Abstract
The CLAVATA1 (CLV1) gene encodes a putative receptor kinase required for the proper balance between cell proliferation and differentiation in Arabidopsis shoot and flower meristems. Impaired CLV1 signaling results in masses of undifferentiated cells at the shoot and floral meristems. Although many putative receptor kinases have been identified in plants, the mechanism of signal transduction mediated by plant receptor-like kinases is largely unknown. One potential effector of receptor kinase signaling is kinase-associated protein phosphatase (KAPP), a protein that binds to multiple plant receptor-like kinases in a phosphorylation-dependent manner. To examine a possible role for KAPP in CLV1-dependent plant development, the interaction of CLV1 and KAPP was investigated in vitro and in vivo. KAPP binds directly to autophosphorylated CLV1 in vitro and co-immunoprecipitates with CLV1 in plant extracts derived from meristematic tissue. Reduction of KAPP transcript accumulation in an intermediate clv1 mutant suppresses the mutant phenotype, and the degree of suppression is inversely correlated with KAPP mRNA levels. These data suggest that KAPP functions as a negative regulator of CLV1 signaling in plant development. This may represent a general model for the interaction of KAPP with receptor kinases.
The shoot meristem in higher plants is the source for all aboveground organs and tissues (Steeves and Sussex, 1989). To continuously generate new organs, the shoot meristem maintains a population of undifferentiated cells at the center of the meristem while directing appropriately positioned progeny cells toward differentiation. Identical processes occur during organ formation at the flower meristem (Steeves and Sussex, 1989; Weigel and Clark, 1996). Proper balance between cell proliferation and differentiation requires the Arabidopsis CLAVATA1 (CLV1) gene. clv1 mutants accumulate undifferentiated cells at both the shoot and flower meristems, leading to disrupted organ placement, enlarged stems, and additional organs generated on the larger clv1 flower meristem (Leyser and Furner, 1992; Clark et al., 1993, 1995). CLV1 has been postulated to either inhibit proliferation of undifferentiated cells at the meristem or promote the transition of these cells toward differentiation. CLV1 encodes a putative receptor kinase (Clark et al., 1997) with a predicted extracellular domain composed of 21 tandem LRRs similar to those in mammalian glycoprotein hormone receptors (Jiang et al., 1995), a hydrophobic membrane-spanning domain, and a predicted intracellular domain containing all of the conserved residues found among Ser/Thr protein kinases (Hanks and Hunter, 1995).
A number of RLKs have been identified in plants. The plant RLKs are predicted to have Ser/Thr specificity (Braun and Walker, 1996), in contrast to the majority of animal RTKs, with which RLKs share structural similarity. The functions of plant RLKs are largely unknown; however, LRR-RLKs have been implicated in developmental processes (Torii et al., 1996; Clark et al., 1997), disease resistance (Song et al., 1995), and hormone response (Li and Chory, 1997).
A LRR-RLK very similar to CLV1 but of unknown function, RLK5, was used to isolate an interacting protein that was named KAPP (Stone et al., 1994). KAPP has three functional domains: an N-terminal type I signal anchor, a KI domain, and a type 2C protein phosphatase catalytic region. Interaction between KAPP and RLK5 is mediated by the KI domain and is dependent on phosphorylation (Stone et al., 1994). KAPP interacts in vitro with a growing subset of plant RLKs (Braun et al., 1997), but the in vivo relevance of KAPP-RLK interactions is unclear. Because of the similarity of CLV1 with these KAPP-interacting RLKs, we hypothesized that KAPP might also interact with CLV1.
In this report we demonstrate that KAPP and CLV1 interact directly using recombinant fusion proteins and co-immunoprecipitation from plant extracts. Furthermore, the availability of mutants impaired in CLV1 signaling permits us to determine whether KAPP participates in the CLV1 signal transduction pathway. Analysis of transgenic clv1 mutant plants with altered levels of KAPP mRNA demonstrated that the clv1 mutant phenotype can be rescued by reducing KAPP mRNA levels. These data support a model for KAPP's functioning as a negative regulator of CLV1 signaling. In an independent study using alternative approaches to examine KAPP-CLV1 interactions, Williams et al. (1997) came to a similar conclusion. This work provides complementary yet distinct evidence for the negative regulation of CLV1 signal transduction by KAPP.
MATERIALS AND METHODS
Recombinant Proteins and Antibody Production
MBP fusions were produced using a modified version of pMalcRI (New England Biolabs), pMalK, and GST fusions were made with a modified version of pGEX-2T (Pharmacia), GTK. Both vectors were modified to contain a protein kinase A recognition site at the junction to allow 32P labeling of the proteins. For in vitro-binding studies, the protein kinase catalytic domain of CLV1 (amino acids 665–980; accession no. U96879) was subcloned into pMalK. Site-directed mutagenesis to produce a single amino acid substitution at the conserved Lys required for phosphotransfer (Lys-720 to Glu-720; K720E) was made using a PCR-based mutagenesis strategy. Oligonucleotide primers were 5′-TAGACGTCGCGATTAACCGACTCGTTGGCCGT-3′ and 5′-ACGGCCAACGAGTCGGTTAATCGCGACGTCTA-3′.
For antibody production, oligonucleotides primers AntiB (5′-ATGAATTCGGAGTGGTTTTGTTGGAGT-3′) and KinC1 (5′-ATCTAGATTCAGAACGCGATCAAGTT-3′) were used to amplify a 315-bp fragment of the CLV1 C terminus encoding a 10.9-kD polypeptide, which was subcloned into pMalK. This region of CLV1 was predicted to generate antibodies specific to CLV1, because only CLV1 is detected on low-stringency Southern-blot analysis using this fragment as a hybridization probe (Clark et al., 1997). Primer 5D2 (5′-GGGAATTCCTGGAAAAGGATCGA-3′) and a universal primer were used to amplify KAPP (amino acids 162–581), which was subcloned into pMalcRI. KAPP antibodies were affinity purified on a column with immobilized GST fusion to the KI domain (KAPP amino acids 99–337; accession no. U09505), which was the same construct used for the protein probe for in vitro-binding studies. Recombinant MBP and GST fusion proteins were expressed in Escherichia coli and purified by affinity chromatography on amylose-agarose resin or glutathione-agarose resin, respectively, essentially as described previously (Horn and Walker, 1994). Protein concentration was determined by the Bradford method (Bradford, 1976).
