Abstract
The transcription factor NF-κB has been causally linked to inflammatory lung diseases. Recent studies have unraveled the complexity of NF-κB activation by identifying two parallel activation pathways: the classical NF-κB pathway, which is controlled by IκB kinase complex–β (IKKβ) and RelA/p50, and the alternative pathway, which is controlled by IKKα and RelB/p52. The alternative pathway regulates adaptive immune responses and lymphoid development, yet its role in the regulation of innate immune responses remains largely unknown. In this study, we determined the relevance of the alternative NF-κB pathway in proinflammatory responses in lung epithelial cells. The exposure of C10 murine alveolar lung epithelial cells to diverse stimuli, or primary murine tracheal epithelial cells to LPS, resulted in the activation of both NF-κB pathways, based on the nuclear translocation of RelA, p50, RelB, and p52. Increases in the nuclear content of RelA occurred rapidly, but transiently, whereas increases in nuclear RelB content were protracted. The small interfering (si) RNA–mediated knockdown of IKKα, RelA, or RelB resulted in decreases of multiple LPS-induced proinflammatory cytokines. Surprisingly, the siRNA ablation of IKKα or RelB led to marked increases in the production of IL-6 in response to LPS. The simultaneous expression of constitutively active (CA)-IKKα and CA-IKKβ caused synergistic increases in proinflammatory mediators. Lastly, the disruption of the IKK signalsome inhibited the activation of both NF-κB pathways. These results demonstrate that the coordinated activation of both NF-κB pathways regulates the magnitude and nature of proinflammatory responses in lung epithelial cells.
Keywords: lung, IκB kinase–β, IκB kinase–α, RelA, RelB
Clinical Relevance
These studies demonstrate that the alternative NF-κB pathway plays a critical role in orchestrating inflammatory responses in lung epithelial cells. Furthermore, our results demonstrate that IκB kinase complex–α (IKKα) and IKKβ, kinases in the alternative and classical NF-κB pathways, respectively, synergize to induce proinflammatory responses. These findings provide insights into the complexity of NF-κB signaling in the lung epithelium, and elucidate further possible mechanisms of lung inflammation.
NF-κB is a transcription factor that plays a cardinal role in multiple cellular processes, including survival, proliferation, and inflammation. In unstimulated cells, NF-κB dimers RelA and p50 are sequestered in the cytosol by the inhibitor of κB (IκBα). The IκB kinase complex (IKK) consists of two catalytic subunits, IKKβ and IKKα, and the regulatory protein, IKKγ (also known as NF-κB essential modulator). Upon ligation by a variety of stimuli such as TNF-α or Toll-like receptor agonists, IKKβ is phosphorylated and in turn phosphorylates IκBα, leading to its subsequent ubiquitination and degradation by the 26S proteasome (1, 2). The processing of IκBα promotes the nuclear translocation of RelA/p50, leading to the transcriptional activation of NF-κB–dependent genes. NF-κB activates the transcription of many proinflammatory cytokine and chemokine genes that initiate and propagate innate immune responses (1, 2).
The airway epithelium, classically regarded as the first line of defense against inhaled agents, toxic factors, and physical trauma, is now recognized as a key component of the innate immune system, and plays an active role in the orchestration of acute inflammatory and adaptive immune responses (3, 4). Upon stimulation, epithelial cells secrete proinflammatory mediators such as IL-6, keratinocyte-derived chemokine (KC), regulated-on-activation normal T cells expressed and secreted (RANTES), granulocyte monocyte–colony stimulating factor (GM-CSF), CCL-20, and many others, all of which have been implicated in the pathogenesis of inflammatory disorders via interactions with dendritic cells, macrophages, T cells, and B cells (3). NF-κB activation in lung epithelial cells has been shown to be crucial in regulating these proinflammatory responses (5–9).
In addition to the classical NF-κB pathway, a parallel, alternative NF-κB activation pathway has been identified. The alternative NF-κB pathway is activated by distinct ligands such as the CD40 ligand and B-cell–activating factor (BAFF) via the NF-κB–inducing kinase (NIK)–induced phosphorylation of IKKα. IKKα in turn phosphorylates the IκB domain of p100, leading to its partial processing and release of RelB and p52 into the nucleus, along with the transcriptional activation of unique subsets of NF-κB–dependent genes important in the adaptive immune response (10, 11). Classically, the alternative pathway has been described as important in the development of B and T lymphocytes and in the formation of peripheral lymphoid organs (12). However, its role in orchestrating proinflammatory responses in lung epithelial cells remains largely unknown.
Given the importance of the classical NF-κB pathway in driving the expression of proinflammatory mediators in epithelial cells, and recent reports demonstrating crosstalk between both NF-κB activation pathways (13–16), we sought to determine whether the alternative NF-κB pathway is activated in lung epithelial cells upon stimulation with a variety of agonists. Furthermore, we evaluated the functional importance of classical and alternative NF-κB pathway activation in LPS-induced proinflammatory responses, and whether crosstalk between the activation of both NF-κB activation pathways exists in lung epithelial cells.
