Abstract
Purpose
Puromycin aminonucleoside (PAN) specifically injures podocytes, leading to foot process effacement, actin cytoskeleton disorganization, and abnormal distribution of slit diaphragm proteins. p130Cas is a docking protein connecting F-actin fibers to the glomerular basement membrane (GBM) and adapter proteins in glomerular epithelial cells (GEpCs; podocytes). We investigated the changes in the p130Cas expression level in the PAN-induced pathological changes of podocytes in vitro.
Methods
We observed changes in the p130Cas expression in cultured rat GEpCs and mouse podocytes treated with various concentrations of PAN and antioxidants, including probucol, epigallocatechin gallate (EGCG), and vitamin C. The changes in the p130Cas expression level were analyzed using confocal immunofluorescence imaging, Western blotting, and polymerase chain reaction.
Results
In the immunofluorescence study, p130Cas showed a diffuse cytoplasmic distribution with accumulation at distinct sites visible as short stripes and colocalized with P-cadherin. The fluorescences of the p130Cas protein were internalized and became granular by PAN administration in a dose-dependent manner, which had been restored by antioxidants, EGCG and vitamin C. PAN also decreased the protein and mRNA expression levels of p130Cas at high doses and in a longer exposed duration, which had been also reversed by antioxidants.
Conclusion
These findings suggest that PAN modulates the quantitative and distributional changes of podocyte p130Cas through oxidative stress resulting in podocyte dysfunction.
Keywords: Cytoskeleton, p130Cas, Podocytes cell, Puromycin aminonucleoside
Introduction
Proteinuria is the main clinical manifestation of podocyte diseases and an important predictor of outcome in glomerular disease1). Proteinuria demonstrates an increase in glomerular permeability caused by ultrastructural and componental changes in podocytes and the slit diaphragm (SD), showing retraction and effacement of the interdigitating foot processes (FPs)1-3).
The specialized, highly differentiated podocytes consist of three morphologically and functionally different segments: cell body, major processes and extending FPs, which are separated by a filtration slit that is 25 to 60 nm wide and covered by the SD2-4). The FPs contain an actin-based cytoskeleton that is linked to the glomerular basement membrane (GBM) in focal contacts of the basal membrane domain and the apical membrane domain proteins, such as podocalyxin3-5). Therefore, podocyte actin cytoskeleton is closely connected to these three FP membrane domains, including SD, basal, and apical, which maintains meticulous glomerular filtration. Interference with any of the three FP domains leads to the changes of actin cytoskeleton from parallel contractile bundles into a dense network with FPs effacement, therefore, FPs effacement requires the active reorganization of actin filaments3,5). As a reverse pathologic pathway, any alterations in the cytoskeleton could also lead to changes in the structure and function of podocytes, resulting in proteinuria5,6).
p130Cas is a protein of Cas (Crk-associated substrate) family that might serve as a ubiquitous docking protein in various tissues and cellular structures including podocytes for actin cytoskeleton-dependent signaling networks. The interaction of p130Cas with other adjacent proteins in normal and pathologic cells modulates cell migration, survival and proliferation7,8). In podocytes, p130Cas localizes diffusely to the cytoplasm with accumulation at ends of F-actin stress fibers in FPs, where focal adhesion proteins and kinases connect docking proteins including integrin and p130Cas to the GBM7), and CD2AP and p130Cas to the SD insertion site9). Therefore, p130Cas protein also plays an important role in maintaining the glomerular permeability by connecting podocyte actin cytoskeleton to GBM and SD.
Puromycin aminonucleoside (PAN) specifically injured podocytes, leading to a flattening of podocyte FPs, focal detachment from the GBM, actin cytoskeleton disorganization, and decreased expression and abnormal distribution of the SD proteins, which coincided with the onset of proteinuria10-12). Therefore, PAN-induced nephrosis has been used extensively as a model of proteinuria. In this study, we applied an in vitro PAN model and found that PAN disregulaed the podocytes p130Cas and might subsequently induce the phemotypical changes of podocytes.