The antigen for CLV1 antibody production was purified as a MBP fusion and subjected to 15% SDS-PAGE (Laemmli, 1970) before injection into rabbits by Cocalico Biologicals, Inc. (Reamstown, PA). The KAPP antibodies were generated in rabbits by the University of Missouri Animal Care Facility using MBP-KAPP (5D2) antigen directly after purification. CLV1 preimmune and immune sera were used directly for immobilization (see “Immunoprecipitations”), whereas the KAPP antisera were first subjected to affinity purification against GST-KID (Koff et al., 1992).
Autophosphorylation
For autophosphorylation experiments, 1 μg of affinity-purified recombinant fusion protein was incubated with [γ-32P]ATP in kinase buffer (50 mm Hepes, pH 7.4, 10 mm MgCl2, 10 mm MnCl2, 1 mm DTT, and 10 μm cold ATP) for 1 h at room temperature. Reaction products were separated by 10% SDS-PAGE (Laemmli, 1970), dried, and exposed to film.
PAA Analysis
The PAA content of autophosphorylated MBP-CLV1CAT was analyzed essentially as described previously (Boyle et al., 1991). Samples were acid hydrolyzed in 6 n HCl (Pierce) for 1 h at 110°C, repeatedly lyophilized to remove the HCl, resuspended in pH 1.9 electrophoresis buffer containing PAA standards, and applied to TLC plates (Merck, Darmstadt, Germany). Samples were electrophoresed at 1.5 kV for 30 min in pH 1.9 buffer (0.22% formic acid, 0.78% acetic acid) in the first dimension, followed by electrophoresis in pH 3.5 buffer (0.5% acetic acid, 0.05% pyridine) at 1.3 kV for 25 min in the second dimension using a TLE system (model HTLE 7000, C.B.S. Scientific Co., Del Mar, CA). PAA standards corresponding to phospho-Ser, phospho-Thr, and phospho-Tyr (Sigma) were visualized by spraying the plates with 0.25% ninhydrin in acetone. Plates were exposed to imaging plates (Bas-IIIS, Fuji Photo Film Co., Tokyo, Japan) to detect 32P.
Two-Dimensional TLE/TLC
Tryptic phosphopeptides were analyzed by two-dimensional TLE/TLC essentially as described previously (Boyle et al., 1991). Autophosphorylated and trypsin-treated samples applied to TLC plates were separated by electrophoresis for 40 min at pH 1.9. The second-dimension separation was achieved by ascending chromatography in phosphochromatography buffer (37.5% n-butanol, 25% pyridine, 7.5% acetic acid) for 16 h, followed by exposure to film.
In Vitro-Binding Assays
The GST-KID fusion was expressed in E. coli, purified, and labeled with [γ-32P]ATP as described previously (Stone, 1997). Purified recombinant fusion proteins (1 μg) were separated on 10% SDS-PAGE gels and transferred to PVDF membranes (Harlow and Lane, 1988). PVDF membranes were blocked for 4 h at 4°C in 25 mm Hepes, pH 7.5, 5 mm MgCl2, 1 mm KCl, and 5% nonfat dry milk, and then incubated overnight with approximately 2.5 × 105 cpm mL−1 32P-labeled GST-KID in B buffer (25 mm Hepes, pH 7.5, 7.5 mm KCl, 0.1 mm EDTA, 2.5 mm MgCl2, and 1% nonfat dry milk). Filters were washed three times in B buffer for 10 min, dried, and exposed to radiographic film.
Immunoprecipitations
CLV1 UM174 immune and preimmune sera (200 μL each) were dialyzed (10,000 Mr cutoff) against 0.1 m NaHCO3 and 0.5 m NaCl. Protein concentration was determined by A280. Dialyzed preimmune and immune sera were cross-linked to cyanogen bromide-activated Sepharose 4 Fast-Flow beads (Pharmacia) according to the manufacturer's recommendations and stored in TSA solution (0.01 m Tris-Cl, pH 8.0, 0.14 m NaCl, and 0.025% NaN3) at 4°C.
Cauliflower (Brassica oleracea) meristem tissue (50 g) was ground in a Waring prechilled blender with 100 mL of 50 mm Hepes, pH 7.4, 10 mm EDTA, 0.1% Triton X-100, 0.1 mm PMSF, 5 μg mL−1 aprotinin, 10 μg mL−1 chymostatin, and 1 μg mL−1 leupeptin. The extract was centrifuged (3000g for 10 min at 4°C) repeatedly until all flocculate was removed from the supernatant, which was stored at −20°C.
Cauliflower extract (50 mL) was passed through a 0.45 μm filter and incubated with approximately 5 mL of swelled (1 g dry weight) preimmune coupled beads at 4°C for 2 h. The reaction was spun at 1000g for 1 min at 4°C. One-half of the supernatant was then incubated with immune beads and the other half with fresh preimmune beads for 2 h at 4°C. Both reactions were centrifuged at 1000g for 1 min. Beads were washed once each in wash buffer (10 mm Tris-Cl, pH 8.0, 140 mm NaCl, 0.025% NaN3, 0.5% Triton X-100, and 0.5% SDS) and Tris/Triton/NaCl (50 mm Tris-Cl, 0.1% Triton X-100, and 0.5 m NaCl) buffer, pH 8.0 and 9.0. The beads were eluted twice with 5 mL of triethanolamine solution (50 mm triethanolamine, pH approximately 11.5, 0.1% Triton X-100, and 150 mm NaCl) into tubes containing 0.2 volume of 1 m Tris-Cl, pH 6.7.
