Abstract
Introduction
Older breast cancer survivors (BCS) report more falls and functional limitations than women with no cancer history. Exercise training could reduce risk factors for future falls and disability.
Methods
We conducted a randomized, controlled trial in 106 early-stage, postmenopausal BCS who were ≥ 50 years old at diagnosis and post-treatment. Women were randomly assigned to a 1-year resistance + impact exercise program or a stretching placebo program. Endpoints were 1-repetition maximum bench press and leg press strength, timed 5-chair stands, 4m usual walk speed, timed stance tests, handgrip strength, self-report physical function, and fatigue. We also examined the influence of age, adjuvant hormone therapy use and exercise adherence on study outcomes.
Results
Women in the resistance + impact training program significantly improved maximal leg (p=.04) and bench (p=.01) press strength compared to the stretching group. Women who attended 50% or more of prescribed resistance training sessions had significantly better changes in maximal strength measures compared to less adherent women.
Conclusions
Resistance + impact exercise is superior to stretching at improving maximal muscle strength and exercise adherence contributes to the degree of improvement.
Implications for Cancer Survivors
Older BCS can safely engage in resistance exercise that improves lower and upper body strength, thereby reducing a risk factor for falls and future disability. However, the ability of resistance training to shift other indices of fall and disability risk, i.e., balance and function is unclear. Strategies to promote adherence to resistance training could lead to greater improvements in strength.
Keywords: neoplasm, muscle strength, aged, balance, fatigue, adherence
INTRODUCTION
Women who have had breast cancer are significantly more likely to fall and to report more functional limitations than women who have not had cancer [1–4]. Falls are strongly associated with fractures in older adults [5] and can have other serious consequences including disability and death [6]. About one-third of older adults who fall will require assistance with activities of daily living after a fall, and over half (58%) of those persons will need help for more than 6 months [7]. In addition to falls, declines in physical functioning can also threaten independence [8] and changes associated with aging appear to be accelerated in BCS [9]. Sweeney et al identified a greater prevalence of self-report functional limitations among older female cancer survivors within 5 years of diagnosis compared to older women with no cancer history [1]. In subgroup analyses by cancer type, older BCS were more likely to report a functional limitation, such as difficulty walking up and down stairs or doing heavy household chores, than cancer-free peers (OR for any limitation: 1.37, 95% CI = 1.14, 1.65).
Falls and disability share overlapping risk factors that typically increase with age. Muscle weakness, altered gait and instability are independently associated with increased fall risk and lower functional capacity for performing daily tasks such as lifting objects [10, 11]. Age-related sarcopenia leads to muscle weakness that is linked to poor balance [12] and falls [13]. Cancer treatment can cause muscle wasting that does not reverse in recovery [14–18] and when combined with deconditioning and fatigue that can accompany treatment [19, 20], may place older BCS at greater risk of falls and functional decline than women without cancer. BCS who fall exhibit significantly lower leg strength [4] and worse balance [3] compared to non-fallers, thus strength and balance are likely to be key risk factors for falls in BCS.
Resistance training can reverse muscle weakness, restore balance, and reduce falls and functional decline in older adults without cancer [21–23]. To date, only 3 trials have tested the efficacy of resistance training to improve muscle strength in post-treatment BCS. All reported improvements in lower body muscle strength [24–26], with Twiss et al also reporting improvements in a measure of dynamic balance [24]; however, none of these trials specifically targeted older BCS. We have developed a resistance + impact exercise program that improves risk factors for falls and fractures (e.g., increased bone density, muscle strength, gait and balance) in women without cancer [27, 28]. Recently, we reported that our program prevented loss of bone density at the spine in older, postmenopausal BCS but had little effect on muscle mass [29]. The primary aim of this study is to report additional outcomes of muscle strength and both objective and self-report physical function from a 12-month randomized, controlled trial of resistance + impact training compared to a stretching control condition in postmenopausal, post-treatment BCS over 50 years of age at diagnosis. A secondary aim was to explore whether or not age, adjuvant hormone therapy, and/or exercise adherence influenced the response to resistance + impact training.
METHODS
Design
We conducted a 1-year single-blind randomized controlled trial comparing two parallel groups equally allocated (1:1 ratio) to either: 1) progressive, moderate-intensity resistance + impact training or 2) low-intensity stretching (placebo exercise). Primary outcomes were measured at baseline, 6 and 12 months. All testing and exercise training took place at Oregon Health & Science University (OHSU). Study recruitment began in October 2006 and concluded when the target sample was accrued in December 2007. Exercise training ended in December 2008 and testing finished by January 2009. The OHSU Institutional Review Board approved the study procedures. The trial is registered with ClinicalTrials.gov (NCT00591747).
Participants
Women were recruited through the Oregon State Cancer Registry, clinician referral, community events, study advertisements and information sessions. Interested women were screened to determine if they met the following eligibility criteria: diagnosis of stage 0–3a breast cancer at or after age 50, postmenopausal, ≥ 1 year post chemotherapy or radiotherapy, non-osteoporotic, physician clearance to exercise, no regular participation in resistance and/or impact exercise (< two, 30-min sessions per week) in the past month and, physical and cognitive ability to complete study testing.
The PASS 2000 program [30] was used to conduct a power analysis based on a 2 × 3 mixed-design analysis of variance. At n=33 per group, we had power of .81 to .99 to detect a significant group by time interaction for outcomes of muscle strength, fatigue and self-report physical function at α<0.01. To protect against 20% attrition [28, 27] we planned to randomize at least 41 participants per group.
Study Interventions
The study interventions have been described in detail in an earlier publication reporting body composition outcomes from the study [29]. Briefly, the participants in both groups were prescribed an exercise program consisting of two 1-hr supervised classes and one 1-hr home-based session per week for 1 year. If necessary, adjustments in the training program were made on an individual basis during supervised sessions and were later recorded. Symptoms of lymphedema were monitored by the exercise trainer and upper extremity circumferences measured regularly [31]. Five women wore prescribed compression sleeves for lymphedema during exercise training.
