-27a-ZBTB10-Sp and miR-155-SHIP1-PIP3-AKT-NF-kB interactions.
Acknowledgement
We would like to thank Dr. Weston Porter Department Veterinary Integrated Bioscience, at Texas A&M University, College Station, and Texas for providing imaging equipment. Lastly we would like to thank Stefan Wypyszyk at Stiebs LLC (Kirkland, WA) for kindly supplying the pomegranate juice. Financial support for this research has been provided by the National Institutes of Health (KOIATOO 4597 to SMT).
Footnotes
Conflict of interest. The authors have no conflicts of interest to declare.
References
- 1.Prakobwong SGSC, Kim JK, Sung B, Pinlaor P, Hiraku Y, Wongkham S, Sripa B, Pinlaor S, B.B A. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis. 2011;32:1372–1380. doi: 10.1093/carcin/bgr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim NDMR, Yu W, Neeman I, Livney T, Amichay A, Poirier D, Nicholls P, Kirby A, Jiang W, Mansel R, Ramachandran C, Rabi T, Kaplan B, Lansky E. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res and Treat. 2002;71:203–217. doi: 10.1023/a:1014405730585. [DOI] [PubMed] [Google Scholar]
- 3.Khan NAF, Kweon MH, Kim K, Mukhtar H. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007;67:3475–3482. doi: 10.1158/0008-5472.CAN-06-3941. [DOI] [PubMed] [Google Scholar]
- 4.Khan NHN, Afaq F, Syed DN, Kweon MH, Mukhtar H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;1:163–173. doi: 10.1093/carcin/bgl145. [DOI] [PubMed] [Google Scholar]
- 5.Khan GNGMA, Rosenthal D, Pan Q, Bao LW, Wu ZF, Newman RA, Pawlus AD, Yang P, Lansky EP, Merajver SD. Pomegranate Fruit Extract Impairs Invasion and Motility in Human Breast Cancer. Integr Cancer Ther. 2009;8:242–253. doi: 10.1177/1534735409341405. [DOI] [PubMed] [Google Scholar]
- 6.Jutooru ICG, Lei P, Safe S. Inhibition of NFkB and Pancreatic Cancer Cell and Tumor Growth by Curcumin Is Dependent on Specificity Protein Down-regulation. J Biol Chem. 2010;285:25332–25344. doi: 10.1074/jbc.M109.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams LSZY, Seeram NP, Heber D, Chen S. Pomegranate Ellagitannin-Derived Compounds Exhibit Antiproliferative and Anti-aromatase Activity in Breast Cancer Cells In Vitro. Cancer Prev Res (Phila) 2010;3:108–113. doi: 10.1158/1940-6207.CAPR-08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakimuddina FTK, Paliyathb G, Meckling K. Grape and wine polyphenols down-regulate the expression of signal transduction genes and inhibit the growth of estrogen receptor–negative MDA-MB231 tumors in nu/nu mouse xenografts. 2008 doi: 10.1016/j.nutres.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R, 3rd, Li X, Safe S. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–5354. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adhami VMKN, Mukhtar H. Cancer Chemoprevention by Pomegranate: Laboratory and Clinical Evidence. Nutr Cancer. 2009;61:811–815. doi: 10.1080/01635580903285064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhami VMSI, Syed DN, Lall RK, Mukhtar H. Oral infusion of pomegranate fruit extract inhibits prostate carcinogenesis in the TRAMP model. Carcinogenesis. 2012;33:644–651. doi: 10.1093/carcin/bgr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 14.Kang NJ, Shin SH, Lee HJ, Lee KW. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol Ther. 130:310–324. doi: 10.1016/j.pharmthera.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Noratto GD, Kim Y, Talcott ST, Mertens-Talcott SU. Flavonol-rich fractions of yaupon holly leaves (Ilex vomitoria, Aquifoliaceae) induce microRNA-146a and have anti-inflammatory and chemopreventive effects in intestinal myofibroblast CCD-18Co cells. Fitoterapia. 2011;82:557–569. doi: 10.1016/j.fitote.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee SKS, Banerjee SK, Haque I. Pomegranate sensitizes Tamoxifen action in ER-α positive breast cancer cells. J Cell Commun Signal. 2011 doi: 10.1007/s12079-011-0138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossmann MKMNK, Schuster T, Cleary MP. Punicic acid is an ˆ-5 fatty acid capableof inhibiting breast cancer proliferation. INTERNATIONAL JOURNAL OF ONCOLOGY. 2010;36:421–426. [PubMed] [Google Scholar]