Materials and methods
1. Cell culture of rat glomerular epithelial cell (GEpC) and mouse podocytes
Rat GEpCs, cloned from primary rat glomerular cultures, were characterized and provided by Kreisberg et al.13). They were characterized by sensitivity to PAN, positive staining for Heymann antigen (gp330) and podocalyxin, whereas negative staining for factor VIII13,14). The GEpCs were maintained as previously described15). Experiments were performed with cells between passages 15 and 18. Cells were treated with 1 to 50 µg/mL PAN (Sigma Chemical Co., St. Louis, MO, USA) dissolved in ethanol for 24 and/or 48 hours. For the protective effect of antioxidants, podocytes were co-treated with the various concentrations of PAN and 50 µM epigallocatechin gallate (EGCG) (Sigma Chemical Co.), 50 µM probucol (Sigma Chemical Co.), or 30 µM vitamin C (Amresco Inc., Solon, OH, USA). Controls were treated with vehicle only.
2. Confocal image analysis of p130Cas
The rat GEpCs and mouse podocytes that were prepared as before on type I collagen-coated glass cover slips incubated for 24 hours were fixed in 4% paraformaldehyde, permeabilized in phosphate buffered saline (PBS), blocked with 10% normal goat serum, and labeled with polyclonal rabbit anti-rat p130Cas (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and polyclonal rabbit anti-P-cadherin (Santa Cruz Biotechnology Inc.). Primary antibody-bound specimens were incubated with 1:500 (v/v) Alexa 488 for green and Alexa 594 for red (Invitrogen, Carlsbad, CA, USA)-conjugated respective secondary antibodies at room temperature for 1 hour. F-actin was visualized with fluorescein isothiocyanate (Sigma Chemical Co.). Coverslips were mounted in aqueous mountant and viewed with a confocal laser scanning microscope (TCS SP2 AOBS, Leica Microsystems, Wetzlar, Germany).
3. Western blotting of p130Cas
At the end of 48-hour incubation with additives, the confluently grown cell layers were washed twice with PBS and subsequently extracted in 4M guanidinium-HCl, 2% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate hydrate, and protease inhibitors containing 100 mM 6-aminohexanoic acid, 10 mM benzamidine HCl, and 1 mM phenylmethylsulfonyl fluoride at 4 overnight and stored at -20℃ till further analysis. Protein concentrations were determined with a Bio-Rad kit (Bio-Rad Laboratories, Hercules, CA, USA). Thirty µg of boiled extracts were applied on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories). Then, the membranes were air-dried and blocked in 3% fat-free milk before incubation with polyclonal rabbit anti-rat p130Cas (Santa Cruz Biotechnology Inc.). After incubation with horseradish peroxidase-conjugated secondary antibodies, bands were detected by using the enhanced chemiluminescence chemiluminescence system (Amersham Biotech Ltd., Bucks, UK).
4. Reverse transcription-polymerase chain reaction (RT-PCR) analysis of p130Cas
Total RNA was extracted from cultured rat GEpC incubated for 48 hours. After estimating its concentration by ultraviolet spectrophotometry, 5 µg of total RNA was used for first-strand cDNA synthesis. Aliquots of the cDNA were amplified using primers for p130Cas: sense 5'-CATTGTGCCTGGTAACCG-3' and anti-sense 5'-TGGCACCTGGTAAATGTC-3'. The expression of GAPDH as a housekeeping gene was analyzed employing the following primers: sense 5'-TCTACCCACGGCAAGTTCAA-3' and anti-sense 5'-GGATGACCTTGCCCACAGC-3'. PCR products were visualized on 1.5% agarose gels, and band density was measured using densitometry program (LabWorks 4.0, UVP Inc., Upland, CA, USA).
5. Statistical analysis
The results are presented as mean±standard deviation, as required under different conditions. The statistical significance was assessed by nonparametric Kruskal-Wallis analysis of variance analysis or Student's t-test. P values less than 0.05 were considered significant.