The resistance plus impact intervention (POWIR: Prevent Osteoporosis With Impact + Resistance) used in this study was based on our prior interventions in women without cancer [32, 33], originally applied with bone outcomes in mind, but also designed to improve strength and function. POWIR complied with the American College of Sports Medicine (ACSM) exercise guidelines for cancer survivors [34] and with ACSM recommendations for progressive resistance training for novice weightlifters and older adults for 1–3 sets of 8–10 exercises at a weight that can be done for 8–12 repetitions (approximately 60–80% of 1-rep max) with 1–2 min rest between sets [35, 36]. Training was progressive, used a combination of dumbbells, barbells and weighted vests to apply resistance and focused on exercises that targeted the leg, hip, chest and back and using movement patterns similar to those used in activities of daily living [29]. Two-footed jumps from the ground to a target height 1″ from the floor were performed with weighted vests. Women followed a training manual for the home-based program that consisted of the same exercises that were performed in class, but without weight vests and replacing free weights with resistance bands, for both convenience and safety reasons. The deadlift move was omitted from the home program because this exercise requires supervision and heavy weight equipment.
Participants in the placebo exercise group (FLEX) performed a series of whole body stretching and relaxation exercises in a seated or lying position. Exercises were selected to minimize muscular forces so that little stimulus to the musculoskeletal system was applied. An exercise placebo group was used as a control rather than a sedentary usual care group so that the attention from the study team and social interactions among participants would be similar across groups and because we felt it would be unethical to ask the control group to remain sedentary because of the known risks of inactivity for cancer survivors [34].
Procedures
At baseline, written informed consent was obtained followed by completion of questionnaires and physical performance testing. Tests were administered by trained technicians blinded to group assignment and were repeated at 6 and 12 months. A statistician used a computer-generated random numbers table (MS Excel) to allocate participant ID numbers to intervention groups. The statistician provided the numbers table to the project director who placed individual assignments into sealed envelopes prior to enrollment of each participant. Randomization was stratified by adjuvant hormone therapy use (AI or SERM vs none) and current aerobic activity (≥ vs < 90 min/week). Group assignments were placed in sealed, sequentially numbered envelopes and opened by the participant following the completion of baseline testing.
Demographics and health status including breast cancer stage, treatment type, diagnosis and treatment dates, medication use and health history were obtained by self-report. Chronic medical conditions were assessed by the Charlson Comorbidity Index, with higher scores indicating worse health [37]. Physical activity was measured with the Community Health Activity Model Program for Seniors (CHAMPS) physical activity questionnaire for older adults [38] to describe our sample and to monitor for changes in outside physical activity across the intervention period. CHAMPS asks about sedentary, low, moderate, and vigorous activities during the last 4 weeks and calculates weekly energy expended in moderate-vigorous and low-vigorous intensity activities (kcal/wk).
Maximal muscle strength of the upper and lower body was evaluated by a 1-repetition maximum leg press and chest press (1-RM; kg), according to standard protocols [39]. The 1-RM test has a low potential for injury and has been used in prior studies to evaluate maximal muscle strength in cancer survivors, including BCS [25, 26]. Our in-house coefficients of variation (CV), using a subsample of older women, for leg and chest press are 6.6% and 7.5%, respectively.
Grip strength was evaluated by hand grip dynamometry (Takei Scientific Instruments Co., Tokoyo, Japan). Grip strength is a predictor of future onset disability in older adults [40, 41] and can discriminate between those able or unable to perform heavy tasks with their hands [42]. Maximal isometric grip strength (kg) was determined for each right and left hands by recording the highest force attained during a 30 second maximal grip. The best of 3 trials for each hand was used for analysis. In-house CV for this measure is 7.2%
Objective physical function was assessed by the Physical Performance Battery (PPB). The PPB consists of 3 timed performance tests: 5 repeated chair stands, standing balance, and usual gait speed over 4 meters (fastest time of two trials). Each test is scored 0 (unable) to 4, based on quartiles of performance [43], then scores are summed. Higher scores indicate better physical function and low scores on the PPB have been shown to predict ADL disability, hospitalization, admission to a nursing home, and mortality [44, 43, 45, 46]. To specifically evaluate intervention effects on individual components of physical function and risk factors for falls, we evaluated changes in the individual chair stand (sec) and gait speed (m/sec) tests. We also administered an additional balance test separate from the PPB stance test because the latter has not been examined in relation to fall risk [47]. We administered the one-leg stance test that involves standing on one leg in two conditions: eyes open and eyes closed. The time each position was maintained was recorded in sections up to a maximum of 30 seconds. After 30 seconds the test begins to measure muscle endurance rather than static balance. Our in-house CVs for chair stand, gait speed and one-leg stance tests are 5.9%, 3.1% and 14.9%, respectively.
Self-report physical function was determined from the Late-Life Function and Disability Instrument (LLFDI) and the SF-36 Physical Function scale. The LLFDI is a valid and reliable instrument and contains subscales of function including basic and advanced function of the lower and upper extremities [48, 49]. The SF-36 is a 36-item instrument that measures eight important health concepts (physical functioning, bodily pain, general health, vitality, social functioning, impact of health or emotion on role functioning, and mental health) and has been used frequently in studies of BCS to evaluate perceived physical function [50–53]. Of interest in this study were changes in the physical function scale of the SF-36, though we report the baseline physical and mental component summary scores (PCS and MCS) to describe our sample. Scales on both the LLFDI and SF-36 are scored 0–100, with higher scores indicating better function.
Fatigue was measured using the Schwartz Cancer Fatigue (SCF) scale, a 6-item scale that assesses the level of current fatigue specific to the cancer experience [54]. Scores range from 6–36 with higher scores indicating worse fatigue.
Statistical Analysis
To characterize the sample, we computed means and standard deviations for continuous variables and frequencies and percentages for categorical variables. To account for the potential influence of age, time since diagnosis and adjuvant hormone therapy on changes in outcome variables over time, we included these variables as covariates in analyses for the primary study aim. The intent-to-treat (ITT) analysis was performed using Hierarchical Linear Modeling (HLM; HLM 6.08 software) which [55] analyzes each participant according to her originally assigned group and regardless of missing 6 or 12-month data. HLM uses maximum likelihood estimation for handling missing data, which is superior to many other approaches in handling missing data, such as mean imputation or last observation carried forward [56, 57]. We also performed a per protocol analysis using data from participants with complete baseline and 12 month data so that we could evaluate intervention effects in participants who completed the study. We conducted per protocol analyses using separate 2 (group) × 3 (time) mixed-design analysis of covariance (MD-ANCOVA) on each outcome. Of interest were significant group x time interactions; in cases when the interaction was not significant, we examined the main effect of time to determine whether any form of exercise (i.e., resistance or stretching) might change study outcomes. To examine potential effect modification of age and adjuvant hormone therapy use (no use, SERM or AI) we performed additional analysis using HLM and MD-ANCOVA to test for significant 3-way (group x time x age or adjuvant hormone use) interactions. For MD-ANCOVA the effect of age was explored by comparing women 60 years of age or older to women younger than 60. The effect of adherence to the experimental intervention was explored within POWIR using both statistical approaches to test for significant 2-way (adherence x time) interactions. For MD-ANCOVA, we compared outcomes between women who attended 50% or more of class sessions to women who attended less often. We evaluated all hypotheses using an alpha level of 0.05.