Eluates were concentrated to approximately 100 μL in Centricon 3000 columns. Preimmune and immune eluates (30 μL) were separated by 12.5% SDS-PAGE (Laemmli, 1970), and transferred to nitrocellulose with a semidry transfer cell (Bio-Rad) for 20 min at 12 V (Harlow and Lane, 1988). Nitrocellulose filters were blocked overnight in 2.5% dry milk in PBST (1× PBS and 0.5% Tween 20) and washed three times in PBST. Filters were incubated with a 1:500 dilution of affinity-purified M12 KAPP antibody or CLV1 UM174 antibody for 2 h. Each filter was washed three times in PBST, incubated for 1 h with a 1:30,000 dilution of goat anti-rabbit IgG horseradish peroxidase conjugate (Bio-Rad), again washed three times in PBST, and then incubated for 1 min in 2 mL of oxidizing reagent plus 2 mL of luminol reagent from a western chemiluminescent kit (Renaissance, NEN-DuPont). Filters were then exposed to film for 1 to 2 min.
Transgenic Plant Generation
A construct consisting of the cauliflower mosaic virus 35S promoter fused to the KAPP cDNA in the sense orientation with a nopaline synthase 3′ terminator was subcloned into the binary vector pGA482 (An et al., 1985). Transformation of Agrobacterium tumefaciens strain GV3101 was accomplished by electroporation and confirmed by Southern-blot analysis. Arabidopsis (Landsberg erecta clv1-1 and clv1-6) plants were transformed with A. tumefaciens by the vacuum-infiltration method, and transformants were selected by sowing the resulting seeds on kanamycin-containing plates (Bechtold et al., 1993). Several independent lines were examined for a suppression of the clv1 phenotype.
Northern-Blot Analysis
RNA was isolated using the RNeasy Plant Mini Kit (Qiagen, Chatsworth, CA) from 100 mg of inflorescence tissue. Concentration was determined by A260, and 10 μg of RNA from each sample was separated on a 1.0% agarose-formaldehyde gel (Brown and Mackey, 1993). RNA was transferred to Hybond-N membranes overnight using 10× SSC. The membrane was baked in a vacuum oven at 80°C for 2 h and incubated for 2 h at 37°C in 5× SSPE, 50% formamide, 0.5% SDS, and 5× Denhardt's reagent. KAPP cDNA was used as a template for a PCR reaction that amplified a 715-bp fragment from oligonucleotide primers 5′-GGGATTTGCAGAGACCA-3′ and 5′-CTTTGTTGTTGT TCCCA-3′, used as a template in a random-primed labeling reaction in the presence of [α-32P]dCTP. Unincorporated nucleotides were removed using a nucleotide-removal kit (Qiagen). 18S rRNA probe was generated by random-primed labeling of a DNA fragment corresponding to the 18S rRNA sequence (accession no. X02623). The membrane was hybridized overnight at 37°C, washed once in 5× SSPE and 0.5% SDS at 37°C for 30 min, washed twice in 2× SSPE at 37°C for 30 min each, and exposed in a molecule-imaging system (model GS363, Bio-Rad). The amount of signal in each band was determined using the Molecular Analyst program (Bio-Rad).
RESULTS
CLV1 and KAPP Interact in Vitro
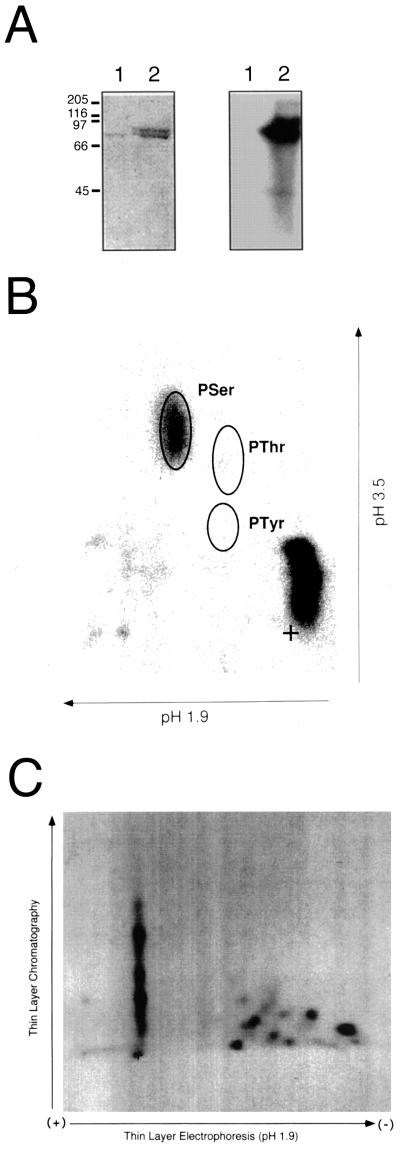
We first established that CLV1 encodes a functional protein kinase. The CLV1 protein kinase catalytic domain, expressed as a recombinant fusion protein (MBP-CLV1) in E. coli, was capable of autophosphorylation when incubated with [γ-32P]ATP. Site-directed mutagenesis of a conserved active-site residue (K720E) abolished this activity, indicating that incorporation of 32P requires an active kinase domain (Fig. 1A). PAA analysis demonstrated that autophosphorylation occurred exclusively on Ser residues (Fig. 1B), and analysis of CLV1 phosphopeptides generated by trypsin digestion indicated that multiple sites were phosphorylated (Fig. 1C).
Figure 1.
