Abstract
Cancer stem-like cell (CSC; also known as tumor initiating cell) is defined as a small subpopulation of cancer cells within a tumor and isolated from various primary tumors and cancer cell lines. CSCs are highly tumorigenic and resistant to anticancer treatments. In this study, we found that prolonged exposure to tumor necrosis factor alpha (TNFα), a major proinflammatory cytokine, enhances CSC phenotype of oral squamous cell carcinoma (OSCC) cells, such as an increase in tumor sphere-forming ability, stem cell-associated genes expression, chemo-radioresistance, and tumorigenicity. Moreover, activation of Notch1 signaling was detected in the TNFα-exposed cells, and suppression of Notch1 signaling inhibited CSC phenotype. Furthermore, we demonstrated that inhibition of a Notch downstream target, Hes-1, led to suppression of CSC phenotype in the TNFα-exposed cells. We also found that Hes1 expression is commonly upregulated in OSCC lesions compared to precancerous dysplastic lesions, suggesting the possible involvement of Hes1 in OSCC progression and CSC in vivo. In conclusion, inflammatory cytokine exposure may enhance CSC phenotype of OSCC, in part by activating the Notch-Hes1 pathway.
Keywords: TNFα, OSCC, cancer stem cells, Notch, Hes1
1. Introduction
OSCC is an important problem not only because of the significant mortality associated with the disease, but also because of the functional defects and disfigurement often associated with its treatment [1,2]. Like other cancers, the development of OSCC is a multistep process with accumulation of genetic and epigenetic changes [3].
Recent studies have uncovered and validated the pathophysiologic role of self-renewing cells, namely CSC (also called tumor-initiating cells), in long-term sustenance of cancers [4]. CSCs share many molecular similarities to embryonic and normal adult stem cells. Many molecular determinants of normal stem cells, such as self-renewal ability and multi-lineage differentiation capacity, are retained in CSCs [5]. CSCs have been isolated from various primary tumors and established cancer cell lines via cell surface markers, and they typically have the following properties: high tumorigenicity upon injection in immunodeficient mice, the ability to grow as tumor spheres in undifferentiating medium and resistance to cancer therapeutic agents [6]. Similarly, the existence of CSCs has been demonstrated in primary OSCC and cell lines [7]. Therefore, CSCs drive the perpetuity of the disease while producing cellular heterogeneity of cancer tissues, and are becoming new targets of anti-cancer therapies [8]. The phenotypes of CSC have been reported to be maintained by several endogenous signaling pathways, such as Notch, Hedgehog, and Wnt [9–11]. Activation of those pathways is frequently found in human cancers [11–13]. In addition to endogenous pathways regulating stem cell functions, CSCs could be enriched by exogenous carcinogenic factors. For instance, nicotine enhanced CSC population in human breast cancer [14]. Furthermore, recent studies demonstrated that proinflammatory cytokines, TGFβ and TNFα, generated CSC in breast cancer, suggesting a possible link between CSC and inflammation [15, 16].
There is increasing evidence of chronic inflammation-associated tumorigenesis [17]. Although the molecular and cellular mechanisms linking chronic inflammation to tumorigenesis have not been fully understood, TNFα, a major mediator of inflammation, is known to play a crucial role in the inflammation-associated cancer development. Disruption of the TNFα signaling pathway could significantly inhibits chemical induced-carcinogenesis in skin [18,19]. Many studies suggested that TNFα promotes inflammation-associated tumorigenesis by activating the nuclear factor-κB (NF-κB) signaling, which inhibits the death of precancerous or transformed cells during the development of inflammation-associated cancers [20,21]. In addition, TNFα is a potential mutagen that causes DNA damage through the induction of reactive oxygen species [22].
In the present study, we investigated the effect of TNFα on CSC property in OSCC. Our study revealed that prolonged exposure to TNFα enhanced CSC property, i.e., tumor sphere-forming ability, stem cell-associated genes expression, and chemo-radioresistance, in OSCC cells. Moreover, the TNFα-exposed cells with elevated CSC phenotype showed higher tumorigenic potential than the control cells. Finally, our study also demonstrated that the enriched CSC phenotype in the TNFα-exposed OSCC cells was attributed to activation of Notch-Hes1 pathway.
