Abstract
Purpose
Neuropeptide Y (NPY) level is elevated in allergic asthmatic airways and activation of NPY receptor-1 (NPY-Y1) on antigen presenting cells (APCs) is essential for T-cell priming. Paradoxically, NPY-Y1 modulates hyper-responsiveness in T cells, suggesting a bimodal role for NPY in APCs and T-cells. Therefore, determination of the temporal and spatial expression pattern of NPY and its receptors in asthmatic airways is essential to further understand the role of NPY in allergic asthma.
Methods
Lungs were isolated from control and acute and chronic stages of OVA-sensitized and challenged mice (OVA). Stains, including H&E, PAS, and trichrome, were used to determine the severity of lung pathology. The expression patterns of NPY and NPY-Y receptors in the airways were determined using ELISA and immunofluorescence. Cytokine levels in the BALF were also measured.
Results
NPY levels were undetectable in the BALF of control mice, but significantly increased in the OVA group at day 80. Levels of IL-4, TGF-β1 and TGF-β2, significantly increased and peaked on day 45 and decreased on day 80 in the OVA group, exhibiting an inverse correlation with NPY levels. NPY expression was localized to macrophage-like cells in the peri-bronchial and peri-vascular areas in the lung tissue. NPY-Y1 and -Y5 receptors were constitutively expressed by both structural and inflammatory cells in the lung tissue.
Conclusions
NPY produced by activated macrophage-like cells may be involved in regulating cytokine production and cellular activities of immune cells in asthma. However, it remains unclear whether such an increase in NPY is a defensive/compensatory mechanism to modulate the effects of inflammatory cytokines.
Keywords: Allergic asthma, antigen presenting cells (APCs), macrophage, neuropeptide Y (NPY), NPY receptor-Y1, transforming growth factor beta (TGF-β)
Introduction
In recent years, neuropeptide (NPY) has emerged as a molecule potentially playing an important role in the regulation of a number of immune responses by acting on a variety of immune cells (El Karim et al., 2008; Ferreira et al., 2010; Wheway et al., 2007). NPY is a 36-amino acid residue polypeptide and is widely distributed in the central and peripheral nervous systems (Michel et al., 1998). Originally isolated from the porcine brain (Tatemoto et al., 1982), it is now known to be ubiquitous also outside the nervous system, in the endothelium, platelets, immune cells, megakaryocytes and the gut (Ericsson et al., 1991; Michel et al., 1998; Myers et al., 1988; Myers et al., 1993; Ogawa et al., 1989). The pleiotropic action of NPY is mediated by the multiplicity of Gi/Go-coupled NPY receptor subtypes (Y1, Y2, Y4, Y5 and Y6), which exert their specific action depending on the target tissue, the status of the cell activation, the presence of degrading enzymes and the presence or absence of other co-regulatory factors including neuroimmune co-transmitters (Michel et al., 1998). NPY can regulate immune cell distribution and release of mediators from T helper (TH) cells and macrophages (Bedoui et al., 2004; Ferreira et al., 2010; Wheway et al., 2007). NPY links the sympathetic nervous system to the immune system, as NPY-releasing sympathetic nerves innervate lymphoid organs (Romano et al., 1991). Moreover, the Y1 receptor for NPY is widely expressed on leukocytes, including T cells, B cells and APCs such as DCs and macrophages (Wheway et al., 2007; Wheway et al., 2005).
Role of NPY in the induction of aberrant TH2 immune response in allergic asthma is controversial (Macia et al., 2011; Wheway et al., 2007). A bimodal role for Y1 on immune cells have been uncovered from results obtained using Y1-deficient mice (Y1-/-) (Wheway et al., 2007; Wheway et al., 2005). Y1 expression on antigen-presenting cells (APC) is essential for their function as T cells priming elements. Conversely, Y1 signaling in T cells plays a regulatory role without which T cells are hyper-responsive (Wheway et al., 2007). The opposite role of NPY on antigen presenting cells (APCs) and T cells justify the goal of this study to determine expression pattern of NPY and its receptors in acute and chronic asthmatic airways. Knowing the expression pattern of NPY and NPY receptors is essential in attributing a potential role for NPY/NPYR autocrine and/or paracrine signaling axis at different stages of allergic asthma. In this study, we determined the expression pattern of NPY and NPY receptors and their correlation with the severity of inflammation in the lung of ovalbumin-sensitized and challenged (OVA) mice.
Materials and Methods
Animals
We purchased BALB/c mice (aged 4-5 weeks) from Harlan Laboratories (Indianapolis, IN) and maintained in a pathogen-free environment at Creighton University. Food and water were provided ad libitum. The research protocol of this study was approved by Institutional Animal Care and Use Committee of Creighton University.