CLV1 encoded an active protein kinase that autophosphorylates on multiple Ser residues. A, MBP fusions to CLV1CAT and CLV1CAT(K720E), which contains a point mutation at the conserved Lys required for phosphotransfer, were expressed in E. coli. Recombinant MBP-CLV1CAT is capable of incorporation of 32P when incubated with [γ-32P]ATP, indicating that it autophosphorylates (lanes 2), whereas the mutated version, MBP-CLV1CAT(K720E), is inactive (lanes 1). On the left is a Coomassie blue-stained SDS-PAGE gel and on the right the corresponding autoradiogram. Sizes of molecular mass standards in kilodaltons are indicated on the left. B, Autophosphorylation of MBP-CLV1CAT occurred on Ser residues. Autophosphorylated MBP-CLV1CAT was excised from an SDS-PAGE gel, hydrolyzed to individual amino acids in 6 n HCl, separated by two-dimensional TLE, and exposed to film. Positions of PAA standards (PSer, PThr, and PTyr) are indicated. C, Autophosphorylation of MBP-CLV1CAT occurred at multiple sites. Autophosphorylated MBP-CLV1CAT was excised from an SDS-PAGE gel, treated with trypsin, separated by two-dimensional TLE/TLC, and exposed to film. Tryptic peptides were applied to a cellulose plate (+) and resolved by electrophoresis in pH-1.9 buffer, followed by ascending chromatography. {/ANNT;;;left;top}
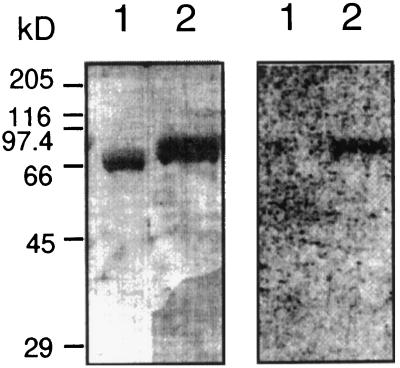
To determine whether CLV1 interacts with the KI domain of KAPP, active and inactive CLV1 fusion proteins were transferred to a PVDF membrane and incubated with 32P-labeled, KI-containing fusion protein. Interaction was observed only between the KI domain and the active, phosphorylated CLV1 kinase domain (Fig. 2). These results are consistent with the phosphorylation-dependent interaction observed with other RLKs (Stone et al., 1994; Braun et al., 1997).
Figure 2.
The KI domain of KAPP interacted with the autophosphorylated form of CLV1. Affinity-purified recombinant fusion proteins were subjected to SDS-PAGE, electrophoretically transferred to PVDF, and probed with 32P-labeled fusion protein containing the KI domain. Molecular mass markers are indicated on the left in kilodaltons. On the left is a gel stained with Coomassie blue and on the right the corresponding autoradiogram after probing with GST-KI. The KI domain was capable of interacting with the catalytic domain of CLV1 (MBP-CLV1CAT) (lanes 2); however, the KI domain could not bind to the mutated form of CLV1 (MBP-CLV1CAT[K720E]) (lanes 1), which was incapable of autophosphorylation. Control blots probed with 32P-labeled GST showed no binding.
CLV1 and KAPP Interact in Vivo
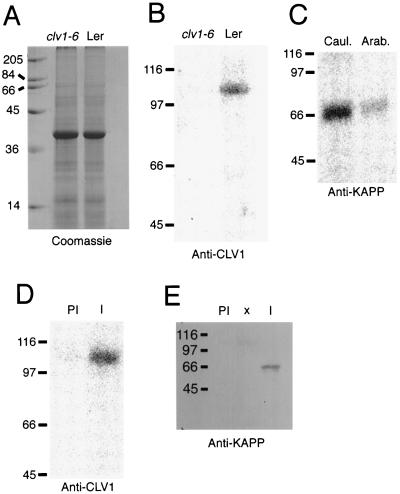
To demonstrate that this interaction occurs in plant cells, polyclonal antibodies were generated to portions of KAPP and CLV1 expressed as recombinant proteins. Specificity of the CLV1 antibodies was assessed by comparing the signals on immunoblots of extracts from wild-type (Landsberg erecta) and clv1-6 plants. No immunoreactive polypeptides within the range of detection were expected in extracts from clv1-6 plants, because the mutation in this allele is predicted to result in a truncated protein lacking the C-terminal protein kinase domain (Clark et al., 1997) to which the CLV1 antisera was directed. Although equivalent amounts of protein were analyzed (Fig. 3A), no signal was present in extracts from clv1-6 plants, whereas a band corresponding to the predicted molecular mass of CLV1 (105 kD) was detected in wild-type plant extracts (Fig. 3B).
Figure 3.
KAPP and CLV1 associated in vivo. Antibodies raised to recombinant KAPP and CLV1 were used to co-immunoprecipitate the proteins from cauliflower meristem extracts. A and B, Demonstration of the specificity of CLV1 antibodies. Extracts (25 μg lane−1) from mutant (clv1-6) and wild-type (Landsberg erecta [Ler]) Arabidopsis inflorescence tissue, including mature flowers, cauline leaves, and some stem tissue, were resolved by SDS-PAGE. A, Coomassie blue-stained gel. B, Results of chemiluminescence detection of immunoreactive polypeptides after incubation with CLV1 antisera. C, Proteins extracted from cauliflower (Caul.) and Arabidopsis (Arab.) were subjected to immunodetection using affinity-purified KAPP antibodies. D and E, Cauliflower meristematic tissue extracts were incubated with immobilized preimmune (PI) or immune (I) antisera against CLV1. Immunocomplexes were eluted, resolved by SDS-PAGE, and immunodetected with immune antisera against CLV1 (D) or KAPP (E). The positions of molecular mass standards are indicated on the left of each panel in kilodaltons.
Because of the limited range of expression of CLV1 (Clark et al., 1997) and the small size of Arabidopsis meristems, co-immunoprecipitation experiments were performed with extracts from cauliflower meristematic tissue. Affinity-purified KAPP antibodies recognized a single polypeptide of the predicted molecular mass (65 kD) in extracts from both Arabidopsis and cauliflower (Fig. 3C). When cauliflower extracts were incubated with immobilized serum, CLV1 immune serum, but not preimmune serum, precipitated an immune complex containing a 105-kD polypeptide detected by CLV1 antiserum (Fig. 3D) and a 65-kD protein recognized by the KAPP antibodies (Fig. 3E). These data demonstrate that KAPP and CLV1 associate in vivo as well as in vitro.