RESULTS
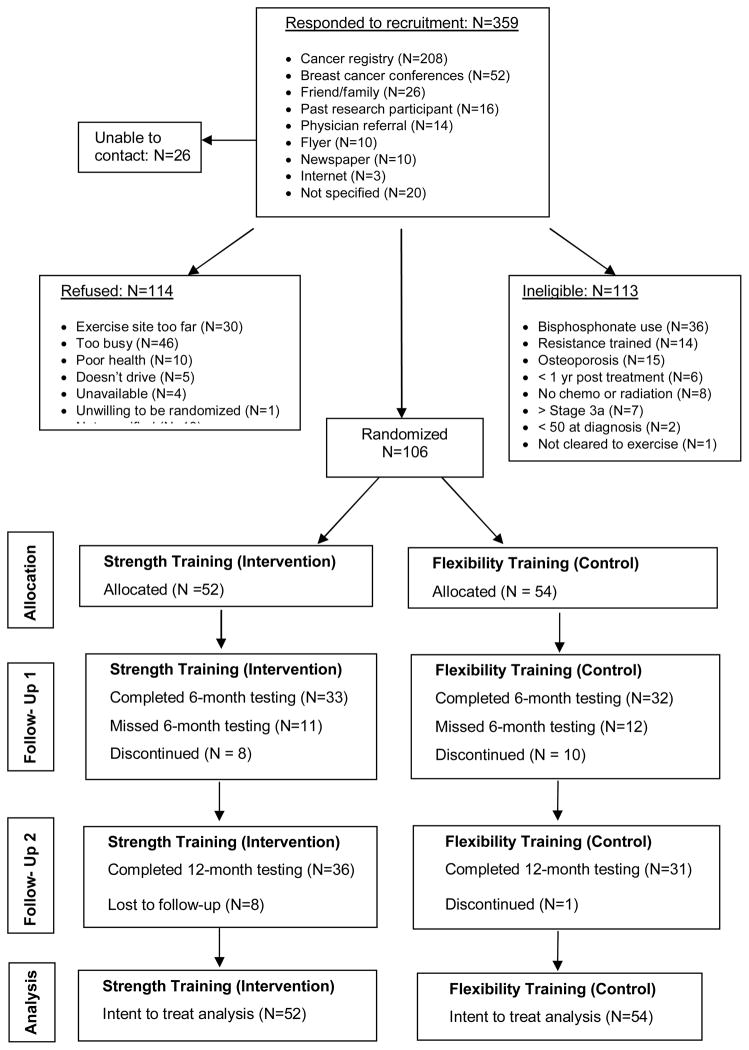
Of 359 women who showed interest in the study, 106 women enrolled in the trial and were randomized to POWIR (n=52) or FLEX (n=54). On average, participants were over 60 years of age and overweight, but in good health based on their low comorbidity index score (Table 1). Most women had stage I or II breast cancer and had received radiation therapy, but adjuvant chemotherapy and hormone therapy were also common. Women reported an average of 447 kcals per day spent in low-vigorous intensity physical activities performed over the month prior to enrollment. Intervention groups were not different at baseline on any demographic or health history characteristic. Participant flow throughout the trial is outlined in Fig 1. Participant retention over 12 months was 85% in POWIR and 80% in FLEX, though a number of women were unable to come in for 12 month testing visits reducing the sample for per protocol analyses to N=36 for POWIR and N=31 for FLEX. Compared to women who remained in the study, those who withdrew from the program were more likely to be closer in time to their cancer diagnosis (43.2 ± 21.5 months vs. 64.2 ± 33.2 months for dropouts and completers, respectively, p<0.05) and to self-report lower mental health based on SF-36 MCS scores (46.6 ± 9.4 vs. 52.6 ± 9.1, p< 0.02) and more difficulty with daily activities based on the LLFDI disability scale (75.1 ± 16.2 vs. 83.7 ± 15.7, p<0.05). However, scores on physical performance measures did not significantly differ between dropouts and completers. Ten women, 5 in each intervention group, changed their adjuvant hormone therapy regimen during the 1-year intervention. Statistical outcomes were unchanged when removing these cases from analyses.
Table 1.
Baseline clinical characteristics of enrolled participants (N=106). Data are expressed as mean (SD) for continuous data or % of sample for categorical data.
| Characteristic | POWIR (N=52) | FLEX (N=54) | Range | p-value |
|---|---|---|---|---|
| Mean (SD) or % of sample | Mean (SD) or % of sample | |||
| Age (yrs) | 62.3 (6.7) | 62.2 (6.7) | 53–83 | 0.97 |
| Comorbidity Index | 1.9 (1.7) | 1.6 (1.7) | 0–9 | 0.29 |
| BMI (kg/m2) | 29.5 (5.8) | 29.5 (5.6) | 20.6–48.1 | 0.98 |
| Time since diagnosis (months) | 56.5 (39.9) | 64.5 (35.4) | 15–259 | 0.28 |
| Stage 0 (%) | 7.7% | 3.7% | 0.37 | |
| Stage I (%) | 38.5% | 40.7% | 0.81 | |
| Stage II (%) | 48.1% | 35.2% | 0.18 | |
| Stage IIIa (%) | 1.9% | 9.3% | 0.10 | |
| Received chemotherapy (%) | 61.5% | 59.3% | 0.81 | |
| Received radiation therapy (%) | 92.3% | 83.3% | 0.16 | |
| Currently taking AI (%) | 42.3% | 40.7% | 0.87 | |
| Currently taking SERM (%) | 17.3% | 13.0% | 0.53 | |
| SF-36 PCS | 49.7 (7.21) | 52.0 (8.17) | 25.2–69.1 | 0.53 |
| SF-36 MCS | 53.3 (8.22) | 52.0 (9.82) | 23.4–66.1 | 0.35 |
| Energy expenditure (kcal/d) | 434.3 (300.1) | 458.9 (346.4) | 0–2223 | 0.65 |
Energy expenditure calculated from CHAMPS physical activity survey and includes energy expended in activities ranging from low to vigorous intensity per week
Abbreviations: AI: Aromatase inhibitor; SERM: selective estrogen receptor modulator; PCS: Physical Component Summary; MCS: Mental Component Summary
Figure 1.
