Abstract
SUMMARY
Background
Thiazolidinediones (TZDs) have been used in the treatment of non-alcoholic steatohepatitis (NASH). However, the magnitude of treatment response associated with TZDs in improving liver histology in NASH has not been quantified systematically.
Aim
To conduct a meta-analysis of randomised, placebo-controlled clinical trials (RPCTs) using pioglitazone and rosiglitazone in the treatment of NASH.
Methods
Pubmed/MEDLINE and Cochrane Central Register of Controlled Trials 2010 were searched until September 2010 and four RPCTs were identified. Peto odds ratios (ORs) and their respective 95% confidence intervals (CIs) were used to assess the efficacy of TZDs in improving liver histological parameters.
Results
Four good quality RPCTs derived from three continents were included. The meta-analysis showed that TZDs (n = 169) were significantly better than placebo (n = 165) in improving ballooning degeneration, lobular inflammation and steatosis with combined ORs of 2.11 (95% CI, 1.33–3.36), 2.58 (95% CI, 1.68–3.97) and 3.39 (95% CI, 2.19–5.25) respectively. The improvement in combined necroinflammation with TZD (n = 58) vs. placebo (n = 52) was also statistically significant (combined OR 6.52[95% CI, 3.03–14.06]), but improvement in fibrosis was not. When pioglitazone (n = 137) was analysed alone, the improvement in fibrosis with pioglitazone (n = 137) vs. placebo (n = 134) (combined OR 1.68 [95% CI, 1.02–2.77]) was statistically significant. The total body fat slightly decreased in the control, while it markedly and highly significantly increased with TZD treatment.
Conclusions
Thiazolidinediones significantly improve ballooning degeneration, lobular inflammation, steatosis and combined necroinflammation in patients with NASH. Pioglitazone may improve fibrosis. Larger randomised, placebo-controlled clinical trials are needed to examine the efficacy of thiazolidinediones in improving NASH fibrosis.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a common cause of elevated liver enzymes and chronic liver disease in Western countries. The NAFLD is characterised by hepatic steatosis in the absence of significant alcohol use or other known liver disease.1 The term NAFLD comprises a spectrum of diseases which range from fat accumulation in hepatocytes with no associated inflammation or fibrosis (simple steatosis) or steatosis with inflammation to non-alcoholic steatohepatitis (NASH) that includes macrovesicular steatosis, lobular inflammation, balloon degeneration of hepatocytes, and zone 3 pericellular fibrosis.2 Non-alcoholic steatohepatitis, as a subset of NAFLD, is associated with progressive liver disease and can ultimately lead to cirrhosis3 and / or hepatocellular carcinoma.4
Although a number of therapeutic agents have been studied in the treatment of NASH, there is currently no US Food and Drug Administration approved therapy. As the prevalence/incidence of conditions associated with NAFLD and NASH, such as obesity5and diabetes6 continue to rise, it is anticipated that NASH will become an increasingly important public health concern. It is thus paramount to identify effective treatments to prevent and treat this disease.
The pathogenesis of NASH is not fully understood. A well-supported theory describes peripheral insulin resistance (IR) leading to hepatic steatosis and steatosis, which subsequently sensitising the liver to different metabolic injuries leading to necroinflammation and fibrosis.7 As insulin resistance is thought to be the inciting factor leading to hepatic steatosis through its effects of increased lipolysis and delivery of free fatty acids to the liver,7 a potential intervention in NASH would be to target insulin resistance. One important mechanism of insulin resistance is the down-regulation of insulin receptor substrate-1 (IRS-1) signalling by excess free fatty acids, which have been shown to impair the tyrosine phosphor-ylation of IRS-1.8 Impaired tyrosine phosphorylation of serine residues has the effect of deactivating IRS-1, which leads to IR. One class of insulin-sensitising agents are the thiazolidinediones (TZDs), which are selective ligands of the nuclear transcription factor peroxisome proliferator-activated receptor γ (PPAR- γ).9 The TZDs bind to and activate PPAR-γ, which in-turn up-regulates fatty acid disposal and facilitates insulin responsiveness. In addition, they are thought to promote fatty acid uptake and storage in adipose tissue, sparing other insulin-sensitive tissues, such as skeletal muscle and the liver, thus reversing the down-regulation of IRS-1.9
The aims of the present study were to perform a meta-analysis to examine the efficacy of TZDs vs. placebo in improving liver histology in patients with NASH and to conduct a pooled analysis of the effect of the selected TZDs, pioglitazone and rosiglitazone, on pertinent anthropometric, biochemical and histological parameters in patients with NASH. This would assist clinicians in better assessment of the benefits of TZDs in NASH and would help design future comparative effectiveness studies in NASH.