2. Materials and Methods
2.1. Cell Culture and chronic exposure of cells to TNFα
The SCC-4, SCC-9, and HEp2 cancer cell lines were purchased from the American Type Culture Collection (ATCC). SCC-4 and SCC-9 were cultured in DMEM/Ham’s F12 (Invitrogen) supplemented with 10% FBS (Gemini Bioproducts) and 0.4 μg/ml hydrocortisone (Sigma-Aldrich), while HEp2 was grown in MEM (Invitrogen) supplemented with 10% FBS. The cell lines were treated with 5ng/ml of TNFα (Sigma-Aldrich) for extended periods.
2.2. Tumor sphere formation assay
The cells were grown in DMEM/F12 media with 1:50 B27 (Invitrogen), 20 ng/mL EGF, 20 ng/mL, 10μg/mL insulin, penicillin, streptomycin, and amphotericin B in 6-well plates at a density of 1,000 cells/mL. Additional 0.5ml of media was added every 5 days for 15 days. The number of tumor spheres formed were observed and counted under a microscope.
2.3. Quantitative real-time PCR (qPCR)
cDNA was synthesized from 5 μg of total RNA using SuperScript first-strand synthesis system (Invitrogen). We used 1 μl cDNA for qPCR amplification using SYBR Green I Master mix (Roche) and LightCycler 480 II (Roche). The primer sequences were obtained from the Universal Probe Library (Roche), and the sequences can be available upon request. Second derivative Cq value determination method was used to compare fold-differences according to the manufacturer’s instructions.
2.4. Chemo-radiosensitivity assays
Chemosensitivity of cells was determined by measuring cell viability using the tetrazolium salt (MTT) cell proliferation assay kit (ATCC). The cells were plated at 2 × 103 cells per well into 96-well plate and incubated in culture medium containing 40μ etoposide (Sigma-Aldrich) for 2, 4, and 6 days. Absorbance at 570nm was determined using a microplate reader.
For radiosensitivity assay, the cells were irradiated with 0, 5Gy, 10 using Mark I-30 Cesium-137 irradiator (JL Shepherd & Assoc.) with the delivery rate of 4.86 Gy/min. Cells were seeded in 6-well plates at low density (200 cells per well). The cells were maintained in culture for 10 additional days to allow colony formation. Visible colonies consisting of at least 50 cells were stained with Giemsa stain (Sigma) and counted.
2.5. Anchorage-independent growth
To determine colony-forming efficiency in semi-solid medium, 1 × 104 cells were plated in culture medium containing 0.3% agarose over a base layer of serum-free medium containing 0.5% agarose. Three weeks after incubation, colonies were counted. The experiment was performed in triplicates with 60-mm dishes.
2.6. Western blotting
Whole cell extracts were isolated using the lysis buffer (1% Triton X-100, 20 mM Tris –HCl pH 7.5, 150 mM NaCl, 1mM EDTA, 1mM EDTA, 2.5 mM sodium pyrophosphate, 1 μM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mg/ml PMSF). The extracts were then fractionated by SDS-PAGE and transferred to Immobilon protein membrane (Millipore). The membranes were incubated successively with the primary and the secondary antibodies, and exposed to the chemiluminescence reagent (Amersham) for signal detection. We used the following primary antibodies for this study: NICD (Val 1744; Cell signaling), Hes1 (H-20; Santa Cruz), and GAPDH (Santa Cruz). Horse radish peroxidase (HRP)-conjugated secondary antibodies were obtained from Santa Cruz.
2.7. Organotypic raft cultures
One million cells were seeded on the submucosal equivalents consisting of type I collagen and normal human oral fibroblasts. The cells were grown to confluence, submerged in the culture medium, and then exposed to the liquid-air interface by lowering the medium level. The cultures were maintained in this “rafting” fashion for 14 days and were harvested by fixing in 10% buffered formalin. Subsequently, hematoxylin-eosin (H&E) staining was performed on thick (6 μm) sagittal sections of each reconstructs to reveal the histological features. Sample processing, paraffin-embedding, sectioning, and H&E staining were performed at UCLA’s TPCL.