Participant flow across the trial
Adherence, defined as the % of prescribed sessions attended, to supervised training sessions was similar between groups, but adherence to home sessions was significantly better in FLEX than POWIR (p<0.01) (Table 2). Adherence to both supervised and home training sessions decreased from the first to second halves of the yearlong intervention. Compliance to the training protocol was defined as the percent of participants who completed the study exercises without significant modification for 6 months or more. Using this definition, 98% of participants were compliant with the prescribed program. No injuries or adverse events were reported from participation in either study program. Our indicator of lymphedema, upper-extremity circumference measures, did not change differentially between groups over time (mean 0–12 month changes in side-to-side % difference in finger, wrist and arm circumferences for POWIR vs. FLEX were -0.5±2.9 vs.−0.2±2.3 (p=0.8), 0.5±2.0 vs. 0.0±1.7 (p=0.4), 0.5±1.7 vs. −0.1±2.3 (p=0.7), respectively)
Table 2.
Percent completion of study POWIR or FLEX (control) exercise programs at the mid-point (6 months) and across the entire intervention period (12 months).
| Study Group | Timeframe | Supervised | Home | Total |
|---|---|---|---|---|
| POWIR | 0–6 months | 82% | 27% | 63% |
| 6–12 months | 62% | 18% | 54% | |
| 0–12 months | 76% | 23% | 57% | |
| FLEX | 0–6 months | 80% | 47% | 69% |
| 6–12 months | 69% | 41% | 51% | |
| 0–12 months | 72% | 44% | 62% |
Using the ITT approach, there were significant differences over time between POWIR and FLEX groups for maximal leg press strength (Coefficient for slope of time=9.96, SE=4.06, t(99)=2.45, p<0.02) and maximal bench press strength (Coefficient for slope of time=2.13, SE=0.87, t(99)=2.46, p<0.02). There were no significant group differences over time on the remaining outcomes.
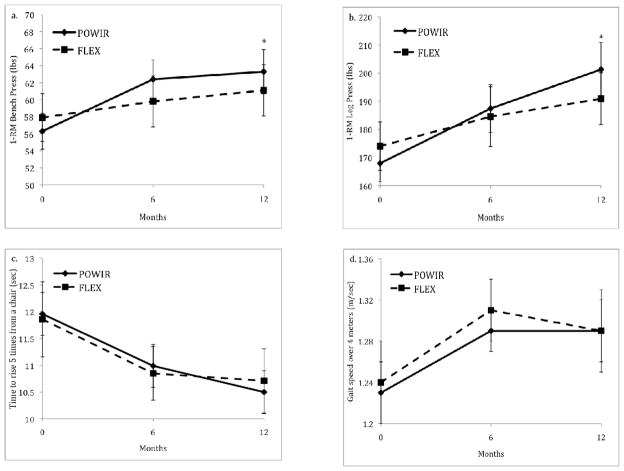
Restricting analyses to women who completed all study visits yielded similar results (Table 3) and provided the opportunity to examine patterns of changes in study outcomes across the intervention period. Muscle strength increased linearly across the intervention period for both upper and lower body strength (Fig 2a–b), though the rate of increase in upper body strength decreased somewhat over the second half of the intervention. Like the ITT results, objective measures of physical function that included the chair stand and usual walk tests did not demonstrate differential change over time by group (Figs 2c–d). However, there was a significant main effect of time for gait speed where both POWIR and FLEX improved over time (p<0.04). Walk speed improved initially over the first 6 months then slightly decreased at 12 months, but stayed above baseline levels. Chair stand time improved similarly in both groups within the first 6 months and improved slightly over the next 6 months in POWIR.
Table 3.
Initial and final values on outcomes by exercise group from baseline to 12 months. Data presented as unadjusted mean (SD) for participants with complete data sets for both time points.