Induction of allergic airway disease
We sensitized mice by 20 μg i.p. injections of ovalbumin (OVA; Sigma-Aldrich) emulsified in 2.25mg of Imject alum (Pierce Biotechnology) on day 0 and 14 (Fig. 1). Animals were challenged with 1% OVA on day 30, and 5% OVA on day 32. On day 33, specific airway resistance (RL) in tracheostomized mice in response to aerosolized acetyl β-methylcholine (methacholine; Sigma-Aldrich) was measured in randomly selected animals (Fig. 2A) using RC system (Buxco Electronics, Troy, NY). The remaining mice with established airway hyperresponsiveness were challenged with 5% OVA by aerosol on day 44. On day 45, specific airway resistance (RL) in tracheostomized mice in response to aerosolized acetyl β-methylcholine was measured in randomly selected animals (Fig. 2A). Remaining Animals were challenged with 5% OVA every three days and 5% OVA on day 79. On day 80, specific airway resistance (RL) in tracheostomized mice in response to aerosolized methacholine was measured in remaining animals (Fig. 2A).
Figure 1.
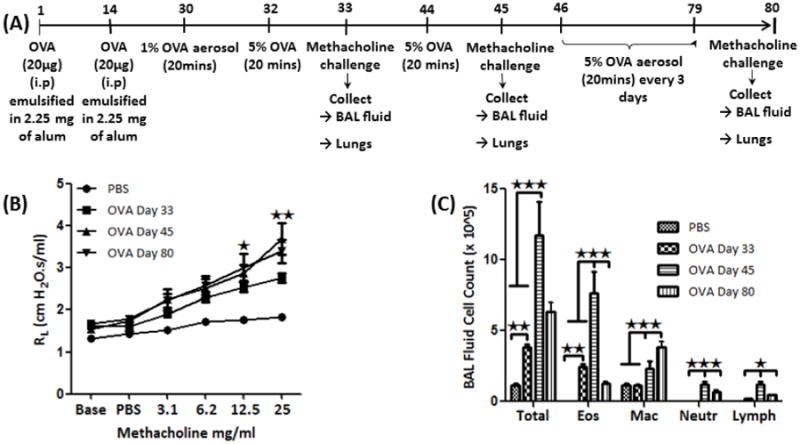
(A) OVA antigen sensitization protocol; (B) AHR analysis: Graph showing the specific airway resistance (RL) in tracheostomized mice in response to aerosolized methacholine in PBS-treated and OVA-sensitized and -challenged mice; (C) Bronchoalveolar lavage (BAL) fluid cell analysis: Showing graph of total and differential inflammatory cell count in BAL fluid of PBS and OVA-sensitized and -challenged mice. Data are shown as mean ± SEM of values of eight mice per group. *P<0.05; **P0.001.
Figure 2.
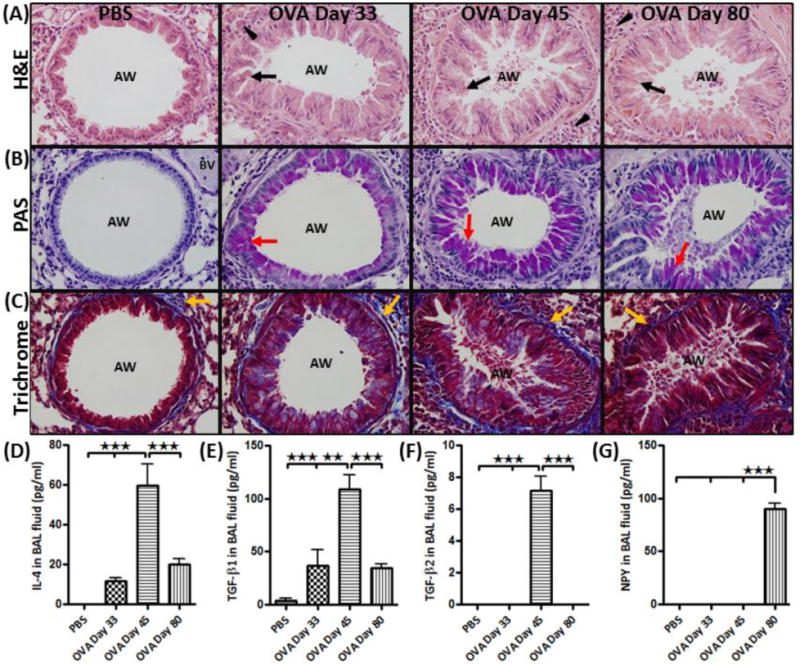
(A) H&E staining: showing infiltration of inflammatory cells (arrow head) and hypertrophy of structural cells (arrow); (B) PAS staining: showing mucous cells (purple color) (red arrow); and (C) Trichrome staining: showing subepithelial collagen deposition (blue color stain) (yellow arrow) in the airway (AW) of PBS and of OVA-sensitized and challenged mice on days 33, 45 and 80. All pictures are representative of each staining technique. BAL fluid analysis: Showing graphs of differential (D) IL-4; (E) TGFβ1; (F) TGFβ2; and (G) NPY levels in BAL fluid using ELISA. Data are shown as mean ± SEM of values of five measurements in each group. *P<0.05; **P0.001.