2.8. Determination of tumorigenicity in vivo
Four million cells were subcutaneously injected into the flank of immunocompromised mice (strain nu/nu, Charles River Laboratories). The animal study was performed according to the protocol approved by UCLA Animal Research Committee. The kinetics of tumor growth was determined by measuring the volume in three perpendicular axes of the nodules using micro-scaled calipers.
2.9. Knockdown of endogenous Hes1 by siRNA
Hes1 expression was knocked down with duplex siRNA targeting Hes1 or the control, scrambeled siRNA (Santa Cruz), which was introduced using Lipofectamine (Invitrogen). SCC9/TNF cells (2 × 105) were plated in 60-mm dishes and transfected with 15 μg siRNA. The cultures were harvested after three days post-transfection for biochemical analyses.
3. Results
3.1. TNFα increases CSC property in OSCC
In order to investigate the effect of TNFα on CSC phenotype of OSCC, we treated three OSCC cell lines (SCC4, SCC9, and HEp2) with TNFα (5–10ng/ml) for extended periods and examined its effects on undifferentiated tumor sphere formation, which is considered as CSC population and property [23]. Two months after the exposure, all tested cell lines showed enhanced tumor sphere-forming ability (Fig. 1A). Increased tumor sphere formation was first evident after 2 month exposure. Importantly, it should be noted that after the 2 month exposure, we withdrew TNFα from culture medium and performed all the experiments. In doing so, we may exclude the immediate effect of TNFα on biological behaviors of tested cell lines. Interestingly, chronic TNFα induced a robust increase in tumor sphere-forming capacity of SCC9. SCC9 displayed negligible or no tumor sphere-forming ability, whereas the TNFα-exposed SCC9 (SCC9/TNF) acquired significant sphere-forming capacity even compared to SCC4 and HEp2 (Fig. 1A and 1B). Because of these results, further experiments were focused using SCC9/TNF compared to SCC9.
Fig. 1.
TNFα enhanced CSC phenotype in OSCC. Three OSCC cell lines (SCC4, SCC9, and HEp2) were exposed to 5–10ng/ml of TNFα for 2 months. (A) Tumor sphere formation assay was performed in triplicate. P values were obtained against untreated corresponding control. (B) Representative image of tumor spheres formed by SCC9 and TNFα-exposed SCC9 (SCC9/TNF). The photographs were taken at a magnification of 40X. (C) qPCR analysis of stemness- associated genes in SCC9 and SCC9/TNF. Levels of the stemness-associated genes in SCC9/TNF were plotted as fold induction against those in SCC9. (D) Chemo-radioresensitivity assay. Cells were treated with 40μM of etoposide for 2, 4, and 6 days, and their viability was measured using MTT assay (left). For clonogenic survival assay (right), cells were irradiated with 5 and 10 Gy, and surviving colonies were stained and counted (right). Data are expressed as the mean ± SD of triplcate.
Quantitative PCR pathway analysis showed enriched expression of stem cell-associated genes, including cyclin D2 (CCND2), epithelial cell adhesion molecule (EPCAM), lin-28 homolog B (Lin28B), CD44, Sox2, Myc, aldehyde dehydrogenase 1 (ALDH1) in SCC9/TNF (Fig. 1C). An important characteristic of CSCs is their resistance to chemo-radiotherapy [24]. The SCC9/TNF cells displayed increased resistance to etoposide (Fig. 1D left) and ionizing radiation (Fig. 1D right) than the control SCC9 cells. These findings suggest that TNFα may increases CSC population and property in OSCC.