| Characteristic | POWIR (n=36) | FLEX (n=31) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | % change | Baseline | 12 months | % change | p-valuea | Effect estimateb (POWIR – FLEX) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (95% CI) | ||||
| Bench press 1RM (lbs) | 56.3 (12.8) | 63.3 (15.3) | 12.4 | 57.9 (15.8) | 61.1 (16.8) | 5.5 | 0.01 | 3.9 (0.5 to 7.3) |
| Leg press 1RM (lbs) | 167.9 (39.4) | 201.3 (57.4) | 19.9 | 174.0 (47.9) | 191.0 (51.7) | 9.8 | 0.04 | 16.4 (−0.1 to 33.0) |
| Grip strength left hand (kg) | 24.4 (5.06) | 24.8 (5.20) | 1.6 | 23.6 (5.02) | 23.8 (5.58) | 0.8 | 0.92 | 0.2 (−1.2 to 1.6) |
| Grip strength right hand (kg) | 26.0 (5.49) | 25.7 (5.28) | −1.2 | 25.5 (5.73) | 25.3 (5.70) | −0.8 | 0.93 | −0.1 (−1.5 to 1.4) |
| Chair stand (sec) | 12.0 (2.6) | 10.5 (1.7) | −12.5 | 11.9 (3.27) | 10.7 (3.2) | −10.1 | 0.70 | −0.3 (−1.1 to 0.5) |
| Best 4m usual walk (m/sec) | 1.2 (0.2) | 1.3 (0.2) | 1.6 | 1.2 (0.2) | 1.3 (0.2) | 4.0 | 0.76 | 0.0 (−0.1 to 0.1) |
| 1 leg standing balance – EO (sec) | 21.9 (10.2) | 22.4 (9.5) | 2.3 | 25.4 (8.9) | 24.0 (9.8) | −5.5 | 0.48 | 1.8 (−1.6 to 5.1) |
| 1 leg standing balance – EC (sec) | 6.98 (6.9) | 6.70 (7.5) | −4.0 | 6.6 (6.5) | 8.24 (7.7) | 25.4 | 0.24 | −1.5 (−3.5 to 0.5) |
| PPB | 11.1 (1.0) | 11.6 (0.6) | 4.5 | 11.1 (1.0) | 11.6 (0.8) | 4.5 | 0.92 | 0.0 (−0.4 to 0.4) |
| SF-36 Physical Function | 50.3 (5.1) | 51.7 (6.2) | 2.8 | 51.8 (6.4) | 52.3 (5.9) | 1.0 | 0.59 | 2.4 (−3.7 to 8.5) |
| LLFDI function upper extremities | 85.0 (12.1) | 82.7 (9.9) | −2.7 | 81.2 (12.1) | 79.5 (13.0) | −2.1 | 0.87 | −0.6 (−5.5 to 4.3) |
| LLFDI function lower extremities | 84.5 (12.7) | 87.2 (12.5) | 3.2 | 85.4 (12.7) | 87.9 (14.7) | 2.9 | 0.71 | 0.2 (−4.6 to 5.1) |
| LLFDI advanced lower extremities | 66.3 (12.6) | 67.2 (13.3) | 1.4 | 70.1 (15.3) | 70.4 (15.9) | 0.4 | 0.81 | 0.5 (−4.2 to 5.1) |
| LLFDI disability limitation score | 84.5 (16.4) | 84.9 (13.5) | 0.5 | 85.5 (13.7) | 87.5 (14.7) | 2.3 | 0.61 | −1.5 (−6.4 to 3.3) |
| Schwartz cancer fatigue scale | 9.9 (3.3) | 10.1 (4.7) | 2.5 | 9.3 (3.1) | 9.0 (3.21) | −2.5 | 0.90 | 0.5 (−1.3 to 2.2) |
1RM: 1-repetition maximum; EO: eyes open condition; EC: eyes closed condition; PPB: Physical performance battery; SF-36: short-form 36; LLFDI: Late life function and disability, CI: Confidence interval
p-value from RM-ANCOVA that included baseline, 6 and 12 month time points and controlled for the following: age, time since diagnosis, current adjuvant hormone therapy use.
Effect estimate determined from unadjusted mean difference in group change scores from baseline to 12 months.
Figure 2.
Figures 2a–d Changes in upper and lower body muscle strength, chair stand and gait speed tests between POWIR and FLEX across 12-month intervention. Bars represent standard error.
* Changes in POWIR significantly different from changes in FLEX, p<0.05
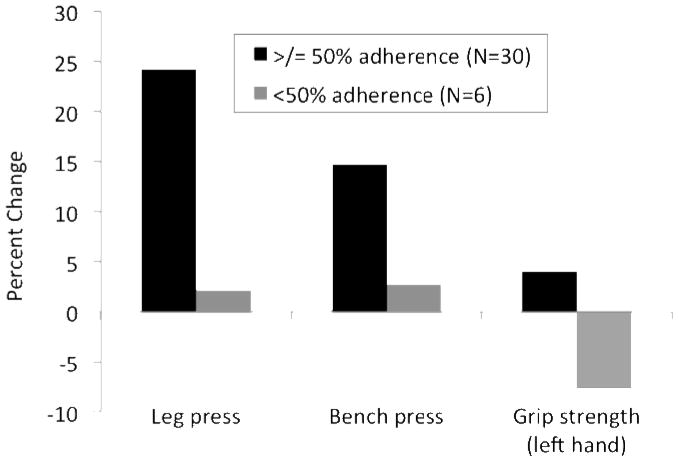
There was no evidence of effect modification from adjuvant hormone therapy use or age on study outcomes using either ITT or per protocol analytic approaches (data not shown). However, HLM analysis revealed significant effect modification of adherence to the POWIR program on both measures of upper body strength (grip strength (p=0.045) and maximal bench press (p=0.023)). When using 2 × 3 MD-ANCOVA to evaluate study completers only, those attending 50% or more of prescribed resistance + impact exercise sessions had better changes in grip strength (p<0.01), bench press (p=0.03) and leg press (p<0.02) tests than women who attended classes less 50% of the time (Fig 3).
Figure 3.
Comparison of percent changes in maximal strength measures between POWIR participants who adhered to 50% or more of prescribed training sessions and POWIR participants who adhered less than 50% of the time
DISCUSSION
Our program of resistance + impact exercise produced statistically significant improvements in upper and lower body maximal strength compared to a control program of low-intensity stretching in BCS who were older than 50 years of age and postmenopausal at diagnosis. However, the POWIR program did not appear to improve either objective or subjective measures of physical function more than the control condition. In fact, both groups improved significantly over time in a measure of mobility, e.g., gait speed. Women were able to perform either study program as prescribed. Within the POWIR program, higher attendance was associated with greater improvements in maximal upper and lower body strength.
Our study is the first to report on a resistance training program specifically for women who were older when they were diagnosed and treated for breast cancer. Approximately 85% of women who receive a first diagnosis of breast cancer are aged 50 and over, thus older women constitute the largest group of BCS [58]. While other resistance training trials have included older women as part of a broader age range of BCS [25, 26, 24], these studies cannot evaluate the specific capacity of older BCS to tolerate and respond to rehabilitative interventions. We found that older BCS could tolerate a moderate-vigorous resistance training program as evidenced by compliance to the prescribed training program and the absence of program-related injuries. We are also among the first to examine physical function using objective measures rather than self-report [59]. Objective measures of physical function, particularly those that can capture different domains of function such as balance and mobility, overcome limitations of self-reported physical function because objective tests can indicate which body systems underlie limitations and detect declines before a woman recognizes a change in her abilities [8].