- 18.Jurenka J. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- 19.Afaq FSM, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and Hydrolyzable Tannin-Rich Pomegranate Fruit Extract Modulates MAPK and NF-kB Pathways and Inhibits Skin Tumorigenesis in CD-1 Mice. Int J Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 20.Seeram NPAL, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Kawaii S, Lansky EP. Differentiation-promoting activity of pomegranate (Punica granatum) fruit extracts in HL-60 human promyelocytic leukemia cells. J Med Food. 2004;7:13–18. doi: 10.1089/109662004322984644. [DOI] [PubMed] [Google Scholar]
- 22.Sreejaa SKTRS, Lakshmib BS, Sreeja S. Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation. Journal of Nutritional Biochemistry. 2011 doi: 10.1016/j.jnutbio.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Bishayee ABD, Thoppil RJ, Darvesh AS, Nevo E, Lansky EP. Pomegranate-mediated chemoprevention of experimental hepatocarcinogenesis involves Nrf2-regulated antioxidant mechanisms. Carcinogenesis. 2011;32:888–896. doi: 10.1093/carcin/bgr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohno HSR, Yasui Y, Hosokawa M, Miyashita K, Tanaka T. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004;95:481–486. doi: 10.1111/j.1349-7006.2004.tb03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik AAF, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. PNAS. 2005;102:14813–14818. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar APBS, Ganapathy M, Crosby K, Davis MD, Kochunov P, Schoolfield J, Yeh IT, Troyer DA, Rita Ghosh R. Akt/cAMP-Responsive Element Binding Protein/Cyclin D1 Network: A Novel Target for Prostate Cancer Inhibition in Transgenic Adenocarcinoma of Mouse Prostate Model Mediated by Nexrutine, a Phellodendron Amurense Bark Extract. Clin Cancer Res. 2007;13:2784–2794. doi: 10.1158/1078-0432.CCR-06-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, Yao J, Ajani J, Xie K. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–6380. [PubMed] [Google Scholar]
- 28.Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, Mansfield P, Ajani J, Xie K. Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res. 2004;10:4109–4117. doi: 10.1158/1078-0432.CCR-03-0628. [DOI] [PubMed] [Google Scholar]
- 29.Hosoi Y, Watanabe T, Nakagawa K, Matsumoto Y, Enomoto A, Morita A, Nagawa H, Suzuki N. Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. Int J Oncol. 2004;25:461–468. [PubMed] [Google Scholar]
- 30.Mertens-Talcott SUCS, Li X, 1, Safe S. The Oncogenic microRNA-27a Targets Genes That Regulate Specificity Protein Transcription Factors and the G 2-M Checkpoint in MDA-MB-231 Breast Cancer Cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. 2007;67:11001–11011. [DOI] [PubMed] [Google Scholar]