Bronchoalveolar lavage fluid and cytokine measurement
After sacrifice, lungs were gently lavaged with 1ml of warm saline (37°C) via a tracheal cannula. Total cell counts were performed with a Coulter counter (Beckman and Coulter, Fullerton, CA). All samples were centrifuged at 400rpm for 10 min, and the supernatant was stored in a −80°C freezer until Enzyme-linked immunosorbent assays (ELISA) were performed. Mouse IL-4, TGF-β1, TGF-β2 and NPY ELISA detection kits (Millipore) were used to measured levels of mediators according to manufacturer's protocol.
Preparation and staining of Lung tissue
Lung lobes were fixed in 4% formalin and paraffin embedded. These lung sections were stained with hematoxylin and eosin (H&E) following manufacturer's standard protocol (Newcomer/supply). Mucus secretion was identified by periodic acid-Schiff (PAS) reaction using standard protocol recommended by the manufacturer (Sigma Aldrich). Trichrome staining was used to identify collagen following standard protocol recommended by the manufacturer (IMEB Inc.).
Immunofluorescence (IF)
We performed deparaffinization, rehydration and antigen retrieval prior to immunostaining. The slides were incubated for 2hrs in block/permeabilizing solution containing 5% (v/v) of appropriate serum with 0.25% Triton X-100 and 0.1% bovine serum albumin (BSA) when goat serum was used. The slide slides were subsequently incubated with primary antibody including rat anti-EpCAM (eBioscience), rat anti-MHCII (eBioscience) rat anti-F4/80 (SantaCruz Biotech), rabbit anti-NPY (Novus Biological), goat anti-alpha actin (Novus Biological), rabbit anti-Y5 (Novus Biological), and sheep anti Y1 (Gentex). The sections were washed and incubated with Cyanine 3 (Cy3) or Cy2 or Cy5 donkey anti-rabbit IgG, donkey anti-rat IgG and donkey anti-goat hamster IgG (Jackson Immuno) as appropriate. Nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI). Fluorescent analysis was performed with a confocal microscope.
Statistical analysis
In all the studies, each experimental group consisted of 8 mice, as determined by power analysis for sample size. Data are presented as means ± SEM and were analyzed using GraphPad Prism. One-way ANOVA was used when one parameter was tested. However, in conditions which required the evaluation of two parameters simultaneously, the two-way ANOVA was used. Multiple group comparisons were performed by Bonferroni's multiple comparison test. A value of p<0.05 was considered significant.
Results
Establishment of AHR to methacholine and airway inflammation
OVA-sensitized and challenged mice (Fig. 1A) demonstrated AHR to methacholine with a marked increase in specific lung resistance on days 33, 45 and 80 (Fig. 1B). AHR peaked on day 45, remains elevated on day 80 (Fig. 1B). There was significant increase in total number of cells, eosinophils, and lymphocytes in the BAL fluid of OVA-sensitized and challenged mice (OVA mice) compared to PBS (control) group, peaking at day 45, followed by a decrease on day 80 (Fig. 1C). Long term exposure to OVA resulted in significant increase in the number of macrophages on day 80 (Fig. 1C). There were no lymphocytes in the BALF of control group (Fig. 1C).
Airway remodeling following OVA-sensitization and challenge
Airways of the control mice exhibited normal parenchyma with no mucus staining and little collagen staining around the respiratory epithelium and vascular basement membrane (Fig. 2A-C). On day 33, the lung of OVA mice exhibited the signs of mild airway remodeling showing airway epithelial cell hypertrophy, mucus staining and sparse collagen deposition (Fig. 2A-C). However, on day 45 and day 80, features of severe airway remodeling were present following additional antigen challenge (Fig. 2A-C).