3.2. TNFα further enhances tumorigenicity of OSCC in vitro and in vivo
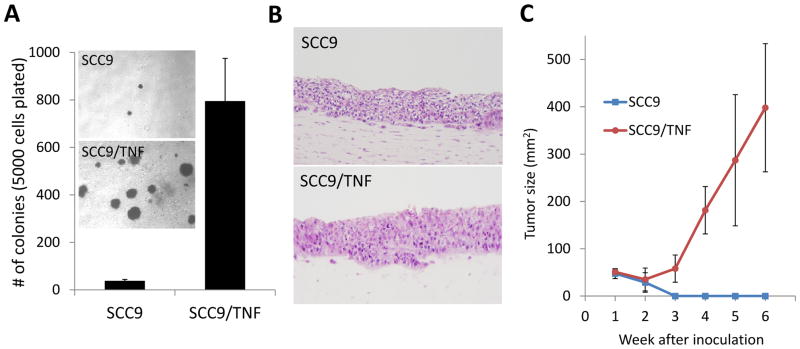
Hallmarks of CSC include high tumorigenic potential. Therefore, we examined whether chronic TNFα enhances anchorage independent growth ability of SCC9. The soft agar assay revealed that SCC9/TNF showed greatly increased anchorage independent growth ability compared to SCC9 (Fig. 2A). Also, SCC9/TNF formed bigger cell colonies than the control SCC9 cells (Fig. 2A). Consistent with previous report, the control SCC9 cells showed extremely low anchorage independent growth ability [25]. We also employed an in vivo-like 3D organotypic cell culture system. To recapitulate the tissue microenvironment, SCC9 and SCC9/TNF were overlaid on an extracellular matrix gel mixed with normal human oral fibroblasts, and after 14 days, a liquid-air interface was created, thereby leading to stratification of the squamous epithelium, which resembles the human squamous epithelium [26]. Organotypic culture of SCC9/TNF demonstrated malignant histomorphology with invasive characteristics into subepithelial layer and increased epithelial thickness, and mitotic cells compared to organotypic culture of SCC9 (Fig. 2B). Furthermore, we evaluated the tumorigenicity of SCC9 and SCC9/TNF in vivo. We injected SCC9 and SCC9/TNF into nude mice and monitored tumor formation. SCC9/TNF developed tumor in 2 of 5 mice, whereas SCC9 failed to form tumor in tested animals (Fig. 2C). Our data indicate that TNFα enhances tumorigenicity of SCC9.
Fig. 2.
TNFα increased tumorigenicity of SCC9. (A) Anchorage independent growth assay.. Five thousand cells were plated in semi-solid agar, and colonies were counted for three weeks. The assay was performed in triplicate with 60-mm dishes. The photographs were taken at a magnification of 40X. (B) Ex vivo three dimensional organotypic raft cultures. Organotypic “raft” cultures were established with SCC9 and SCC9/TNF. (C) In vivo tumorigenic assay. SCC9 and SCC9/TNF were injected subcutaneously into five nude mice. The kinetics of tumor growth was determined by measuring the volume in three perpendicular axes of the nodules using micro-scaled calipers.
3.3. TNFα activates Notch1 signaling pathway
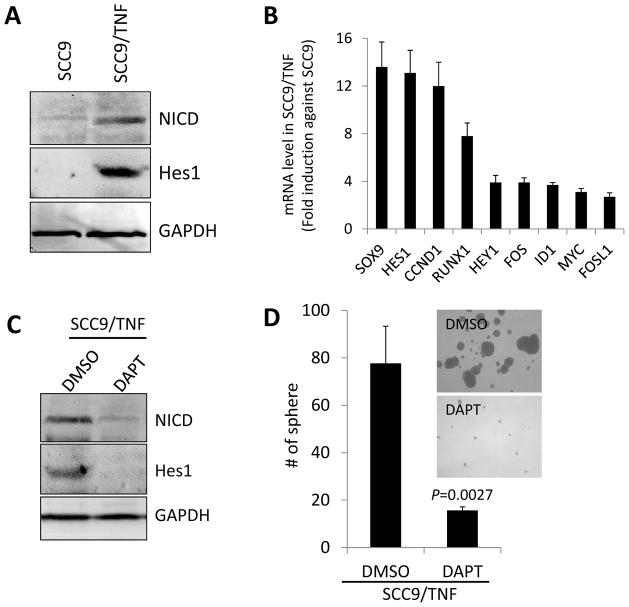
Activation of Notch1 signaling pathway is critical for the maintenance of CSC and requires binding of its ligands, Jagged 1 (JAG1), JAG 2 and Delta-like (DLL), followed by proteolytic release of Notch intracellular domain (NICD) and the activation of NICD downstream target genes [27]. Since our data showed increased expression of Notch ligands, i.e, DLL1, JAG1, and JAG2 (Fig. 1C) in SCC9/TNF, we explored activation status of Notch1 pathway in SCC9/TNF by examining the expression of NICD, an activated form of Notch protein. Western blot revealed that NICD was significantly increased in SCC9/TNF compared to SCC9 (Fig. 3A). Furthermore, various NICD downstream target genes, including Hes1 (hairy and enhancer of split 1), were upregulated in SCC9/TNF compared to SCC9 (Fig. 3A and 3B). These findings clearly indicate the activation of Notch1 signaling pathway by TNFα in SCC9.