METHODS
Search strategy and selection criteria
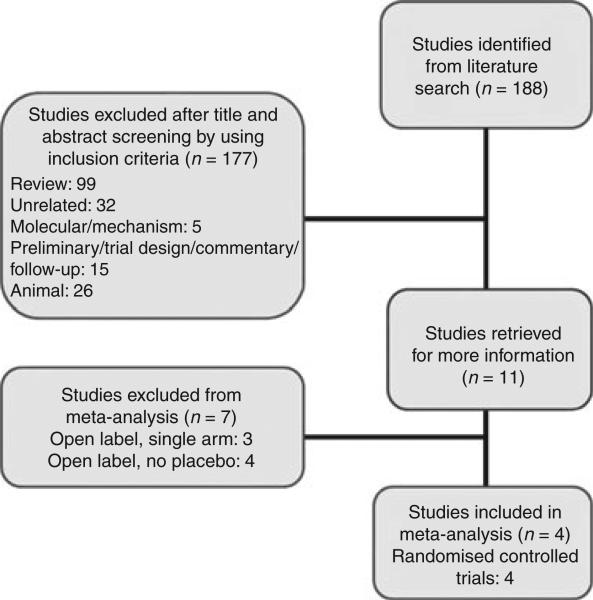
As detailed in Figure 1, the following databases were searched from their inception until 9/25/10: PubMed, MEDLINE and Cochrane Controlled Register of Controlled Trials 2010. Indexing terms included non-alcoholic steatohepatitis or non-alcoholic steatohepatitis or NASH in combination with Pioglitazone, non-alcoholic steatohepatitis or non-alcoholic steatohepatitis or NASH in combination with Rosiglitazone or non-alcoholic steatohepatitis or non-alcoholic steatohepatitis or NASH in combination with insulin sensitizers. Although the TZD troglitazone has been studied in NASH,10 it was taken off the market for severe hepatotoxicity,11 thus was not included in the search. Limits of humans and English language were included in the search. A manual review of the bibliographies of seminal primary and review articles was also performed to identify additional studies.
Figure 1.
Literature search protocol and derivation of studies included in the meta-analysis.
Inclusion criteria for meta-analysis included (i) randomised placebo-controlled clinical trials (RPCTs) in patients with NASH, (ii) minimum of 15 patients, (iii) minimum of 24 weeks of treatment and (iv) well-defined treatment outcomes, defined by reporting at least one of the following: changes in serum ALT or AST or liver histological parameters related to NASH.
As the number of randomised controlled trials for NASH was small, we included description of pilot studies in the research synthesis to capture the entire spectrum of treatment experience in NASH with pioglitazone and rosiglitazone. Case reports or series were excluded as were review articles. Trials were also excluded if relevant data were not extractable or if the trial lacked inter-independence with other trials, or lacked peer review.
Data extraction and quality assessment
One investigator (EB) carried out the literature search and data extraction. Two investigators (FP and RL) independently reviewed the studies and determined whether they met prespecified criteria in addition to verifying the extracted data with complete agreement. Utilising the techniques described previously,12 quality was assessed based on the design of the studies and is described in Supporting Table S1. The studies included in the meta-analysis were in addition assessed for quality based on the Jadad 3-point scale [Was the study described as randomised (this includes words, such as randomly, random and randomisation)? Yes = 1, No = 0. Was the method used to generate the sequence of randomisation described and appropriate (table of random numbers, computer-generated, etc.)? Yes = 1, No = 0. Was the study described as double-blind? Yes = 1, No = 0].13
Histological parameters
Three well-described scoring systems, including the systems described by Brunt, et al., Promrat, et al., and Kleiner, et al.,14–16 were used to assess liver histology in various publications. Data used in our meta-analysis were extracted as reported by the authors based on the assumption that the improvement or worsening will be captured uniformly by all the three systems as previously shown.12, 17
Meta-analysis
For each eligible study, odds ratios (ORs) and their respective 95% confidence intervals (CIs) were estimated to evaluate effect sizes of the primary outcomes (liver histology parameters). As some of the trials reported few events of interest, the Peto method was used.18 An OR greater than 1.00 indicated improved histological findings with TZD use compared to placebo. As patient populations may have differed among studies (e.g. treatment regimens, lifestyle modifications and populations), a random-effects model incorporating the variance between study findings in a weighted average of rate ratios (weighted according to sample size), was used to estimate the combined (summary) OR and its 95% CI. As confirmation, OR estimates, P-values and confidence limits were also computed using exact stratified methods with statxact software, version 6 (Cytel Inc., Cambridge, MA). The Cochran Q statistic19 and Inconsistency Index (I2) were used for assessing heterogeneity among studies for which results from three or more trials could be pooled.20 Publication bias was examined by visual inspection of funnel plots for symmetry and using the Egger test to determine whether there is an association between test accuracy estimates and their precision. Except for the exact methods, all statistical procedures were performed using Comprehensive Meta Analysis software, version 2 (Biostat, Englewood, NJ).21 Statistical significance for the two-sided P-values was set, a priori, as <0.05.