Results
1. Changes in localization of p130Cas by PAN
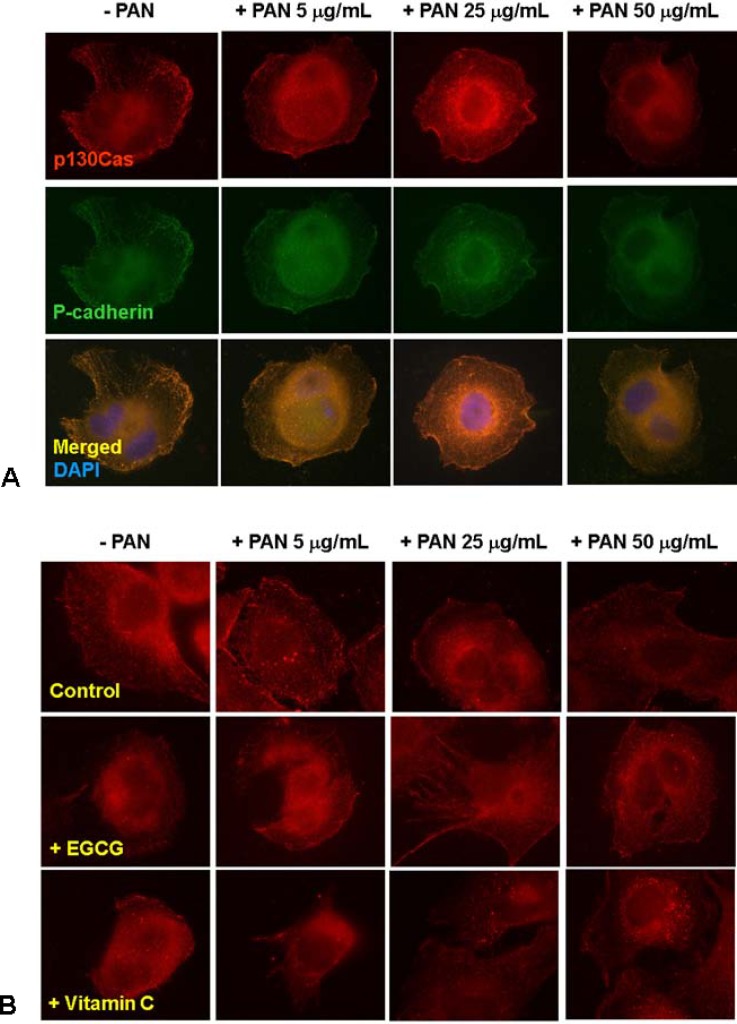
In immunofluorescence study, p130Cas showed a diffuse cytoplasmic distribution with accumulations at distinct peripheral areas visible as short stripes in physiologic condition and colocalized with P-cadherin (Fig. 1A left column). The fluorescences of p130Cas protein were internalized into perinuclear areas and became granular by PAN in a dose-dependent manner (Fig. 1A, B upper row). Such distributional changes were reversed by 50 µM EGCG and 30 µM vitamin C (Fig. 1B), recovering peripheral linear stainings. Subtitles of A and B of all figures in these results denote rat GEpCs and mouse podocytes, respectively.
Fig. 1.
Distributional changes of p130Cas. p130Cas showed a diffuse cytoplasmic distribution with accumulation at distinct peripheral areas visible as short stripes in physiologic condition and colocalized with P-cadherin. The fluorescences of p130Cas protein were internalized and became granularly by PAN in a dose-dependent manner (A, rat glomerular epithelial cells). Such pathologic changes were reversed by EGCG and vitamin C (B, mouse podocyte). Magnification, ×400.
2. Western blotting of p130Cas in cultured GEpC
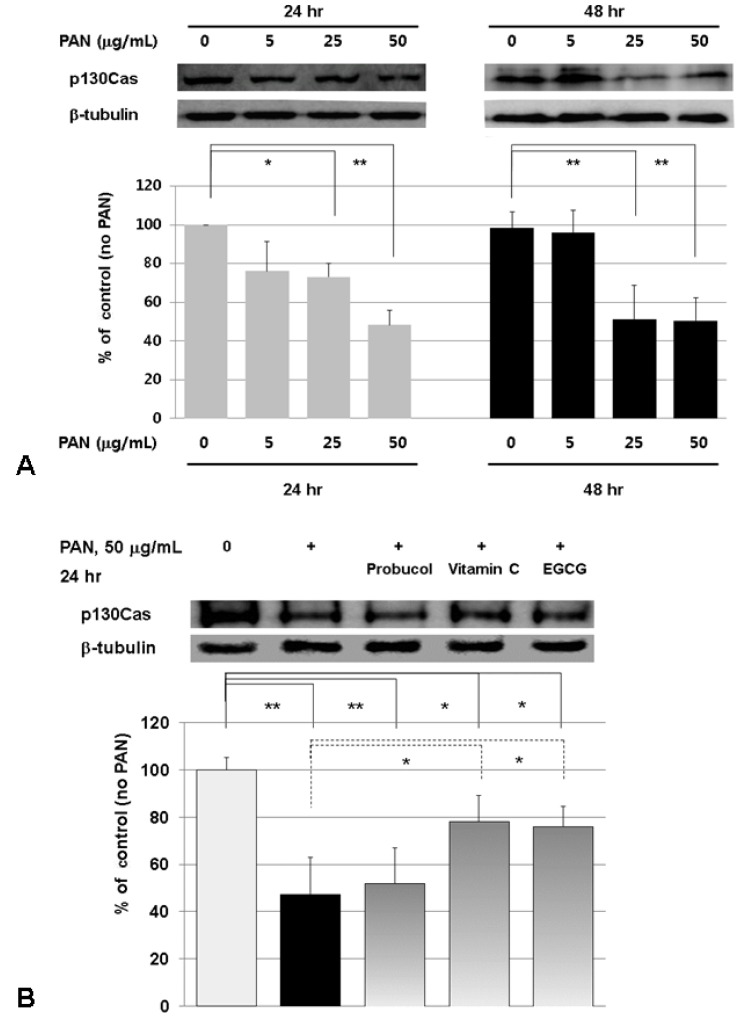
Density values for cellular p130Cas protein of representative immunoblots from each group tended to be reduced by PAN, particularly in a dose-dependent manner at 24 hours. High dose (50 µg/mL) of PAN decreased cellular p130Cas protein levels by 51.7% at 24 hours and by 49.5% at 48 hours (both P<0.01; n=3) (Fig. 2A). Less high amount (25 µg/mL) of PAN also decreased cellular p130Cas protein levels to a less degree, by 26.8% at 24 hours (P<0.05); however, the 48.6% reduction at 48 hours is similar to that of 50 µg/mL of PAN (P<0.01; n=3) (Fig. 2A). The suppression of p130Cas protein by high dose (50 µg/mL) of PAN could be restored by EGCG and vitamin C, but not by probucol at 24 hours (P<0.05; n=3) (Fig. 2B). These results suggest that PAN suppresses p130Cas protein at least by oxidative stress.
Fig. 2.