CLV1 and KAPP Participate in Control of Meristem Development
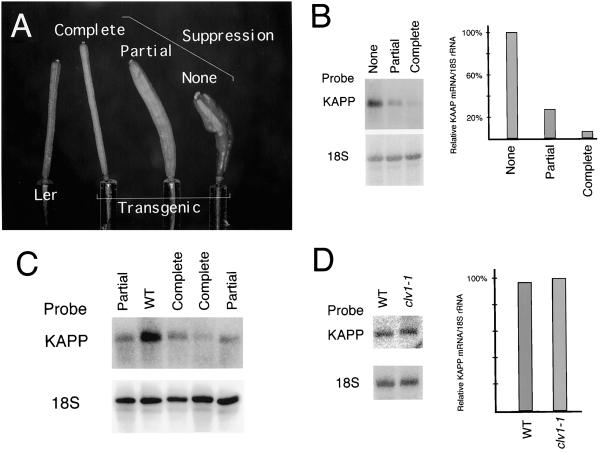
To determine a possible in vivo significance of KAPP-CLV1 interactions, we sought to alter KAPP levels in the partial-loss-of-function clv1-1 and clv1-6 mutant plants in which CLV1 signaling is limited. clv1-1 and clv1-6 plants were transformed with the complete KAPP cDNA under control of the strong, constitutive 35S cauliflower mosaic virus promoter (Bevan et al., 1985). Several independent lines exhibited varying degrees of suppression of the clv1 phenotype. One family of clv1-1 primary transformants displaying heritable phenotypes was further characterized to determine a relationship between the phenotypes and the level of KAPP mRNA. In subsequent generations, individuals with a range of phenotypes were observed and classified into three phenotypic categories: a normal clv1-1 phenotype, a partially suppressed phenotype, and a completely suppressed phenotype (Fig. 4A).
Figure 4.
Suppression of the clv1-1 phenotype correlated with a reduction in KAPP mRNA levels. A, Comparison of typical siliques from a wild-type plant (Landsberg erecta [Ler]) and three clv1-1 plants transformed with a 35S KAPP construct exhibiting varying degrees of suppression of the clv1 phenotype: complete, partial, and none. B, Analysis of RNA isolated from the corresponding transgenic plants shown in A. RNA was isolated from inflorescence tissue, separated in a formaldehyde-containing agarose gel, blotted to Hybond-N membranes, and hybridized with a 32P-labeled KAPP cDNA probe. The membrane was also probed with 18S rRNA as a loading control. On the left is an autoradiogram of the RNA blots and on the right a graph showing the relative levels of KAPP transcript determined by quantitation of the blots. KAPP mRNA levels are expressed as a percentage of that in plants exhibiting no suppression. C, Partially (Partial) and completely (Complete) suppressed 35S KAPP plants accumulated reduced levels of KAPP mRNA compared with untransformed wild-type plants (WT). D, KAPP mRNA levels were equivalent in wild-type (WT) and clv1-1 inflorescence tissues. On the left is an autoradiogram of RNA blots hybridized with KAPP cDNA and 18S rRNA as a loading control and on the right a graph showing the relative levels of KAPP mRNA determined by quantitation of the blots. RNA analysis was repeated with similar results.
To determine if the changes in the clv1-1 phenotype were caused by changes in KAPP transcript levels, RNA was collected from inflorescence tissue of plants from each phenotypic class. Compared with the normal clv1-1 plants by RNA-blot analysis using 18S rRNA as an RNA-loading control standard, the partially suppressed plants exhibited a 70% reduction in KAPP transcript accumulation, whereas the completely suppressed plants exhibited a 94% reduction (Fig. 4B). Direct comparison of KAPP mRNA accumulation in untransformed wild-type plants and several partially suppressed and completely suppressed isolates from the single transformed clv1-1 line indicated that KAPP levels were significantly reduced in the suppressed isolates (Fig. 4C). To confirm that the KAPP mRNA level was not altered by the clv1 mutation, comparison of KAPP transcript accumulation in untransformed wild-type and clv1-1 plants revealed that KAPP was expressed equally in these plants (Fig. 4D). Thus, the differences in KAPP transcript accumulation were not caused by differences in the amount of meristematic tissue present, but appear to have been the result of sense suppression of the endogenous KAPP gene.
Progeny exhibiting suppression of the clv1 phenotype were found to have variable kanamycin resistance. We observed that wild-type plants transformed with CLV1 under the control of the 35S promoter that exhibited sense suppression also had reduced frequency of kanamycin resistance that correlated with the level of sense suppression based on the mutant phenotype. This was perhaps the result of simultaneous suppression of the transgene and the npt II gene that provides kanamycin resistance (A.E. Trotochaud and S.E. Clark, unpublished results). Therefore, kanamycin resistance was compared for the different phenotypic classes. Analysis of kanamycin-resistance rates, which may reflect sense suppression of KAPP, correlated with the level of suppression of the clv1 phenotype (Table I).
Table I.
Kanamycin resistance of control clv1-1 plants and those exhibiting a range of suppression of the clv1 phenotype after transformation with a cauliflower mosaic virus 35S promoter∷KAPP cDNA construct
| Sample | Resistance to Kanamycina |
|---|---|
| % | |
| Control transformedb | 100 |
| Control untransformed | 0 |
| Not suppressedc | 66 |
| Partially suppressedc | 40 |
| Completely suppressedc | 31 |
Resistance is expressed as the percentage of plants that survived treatment on plates containing 50 μg mL−1 kanamycin.
These plants were homozygous for an enhancer trap line 553–643 (Goddijn et al., 1993).
Degree of suppression is based on visual examination of the clv1 phenotype compared with untransformed control clv1-1 plants.
DISCUSSION
CLV1 encodes a receptor kinase required for maintenance of the size of shoot and inflorescence meristems in Arabidopsis. The similarity of CLV1 to other receptor kinases shown to interact with KAPP (Stone et al., 1994; Braun et al., 1997) motivated us to investigate whether KAPP might be involved in CLV1 signal transduction. The availability of clv1 mutants with an easily observable phenotype (Leyser and Furner, 1992; Clark et al., 1993, 1997) permits direct assessment of the in vivo significance of the KAPP-CLV1 interaction.