Fig. 3.
Role of Notch1 signaling pathway in CSC property in the TNFα-exposed SCC9 cells. (A) Western blot analysis of NICD and its target Hes1 in SCC9 and SCC9/TNF. GAPDH was used as a loading control. (B) qPCR analysis of NICD target genes in SCC9 and SCC9/TNF. Levels of the genes in SCC9/TNF were plotted as fold induction against those in SCC9. (C) Synthetic γ-secretase inhibitor, DAPT (5μM) abrogated the expression of NICD and Hes1 protein in SCC9/TNF as determined by Western blot analysis. The cells treated with DMSO were included for comparison. (D) Inhibition of Notch 1 pathway reduced tumor sphere-forming ability of SCC9/TNF. The SCC9/TNF cells were treated with DAPT for 2 days and subjected to tumor sphere-forming assay in triplicate. P value was obtained against DMSO-treated control.
3.4. Effect of Notch-Hes1 pathway on CSC property in the TNFα-exposed cells
To investigate the role of Notch1 signaling pathway in CSC property of SCC9/TNF, we examined the effect of a pharmacological Notch1 inhibitor (γ-secretase inhibitor, DAPT) on tumor sphere-forming ability of SCC9/TNF. Because 5 μM of DAPT has no effect on SCC9/TNF cell growth (data not shown), we treated the cells with the concentration and measured NICD expression. DAPT had significantly reduced NICD expression compared to control DMSO treatment (Fig. 3C). Similarly, DAPT also downregulated the NICD downstream target Hes1 (Fig. 3C). We then assessed tumor sphere formation under DAPT treatment. The assay revealed that DAPT treatment significantly suppressed tumor sphere-forming ability of SCC9/TNF (Fig. 3D). This finding indicates that NICD is required for tumor sphere-forming capacity of SCC9/TNF.
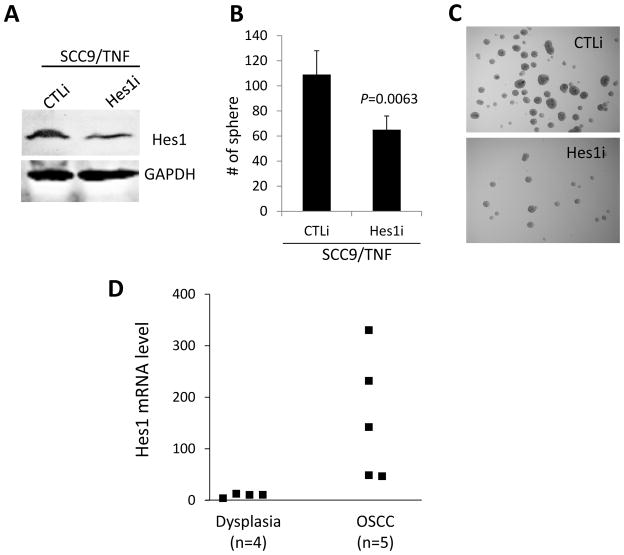
To further understand the role of Hes1 in the CSC property, we suppressed endogenous Hes1 in the SCC9/TNF cells by transfecting them with siRNA against Hes1 (Hes1i) or control siRNA (CTLi) as a control (Fig. 4A). The Hes1i-transfected SCC9/TNF cells formed significantly smaller number of tumor spheres compared to the CTLi-transfected control cells (Fig. 4B and 4C). These data indicate that Hes1is also important regulator for tumor sphere-forming capacity of SCC9/TNF, suggesting that TNFα increases the CSC property via a Notch-Hes1 pathway.