Effects of PAN and antioxidants on the p130Cas protein assayed by Western blotting. Density values for p130Cas protein of representative immunoblots from each group show decreased cellular p130Cas protein levels by PAN (A, rat glomerular epithelial cells). Treatments with EGCG and vitamin C restore the change of p130Cas protein (B, mouse podocytes). Data on the densitometric analysis of p130Cas/β-tubulin ratio are expressed as mean±SD. Control (100%); the value of PAN (-) group. *P<0.05 and **P<0.01 versus control or 50 µg/mL of PAN.
3. Expression of p130Cas mRNA
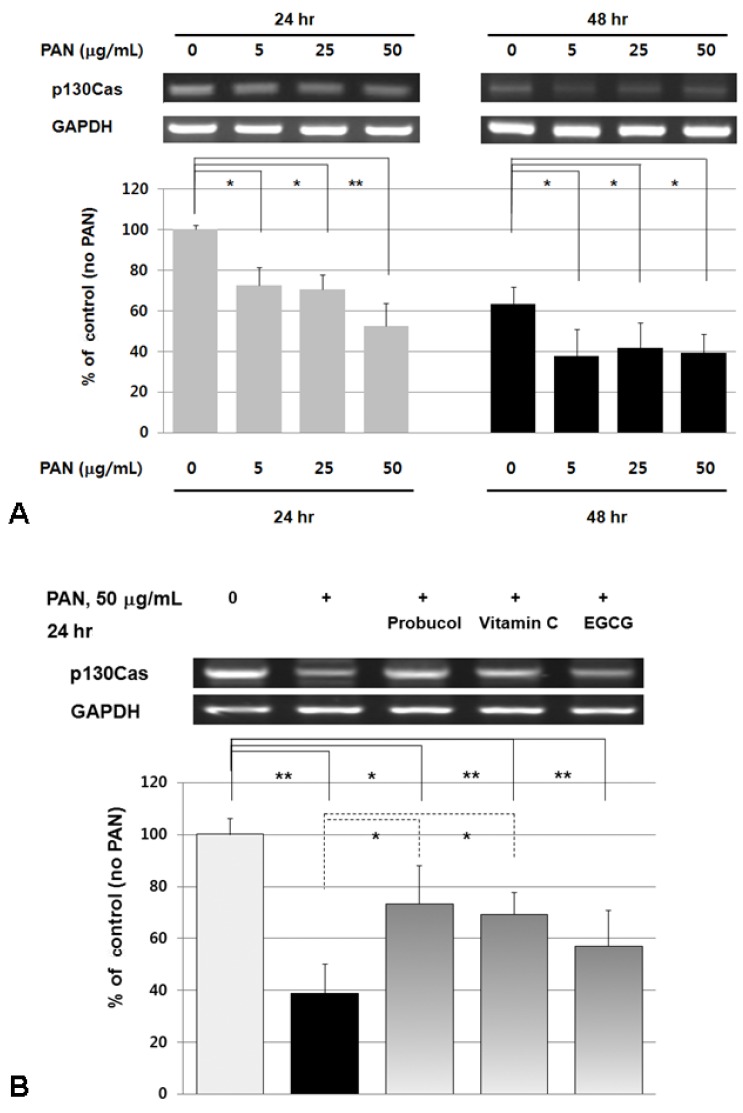
Values for mRNA expression of p130Cas and representative gels showed the decreased RT-PCR products of podocyte p130Cas by PAN significantly at 24 and 48 hours (P<0.05; n=3) (Fig. 3A). At 48 hours, even small amounts of PAN could suppress p130Cas mRNA similar to the results by higher doses, which meant that the suppressive effect of PAN were similar at extended exposure time. The suppression of p130Cas mRNA by PAN could be restored significantly by probucol and vitamin C at 24 hours (P<0.05; n=3) (Fig. 3B). These results suggest that PAN suppresses p130Cas mRNA and subsequent protein by transcriptional modulation at least by oxidative stress.
Fig. 3.
Effects of PAN and antioxidants on the mRNA expression of p130Cas assayed by RT-PCR. Values for mRNA expression of p130Cas and a representative gel show that PAN significantly decrease RT-PCR products of p130Cas (A, rat glomerular epithelial cells) and probucol and vitamin C restore the change of p130Cas mRNA (B, mouse podocytes). Data on the densitometric analysis of p130Cas/GAPDH ratio are expressed as mean±SD. Control (100%); the value of PAN (-) group. *P<0.05 and **P<0.01 versus control or 50 µg/mL of PAN.
Discussion