NPY and inflammatory cytokine levels in the BAL fluid
There was significant increase in IL-4, TGF-β1 and TGF-β2 levels in the BAL fluid (BALF) of OVA mice compared to control group, peaking at day 45, followed by a significant decrease on day 80 (Fig. 2D-F). NPY expression was not detectable in the BALF of control and OVA days 33 and 45 mice. However, long term exposure to OVA resulted in significant increase in NPY expression in the BALF of OVA mice on day 80 (Fig. 2G).
NPY expression in respiratory epithelium and airway smooth muscle cells
NPY expression was not detectable in respiratory epithelium and airway smooth muscle cells of control and OVA mice, as assessed by EpCAM and α-actin as positive markers for airway epithelial and smooth muscle cells, respectively (Fig. 3A). NPY expression was not detectable in control and OVA day 33 and barely detectable in OVA day 45 mice. However, NPY expression was significantly increased in some inflammatory cells of OVA day 80 mice (Figs. 3A&B).
Figure 3.
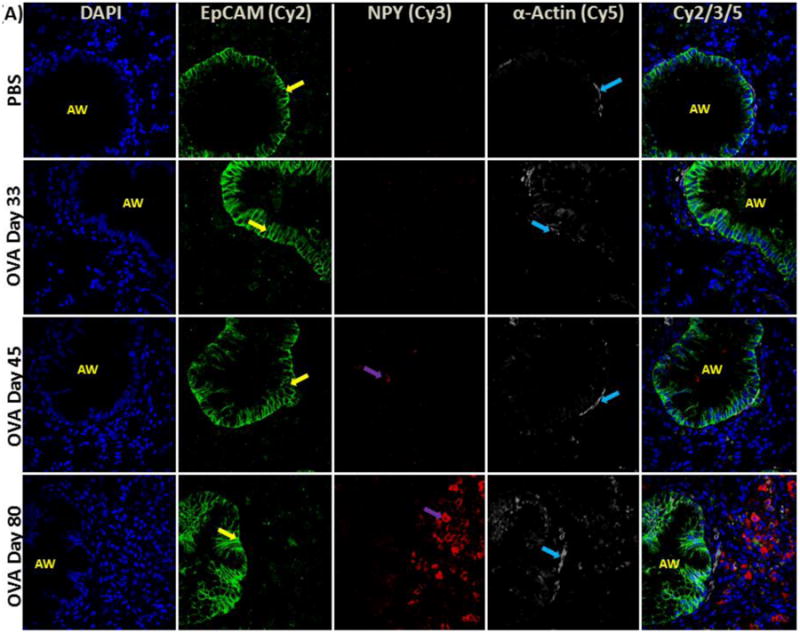
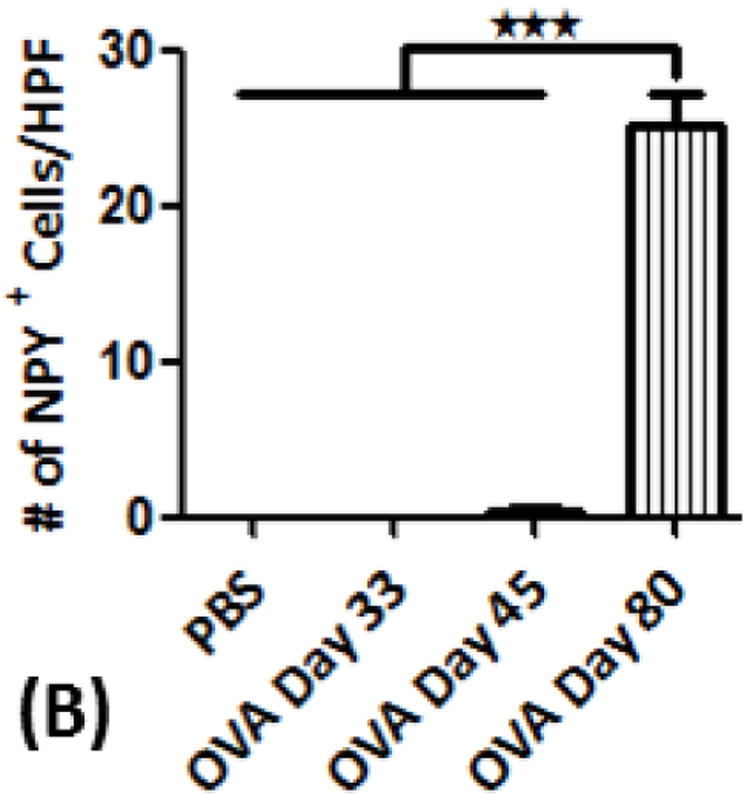
(A) NPY expression in airway epithelial cells and smooth muscle cells: Confocal images (600×) showing immunofluorescent (IF) staining analysis of NPY (Cy3) in airway structural cells using epithelial cell adhesion molecule (EpCAM) (Cy2) as a selective marker for airway epithelial cells and α-actin as a specific marker for smooth muscle cells (Cy5). These are representative images of the IF staining. (B) NPY positive cell analysis: Graph showing number of NPY-positive (+) cells detected as part of the inflammatory cell population in the peri-bronchial area per high powered field (HPF). Data are shown as mean ± SEM of values of eight measurements in each group. *P<0.05; **P0.001.