Fig. 4.
Effect of Hes1 on CSC property of SCC9/TNF and its expression in dysplastic and OSCC tissues. (A) Western blot analysis of Hes1. Endogenous Hes1 was knocked down in SCC9/TNF using siRNA against Hes1 (Hes1i). The cells transfected with control siRNA (CTLi) were included for comparison. (B) Inhibition of Hes1 reduced tumor sphere-forming ability of SCC9/TNF. P value was obtained against CTLi-transfected control. (C) Tumor spheres were photographed at a magnification of 40X. (D) Dysplasia and OSCC tissue were dissected by laser capture microdissection, and RNA was isolated to synthesize cDNA. The level of Hes1 mRNA was quantified using qPCR and normalized with the GAPDH expression.
3.5. Expression of Hes1 in dysplastic and OSCC lesions
To further investigate the importance of Hes1 in oral carcinogenesis and CSC in vivo, we determined the level of Hes1 expression in laser capture microdissected dysplastic and OSCC lesions. We speculate that CSC population is enriched in OSCC lesion compared to dysplastic lesion. Quantitative PCR revealed that Hes1 is commonly upregulated in OSCC compared to precancerous dysplastic lesions (Fig. 4D). Although further analysis with a larger sample size is required, this finding suggests that Hes1 may be associated with the progression of OSCC and the enrichment of CSC population in vivo.
4. Discussion
In this study, we report that prolonged exposure of OSCC cells to proinflammatory cytokine TNFα enhances CSC phenotype and tumorigenicity. TNFα increased the CSC properties of OSCC, such as 1) tumorigenic potential, 2) tumor sphere-forming ability, 3) expression of stem cell-associated genes, and 4) chemo-radioresistance. Subsequently, our study found activation of Notch-Hes1 pathway in the TNFα-exposed OSCC cells. Suppression of Notch-Hes1 pathway inhibited the CSC property of the TNFα-exposed OSCC cells.
TNFα is a major mediator of inflammation. TNFα is not only widely distributed throughout the body in normal physiologic conditions, but it is involved in many pathologic processes including inflammation and carcinogenesis [28]. Indeed, many studies have reported the tumor-promoting effects of TNFα. First, TNFα activated oncogenic pathways [29]. Second, overexpression of TNFα increased malignant behavior of tumor cell lines [30]. Third, TNFα and TNFα receptor 1 knock-out mice are resistant to chemical induced skin carcinogenesis [24,31]. Fourth, TNFα elevated chromosomal instability by virtue of its ability to induce ROS [32]. Consistent with these observations, TNFα further elevated malignant phenotypes of OSCC, i.e., anchorage independent growth ability and in vivo tumorigenic poteintial. We also obtained similar result that chronic TNFα exposure further elevated malignant behavior of non-tumorigenic immortalized oral keratinocytes (unpublished data). Our results suggest that prolonged TNFα exposure mimicking chronic inflammatory microenvironment is an important carcinogenic factor for oral cancer.
CSCs are considered the seed of cancer for their crucial roles in malignant behavior of cancer cells, i.e., migration/invasion, resistance to anticancer therapies, anchorage independent growth ability and in vivo tumorigenic potential. However, the effect of chronic inflammation on CSC remains largely unclear. Our study show that the chronic exposure of OSCC cells to proinflammatory agent TNFα enhances CSC properties. This is important evidence supporting the role of chronic inflammation in OSCC. Our finding is consistent with a recent reports demonstrating that inflammatory cytokines, TGFβ and TNFα, increased CSC population and phenotypes in breast cancer cells [15,16]. Furthermore, we documented that prolonged TNFα treatment results in activation of Notch1 signaling pathway, a critical CSC maintaining pathway [27]. Our data demonstrated that inhibition of Notch1 pathway by DAPT suppressed tumor sphere-forming capacity of the SCC9/TNF cells with concomitant reduction of Notch1 downstream target Hes1, suggesting the importance of Notch-Hes1 pathway in the TNFα-induced CSC property in SCC9 cells. Hes1 was significantly increased in the SCC9/TNF cells, and repressed by the Notch1 inhibitor in the same cells, indicating a direct regulation of Hes1 by activated Notch1 in our cell model. In the present study, the knockdown of endogenous Hes1 in SCC9/TNF significantly inhibits tumor sphere-forming capacity, further confirming the significance of Notch-Hes1 pathway in the CSC phenotype. It is interesting to note that the effect of Hes1 knockdown on tumor sphere formation was smaller than that of Notch1 inhibition (75% vs 36% reduction). This observation suggests the presence of Notch-Hes1 independent pathway/mechanism in the TNFα-induced CSC property in SCC9.