Calculation or estimation of weighted averages
Weighted average was calculated for biochemical and anthropometric parameters extracted from various studies. For each qualifying study, the baseline and end-of-study population means and standard deviations (SD) were estimated. For the means, either the reported sample mean or sample median was used. For standard deviations, the reported value was used if available; if instead the inter-quartile range was available, an estimate of the associated SD was computed; if only the baseline SD was reported, that was also used as the SD for the end-of-study (and vice versa). In the few cases where no SD was reported, the value was calculated from the reported mean difference from baseline to end-of-study, together with the reported P-value and sample size. These separate mean values, from each study, were combined into an overall weighted mean using the individual study sample sizes and SDs. For studies not reporting a paired P-value comparing baseline and end-of-study values, the baseline and end-of-study estimated means and SDs were used to calculate a P-value, assuming a correlation between pre and postvalues typically seen in studies such as these. Finally, the overall pooled one-sided P-value was computed using Fisher method of combining one-sided P-values from each study. The stata statistical software, version 11 (Stata Corp, College Station, TX) was used for these statistical calculations.
RESULTS
Study characteristics
Characteristics of the 11 studies identified by specific criteria on initial screening (Figure 1) are outlined in Supporting Table S1.31–34 Characteristics of the four studies included in the meta-analysis are outlined in Table 1.
Table 1.
Characteristics of studies included in the meta-analysis
| Study | Year | Type of study | Country | Duration, month | Dose, mg/day | Number of patients in treatment group (dropouts) | Number of patients in placebo group (dropouts) | Design | Blinding | Allocation concealment, yes/no | Sample size calculations in article, yes/no | Intention-to-treat analysis, yes/no | Inclusion of diabetics, yes/no | Histological scoring system used |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pioglitazone | ||||||||||||||
| Sanyal et al.24 | 2010 | Multicentre | US | 24 | 30 | 80 (14) | 83 (12) | Randomised, placebo-controlled | Double-blind | Yes | Yes | Yes | No | Kleiner et al.13 |
| Aithal et al.22 | 2008 | Multicentre | UK | 12 | 30 | 37 (6) | 37 (7) | Randomised, placebo-controlled | Double-blind | Yes | Yes | No | No | Promrat et al.12 |
| Belfort et al.23 | 2006 | Multicentre | US | 6 | 45 | 26 (3) | 21 (4) | Randomised, placebo-controlled | Double-blind | Yes | Yes | Yes | Yes | Kleiner et al.13 |
| Rosiglitazone | ||||||||||||||
| Ratziu et al.25 | 2008 | Single centre | France | 12 | 4 × 1 month then 8 × 11 months | 32 (1) | 31 (0) | Randomised, placebo-controlled | Double-blind | Yes | Yes | Yes | Yes | Brunt et al.11 and Kleiner et al.13 |
US, United States; UK, United Kingdom.
Characteristics of study patients
Baseline characteristics of patients in the 11 individual studies are shown in Supporting Table S2. Baseline characteristics of patients in the four studies included in the meta-analysis are shown in Table 2.
Table 2.