Expression of NPY and NPY-Y receptors in inflammatory cells
Airways of the control mice exhibited no detectable localization of F4/80+ cells, However, there was a gradual increase in the number of F4/80+ cells in the peri-bronchial area of OVA mice on days 33, 45 and 80 (Fig. 4A-C). NPY expression was restricted to F4/80+ cells in OVA day 80 mice (Fig. 4A&D). MHCII+ cells were detectable in the peri-bronchial area of control and OVA mice groups, with the highest number of positive cells detected in the OVA day 80 group (Fig. 5A&B). There was a decrease in the level of MHCII expressed on the immune cells in OVA day 33 mice, followed by an increase in the OVA mice on days 45 and 80 as measured by MFI (Fig. 5C). MHCII expression was detectable in about 40% of NPY+ cells in OVA day 80 mice (Fig. 5D). There is constitutive expression of NPY-Y1 and NPY-Y5 in the structural and inflammatory cells in control and OVA group (Fig. 6A&B).
Figure 4.
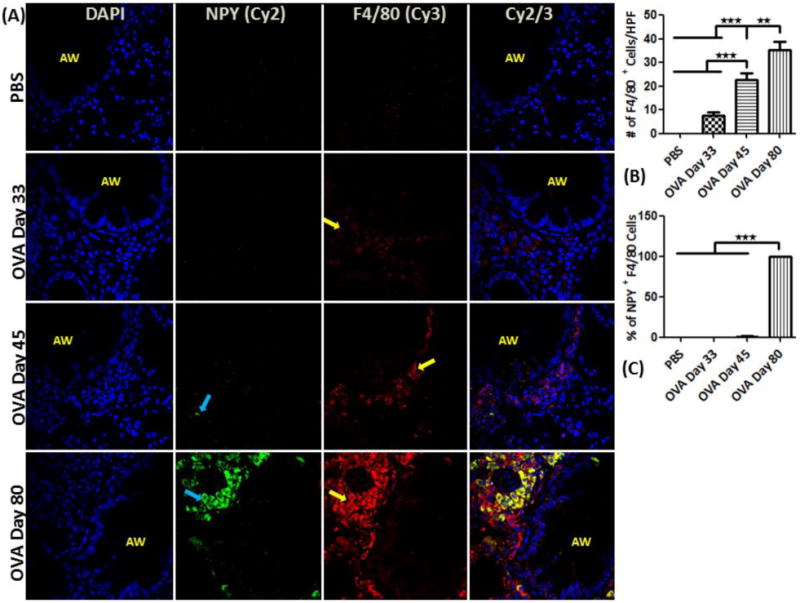
NPY expression in macrophages: (A) Confocal images (600×) showing immunofluorescent (IF) staining analysis of NPY (Cy2) in inflammatory cells using F4/80 (Cy3) as a specific marker for macrophages. These are representative images of the IF staining. (B) F4/80 positive cell analysis: Graph showing number of F4/80-positive (+) cells detected as part of the inflammatory cell population in the peri-bronchial area per high powered field (HPF). (C) Percentage of NPY+ cells that are also positive for F4/80 (double positive) relative to the total number of F4/80+ cells. Data are shown as mean ± SEM of values of eight measurements in each group. *P<0.05; **P0.001.
Figure 5.