Materials and Methods
Cell Culture
Primary murine tracheal epithelial cells (MTECs) were isolated and cultured according to previously described methods (17, 18). MTECs were infected with constitutively active adeno IKKα at 1 × 105 virions per Transwell, and harvested at the indicated times. A spontaneously transformed Type II murine lung alveolar epithelial cell line (19) (C10) was cultured, as described previously (17). Sixteen hours before treatment, cells were starved in medium containing 0.5% FBS. Cells were exposed to 1 μg/ml LPS (List Biological Laboratories, Inc., Campbell, CA), 50 ng/ml IL-17A (R&D Systems, Minneapolis, MN), 1 μg/ml murine TNF-α (Sigma Chemical Co., St. Louis, MO), 100 ng/ml CD-40 ligand (CD-40L; eBioscience, San Diego, CA), 10 μg/ml polyinosinic acid (poly-IC; Calbiochem, Darmstadt, Germany), and 1 μg/ml lipoteichoic acid (LTA; Sigma) for the indicated times. Cells were exposed to 10 μM NEMO-binding domain (NBD) peptides (Calbiochem) or scrambled peptide control samples, 2 hours before the indicated treatments.
Mice
C57Bl/6 mice were anesthetized and received 5 μg LPS oropharyngeally (List Biological Laboratories, Inc.), and 24 hours later were killed by pentobarbital injection. The right lobe was flash-frozen in liquid nitrogen and pulverized, and the left lobe was inflated with 4% paraformaldehyde. Five-micron sections were used to assess the localization of RelB in situ via immunofluorescence, according to previously described methods (6). RelB was detected in nuclear extracts prepared from whole lung, according to previously published procedures (20). All studies were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
Western Blot Analysis
Cells were washed with cold PBS before harvesting. Protein concentrations were determined by a Bio-Rad DC Protein Assay kit (Bio-Rad, Hercules, CA), and 20 μg of protein were used for Western blotting. RelA, RelB, p50, and β-actin antibodies were acquired from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for p100/p52, IκBα, IKKβ, phosphoserine RelA 536, tubulin, and histone H3 were acquired from Cell Signaling Technology (Danvers, MA). The IKKα antibody was purchased from Upstate (Darmstadt, Germany). Cytosolic and nuclear extracts were prepared as previously described (21).
RelA, RelB, and IKKα Small Interfering RNA
C10 cells were incubated with Dharmacon SMARTpool control nontargeting small interfering (si) RNA or Dharmacon SMARTpool siRNA against RelA, RelB, and IKKα (all at 100 nM; Dharmacon, Lafayette, CO), and subsequently harvested and analyzed as indicated.
Transfections and Plasmids
Plasmid transfections were performed with Nanofectin (PAA, Pasching, Austria). Constitutively active (CA)-IKKβ containing serine 177/181 to glutamic acid mutations and CA-IKKα containing serine 176/180 to glutamic acid mutations were cloned into pcDNA3.1 and pRc β-actin plasmids, respectively.
ELISA
C10 cells were treated, and the medium was assessed for macrophage inflammatory protein (MIP-2), KC, RANTES, CCL-20, IL-6, and GM-CSF cytokines with materials from R&D Systems, according to the manufacturer’s instructions.
Gene Expression
Total RNA was isolated from C10 cells using the RNeasy kit (Qiagen, Valencia, CA), and reverse transcribed for TaqMan gene analysis using SYBR green (Bio-Rad).
Primers
Primers for quantitative RT-PCR are listed in the online supplement.
Statistical Analysis
Data were evaluated by GraphPad Prism 5 Software (Graphpad, Inc., San Diego, CA), using one-way ANOVA with the Bonferroni correction to adjust for multiple comparisons. Results at P < 0.05 or less were considered statistically significant.
Results
Activation of Classical and Alternative NF-κB Signaling in Response to Diverse Agonists in Lung Epithelial Cells
Previous work in our laboratory demonstrated the importance of classical NF-κB signaling in lung epithelium in acute inflammatory and allergic disease (6, 7, 9). To date, as far as we are aware, no studies have determined whether the activation of alternative NF-κB signaling occurs in lung epithelial cells. We therefore analyzed the activation of both classical and alternative NF-κB signaling after stimulation with a variety of agonists. C10 cells were exposed to the Toll-like receptor (TLR)–4 agonist LPS for varying times before evaluation of the nuclear content of RelA, RelB, p50, and p52. The results in Figure 1 demonstrate rapid increases in the nuclear content of RelA and p50, components of the classical NF-κB pathway, which decreased by 2 hours. Increases in the nuclear content of RelB/p52, components of the alternative NF-κB pathway, were also observed in response to LPS. Increases in RelB occurred gradually, and were still apparent 8 hours after LPS exposure. To determine whether increases in RelB/p52 are unique to LPS, we exposed C10 lung epithelial cells to the TLR2 agonist LTA, the TLR3 agonist Poly-IC, TNF-α, and IL-17A. The results in Figure 1 demonstrate similar patterns of NF-κB activation in response to all agonists. The densitometric evaluation of RelA or RelB nuclear content, normalized to histone H3, demonstrated that in response to all stimuli, early increases in the nuclear content of RelA were apparent, whereas increases in nuclear RelB tended to be protracted (Figures 1A and 1B). Comparative evaluations of nuclear p50 and p52 demonstrated more variable increases, with apparent biphasic fluctuations in response to all agonists (Figures 1A and 1C). Upon stimulating cells with the CD-40 ligand, a molecule known to activate the alternative pathway in myeloid cells (22), increases in the nuclear content of RelA/p50 and RelB/p52 occurred (Figures 1A–1C), demonstrating that an agonist implicated in the activation of the alternative NF-κB pathway, in fact, induces both NF-κB pathways in epithelial cells.