Baseline characteristics of the subjects included in the meta-analysis
| Study | Age of treatment group ± s.d. [range] | Age of placebo group ± s.d. [range] | Gender of treatment group (M/F) | Gender of placebo group (M/ F) | Race of treatment group | Race of placebo group | Body-mass index of treatment group ± s.d. [range] | Body-mass index of placebo group ± s.d. |
|---|---|---|---|---|---|---|---|---|
| Pioglitazone | ||||||||
| Sanyal et al.24 | 47 ± 12.6 | 45.4 ± 11.2 | 33/47 | 35/48 | White 74/80 | White 74/83 | 34 ± 6 | 35 ± 7 |
| Aithal et al.22 | 52[28-71] | 55[27-73] | 26/11 | 19/18 | NR | NR | 29.8 ± 3.0 | 30.8 ± 4.1 |
| Belford et al.23 | 51 ± 7 | 51 ± 10 | 14/12 | 7/14 | NR | NR | 33.5 ± 4.9 | 32.9 ± 4.4 |
| Rosiglitazone | ||||||||
| Ratziu et al.25 | 53.1 ± 11.5 | 54.1 ± 10.4 | 19/13 | 18/13 | NR | NR | 31.5 ± 6 | 30.5 ± 4.4 |
N/A, not applicable; NR, not recorded.
Meta-analysis: changes in liver histology with TZD
All four studies22–25 included in the Peto OR meta-analysis for assessing changes in liver histology were RCPTs (Table 1). Based on the Jadad 3-item scoring system for quality assessment of RPCTs,13 all studies were of good quality with an overall quality score of 3.00 of a maximum score of 3. One study was conducted in the UK,22 two in the US23, 24 and one in France.25 Three were multicentre studies22–24 and one was conducted at a single centre.25
The average age of patients included in the four studies was 50.8 years for the treatment group and 51.4 years for placebo. A total of 56.2% were male in the treatment group; 46.3% were male in the placebo group.
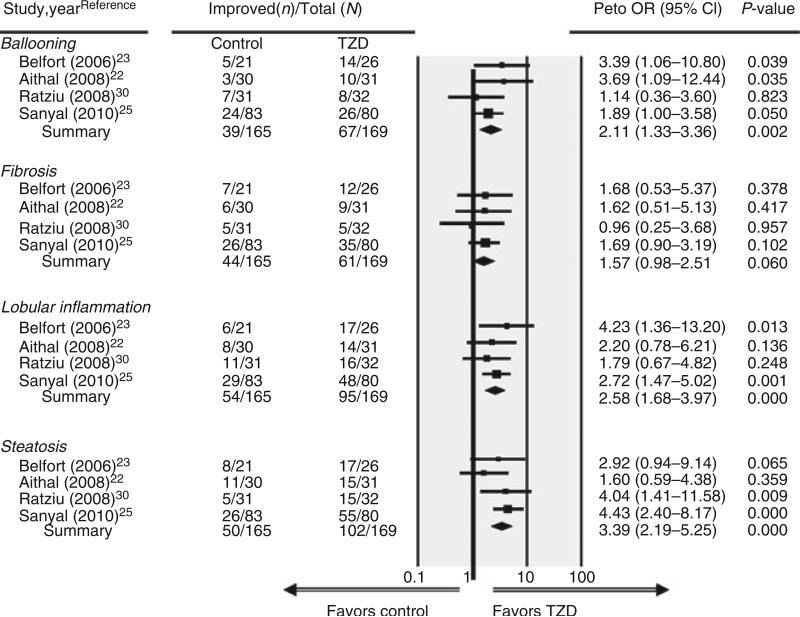
Pooled results from these four trials (Figure 2) found that TZD use (n = 169) was associated with an increased likelihood for improvement in the following histological parameters of the liver: ballooning degeneration (OR, 2.11 [95% CI, 1.33–3.36]), lobular inflammation (OR, 2.58 [95% CI, 1.68–3.97]) and steatosis (OR, 3.39 [95% CI, 2.19–5.25]) compared with placebo (n = 165 patients) (all P ≤ 0.002). A borderline significant trend (P = 0.06) was also observed for improvement in fibrosis (OR, 1.57 [95% CI, 0.98–2.51]). Based on the pooled results from only two studies, TZD use (n = 52) vs. placebo (n = 58) was associated with significant improvement (P < 0.001) for combined necroinflammation (OR, 6.52 [95% CI, 3.03–14.06]),23, 25 while portal inflammation did not change (OR, 1.07 [95% CI, 0.43–2.66]).
Figure 2.