The proteinuric conditions usually demonstrate ultrastructural changes in the GEpCs (podocytes) with retraction and effacement of the highly specialized interdigitating FPs, which were accompanied by the alterations of the SD and linking adapter molecules even before the onset of proteinuria16,17). The FP effacement has been regarded as an abnormal response of the epithelium either to direct injury or to alterations elsewhere in the glomerulus, which means the detachment of podocytes from the GBM and/or FP retraction leading to separation of neighboring podocytes. The FP effacement requires a precise interplay of multiple cellular functions including structural alterations of the cytoskeleton and linking adapter proteins, abnormal movement of FP over the GBM, and reconstruction of the SD1,16,17).
Podocyte FPs form a scaffolding around the capillary loops that are anchored to the GBM via α3β1 integrin and α-/β-dystroglycans2,4). These anchoring molecules connect to the actin cytoskeleton via intracellular macromolecular complex of the focal adhesion kinase (FAK) and molecules, including paxillin, vinculin, p130Cas, and α-actinin2,4,16-18).
p130Cas belongs to a family of docking proteins7,8). On the outer side of p130Cas, proline-rich domains and binding motifs for the SH2 domains of v-Crk and v-Src mediate the adhesion to extracellular matrix via integrin by tyrosine phosphorylation7,8,18-20). On the other cytoplasmic side, p130Cas is connected to FAK and c-Crk, mediating signaling pathways from cell adhesion sites to the inner cytoskeleton7,8,21-23). Activation or overexpression of Cas proteins activates multiple downstream effectors to promote formation of filopodia, lamellipodia, pseudopodia, and induce additional changes in the cytoskeleton that support migration7,8). Therefore, p130Cas protein plays a pivotal role in maintaining the cell adhesion/migration and cellular signaling by connecting extracellular structure and cytoskeleton.
In podocytes, p130Cas localizes diffusely to the cytoplasm with accumulation at ends of F-actin stress fibers in FPs, where p130Cas connects the cytoskeleton to the GBM via FAKs and integrins7), and to the SD via CD2AP and FAKs9). Therefore, p130Cas protein also plays an important role in maintaining the podocyte cytoskeleton and cellular signaling by connecting podocyte actin cytoskeleton to GBM and SD.