The notable limitations of our study were the modest size of the sample and generalizability of the program. Our study may not generalize to the broader population of BCS, since women who dropped out of the program had some different characteristics than women who did not. Women who withdrew from the program were closer to diagnosis and reported more difficulties with activities of daily living and lower mental health scores. It is unlikely that these women dropped out because they could not tolerate the POWIR program since a greater proportion dropped out of the control program due to poor health than the POWIR program (Fig 1); however, dropouts from our study may represent a subgroup of older BCS that have different exercise preferences and warrant further attention. Though our choice against a non-exercise control group may also be perceived as a limitation, we view it as a strength because an exercise placebo group reduces the potential for unequal attrition among participants allocated to a non-exercise group and also keeps the level of attention from instructors and peers similar across groups. We also felt that withholding exercise completely from BCS would be unethical given the recommendations that cancer survivors avoid inactivity [34]. Stretching exercise has been used as a control condition for resistance training studies in older adults and did not improve strength or bone density [60–63], but has been shown to provide other benefits such as increased range of motion [64] that could improve mobility [65]. In fact, the main effects of time on gait speed in our study (Fig 2c) indicate that even low intensity stretching might have had a small effect on function outcomes in BCS, though neither group could be compared to a no exercise condition.
Women in the POWIR program improved upper and lower body muscle strength as evidenced by 12% and 20% increases in 1-RM bench and leg press performance, respectively. Our findings agree with other resistance training trials in BCS but the magnitude of strength increases was slightly lower among our older sample [25, 26, 24]. Differences between those studies and ours may be attributed in part to the different age ranges of participants, but could also reflect differences in strength testing protocols and training programs. Resistance training trials in older adults without cancer consistently report significant strength gains, but those studies comparing the degree of change between young and old participants report similar improvements or slightly less improvement in old versus young adults [66]. Within our sample of BCS ranging in age from 53–83 years old, age did not moderate significant group differences over time suggesting that resistance exercise benefits are similar across this older age span.
In contrast to Twiss et al who reported improvements in dynamic balance after strength training in BCS [24], we did not find differences between POWIR and FLEX on simple static balance tests. We expected that the POWIR program would improve balance based on our prior reports of stability improvements with this program in women without cancer [27, 28] and because muscle strength contributes to balance control [13, 12]. We used a simple clinical test of static balance, the one-leg stance test, whereas other studies used laboratory balance tests [27, 28] or more complex clinical tests of dynamic balance, e.g., timed backward tandem walk [24]. Our simple clinical test may have lacked the specificity and sensitivity to detect balance changes from strength training, particularly attributes most relevant to falls [47], and future trials should select the most appropriate measurement tool for their intervention and population. Another reason that we may have failed to find group differences on either objective or perceived self-report physical function is that we did not select women for low baseline function nor did we exclude aerobically active women (Table 1). We also excluded women who could not engage in moderate-vigorous intensity resistance training sufficient to improve bone health and thus may have inadvertently excluded women who might have been more likely to improve function because of low baseline fitness [67]. Average baseline values for usual gait speed, handgrip strength and the PPB among our participants (Table 3) were at or above values reported in cohort studies of well functioning, community-dwelling older adults [68–70].
Campbell et al. have urged investigators to thoroughly report compliance and adherence to prescribed exercise interventions in cancer survivors so that specific evidence-based exercise prescriptions, administered by healthcare professionals, could be available for BCS [67]. In addition to reporting participant compliance to our prescribed protocol, we evaluated the influence of adherence on study outcomes. Women who came to class an average of 1 or more times per week had significantly greater improvements in maximal upper and lower body strength measures than women attending less often (Fig. 3). These findings are consistent with those of Taafe et al and Di Franco et al who reported significant maximal strength gains among older adults who participated in moderate-vigorous intensity resistance training 1 day per week compared to inactive controls [71]. However, while Taafe and Di Franco reported that strength gains were no better among older adults who resistance trained 2 or 3 days per week compared to 1, our results indicate that adherence rates above an average of 1 time per week promoted greater upper body strength gains. Our study was in women only and the others included men, thus it is possible that in women the upper body may be more responsive to further increases in training than the lower body. This body region may be more sensitive to increased frequency of training in women because faster declines in upper extremity strength than lower extremity strength across the 6th to 9th decades have been reported for community-dwelling women [72]. For these older BCS, an average participation rate equal to one day per week was sufficient to cause gains in muscle strength and this frequency may meet the preferences of older BCS for gradual increases in the level of exercise over time [73]. Our data suggest, though, that even greater gains in upper extremity strength could be achieved by progressing toward ACSM recommendations for twice-weekly resistance training for older adults [36].
Given the known roles of muscle weakness in the etiology of falls and functional decline, improvements in muscle strength could translate to lower fall and disability risk in older BCS. When considering the multi-factorial nature of falls, muscle weakness ranks as the lead risk factor in older adults [11]. In addition to fall risk, muscle weakness is also a precursor to disability onset because it precedes functional limitations that can lead to loss of independence [8, 74, 10]. Muscle weakness is a consistent predictor of ADL disability among community-dwelling older adults [75]. Since we did not measure falls nor disability in our study, we cannot assume that our older BCS who gained strength will fall less or remain independent longer; however, future controlled exercise trials that track falls and disability in adequately powered samples are warranted. In 2011, the first generation of baby boomers will reach 65, and the aging of this generation will contribute to a projected doubling of cancer survivors by 2050 [76]. Developing safe, effective and translatable interventions that optimize function and quality of life specific to older cancer survivors is an important area for future work.
Acknowledgments
Supported by Susan G. Komen Race for the Cure and the National Cancer Institute (1R01 CA120123, to Dr. Winters-Stone) and with partial support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. We thank the Oregon State Cancer Registry for their assistance with recruitment efforts for the study. Thera-band provided elastic bands for home exercise programs. We thank Mr. Nathan Brooks, Ms. Camella Potter, and Mr. Anton Stupnitskiy for their assistance with data collection. We also thank Ms. Janice Hoffman, Ms. Laurie Iverson, and Ms. Lisa Domenico for their assistance with exercise training.