Forest plot of randomised controlled trials comparing the effect of thiazolidinediones on histological parameters in non-alcoholic steatohepatitis.
Sensitivity analysis
We performed a sensitivity analysis using an exact stratified method for calculating ORs and the histological results were similar: ballooning degeneration (OR, 2.14 [95% CI, 1.30–3.57]), lobular inflammation (OR, 2.63 [95% CI, 1.65–4.23]), steatosis (OR, 3.56 [95% CI, 2.20–5.81]), fibrosis (OR, 1.57 [95% CI, 0.95–2.61]), combined necroinflammation (OR, 7.80 [95% CI, 3.02–22.00]) and portal inflammation (OR, 1.07 [95% CI, 0.39–3.98]).
Assessment of heterogeneity and publication bias
For primary outcomes in which the results from all four trials could be pooled, the P-values for the Q statistic were nonsignificant (all P ≥ 0.387), indicating a lack of heterogeneity across studies. Although a sample of only four studies was small, there was no obvious publication bias for any of these outcomes of interest among the studies, based on visual inspection of funnel plots and the Egger regression method (all P ≥ 0.371).
Sub-analysis of pioglitazone vs. placebo
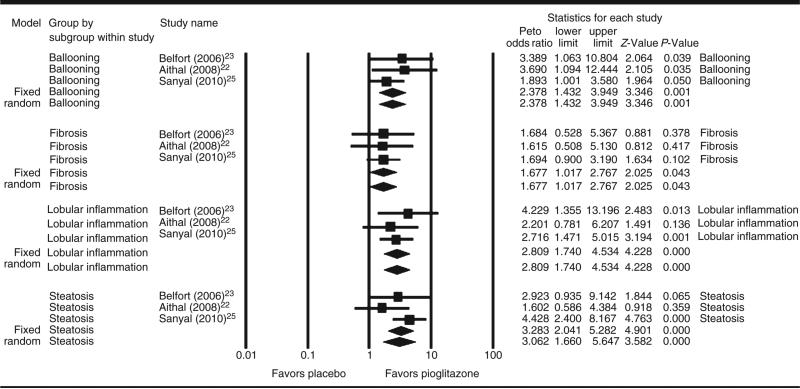
We performed a sub-analysis including the three RPCT comparing pioglitazone vs. placebo. Pooled results from these three trials (Figure 3) found that pioglitazone (n = 137) was associated with an increased likelihood for improvement in the following histological parameters : ballooning degeneration (OR, 2.39 [95% CI, 1.43–3.95]), lobular inflammation (OR, 2.81 [95% CI, 1.74–4.53]), steatosis (OR, 3.28 [95% CI, 2.04–5.28]) and fibrosis (OR, 1.68 [95% CI, 1.02–2.77] compared with placebo (n = 134) (all P ≤ 0.05).
Figure 3.
Forest plot of randomised controlled trials comparing the effect of pioglitazone on histological parameters in non-alcoholic steatohepatitis.
Changes in biochemical parameters in patients with TZD
Pooled analysis of the changes in biochemical parameters was performed by calculating or estimating the weighted averages. These changes are summarised in Tables 3 and 4.
Table 3.
Pre-treatment and post-treatment weighted means of biochemical variables in TZD-treated and placebo patients with NASH
| Parameter | Arm | Weighted mean pre-treatment | Weighted mean post-treatment | P-value |
|---|---|---|---|---|
| BG (mg/dL) | Control | 98.51 (n = 221) | 100.02 (n = 212) | <0.0003 |
| TZD | 97.78 (n = 319) | 93.61 (n = 304) | <0.0001 | |
| HgbAlc (%) | Control | 5.77 (n = 89) | 5.56 (n = 89) | 0.2 |
| TZD | 6.08 (n = 185) | 5.71 (n = 180) | <0.0001 | |
| Fasting insulin (μU/mL) | Control | 15.04 (n = 138) | 14.12 (n = 138) | <0.001 |
| TZD | 15.89 (n = 239) | 10.75 (n = 234) | <0.0001 | |
| Total-C (mg/dL) | Control | 204.81 (n = 190) | 195.48 (n = 181) | <0.002 |
| TZD | 195.76 (n = 287) | 200.27 (n = 272) | <0.04 | |
| LDL-C (mg/dL) | Control | 125.06 (n = 141) | 119.43 (n = 132) | 0.14 |
| TZD | 121.56 (n = 221) | 124.30 (n = 211) | 0.047 | |
| HDL-C (mg/dL) | Control | 44.00 (n = 172) | 44.46 (n = 163) | 0.4 |
| TZD | 44.28 (n = 253) | 46.53 (n = 243) | <0.001 | |
| Trig (mg/dL) | Control | 163.85 (n = 190) | 155.12 (n = 212) | 0.2 |
| TZD | 169.29 (n = 319) | 157.27 (n = 304) | <0.001 | |
| AST (U/L) | Control | 44.69 (n = 153) | 33.80 (n = 144) | <0.0001 |
| TZD | 38.83 (n = 250) | 30.28 (n = 235) | <0.0001 | |
| ALT (U/L) | Control | 77.23 (n = 221) | 49.44 (n = 212) | <0.0001 |
| TZD | 56.33 (n = 319) | 37.65 (n =272 | <0.0001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BG, blood glucose; HgbA1c, haemoglobin A1C; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; NASH, non-alcoholic steatohepatitis; Total-C, total cholesterol; Trig, triglycerides; TZD, thiazolidinediones.