There is increasing evidence of the importance of Hes1 in the maintenance of progenitor cell fate. For instance, Hes1 is commonly expressed in most undifferentiated cell types in the developing mouse embryo. Moreover, Hes1 deficient mice displayed premature differentiation, progenitor cell depletion, and a consequent lethality [33]. Many studies also demonstrated the implication of Hes1 in tumorigenesis. In breast and pancreatic endocrine tumors, Hes1 was commonly repressed, whereas Hes1 is increased in osteosarcomas [34–36]. These conflicting reports suggest that Hes1 may have dual role as a tumor suppressor or an oncogene depending on the tumor types and the stages of cancer progression. Our study reports for the first time that Hes1 is overexpressed in the TNFα-exposed OSCC cells with increased CSC property. Although the sample size was limited, Hes1 is greatly increased in OSCC lesions compared to precancerous lesions. Our data suggest that Hes1 may be associated with cancer progression and CSC phenotype in OSCC. This also suggests the possible oncogenic role of Hes1 in oral carcinogenesis. Thus, Hes1 could be considered for a therapeutic target for CSCs in OSCC.
In conclusion, this study provides novel information of the role of proinflammatory cytokine in OSCC progression. Our results reveal a molecular mechanism that TNFα enhances OSCC stem cells-like phenotype, which is associated with activation of Notch-Hes1 pathway. Therefore, we speculate that the challenge of OSCC cells with inflammatory microenvironment, e.g., chronic inflammation, may further increase CSC population/phenotype and allow CSCs to subvert immune-mediated elimination, thereby permitting long term survival, which would promote malignancy of OSCC.
Research highlights.
TNFα increases CSC phenotype and tumorigenicity of oral squamous cell carcinoma (OSCC).
TNFα enhances CSC property of OSCC by activating Notch-Hes1 pathway.
Hes1 is upregulated in OSCC tissue in vivo.
Footnotes
This study was supported by UCLA School of Dentistry faculty seed grants (K.-H. Shin), Department of Pathology and Laboratory Medicine Research Service Fund (K.-H. Shin), NIDCR R01DE18295 (M.K. Kang), NIDCR K02DE18959 (M.K. Kang) and NIDCR K08DE17121 (R.H. Kim).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein JB, Zhang L, Rosin M. Advances in the diagnosis of oral premalignant and malignant lesions. J Can Dent Assoc. 2002;68:617–621. [PubMed] [Google Scholar]
- 2.Farah CS, McCullough MJ. Oral cancer awareness for the general practitioner: new approaches to patient care. Aust Dent J. 2008;53:2–10. doi: 10.1111/j.1834-7819.2007.00002.x. [DOI] [PubMed] [Google Scholar]
- 3.Kang MK, Park N-H. Conversion of normal to malignant phenotype: telomere shortening, telomerase activation, and genomic instability during immortalization of human oral keratinocytes. Crit Rev Oral Biol Med. 2001;12:38–54. doi: 10.1177/10454411010120010301. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells--old concepts, new insights. Cell Death and Diff. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- 6.Albers AE, Chen C, Köberle B, Qian X, Klussmann JP, Wollenberg B, Kaufmann AM. Stem cells in squamous head and neck cancer. Crit Rev Oncol Hematol. 2012;8:224–240. doi: 10.1016/j.critrevonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, et al. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 8.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:605–615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochimica Biophysica Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 12.Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12:5924–5928. doi: 10.1158/1078-0432.CCR-06-1736. [DOI] [PubMed] [Google Scholar]
- 13.Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 14.Hirata N, Sekino Y, Kanda Y. Nicotine increases cancer stem cell population in MCF-7 cells. Biochem Biophys Res Comm. 2010;403:138–143. doi: 10.1016/j.bbrc.2010.10.134. [DOI] [PubMed] [Google Scholar]