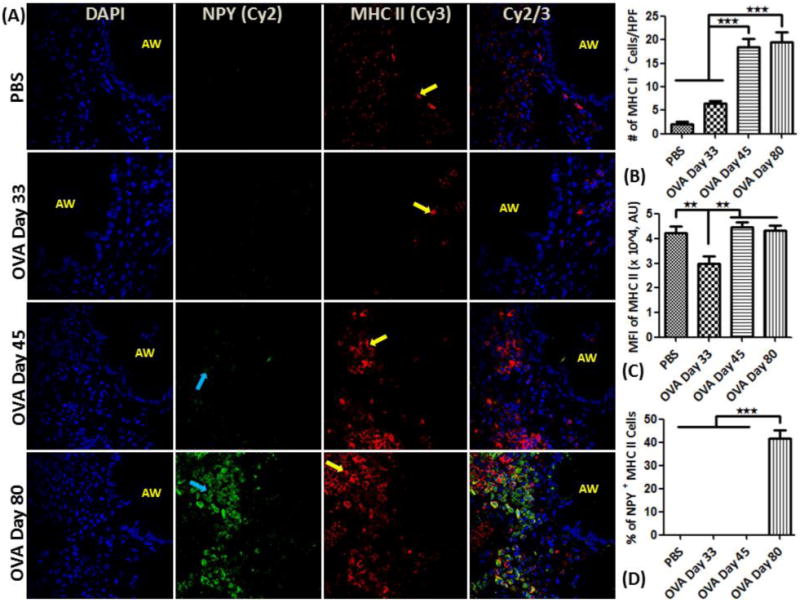
NPY expression in APC-like cells: (A) Confocal images (600×) showing immunofluorescent (IF) analysis of NPY (Cy2) in inflammatory cells using MHC class II (Cy3) as a selective marker for APC-like cells. These are representative images of the IF staining. (B) MHC II positive cell analysis: Graph showing number of MHCII-positive (+) cells detected as part of the inflammatory cell population in the peri-bronchial area per high powered field (HPF). (C) Mean fluorescent intensity (MFI) in arbitrary units (using NIH Image J software) of MHCII expression in MHCII+ cells. (D) Percentage of NPY+ cells that are also positive for MHC II (double positive) relative to the total number of MHCII+ cells. Data are shown as mean ± SEM of values of eight measurements in each group. *P<0.05; **P0.001.
Figure 6.
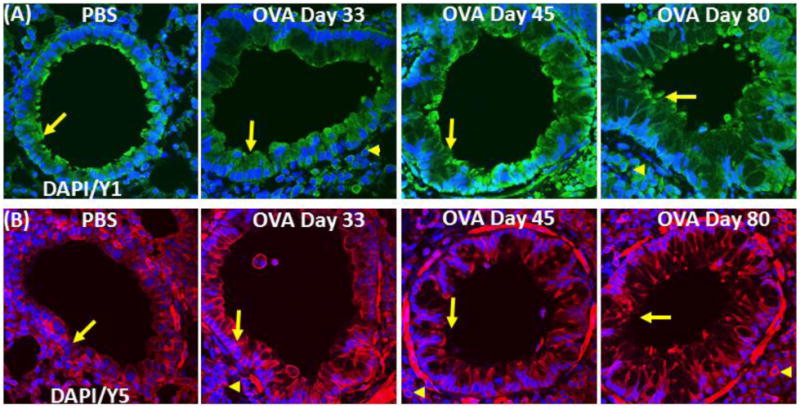
NPY receptor expression in airway: (A) Confocal images (600×) showing immunofluorescent (IF) staining analysis of (A) NPY receptor 1 (NPY1R) (Cy2) and (B) NPY receptor 5 (NPY5R) (Cy3) in the airway. These are representative images of the IF staining. These are representative images of the IF staining.
Discussion
In this study, we demonstrated that both structural and inflammatory cells express NPY-Y receptors and that NPY is elevated in the allergic airways. Importantly, NPY is elevated at the latter stages of the inflammatory response, corresponding to a more severe phenotype of the disease, suggesting that NPY potentially plays an important role in chronic asthma. Additionally, NPY level inversely correlates with the level of inflammatory mediators in the airways and expression is restricted to macrophage-like cells.
Many non-neuronal cells express NPY either constitutively or inducibly, in the endothelium, platelets, immune cells, megakaryocytes and the gut (Ericsson et al., 1991; Michel et al., 1998; Myers et al., 1988; Myers et al., 1993; Ogawa et al., 1989; Schwarz et al., 1994). Like in the sympathetic nervous system, NPY levels in immune cells vary depending on their activation states. In monocytes and macrophages, NPY mRNA expression is upregulated by phorbol myristate acetate (Schwarz et al., 1994). As indicated by the change in cytokine levels at different stages of inflammation (Fig 2.), it is possible that extrinsic factors present at the chronic stage of the disease are inducing the NPY expression in inflammatory macrophages. Identification of such mediators would shed light on the regulation of NPY expression in these cells.