Significant p-values are in bold.
Table 4.
Pre-treatment and post-treatment weighted means in anthropometric variables in TZD and placebo treated patients with NASH
| Parameter | Arm | Weighted mean pre-treatment | Weighted mean post-treatment | P-value |
|---|---|---|---|---|
| Wt (kg) | Control | 92.48 (n = 166) | 91.65 (n = 157) | 0.3 |
| TZD | 90.18 (n = 251) | 94.17 (n = 236) | <0.0001 | |
| BMI (kg/m2) | Control | 31.80 (n = 190) | 31.16 (n = 181) | <0.01 |
| TZD | 31.12 (n = 287) | 31.85 (n = 272) | <0.0001 | |
| Total body fat (%) | Control | 37.64 (n = 129) | 37.09 (n = 120) | 0.05 |
| TZD | 34.60 (n = 209) | 36.40 (n = 199) | <0.0001 | |
| Liver fat (%) | Control | 17.20 (n = 21) | 17.20 (n = 21) | 0.5 |
| TZD | 21.28 (n = 44) | 10.08 (n = 44) | <0.0001 | |
| Fasting FFA (μEq/L) | Control | 720.00 (n = 21) | 798.00 (n = 21) | 0.07 |
| TZD | 735.60 (n = 44) | 606.99 (n = 44) | <0.003 | |
| Waist circumf (cm) | Control | 105.99 (n = 108) | 103.81 (n = 99) | 0.06 |
| TZD | 101.00 (n = 183) | 100.17 (n = 173) | 0.05 | |
| Waist-hip ratio | Control | 0.97 (n = 37) | 0.96 (n = 37) | 0.06 |
| TZD | 0.96 (n = 115) | 0.92 (n = 115) | <0.002 |
BMI, body-mass-index; FFA, fasting free fatty acids; fasting waist circumf, waist circumference; NASH, non-alcoholic steatohepatitis; TZD, thiazolidinediones; Wt, weight.
Significant p-values are in bold.
Both AST and ALT consistently improved with time and the decreases were highly significant both in control and TZD-patients. Although far fewer studies reported extractable data for total bilirubin, a significant decrease occurred only in TZD-treated patients.
Fasting plasma glucose slightly but significantly increased in control patients, whereas it slightly but significantly decreased in those with TZD treatment. Fasting plasma insulin significantly decreased both in the control and TZD-treated patients, but the magnitude of change was apparently greater in those receiving TZD. A decrease in haemoglobin A1c (HbA1c) was significant only in TZD-treated patients. The concentration of free (nonesterified) fatty acids (FFA or NEFA) showed a borderline significant increase with time in the controls, whereas FFA significantly decreased in TZD-treated patients.
The total cholesterol (TC) significantly decreased, whereas the low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C) and triglycerides (TG) did not change significantly with time in control patients. The TC and LDL-C borderline significantly decreased, whereas HDL-C significantly increased and TG significantly decreased with TZD treatment.
Changes in Body Fat and BMI in patients with TZD
The average increase in body weight (kg), BMI, % body fat with TZD use was 3.99, 0.73 and 1.8, respectively; these were all statistically significant. Liver fat decreased by a total of 11.2% (pre-treatment: 21.3% vs. post-treatment 10.1%, P-value < 0.0001) with TZD use.