- 31.Mertens-Talcott SUNG, Li X, Angel-Morales G, Bertoldi MC, Safe S. Betulinic Acid Decreases ER-Negative Breast Cancer Cell Growth In Vitro and In Vivo: Role of Sp Transcription Factors and MicroRNA-27a:ZBTB10. Mol Carcinog. 2012;10 doi: 10.1002/mc.21893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chintharlapalli SPS, Ramaiah SK. Betulinic Acid Inhibits Prostate Cancer Growth through Inhibition of Specificity Protein Transcription Factors. Cancer Res. 2007;67:2816–2823. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 33.Chintharlapalli SPS, Abdelrahim M, Abudayyeh A, Jutooru I, Chadalapaka G, Wu F, Mertens-Talcott S, Vanderlaag K, Cho SD, et al. Oncogenic microRNA-27a is a target for anticancer agent methyl 2-cyano-3,11-dioxo-18 beta-olean-1,12-dien-30-oate in colon cancer cell. International Journal of Cancer. 2009;125:1965–1974. doi: 10.1002/ijc.24530. , s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chintharlapalli SPS, Lei P, Pathi S, Safe S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer. 2011;11 doi: 10.1186/1471-2407-11-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li XM-TS, Zhang S, Kim K, Ball J, Safe S. MicroRNA-27a Indirectly Regulates Estrogen Receptor {alpha} Expression and Hormone Responsiveness in MCF-7 Breast Cancer Cells. Endocrinology. 2010;151:2462–2473. doi: 10.1210/en.2009-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisburg H, Alyssa; JGS, Malki S. Silverman, Channa G. Ovits-Levy, Loriel J. Solodokin, Harriet L. Zuckerbraun, Harvey Babich. Pomegranate Extract, A Prooxidant with Antiproliferative and Proapoptotic Activities Preferentially Towards Carcinoma Cells. Anti-Cancer Agents in Medicinal Chemistry. 2010;10:634–643. doi: 10.2174/187152010794474000. [DOI] [PubMed] [Google Scholar]
- 37.Prasad S, Ravindran J, Aggarwal BB. NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem. 336:25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baran CP, Tridandapani S, Helgason CD, Humphries RK, Krystal G, Marsh CB. The inositol 5'-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor-induced Akt activity. J Biol Chem. 2003;278:38628–38636. doi: 10.1074/jbc.M305021200. [DOI] [PubMed] [Google Scholar]
- 39.Chao X, Zao J, Xiao-Yi G, Li-Jun M, Tao S. Blocking of PI3K/AKT induces apoptosis by its effect on NF-kappaB activity in gastric carcinoma cell line SGC7901. Biomed Pharmacother. 64:600–604. doi: 10.1016/j.biopha.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Boesch-Saadatmandia CLA, Wagnera AE, Stachurskab A, Jozkowiczb A, Dulakb J, Döringa F, Wolfframc S, Rimbacha G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide oninflammatory gene expression: role of miR-155. Journal of Nutritional Biochemistry. 2011;22:293–299. doi: 10.1016/j.jnutbio.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Tili EMJ-J, Adair B, Alder H, Limagne E, Taccioli C, Ferracin M, Delmas D, Latruffe N, Croce CM. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis. 2010;31:1561–1566. doi: 10.1093/carcin/bgq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner AEB-SC, Dose J, Schultheiss G, Rimbach G. Anti-inflammatory potential of allyl-isothiocyanate-role of Nrf2, NFκB and microRNA-155. Journal of Cellular and Molecular Medicine. 2011:1582–4934. doi: 10.1111/j.1582-4934.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleemann RVL, Morrison M, Zadelaar S, J.vanErk M, Wielinga PY, Kooistra T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharyya SBN, Dalgard C, Gutti U, Armistead D, Jozwik C, Srivastava M, Pollard HB, Biswas R. Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J Biol Chem. 2011 Apr 1;286(13):11604–11615. doi: 10.1074/jbc.M110.198390. 2011, Epub 2011 Jan 3 13:11604–11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gil MITs-BnFA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 46.Brauns SC, Dealtry G, Milne P, Naude R, Van de Venter M. Caspase-3 activation and induction of PARP cleavage by cyclic dipeptide Cyclo(Phe-Pro) in HT-29 cells. Anticancer Res. 2005;25:4197–4202. [PubMed] [Google Scholar]
- 47.Ruan KFX, Ouyang G. MicroRNAs: Novel regulators in the hallmarks of human cancer. Cancer Letters. 2009;285:116–126. doi: 10.1016/j.canlet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 48.WVaW W. MicroRNA: A New Player in Breast Cancer Development. Journal of Cancer Molecules. 2007;3:133–138. [Google Scholar]