The proteinuric conditions are usually associated with retraction and effacement of the highly specialized interdigitating FPs of podocytes, which are accompanied by the alterations of the SD and docking adapter molecules16,17). However, there are very limited reports on the change of p130Cas in pathologic conditions till now. The immunofluorescent stainings of p130Cas increased around the glomerular capillary loop of human membranous nephropathy, however, not of minimal change disease24). They suggested that the increased p130Cas might be a result of tyrosine phosphorylation of constituent proteins. Although both diseases are podocyte diseases, the pathophysiologic mechanisms leading to membranous nephropathy and minimal change disease are different. Membranous nephropathy is caused by an accumulation of immune deposits on the outer aspect of the GBM, however, minimal change disease is characterized by podocyte phenotypical changes caused by plasma permeability factors25). In patients and animal models with immune-mediated glomerular diseases, increased tyrosine phosphorylation within focal adhesion proteins, increased Pyk2, and FAK activation have been reported25-28). Therefore, the expression of podocyte p130Cas could be different according to the pathophysiologic mechanisms leading to podocytopathy. In this study we found a reduced expression of p130Cas by PAN which could induce the similar pathologic findings of minimal change disease. Oxidative stress induced by H2O2 concentration dependently inhibited carbachol-induced tyrosine phosphorylation of the adhesion-related proteins, including FAKs and p130Cas in human neuroblastoma SH-SY5Y cells29).
As p130Cas is associated with focal adhesions as an adaptor protein and integrin-mediated cell adhesion results in tyrosine phosphorylation of p130Cas and several other focal adhesion proteins at the cell membrane, tyrosine phosphorylation of their constituent proteins including p130Cas could relate to the supramolecular assembly and the formation of new focal adhesions7,23,30). As a consequence of its phosphorylation, p130Cas is involved in the linkage of actin cytoskeleton to the extracellular matrix during cell migration, cell invasion, and cell transformation18,20-23). Pro-apoptotic stimuli including oxidative stress trigger dephosphorylation and cleavage of Cas proteins, leading to direct dominant-negative mediated focal adhesion disassembly, altering the transcriptional balance between Cas-dependent pro- and anti-survival factors31). Therefore, we speculate that PAN-induced oxidative stress could inhibit tyrosine phosphorylation and suppress the expression of p130Cas, then, subsequently may disrupt podocyte adhesion to neighbor structures, such as, adaptor proteins, GBM, etc.
Recently, we reported that the exposure of podocytes to PAN in vitro relocated β-catenin, an adapter protein linking the SD and cytoskeleton, internally and reduced β-catenin mRNA and protein expression32), similar to the changes of 130Cas in this study. Taking together, we speculate that PAN suppresses the expression of adapter proteins, including p130Cas and β-catenin, via cytotoxic, oxidative, or consumptive effects, therefore, leading to disrupt podocyte architecture and filtration function. Further investigations for the quantitative and qualitative changes and tyrosine phosphorylation of related focal adhesion proteins and FAK in PAN-induced nephropathy and various human proteinuric diseases are needed.
In conclusion, this study suggests that PAN induces the distributional and quantitative changes of podocyte p130Cas, leading to disrupt podocyte adhesions to neighbor structures via oxidative stress, then, develop proteinuria in experimental PAN-induced nephropathy.
Acknowledgment
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007-313-E00269) and the grants from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012-0004731). The authors thank E-M Ahn for their technical assistances and Dr. Peter Mundel for mouse podocytes.