Adverse events in patients with TZD
Supporting Table S3 lists the most common adverse events. Most reported adverse events in the treatment groups were mild and related to gastrointestinal upset, lower extremity oedema and generalised fatigue. Only four patients in two studies15, 24 reported serious adverse events that were thought to be unrelated to the treatment medication (myocardial infarction, recurrent autoimmune uveitis, arthritis, transient ischaemic attack).
DISCUSSION
The main finding of the meta-analysis was that the histological parameters of ballooning necrosis, combined necroinflammation, steatosis, lobular inflammation and to a lesser degree, fibrosis, are all improved with the TZD vs. control group. In a sub-analysis, pioglitazone showed significant improvement in improving fibrosis compared with placebo. This is in contrast to a previous meta-analysis of randomised trials for the treatment of nonalcoholic fatty liver disease35 which not only included three22, 23, 30 of the four22, 23, 25, 30 studies included in this meta-analysis but also included two open label trials24, 27 and found that TZDs improved histological steatosis and inflammation but not fibrosis. An additional meta-analysis of randomised trials28 which included all four22–25 studies included in this meta-analysis but included two open label trials26, 27, found a reduction in steatosis and ballooning, but no improvement in lobular inflammation or fibrosis compared with control. In this meta-analysis, we excluded open label trials, and studies in which the control group received active treatment. Furthermore, we believe that our results are different from these two previous meta-analyses because we conducted a sub-group analysis to assess the efficacy of pioglitazone alone. Therefore, we included three randomised placebo-controlled trials that compared the efficacy of pioglitazone vs. placebo, and for this subgroup analysis, we excluded the RCT evaluating the efficacy of rosiglitazone vs. placebo.
The main finding of the pooled analysis is that serum AST (mean reduction of 8.55 U/L) and ALT (mean reduction of 18.65 U/L) levels declined significantly in NASH patients treated with TZDs compared with controls. In terms of anthropometric findings, in TZD-treated patients, there was significant weight gain (mean increase in body weight was 3.99 kg) associated with significant increase in BMI. The percentage total body fat markedly and highly significantly increased with TZD treatment (mean increase in body fat was 1.8%).
There is currently no widely accepted treatment for non-alcoholic steatohepatitis. Many pharmacological agents have been studied, including the class of antidiabetic agents, such as the TZDs. Over the past several years, studies with TZDs in NASH patients have yielded promising results. The results of this study indicate that while TZDs may be beneficial in the treatment of NASH, weight gain and increase in total body fat among subjects receiving these medications may detract from their usefulness.
Strengths of this study included the good quality of the studies included in both the pooled results and the meta-analysis and unidirectional nature of the study findings. The patients included in the pooled analysis were derived from three continents (North America, Europe and Asia). The treatment duration of the majority of the studies was at least 6 months or greater. There were few dropouts in the trials, and follow-up was adequate. In addition, the results of the meta-analysis were uniform across studies with just minor variability. All these suggest good generalisability of these reported findings.
Limitations of this study included the variation in study design and in reporting of both biochemical and histological parameters. In terms of study design, there were varying doses of the study medication, and some of the trials enforced strict diet and exercise regimens in addition to or in lieu of the treatment, while others did not incorporate any lifestyle modification into the design. In addition, inclusion criteria varied with some trials allowing type 2 diabetics, while others excluding such patients. In reporting of biochemical parameters, although ALT and AST were consistently reported, most other biochemical parameters were not recorded across all studies and this made pooling data difficult. In regard with the histological parameters, there were three scoring systems used to report the liver biopsies.15, 16, 29 The score range for ballooning necrosis, steatosis, hepatocellular injury, lobular inflammation and portal inflammation differed between these systems, making difficult to compare the degree of changes in these parameters. In addition, the recorded results varied extensively with some studies, only reporting the percentage of patients with an improvement in score, while others reporting the change in score. This indicates a need for standardisation of study design, outcomes, histological scoring and reporting in future NASH clinical trials as underscored by a recent expert panel recommendations.30
CONCLUSIONS
In conclusion, serum ALT levels and histological parameters improve in NASH patients treated with the TZDs. There is a suggestion that pioglitazone might reverse fibrosis in NASH. Further studies are needed to assess antifibrotic effects of TZDs. Results of the current study suggest that future trials in NASH would benefit from standardising study design, treatment outcomes and histological scoring.