We demonstrate that KAPP and CLV1 interact in vitro by binding KAPP to immobilized CLV1 recombinant protein. Moreover, the observed interaction is dependent on a functional protein kinase domain, i.e. the KI domain of KAPP fails to bind to an inactive mutant version of CLV1. This is consistent with other previously observed interactions between KAPP and other protein kinases that have been demonstrated to be phosphorylation dependent (Stone et al., 1994; Braun et al., 1997). Using these assay conditions, KAPP has previously been shown to interact with several RLKs in vitro, but it exhibits some specificity and does not interact with several phosphorylated RLKs (Braun et al., 1997; J.M. Stone and J.C. Walker, unpublished data). This in vitro interaction is direct and not dependent on other factors, because the KAPP and CLV1 recombinant proteins were purified before analysis. Although our findings are consistent with separate observations of KAPP and CLV1 interaction using in vitro-translated KAPP and CLV1 (Williams et al., 1997), the association in vitro is not a guarantee of in vivo interaction. For example, MADS-box-containing proteins appear very promiscuous in their binding to other MADS-box proteins, co-precipitating with proteins in vitro that they are unlikely to interact with in vivo (Riechmann et al., 1996).
To overcome the difficulty in interpreting in vitro associations, we sought to determine if CLV1 and KAPP associate in vivo. Because CLV1 is expressed in a small number of cells of the shoot meristem, biochemical analysis of CLV1 from Arabidopsis is less than ideal. Instead, we generated extracts from cauliflower heads, which are composed almost entirely of reiterative meristems and are therefore likely to be an excellent system in which to study CLV1 signaling. Using this system we were able to demonstrate that KAPP and CLV1 co-immunoprecipitate, suggesting that KAPP and CLV1 are associated in vivo, and providing a strong argument for the significance of the in vitro studies that we and others (Williams et al., 1997) have performed. Although co-immunoprecipitation is not evidence of direct interaction, and it is quite likely that other proteins may be associated with the KAPP/CLV1 immunocomplex, in vitro experiments suggest that the interaction is direct. Identification of other proteins in the KAPP/CLV1 signaling complex might be facilitated by the cauliflower extract system. It will be interesting to determine whether KAPP is constitutively associated with CLV1, or if it only does so in response to CLV1 activation. The fact that the CLV1 antibody, which we demonstrated is specific for CLV1, and the affinity-purified KAPP antibody each recognize a protein from cauliflower extracts of the appropriate mass indicates that these proteins are sufficiently conserved in the closely related Arabidopsis and Brassica genera. Furthermore, homologs have also been reported in more divergent plants, such as maize (Braun et al., 1997; Clark et al., 1997), suggesting that CLV1 and KAPP signal transduction pathways may be common to higher plants.
The CLV1 signaling pathway is an excellent system in which to study KAPP participation in receptor kinase-mediated signal transduction, because multiple mutant alleles of clv1 have been isolated that exhibit phenotypes ranging from barely detectable (clv1-7) to possibly null (clv1-4) (Clark et al., 1993). In the weak and intermediate mutant alleles (such as clv1-1), CLV1 signaling is rate limiting, meaning that any changes in activity should be translated into an enhanced or suppressed mutant phenotype. This is evident by the fact that normally recessive alleles such as clv3-1 and stm-1 become dominant in a clv1-1 background (Clark et al., 1995, 1996). Thus, in a clv1-1 mutant, CLV1 signaling is intrinsically different from other receptor-kinase signaling pathways, in that in other systems significant alterations in the level of signaling will not necessarily lead to changes in phenotype. For example, mutations in or absence of other putative receptor kinases involved in disease resistance and developmental control are recessive (Song et al., 1995; Torii et al., 1996), indicating that a 50% reduction in the amount of receptors likely has no effect on phenotype. This was the rationale for using clv1 mutant backgrounds for generating transgenic plants over- and underexpressing KAPP. We hypothesized that if KAPP is involved in multiple signaling pathways, over- or underexpression of KAPP at levels sufficient to induce clv1 mutant phenotypes might also lead to defects in other signaling pathways, which could result in gamete inviability.
Transgenic experiments were performed in plants carrying the intermediate clv1-1 and clv1-6 alleles. Theoretically, if KAPP is involved in CLV1 signal transduction, a change in KAPP levels in a genetic background in which signal from CLV1 is rate-limiting should lead to an alteration of the clv1 phenotype. For example, if KAPP positively regulates CLV1 signaling, reducing KAPP in clv1 plants should enhance the mutant phenotype. If, on the other hand, KAPP negatively regulates CLV1 signaling, reducing KAPP should suppress the clv1 phenotype.
Plants were transformed with a construct containing the entire KAPP cDNA driven by the strong cauliflower mosaic virus 35S promoter. Phenotypes of transformed plants were moderately stable. For example, the progeny of partially suppressed plants were generally partially suppressed, although the full range of phenotypes was observed. In subsequent generations of the suppressed plants and/or in F2 and F3 generations of outcrosses to wild-type plants, a number of phenotypes were consistently observed at low frequency. These included reduced fertility, the absence of cauline leaves, terminated shoot meristems, shortened and/or absent root hairs, and early flowering. Because KAPP interacts with several kinase domains in vitro (Braun et al., 1997) and may therefore act as a common intermediate in multiple signaling cascades, these additional phenotypes were not unexpected.
Rather than overexpression of KAPP, we found that KAPP mRNA levels were reduced in the lines exhibiting partial to complete suppression of the clv1 mutant phenotype. Furthermore, the degree of suppression of the phenotype was inversely correlated with KAPP mRNA levels, lending greater support for interacting roles of CLV1 and KAPP in meristem development.