References
- 1.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional Limitations in Elderly Female Cancer Survivors. J Natl Cancer Inst. 2006;98(8):521–9. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 2.Chen ZMM, Aragaki AK, Mouton C, Arendell L, Lopez AM, Bassford T, Chlebowski RT. Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: results from the Women’s Health Initiative. Osteoporos Int. 2009;20(4):527–36. doi: 10.1007/s00198-008-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winters-Stone K, Torgrimson B, Horak F, Eisner A, Leo M, Nail L, Chui S, Luoh Shiuh-Wen. Identifying risk factors for falls in postmenopausal breast cancer survivors: a multidisciplinary approach. Arch Phys Med Rehab. 2011;9(4) doi: 10.1016/j.apmr.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winters-Stone KM, Nail L, Bennett JA, Schwartz A. Bone health and falls: fracture risk in breast cancer survivors with chemotherapy-induced amenorrhea. Oncol Nurs Forum. 2009;36(3):315–25. doi: 10.1188/09.ONF.315-325. [DOI] [PubMed] [Google Scholar]
- 5.Frost HM. Should fracture risk-of-fracture analyses include another major risk factor? The case for falls. J Clin Densitom. 2001;4:381–3. doi: 10.1385/jcd:4:4:381. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control aP. Falls among older adults: An overview. 2008 http://www.cdc.gov/ncipc/factsheets/adultfalls/htm.
- 7.Stevens JA, Sogolow ED. Gender differences for non-fatal unintentional fall related injuries among older adults. Inj Prev. 2005;11(2):115–9. doi: 10.1136/ip.2004.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett JA, Winters-Stone K, Nail L. Conceptualizing and measuring physical functioning in cancer survivorship studies. Oncol Nurs Forum. 2006;33(1):41–9. doi: 10.1188/06.ONF.41-49. [DOI] [PubMed] [Google Scholar]
- 9.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Medical hypotheses. 2006;67(2):212–5. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 10.Rantanen T. Muscle strength, disability and mortality. Scandinavian Journal of Medicine & Science in Sports. 2003;13(1):3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 11.American Geriatrics Society and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc. 2001;49(5):664–72. [PubMed] [Google Scholar]
- 12.Orr R. Contribution of muscle weakness to postural instability in the elderly. A systematic review. Eur J Phys Rehabil Med. 2010;46(2):183–220. [PubMed] [Google Scholar]
- 13.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurence of falls. J Gerontol A Biol Sci Med Sci. 1995;50:64–7. doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]
- 14.Freedman RJ, Aziz N, Albanes D, Hartman T, Danforth D, Hill S, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89(5):2248–53. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- 15.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19(9):2381–9. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 16.Harvie MN, Campbell IT, Baildam A, Howell A. Energy balance in early breast cancer patients receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2004;83(3):201–10. doi: 10.1023/B:BREA.0000014037.48744.fa. [DOI] [PubMed] [Google Scholar]
- 17.Cheney CL, Mahloch J, Freeny P. Computerized tomography assessment of women with weight changes associated with adjuvant treatment for breast cancer. Am J Clin Nutr. 1997;66(1):141–6. doi: 10.1093/ajcn/66.1.141. [DOI] [PubMed] [Google Scholar]
- 18.Kutynec CL, McCargar L, Barr SI, Hislop TG. Energy balance in women with breast cancer during adjuvant treatment. J Am Diet Assoc. 1999;99(10):1222–7. doi: 10.1016/s0002-8223(99)00301-6. [DOI] [PubMed] [Google Scholar]
- 19.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–57. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz AL. Fatigue in long-term cancer survivors. Oncology (Williston Park) 2009;23(8 Suppl):27, 33–4. [PubMed] [Google Scholar]
- 21.Hauer K, Rost B, Rutschle K, Opitz H, Specht N, Bartsch P, et al. Exercise training for rehabilitation and secondary prevention of falls in geriatric patients with a history of injurious falls. J Am Geriatr Soc. 2001;49(1):10–20. doi: 10.1046/j.1532-5415.2001.49004.x. [DOI] [PubMed] [Google Scholar]
- 22.Campbell AJ, Robertson MC, Gardner M, Norton RN, Buchner DM. Falls prevention over 2 years: a randomized controlled trial in women 80 years and older. Age Ageing. 1999;28(6):513–8. doi: 10.1093/ageing/28.6.513. [DOI] [PubMed] [Google Scholar]
- 23.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic Review of Progressive Resistance Strength Training in Older Adults. J Gerontol A Biol Sci Med Sci. 2004;59(1):M48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- 24.Twiss JJ, Waltman NL, Berg K, Ott CD, Gross GJ, Lindsey AM. An exercise intervention for breast cancer survivors with bone loss. J Nurs Scholarsh. 2009;41(1):20–7. doi: 10.1111/j.1547-5069.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight Lifting in Women with Breast-Cancer–Related Lymphedema. New England Journal of Medicine. 2009;361(7):664–73. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis-Grant L, Smith R, et al. Weight Lifting for Women at Risk for Breast Cancer–Related Lymphedema. JAMA: The Journal of the American Medical Association. 2010;304(24):2699–705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 27.Shaw JM, Snow CM. Weighted vest exercise improves indices of fall risk in older women. J Gerontol. 1998;53:M53–8. doi: 10.1093/gerona/53a.1.m53. [DOI] [PubMed] [Google Scholar]
- 28.Winters KM, Snow CM. Detraining reverses positive effects of exercise on the musculoskeletal system in premenopausal women. J Bone Miner Res. 2000;15:2495–503. doi: 10.1359/jbmr.2000.15.12.2495. [DOI] [PubMed] [Google Scholar]
- 29.Winters-Stone K, Dobek J, Nail L, Bennett JA, Naik A, Schwartz A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2011;27(2):447–56. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hintze J. PASS 6.0 Power Analysis and Sample Size for Windows. Keysville, Utah: NCSS; 1996. [Google Scholar]
- 31.Bicego D, Brown K, Ruddick M, Storey D, Wong C, Harris SR. Exercise for Women With or at Risk for Breast Cancer–Related Lymphedema. Physical Therapy. 2006;86(10):1398–405. doi: 10.2522/ptj.20050328. [DOI] [PubMed] [Google Scholar]
- 32.Winters-Stone K, Snow C. Site-specific response of bone to exercise in premenopausal women. Bone. 2006;39(6):1203–9. doi: 10.1016/j.bone.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Snow CM, Shaw JM, Winters KM, Witzke KA. Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2000;55(9):M489–91. doi: 10.1093/gerona/55.9.m489. [DOI] [PubMed] [Google Scholar]