Supplementary Material
ACKNOWLEDGEMENTS
Declaration of personal interests: Rohit Loomba has served as a consultant for Corgenix Inc and an advisory board member for Gilead Inc and Daiichi Sankyo Inc, and has received research funding from the AGA-Foundation, Daiichi Sankyo Inc and National Institutes of Health. Declaration of funding interests: This work is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award. Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association to Rohit Loomba, MD, MHSc. This research was funded in part with the support of the UCSD Digestive Diseases Research Development Center, U.S. PHS grant #DK080506. This work was supported in part by the National Institute of Health grants RO1AG28507, R37AG007181, and RO1DK31801 to Elizabeth Barrett-Connor, MD.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 3.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689–92. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 4.Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of non-alcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–40. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 5.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282:1519–22. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 6.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D'Agostino RBS. Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the framingham heart study. Circulation. 2006;113:2914–8. doi: 10.1161/CIRCULATIONAHA.106.613828. [DOI] [PubMed] [Google Scholar]
- 7.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 8.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 9.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:519–25. doi: 10.1111/j.1572-0241.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 11.Menon KVN, Angulo P, Lindor KD. Severe cholestatic hepatitis from troglitazone in a patient with nonalcoholic steatohepatitis and diabetes mellitus. Am J Gastroenterol. 2001;96:1631–4. doi: 10.1111/j.1572-0241.2001.03809.x. [DOI] [PubMed] [Google Scholar]
- 12.Loomba R, Wesley R, Pucino F, Liang TJ, Kleiner DE, Lavine JE. Placebo in nonalcoholic steatohepatitis: insight into natural history and implications for future clinical trials. Clin Gastroenterol Hepatol. 2008;6:1243–8. doi: 10.1016/j.cgh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Jadad AR, Tugwell P. Assessing the quality of randomized controlled trials. Current issues and future directions. Int J Technol Assess Health Care. 1996;12:195–208. doi: 10.1017/s0266462300009570. [DOI] [PubMed] [Google Scholar]
- 14.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 15.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–96. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 18.Cooper NJ, Jones DR, Sutton AJ. The use of systematic reviews when designing studies. Clin Trials. 2005;2:260–4. doi: 10.1191/1740774505cn090oa. [DOI] [PubMed] [Google Scholar]
- 19.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. Available at: http://www.jstor.org/stable/3001666. [Google Scholar]
- 20.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 21.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2. Biostat; Engelwood, NJ: 2005. [Google Scholar]
- 22.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–84. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 23.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steato-hepatitis. N Engl J Med. 2006;355:2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 24.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) trial. Gastroenterology. 2008;135:100–10. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal AJ, Mofrad PS, Contos MJ, et al. A pilot study of vitamin E versus vita-min E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–15. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 27.Idilman R, Mizrak D, Corapcioglu D, et al. Clinical trial: insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;28:200–8. doi: 10.1111/j.1365-2036.2008.03723.x. [DOI] [PubMed] [Google Scholar]
- 28.Rakoski MO, Singal AG, Rogers MAM, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2010;32:1211–21. doi: 10.1111/j.1365-2036.2010.04467.x. [DOI] [PubMed] [Google Scholar]
- 29.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 30.Ratziu V, Caldwell S, Neuschwander-Tetri BA. Therapeutic trials in nonalcoholic steatohepatitis: insulin sensitizers and related methodological issues. Hepatology. 2010;52:2206–15. doi: 10.1002/hep.24042. [DOI] [PubMed] [Google Scholar]
- 31.Akyuz F, Demir K, Ozdil S, et al. The effects of rosiglitazone, metformin, and diet with exercise in nonalcoholic fatty liver disease. Dig Dis Sci. 2007;52:2359–67. doi: 10.1007/s10620-006-9145-x. [DOI] [PubMed] [Google Scholar]
- 32.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–17. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 33.Omer Z, Cetinkalp S, Akyildiz M, et al. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22:18–23. doi: 10.1097/MEG.0b013e32832e2baf. [DOI] [PubMed] [Google Scholar]
- 34.Wang CH, Leung CH, Liu SC, Chung CH. Safety and effectiveness of rosiglitazone in type 2 diabetes patients with non-alcoholic fatty liver disease. J Formos Med Assoc. 2006;105:743–52. doi: 10.1016/S0929-6646(09)60202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.