References
- 1.Smoyer WE, Mundel P. Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med (Berl) 1998;76:172–183. doi: 10.1007/s001090050206. [DOI] [PubMed] [Google Scholar]
- 2.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 3.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl) 1995;192:385–397. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- 5.Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108:289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Neill GM, Fashena SJ, Golemis EA. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- 8.Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Welsch T, Endlich N, Kriz W, Endlich K. CD2AP and p130Cas localize to different F-actin structures in podocytes. Am J Physiol Renal Physiol. 2001;281:F769–F777. doi: 10.1152/ajprenal.2001.281.4.F769. [DOI] [PubMed] [Google Scholar]
- 10.Ryan GB, Karnovsky MJ. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975;8:219–232. doi: 10.1038/ki.1975.105. [DOI] [PubMed] [Google Scholar]
- 11.Caulfield JP, Reid JJ, Farquhar MG. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976;34:43–59. [PubMed] [Google Scholar]
- 12.Guan N, Ding J, Deng J, Zhang J, Yang J. Key molecular events in puromycin aminonucleoside nephrosis rats. Pathol Int. 2004;54:703–711. doi: 10.1111/j.1440-1827.2004.01683.x. [DOI] [PubMed] [Google Scholar]
- 13.Kreisberg JI, Hoover RL, Karnovsky MJ. Isolation and characterization of rat glomerular epithelial cells in vitro. Kidney Int. 1978;14:21–30. doi: 10.1038/ki.1978.86. [DOI] [PubMed] [Google Scholar]
- 14.Singh AK, Mo W, Dunea G, Arruda JA. Effect of glycated proteins on the matrix of glomerular epithelial cells. J Am Soc Nephrol. 1998;9:802–810. doi: 10.1681/ASN.V95802. [DOI] [PubMed] [Google Scholar]
- 15.Ha TS. High-glucose and advanced glycosylation end products increased podocyte permeability via PI3-K/Akt signaling. J Mol Med (Berl) 2010;88:391–400. doi: 10.1007/s00109-009-0575-8. [DOI] [PubMed] [Google Scholar]
- 16.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 17.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 18.Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- 19.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, et al. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 20.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a "molecular switch" for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Nishida J, et al. Characterization, partial purification, and peptide sequencing of p130,the main phosphoprotein associated with v-Crk oncoprotein. J Biol Chem. 1994;269:32740–32746. [PubMed] [Google Scholar]
- 22.Matsuda M, Mayer BJ, Fukui Y, Hanafusa H. Binding of transforming protein, P47gag-crk, to a broad range of phosphotyrosine-containing proteins. Science. 1990;248:1537–1539. doi: 10.1126/science.1694307. [DOI] [PubMed] [Google Scholar]
- 23.Di Stefano P, Cabodi S, Boeri Erba E, Margaria V, Bergatto E, Giuffrida MG, et al. P130Cas-associated protein (p140Cap) as a new tyrosine-phosphorylated protein involved in cell spreading. Mol Biol Cell. 2004;15:787–800. doi: 10.1091/mbc.E03-09-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bains R, Furness PN, Critchley DR. A quantitative immunofluorescence study of glomerular cell adhesion proteins in proteinuric states. J Pathol. 1997;183:272–280. doi: 10.1002/(SICI)1096-9896(199711)183:3<272::AID-PATH914>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. doi: 10.1016/S0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- 26.Takagi C, Ueki K, Ikeuchi H, Kuroiwa T, Kaneko Y, Tsukada Y, et al. Increased expression of cell adhesion kinase beta in human and rat crescentic glomerulonephritis. Am J Kidney Dis. 2002;39:174–182. doi: 10.1053/ajkd.2002.29912. [DOI] [PubMed] [Google Scholar]
- 27.Morino N, Matsumoto T, Ueki K, Mimura T, Hamasaki K, Kanda H, et al. Glomerular overexpression and increased tyrosine phosphorylation of focal adhesion kinase p125FAK in lupus-prone MRL/MP-lpr/lpr mice. Immunology. 1999;97:634–640. doi: 10.1046/j.1365-2567.1999.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma H, Togawa A, Soda K, Zhang J, Lee S, Ma M, et al. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J Am Soc Nephrol. 2010;21:1145–1156. doi: 10.1681/ASN.2009090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jope RS, Song L, Grimes CA, Zhang L. Oxidative stress oppositely modulates protein tyrosine phosphorylation stimulated by muscarinic G protein-coupled and epidermal growth factor receptors. J Neurosci Res. 1999;55:329–340. doi: 10.1002/(SICI)1097-4547(19990201)55:3<329::AID-JNR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 30.Panetti TS. Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front Biosci. 2002;7:d143–d150. doi: 10.2741/A771. [DOI] [PubMed] [Google Scholar]
- 31.Tikhmyanova N, Little JL, Golemis EA. CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci. 2010;67:1025–1048. doi: 10.1007/s00018-009-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JY, Ahn EM, Park HY, Shin JI, Ha TS. The change of podocyte β-catenin by puromycin aminonucleoside. J Korean Soc Pediatr Nephrol. 2011;15:138–145. [Google Scholar]