- 34.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 35.American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Medicine and science in sports and exercise. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 36.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Medicine and science in sports and exercise. 2009;41(7):1510–30. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 37.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 38.Stewart A, Mills K, King A, Haskell W, Gillis D, Ritter P. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 39.American College of Sports Medicine. ACSM’s guildelines for exercise testing and prescription. 7. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 40.den Ouden MEM, Schuurmans MJ, Arts IEMA, van der Schouw YT. Physical performance characteristics related to disability in older persons: A systematic review. Maturitas. 2011;69(3):208–19. doi: 10.1016/j.maturitas.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31(1):3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 42.Wang C-Y, Chen L-Y. Grip Strength in Older Adults: Test-Retest Reliability and Cutoff for Subjective Weakness of Using the Hands in Heavy Tasks. Archives of physical medicine and rehabilitation. 2010;91(11):1747–51. doi: 10.1016/j.apmr.2010.07.225. [DOI] [PubMed] [Google Scholar]
- 43.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 44.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 45.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2000;55(11):M691–7. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 47.Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med. 2010;46(2):239–48. [PMC free article] [PubMed] [Google Scholar]
- 48.Jette AM, Haley SM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57(4):M209–16. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 49.Haley SM, Jette AM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57(4):M217–22. doi: 10.1093/gerona/57.4.m217. [DOI] [PubMed] [Google Scholar]
- 50.McKenzie DC, Kalda AL. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: a pilot study. J Clin Oncol. 2003;21(3):463–6. doi: 10.1200/JCO.2003.04.069. [DOI] [PubMed] [Google Scholar]
- 51.Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, et al. Exercise manages fatigue during breast cancer treatment: A randomized controlled trial. Psychooncology. 2004;14(6):464–77. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- 52.Mock V, Pickett M, Ropka ME, Muscari Lin E, Stewart KJ, Rhodes VA, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001;9(3):119–27. doi: 10.1046/j.1523-5394.2001.009003119.x. [DOI] [PubMed] [Google Scholar]
- 53.Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657–65. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz AL. The Schwartz Cancer Fatigue Scale: testing reliability and validity. Oncol Nurs Forum. 1998;25(4):711–7. [PubMed] [Google Scholar]
- 55.Raudenbush S, Bryk A. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 56.Lane P. Handling drop-out in longitudinal clinical trials: a comparison of the LOCF and MMRM approaches. Pharm Stat. 2008;7:93–106. doi: 10.1002/pst.267. [DOI] [PubMed] [Google Scholar]
- 57.Streiner DL. The case of the missing data: methods of dealing with dropouts and other research vagaries. Can J Psychiatry. 2002;47:68–75. [PubMed] [Google Scholar]
- 58.SEER: Surveillance, Epidemiology, and End Results [database on the Internet] National Cancer Institute; 2005. [Accessed: January 19, 2005]. Available from: http://seer.cancer.gov/faststats/html/pre_breast.html. [Google Scholar]
- 59.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 60.Brochu M, Savage P, Lee M, Dee J, Cress ME, Poehlman ET, et al. Effects of resistance training on physical function in older disabled women with coronary heart disease. J Appl Physiol. 2002;92(2):672–8. doi: 10.1152/japplphysiol.00804.2001. [DOI] [PubMed] [Google Scholar]
- 61.Alexander NB, Gross MM, Medell JL, Hofmeyer MR. Effects of functional ability and training on chair-rise biomechanics in older adults. J Gerontol A Biol Sci Med Sci. 2001;56(9):M538–47. doi: 10.1093/gerona/56.9.m538. [DOI] [PubMed] [Google Scholar]
- 62.Brown M, Sinacore DR, Ehsani AA, Binder EF, Holloszy JO, Kohrt WM. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil. 2000;81(7):960–5. doi: 10.1053/apmr.2000.4425. [DOI] [PubMed] [Google Scholar]
- 63.Barrett CJ, Smerdely P. A comparison of community-based resistance exercise and flexibility exercise for seniors. Aust J Physiother. 2002;48(3):215–9. doi: 10.1016/s0004-9514(14)60226-9. [DOI] [PubMed] [Google Scholar]
- 64.Rider RA, Daly J. Effects of flexibility training on enhancing spinal mobility in older women. J Sports Med Phys Fitness. 1991;31(2):213–7. [PubMed] [Google Scholar]
- 65.Christiansen CL. The Effects of Hip and Ankle Stretching on Gait Function of Older People. Arch Phys Med Rehabil. 2008;89(8):1421–8. doi: 10.1016/j.apmr.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 66.Chodzko-Zajko WJPD, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine Position Stand: Exercise and Physical Activity for Older Adults. Med Sci Sports Exerc. 2009;41(7):1510–30. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 67.Campbell KL, Neil SE, Winters-Stone KM. Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med. 2011 doi: 10.1136/bjsports-2010-082719. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 68.Perera S, Mody SH, Woodman RC, Studensk SA. Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults. Journal of the American Geriatrics Society. 2006;54(5):743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 69.Wennie Huang W-N, Perera S, VanSwearingen J, Studenski S. Performance Measures Predict Onset of Activity of Daily Living Difficulty in Community-Dwelling Older Adults. J Am Geriatr Soc. 2010;58(5):844–52. doi: 10.1111/j.1532-5415.2010.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brach JS, VanSwearingen JM, Newman AB, Kriska AM. Identifying Early Decline of Physical Function in Community-Dwelling Older Women: Performance-Based and Self-Report Measures. Physical Therapy. 2002;82(4):320–8. [PubMed] [Google Scholar]
- 71.Taaffe D, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47(10):1208–14. doi: 10.1111/j.1532-5415.1999.tb05201.x. [DOI] [PubMed] [Google Scholar]
- 72.Sinaki M, Nwaogwugwu NC, Phillips BE, Mokri MP. Effect of gender, age, and anthropometry on axial and appendicular muscle strength. Am J Phys Med Rehabil. 2001;80(5):330–8. doi: 10.1097/00002060-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Whitehead S, Lavelle K. Older Breast Cancer Survivors’ Views and Preferences for Physical Activity. Qual Health Res. 2009;19(7):894–906. doi: 10.1177/1049732309337523. [DOI] [PubMed] [Google Scholar]
- 74.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol Bio Sci Med Sci. 2000;55(1):M43–52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 75.Vermeulen J, Neyens J, van Rossum E, Spreeuwenberg M, de Witte L. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatrics. 2011;11(1):33. doi: 10.1186/1471-2318-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edwards BK, Howe HL, Ries LA, Thun MJ, Rosenberg HM, Yancik R, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94(10):2766–92. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]