Increasing lines of evidence indicate that NPY is an important regulator of immune functions. Indeed, close association of the NPY gene (Npy) with other genes involved in immune regulation, including MHCII, CD36 and NF-κB (Jacob et al., 1992; Musso et al., 1997; Pravenec et al., 1999), is indicative of potential role of NPY during inflammation. During T-cells stimulation, NPY increases the release of cytokines (IL-4) and chemokines (e.g., MIP-1β) and inhibits IFN-γ (Kawamura et al., 1998). However, Y1-/- T cells were hyperresponsive to activation and NPY treatment inhibited T cell proliferation following agonistic anti-CD3 antibody and allogeneic APC stimulation in vitro (Soder and Hellstrom, 1987; Wheway et al., 2005). NPY increased the adhesiveness of human neutrophil and monocyte to endothelial cells (Sung et al., 1991). However, another study revealed that NPY inhibits the interaction of leukocytes with endothelial cells, resulting in their mobilizing from marginal pool. Furthermore, exogenous NPY application reduces tissue immigration of blood monocytes (Nave et al., 2004).
The release of Y1 ligand(s), produced by activated APCs, are required for optimal cytokine production by these cells, suggesting the involvement of an autocrine regulatory mechanism in APCs including macrophages and dendritic cells (DC). Also the ability of NPY-Y1-/-; DCs to take up fluorescently labeled antigen as measured by flow cytometry was significantly reduced compared to that of wild type DCs (Wheway et al., 2007). However, another study showed that NPY inhibited LPS-induced release of IL-1β from microglial cells through NPY-Y1 activation. The same study also showed that NPY inhibited IL-1β-induced nitric oxide (NO) and nitric-oxide synthase (iNOS) production by blocking nuclear translocation of NF-κB (Ferreira et al., 2010). Comparison of these various studies show a degree of receptor and target cell specificity within the neuro-immuno-communication driven by NPY and in particular NPY-Y1 appears to have a bimodal role (Wheway et al., 2005). Signaling via NPY-Y1 receptor acts as a key activator of APC function, while acting as a negative regulator for T cells (Bedoui et al., 2004; Wheway et al., 2007; Wheway et al., 2005).
Bimodal role of NPY-Y1 on APCs and T-cells may help explain the discoveries from our study. In the present study, NPY is elevated at the latter stages of the inflammatory reaction, corresponding to a chronic phenotype of the disease. Additionally, NPY expression inversely correlates with the level of inflammatory mediators in the airway. We hypothesize that the NPY released from the macrophage-like cells may be responsible for attenuating cytokine production and release by the TH2 cells resulting in a decrease in IL-4 level, while enhancing macrophage functions including phagocytosis and cytokine release in an attempt to start the repair process following injury caused by the inflammatory reaction.
Conclusion
Results from our study indicate that there is a correlation between NPY expression and disease progression in asthma. Presence of NPY-Y receptors on structural cells suggests a potential paracrine signaling in these cell types, in addition to the autocrine signaling in activated macrophages. Our results show that NPY may be a potential therapeutic target in chronic asthma.
Potential Limitations
The study was conducted in ovalbumin allergic mice model, so a similar study may need to be done using a more clinically relevant antigen such as cockroach and house-dust mite. NPY-induced signaling may need to be inhibited in the asthmatic mice using a NPY blocker in other to determine the role of NPY in the initiation and sustenance of the inflammatory reaction. Since human are not mice, this work would also need to be verified in asthmatic patients to bolster the translational significance of the data generated.
Highlights.
NPY is undetectable in BALF of normal mice, but high in chronic asthmatic mice.
NPY is localized in macrophages and in the peribronchial area
Expression of inflammatory cytokines inversely correlates with NPY.
NPY-Y1 and -Y5 receptors are expressed in lung structural and inflammatory cells.