- 15.Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL. TGFbeta/TNF(alpha)-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 2011;71:4707–4719. doi: 10.1158/0008-5472.CAN-10-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storci G, Sansone P, Mari S, D’Uva G, Tavolari S, Guarnieri T, et al. TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype. J Cell Physiol. 2010;225:682–91. doi: 10.1002/jcp.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8:3267–3273. doi: 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 19.Scott KA, Moore RJ, Arnott CH, East N, Thompson RG, Scallon BJ, et al. An anti-tumor necrosis factor-α antibody inhibits the development of experimental skin tumors. Mol Cancer Ther. 2003;2:445–451. [PubMed] [Google Scholar]
- 20.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 21.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNF alpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Yan B, Wang H, Rabbani ZN, Zhao Y, Li W, Yuan Y, et al. Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 2006;66:11565–11570. doi: 10.1158/0008-5472.CAN-06-2540. [DOI] [PubMed] [Google Scholar]
- 23.Grimshaw MJ, Cooper L, Papazisis K, Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, Taylor-Papadimitriou J, Burchell JM. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10:R52. doi: 10.1186/bcr2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milas L, Raju U, Liao Z, Ajani J. Targeting molecular determinants of tumor chemo-radioresistance. Seminars in Oncol. 2005;32:S78–81. doi: 10.1053/j.seminoncol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- 26.Shin KH, Bae SD, Hong HS, Kim RH, Kang MK, Park NH. miR-181a shows tumor suppressive effect against oral squamous cell carcinoma cells by downregulating K-ras. Biochem Biophys Res Commun. 2011;40:896–902. doi: 10.1016/j.bbrc.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 27.Bolós V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocrine Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 28.Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Frontiers in Bioscience. 2008;13:5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 29.Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncology. 2003;9:565–573. doi: 10.1016/s1470-2045(03)01196-3. [DOI] [PubMed] [Google Scholar]
- 30.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006;42:745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Bolos V, Blanco M, Medina V, Aparicio G, Diaz-Prado S, Grande E. Notch signalling in cancer stem cells. Clin Transl Res. 2009;11:11–19. doi: 10.1007/s12094-009-0305-2. [DOI] [PubMed] [Google Scholar]
- 32.Beyne-Rauzy O, Recher C, Dastugue N, Demur C, Pottier G, Laurent G, et al. Tumor necrosis factor alpha induces senescence and chromosomal instability in human leukemic cells. Oncogene. 2004;23:7507–7016. doi: 10.1038/sj.onc.1208024. [DOI] [PubMed] [Google Scholar]
- 33.Kageyama R, Ohtsuka T, Tomita K. The bHLH gene Hes1 regulates differentiation of multiple cell types. Mol Cells. 2000;10:1–7. doi: 10.1007/s10059-000-0001-0. [DOI] [PubMed] [Google Scholar]
- 34.Strom A, Arai N, Leers J, Gustafsson JA. The Hairy and Enhancer of Split homologue-1 (HES-1) mediates the proliferative effect of 17beta-estradiol on breast cancer cell lines. Oncogene. 2000;19:5951–5953. doi: 10.1038/sj.onc.1203990. [DOI] [PubMed] [Google Scholar]
- 35.Johansson T, Lejonklou MH, Ekeblad S, Stalberg P, Skogseid B. Lack of nuclear expression of hairy and enhancer of split-1 (HES1) in pancreatic endocrine tumors. Horm Metab Res. 2008;40:354–359. doi: 10.1055/s-2008-1076695. [DOI] [PubMed] [Google Scholar]
- 36.Engin F, Bertin T, Ma O, Jiang MM, Wang L, Sutton RE, et al. Notch Signaling Contributes to the Pathogenesis of Human Osteosarcomas. Hum Mol Genet. 2009;18:1464–1470. doi: 10.1093/hmg/ddp057. [DOI] [PMC free article] [PubMed] [Google Scholar]