A reduction in expression of endogenous KAPP in plants harboring a sense construct may be explained by sense suppression or gene silencing caused by the introduction of homologous DNA sequences, which is a frequently reported phenomenon in plants (Meyer and Saedler, 1996). Low-stringency RNA and DNA analyses suggest that no other genes closely related to KAPP are present in the Arabidopsis genome (data not shown). Therefore, the observed suppression should be restricted to KAPP.
Our observations suggest that KAPP most likely acts as a negative regulator of the CLV1 signaling pathway, because the suppression of the clv1 phenotype correlated with reduced levels of KAPP transcript. CLV1 is predicted to either promote differentiation and organ formation or repress cell division in the meristematic regions; KAPP would therefore function to promote cell division or suppress differentiation. One scenario that is consistent with our observations is that KAPP acts to attenuate the signal through the CLV1 pathway. In wild-type plants CLV1 signaling is appropriately maintained and normal meristems are generated. In clv1 mutant plants CLV1 signaling is impaired, leading to an increased pool of undifferentiated cells. By reducing the level of an attenuator, KAPP, a normal meristem is produced. This model predicts that overexpression of KAPP in wild-type plants would lead to a phenocopy of the clv1 mutant phenotype by promoting cell division or suppressing differentiation in the meristems.
In a recent report, weak, clv1-like phenotypes were produced upon transformation of KAPP driven under the 35S promoter (Williams et al., 1997). However, there was no demonstration that KAPP levels actually increased in the transgenic plants, leaving these results open to a number of interpretations. clv1-Like phenotypes could conceivably result from down-regulation of CLV1, CLV2, or CLV3, as well as positive up-regulation of STM. Cytokinin application has also been shown to result in clv1-like phenotypes (in addition to other effects on organ identity and development) (Venglat and Sawhney, 1996). In the experiments described here, a weak clv1 mutant was used as the starting material for transgenic work. Therefore, as discussed above, CLV1 signaling is fundamentally different from other signaling pathways in these plants. Specifically, a phenotypic change should result from any minor alteration in the level of CLV1 signaling, whereas other pathways are likely to be insensitive to minor changes in the level of signaling. The partially suppressed plants retained 30% of the normal level of KAPP transcript accumulation. This was apparently sufficient for other pathways to behave in a manner phenotypically similar to that of the wild type, while at the same time causing a significant alteration in the clv1-1 phenotype.
The in vitro and in vivo interaction between CLV1 and KAPP is reminiscent of interactions between RTKs and PAA-binding SH2-PTPs. Tyr phosphorylation of RTKs induced by ligand binding recruits SH2-PTPs and other SH2-containing proteins to form an activated receptor complex for signaling. Some SH2-PTPs have been shown to act downstream of multiple RTKs (Perkins et al., 1996; Su et al., 1996), which may also be the case for KAPP. SH2-PTPs have been demonstrated to act as positive regulators, negative regulators, and attenuators of RTK signaling. These different roles can be attributed to the fact that SH2-PTPs can act as substrates for RTKs (Vogel et al., 1993), dephosphorylate the RTK for desensitization (Klinghoffer and Kazlauskas, 1995; Tomic et al., 1995), serve as adaptor molecules (Kharitonenkov et al., 1995), or dephosphorylate downstream signaling components (Herbst et al., 1996; Kharitonenkov et al., 1997). Unlike the RTK/SH2-PTP paradigm, however, CLV1 autophosphorylates on Ser residues (Fig. 1B) and KAPP is a phospho-Ser/phospho-Thr protein phosphatase (Stone et al., 1994).
Although multiple scenarios can be envisioned for the participation of KAPP and CLV1 in the control of meristem development, the simplest model to explain our data, KAPP as a negative regulator of CLV1 signal transduction, is that KAPP could act directly on activated CLV1 to attenuate or desensitize the receptor kinase. KAPP is capable of dephosphorylating recombinant CLV1 protein in vitro (Williams et al., 1997). The work presented here contributes to our understanding of general mechanisms of receptor kinase-mediated signal transduction and control of plant development. Future work should elucidate other components of the CLV1 signal transduction pathway and other pathways in which KAPP might participate.
ACKNOWLEDGMENTS
We acknowledge Tsung-Luo Jinn for preparing the affinity-purified KAPP antibodies and Hannah Alexander for constructing the modified pMalK vector. We also thank members of the Clark and Walker laboratories for comments on the manuscript.
Abbreviations:
- GST
glutathione S-transferase
- KAPP
kinase-associated protein phosphatase
- KI
kinase interaction
- KID
KI domain
- LRR
Leu-rich repeat
- MBP
maltose-binding protein
- PAA
phosphoamino acid
- PTP
phospho-Tyr phosphatase
- RLK
receptor-like kinase
- RTK
receptor Tyr kinase
- SH2
src homology 2
- TLC
thin-layer cellulose
- TLE
thin-layer electrophoresis
Footnotes
This work was supported by the National Science Foundation (NSF) (grant no. MCB-9417732 to J.C.W.), by the University of Missouri Food for the 21st Century Program (grant to J.C.W.), by the Department of Energy (DOE) (grant no. DE-FG02-96ER20227 to S.E.C.), and by the Triagency DOE/NSF/U.S. Department of Agriculture (Plant Biology Grant to Promote Collaboration in Plant Protein Phosphorylation no. 92-37105-7675).