Acknowledgments
This study was supported by the National Institute of Health grant R01HL085680 (to D.K.A). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. All authors declare no conflict of any sort with the content in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedoui S, Miyake S, Straub RH, von Horsten S, Yamamura T. More sympathy for autoimmunity with neuropeptide Y? Trends in immunology. 2004;25:508–512. doi: 10.1016/j.it.2004.08.005. [DOI] [PubMed] [Google Scholar]
- El Karim IA, Linden GJ, Orr DF, Lundy FT. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. Journal of neuroimmunology. 2008;200:11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Hemsen A, Lundberg JM, Persson H. Detection of neuropeptide Y-like immunoreactivity and messenger RNA in rat platelets: the effects of vinblastine, reserpine, and dexamethasone on NPY expression in blood cells. Experimental cell research. 1991;192:604–611. doi: 10.1016/0014-4827(91)90082-6. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Xapelli S, Santos T, Silva AP, Cristovao A, Cortes L, Malva JO. Neuropeptide Y modulation of interleukin-1{beta} (IL-1{beta})-induced nitric oxide production in microglia. The Journal of biological chemistry. 2010;285:41921–41934. doi: 10.1074/jbc.M110.164020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob HJ, Pettersson A, Wilson D, Mao Y, Lernmark A, Lander ES. Genetic dissection of autoimmune type I diabetes in the BB rat. Nature genetics. 1992;2:56–60. doi: 10.1038/ng0992-56. [DOI] [PubMed] [Google Scholar]
- Kawamura N, Tamura H, Obana S, Wenner M, Ishikawa T, Nakata A, Yamamoto H. Differential effects of neuropeptides on cytokine production by mouse helper T cell subsets. Neuroimmunomodulation. 1998;5:9–15. doi: 10.1159/000026321. [DOI] [PubMed] [Google Scholar]
- Macia L, Rao PT, Wheway J, Sierro F, Mackay F, Herzog H. Y1 signalling has a critical role in allergic airway inflammation. Immunology and cell biology. 2011;89:882–888. doi: 10.1038/icb.2011.6. [DOI] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacological reviews. 1998;50:143–150. [PubMed] [Google Scholar]
- Musso R, Grilli M, Oberto A, Gamalero SR, Eva C. Regulation of mouse neuropeptide Y Y1 receptor gene transcription: a potential role for nuclear factor-kappa B/Rel proteins. Molecular pharmacology. 1997;51:27–35. doi: 10.1124/mol.51.1.27. [DOI] [PubMed] [Google Scholar]
- Myers AK, Farhat MY, Vaz CA, Keiser HR, Zukowska-Grojec Z. Release of immunoreactive-neuropeptide by rat platelets. Biochemical and biophysical research communications. 1988;155:118–122. doi: 10.1016/s0006-291x(88)81057-x. [DOI] [PubMed] [Google Scholar]
- Myers AK, Torres Duarte AP, Zukowska-Grojec Z. Immunoreactive neuropeptide Y (NPY) in plasma and platelets of rat and mouse strains and human volunteers. Regulatory peptides. 1993;47:239–245. doi: 10.1016/0167-0115(93)90391-k. [DOI] [PubMed] [Google Scholar]
- Nave H, Bedoui S, Moenter F, Steffens J, Felies M, Gebhardt T, Straub RH, Pabst R, Dimitrijevic M, Stanojevic S, von Horsten S. Reduced tissue immigration of monocytes by neuropeptide Y during endotoxemia is associated with Y2 receptor activation. Journal of neuroimmunology. 2004;155:1–12. doi: 10.1016/j.jneuroim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Kitamura K, Kawamoto M, Eto T, Tanaka K. Increased immunoreactive neuropeptide Y in platelets of spontaneously hypertensive rats (SHR) Biochemical and biophysical research communications. 1989;165:1399–1405. doi: 10.1016/0006-291x(89)92759-9. [DOI] [PubMed] [Google Scholar]
- Pravenec M, Zidek V, Simakova M, Kren V, Krenova D, Horky K, Jachymova M, Mikova B, Kazdova L, Aitman TJ, Churchill PC, Webb RC, Hingarh NH, Yang Y, Wang JM, Lezin EM, Kurtz TW. Genetics of Cd36 and the clustering of multiple cardiovascular risk factors in spontaneous hypertension. The Journal of clinical investigation. 1999;103:1651–1657. doi: 10.1172/JCI6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano TA, Felten SY, Felten DL, Olschowka JA. Neuropeptide-Y innervation of the rat spleen: another potential immunomodulatory neuropeptide. Brain, behavior, and immunity. 1991;5:116–131. doi: 10.1016/0889-1591(91)90011-x. [DOI] [PubMed] [Google Scholar]
- Schwarz H, Villiger PM, von Kempis J, Lotz M. Neuropeptide Y is an inducible gene in the human immune system. Journal of neuroimmunology. 1994;51:53–61. doi: 10.1016/0165-5728(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Soder O, Hellstrom PM. Neuropeptide regulation of human thymocyte, guinea pig T lymphocyte and rat B lymphocyte mitogenesis. International archives of allergy and applied immunology. 1987;84:205–211. doi: 10.1159/000234424. [DOI] [PubMed] [Google Scholar]
- Sung CP, Arleth AJ, Feuerstein GZ. Neuropeptide Y upregulates the adhesiveness of human endothelial cells for leukocytes. Circulation research. 1991;68:314–318. doi: 10.1161/01.res.68.1.314. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Wheway J, Herzog H, Mackay F. The Y1 receptor for NPY: a key modulator of the adaptive immune system. Peptides. 2007;28:453–458. doi: 10.1016/j.peptides.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Wheway J, Mackay CR, Newton RA, Sainsbury A, Boey D, Herzog H, Mackay F. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. The Journal of sexperimental medicine. 2005;202:1527–1538. doi: 10.1084/jem.20051971. [DOI] [PMC free article] [PubMed] [Google Scholar]