- 49.Fu SW, CLMLY miRNA Biomarkers in Breast Cancer Detection and Management. Journal of Cancer. 2011;2:116–122. doi: 10.7150/jca.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.GGPaM RD. MicroRNAs: POTENTIAL BIOMARKERS IN CANCER. Indian Journal of Clinical Biochemistry. 2010;25:4–14. doi: 10.1007/s12291-010-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Connella RMCAA, Raoa DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. PNAS. 2009;106:7113–7118. doi: 10.1073/pnas.0902636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong MYSN, Heber D. Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. J Nutr Biochem. 2008;19:848–855. doi: 10.1016/j.jnutbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nahta OTa. Delphinidin Inhibits HER2 and Erk1/2 Signaling and Suppresses Growth of HER2-Overexpressing and Triple Negative Breast Cancer Cell Lines. Breast Cancer: Basic and Clinical Research. 2011;5:143–154. doi: 10.4137/BCBCR.S7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandey PROHMWSKPWLAKFXKFSH, Cao Deliang, Wilber Andrew C, Kounosuke Watabe TSGWKKMKKSTCMETFSTY-YM. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat. 2011;130:387–398. doi: 10.1007/s10549-010-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu HWH, Liu XP, Euans BR, Medina DJ, Liu CG, Yang JM. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76:582–588. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu TTH, Lang YY, Liu M, Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 2009;2:233–242. doi: 10.1016/j.canlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 57.I. Jutooru GC, Abdelrahim M, Basha MR, Samudio I, Konopleva M, Andreeff M, Safe S. Methyl 2-Cyano-3,12-dioxooleana-1,9-dien-28-oate Decreases Specificity Protein Transcription Factors and Inhibits Pancreatic Tumor Growth: Role of MicroRNA-27a. Molecular Pharmacology. 2010;78:226–236. doi: 10.1124/mol.110.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang YKRJ, Hunter MG, Mitchell CA, Frey RS, Javaid K, Malik AB, Schurmans S, Tridandapani S, Marsh CB. SHIP2 Is Recruited to the Cell Membrane upon Macrophage Colony-Stimulating Factor (M-CSF) Stimulation and Regulates M-CSF-Induced Signaling1. The Journal of Immunology. 2004;173:6820–6830. doi: 10.4049/jimmunol.173.11.6820. [DOI] [PubMed] [Google Scholar]
- 59.Gwenny M, Fuhler GMBR, Bonnie Toms B, Iyer S, Gengo EA, Park MY, Gumbleton M, Viernes DR, Chisholm JD, 4, Kerr WG. Therapeutic Potential of SH2 Domain-Containing Inositol-5′-Phosphatase 1 (SHIP1) and SHIP2 Inhibition in Cancer. Mol Med. 2012;18:6 5–7 5. doi: 10.2119/molmed.2011.00178. 1 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou PKH, Teitelbaum ST, Krystal G, Ross P, Takeshita S. SHIP1 Negatively Regulates Proliferation of Osteoclast Precursors via Akt-Dependent Alterations in D-Type Cyclins and p271. The Journal of Immunology. 2006;177:8777–8784. doi: 10.4049/jimmunol.177.12.8777. [DOI] [PubMed] [Google Scholar]
- 61.Conde CGG, Piette J. Enzymatic and non-enzymatic activities of SHIP-1 in signal transduction and cancer. Biochemical Pharmacology. 2011;82:1320–1334. doi: 10.1016/j.bcp.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 62.Hamiltona MJHVW, Kurodaa E, Ruschmanna J, Antignanoa F, Lama V, Krystal G. Role of SHIP in cancer. Experimental Hematology. 2011;39:2–13. doi: 10.1016/j.exphem.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Thomas SPNN, Vohra N, Jerald M, Pendleton L, Szekeres K, Ghansah T. Murine Pancreatic Adenocarcinoma Dampens SHIP-1 Expression and Alters MDSC Homeostasis and Function. PLoS ONE. 2011;6(11):277–229. doi: 10.1371/journal.pone.0027729. [DOI] [PMC free article] [PubMed] [Google Scholar]