LITERATURE CITED
- An G, Watson BD, Stachel S, Gordon MP, Nester EW. New cloning vehicles for transformation of higher plants. EMBO J. 1985;4:277–284. doi: 10.1002/j.1460-2075.1985.tb03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G. In plantaAgrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci Paris Life Sci. 1993;316:1194–1199. [Google Scholar]
- Bevan MW, Mason SE, Goelet P. Expression of tobacco mosaic virus coat protein by a cauliflower mosaic virus promoter in plants transformed by Agrobacterium. EMBO J. 1985;4:1921–1926. doi: 10.1002/j.1460-2075.1985.tb03871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun DM, Stone JM, Walker JC. Interaction of the maize and Arabidopsis kinase interaction domains with a subset of receptor-like protein kinases: implications for transmembrane signaling in plants. Plant J. 1997;12:83–95. doi: 10.1046/j.1365-313x.1997.12010083.x. [DOI] [PubMed] [Google Scholar]
- Braun DM, Walker JC. Plant transmembrane receptors: new pieces in the signaling puzzle. Trends Biochem Sci. 1996;21:70–73. [PubMed] [Google Scholar]
- Brown T, Mackey K. Analysis of RNA by northern and slot blot hybridization. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1993. , unit 4.9. [Google Scholar]
- Clark SE, Jacobsen SE, Levin J, Meyerowitz EM. The CLAVATA and SHOOT MERISTEMLESS loci competitively regulate meristem activity in Arabidopsis. Development. 1996;122:1567–1575. doi: 10.1242/dev.122.5.1567. [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development. 1993;119:397–418. doi: 10.1242/dev.119.2.397. [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development. 1995;121:2057–2067. [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997;89:575–585. doi: 10.1016/s0092-8674(00)80239-1. [DOI] [PubMed] [Google Scholar]
- Goddijn OJM, Lindsey K, van der Lee FM, Klap JC, Sijmons PC. Differential gene expression in nematode-induced feeding structures of transgenic plants harbouring promoter-gusA fusion constructs. Plant J. 1993;4:863–873. doi: 10.1046/j.1365-313x.1993.04050863.x. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Hunter T. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- Harlow E, Lane D. Immunoblotting. In: Harlow E, Lane D, editors. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. pp. 471–510. [Google Scholar]
- Herbst R, Carroll PM, Allard JD, Schilling J, Raabe T, Simon MA. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase corkscrew and functions during sevenless signalling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- Horn MA, Walker JC. Biochemical properties of the autophosphorylation of RLK5, a receptor-like protein kinase from Arabidopsis thaliana. Biochim Biophys Acta. 1994;1208:65–74. doi: 10.1016/0167-4838(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Jiang S, Dreano M, Buckler DR, Cheng S, Ythier A, Wu H, Henrickson WA, El Tayar N. Structural predictions for the ligand-binding region of glycoprotein hormone receptors and the nature of hormone-receptor interactions. Structure. 1995;3:1341–1353. doi: 10.1016/s0969-2126(01)00272-6. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Schnekenburger J, Chen ZJ, Knyazev P, Ali S, Zwick E, White M, Ullrich A. Adapter function of protein-tyrosine phosphatase 1D in insulin receptor-insulin receptor substrate-1 interaction. J Biol Chem. 1995;270:29189–29193. doi: 10.1074/jbc.270.49.29189. [DOI] [PubMed] [Google Scholar]
- Klinghoffer RA, Kazlauskas A. Identification of a putative syp substrate, the PDGF-beta receptor. J Biol Chem. 1995;270:22208–22217. doi: 10.1074/jbc.270.38.22208. [DOI] [PubMed] [Google Scholar]
- Koff A, Giordano A, Desai D, Yamahita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Furner IJ. Characterization of three shoot apical meristem mutants of Arabidopsis thaliana. Development. 1992;116:397–403. [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Meyer P, Saedler H. Homology-dependent gene silencing in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:23–48. doi: 10.1146/annurev.arplant.47.1.23. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Johnson MR, Melnick MB, Perrimon N. The nonreceptor protein tyrosine phosphatase corkscrew functions in multiple receptor tyrosine kinase pathways in Drosophila. Dev Biol. 1996;180:63–81. doi: 10.1006/dbio.1996.0285. [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Krizek BA, Meyerowitz EM. Dimerization specificity of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA, and AGAMOUS. Proc Natl Acad Sci USA. 1996;93:4793–4798. doi: 10.1073/pnas.93.10.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Wang G, Chen L, Kim H, Pi L, Holsten T, Gardner J, Wang B, Zhai W, Zhu L and others. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804–1806. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- Steeves TA, Sussex IM (1989) Patterns in Plant Development. Cambridge University Press, New York
- Stone JM. Phage-based expression cloning to identify interacting proteins. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1997. , unit 20.3. [Google Scholar]
- Stone JM, Collinge MA, Smith RD, Horn MA, Walker JC. Interaction of a protein phosphatase with an Arabidopsis serine-threonine receptor kinase. Science. 1994;266:793–795. doi: 10.1126/science.7973632. [DOI] [PubMed] [Google Scholar]
- Su L, Zhao Z, Bouchard P, Banville D, Fischer EH, Krebs EG, Shen SH. Positive effect of overexpressed protein-tyrosine phosphatase PTP1C on mitogen-activated signaling in 293 cells. J Biol Chem. 1996;271:10385–10390. doi: 10.1074/jbc.271.17.10385. [DOI] [PubMed] [Google Scholar]
- Tomic S, Greiser U, Lammers R, Kharitonenkov A, Imyanitov E, Ullrich A, Bohmer FD. Association of SH2 domain protein tyrosine phosphatases with the epidermal growth factor receptor in human tumor cells: phosphatidic acid activates receptor dephosphorylation by PTP1C. J Biol Chem. 1995;270:21277–21284. doi: 10.1074/jbc.270.36.21277. [DOI] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell. 1996;8:735–746. doi: 10.1105/tpc.8.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglat SP, Sawhney VK. Benzylaminopurine induces phenocopies of floral meristem and organ identity mutants in wild-type Arabidopsis plants. Planta. 1996;198:480–487. doi: 10.1007/BF00620066. [DOI] [PubMed] [Google Scholar]
- Vogel W, Lammers R, Huang J, Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993;259:1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- Weigel D, Clark SE. Sizing up the floral meristem. Plant Physiol. 1996;112:5–10. doi: 10.1104/pp.112.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Wilson JM, Meyerowitz EM. A possible role for kinase-associated protein phosphatase in the Arabidopsis CLAVATA1 signaling pathway. Proc Natl Acad Sci USA. 1997;94:10467–10472. doi: 10.1073/pnas.94.19.10467. [DOI] [PMC free article] [PubMed] [Google Scholar]