Figure 1.
Activation of classical and alternative NF-κB signaling in response to diverse agonists in lung epithelial cells. (A) Western blot analysis of nuclear extracts from murine Type II alveolar lung epithelial (C10) cells stimulated with LPS (1 μg/ml), lipoteichoic acid (LTA, 1 μg/ml), polyinosinic acid (PolyIC, 10 μg/mL), TNF-α (1 μg/ml), CD-40L (100 ng/ml), or IL-17A (50 ng/ml) for the indicated times. Twenty micrograms of nuclear protein were separated by SDS-PAGE and analyzed for RelA, RelB, p50, p52, or histone H3 (H3, nuclear loading control). (B and C) Densitometric evaluation of nuclear RelA and RelB (B), and of p50 and p52 (C). Data shown are normalized to histone H3. Values on the left y axis correspond to RelA/H3 (B) or p50/H3 (C), and values on the right y axis correspond to RelB/H3 (B) or p52/H3 (C), and reflect arbitrary units.
LPS Activates Classical and Alternative NF-κB Signaling in Primary Murine Lung Epithelial Cells and in the Murine Lung
Given that exposure to LPS resulted in increases in both RelA/p50 and RelB/p52, and that TLR4 signaling is important in acute and allergic inflammation (23, 24), we sought to characterize more fully the relevance of the coordinated activation of both NF-κB pathways in lung epithelial cells in response to LPS. We first determined the timing and duration of increases in RelA/p50 and RelB/p52 content in response to LPS in further detail. The results in Figure 2A demonstrate that increases in nuclear RelA/p50 content occurred robustly by 30 minutes, and strongly decreased toward control levels by 2 hours after LPS administration. Increases in nuclear RelB also occurred by 30 minutes, albeit somewhat less robustly than with RelA, but increases in nuclear RelB were sustained at least for 48 hours after LPS exposure. Increases in p52 nuclear content were observed rapidly in response to LPS, and decreased 16 hours after exposure. To determine whether similar patterns in the activation of classical and alternative NF-κB pathways exist in primary epithelial cells, MTECs were exposed to LPS. The results in Figure 2B demonstrate increases in nuclear RelA and p50 content 15 minutes or 30 minutes, respectively, after the stimulation of MTECs with LPS, with peak increases apparent by 1 hour, and decreases by 4 hours. The nuclear content of RelB and p52 increased after 1 hour of exposure to LPS, and remained elevated by 4 hours. The phosphorylation of RelA at Ser536, reflecting the activation of IKK, was apparent by 15 minutes, and decreased by 2 hours, whereas the degradation of IκBα occurred between 30 minutes and 1 hour after the stimulation of cells with LPS. Although p100 was undetectable in control cells, increases in overall p100 content occurred by 1 hour and were sustained for at least 4 hours after LPS. Overall, these findings demonstrate that in response to a TLR-4 agonist, the activation of both classical and alternative NF-κB pathways occurs in lung epithelial cells, based on increases in nuclear content. Increases in RelA/p50 were transient, whereas increases in RelB and p52 occurred in a protracted manner in response to LPS.
Figure 2.
LPS activates the classical and alternative NF-κB pathways in C10 lung epithelial cells, primary murine tracheal epithelial cells, and lung tissue. (A) Western blot analysis of nuclear extracts from C10 cells treated with LPS for the indicated times. Blots were evaluated for content of RelA, RelB, p50, and p52, and β-actin is shown as a loading control, because of changes in histone H3 content over time in culture. Right: Confirmation of cellular fractionation into nuclear and cytosolic fractions according to analysis of tubulin (cytosol) and histone H3 (nuclear). β-actin served as loading control. (B) Western blot analysis of nuclear and cytosolic extracts prepared from primary murine tracheal epithelial cells (MTECs) treated with 10 μg/ml LPS for the indicated times. Nuclear extracts were evaluated for content of RelA, RelB, p50, p52, and histone H3 as a loading control. Cytosolic extracts were assayed for content of p100, phosphorylated (P) RelA (serine 536), inhibitor of κB (IκBα), and β-actin as a loading control. (C) Western blots of nuclear extracts (20 μg protein per lane) were prepared from lung homogenates of C57Bl/6 mice (n = 3/group) oropharyngeally instilled with 5 μg LPS or with sterile PBS as a control for 24 hours. Blots were analyzed for nuclear content of RelB and histone H3 (nuclear loading control). Samples were run on the same gel and blotted onto the same membrane, and the lanes were reassembled for consistency. Data are representative of at least two separate experiments. (D) Immunofluorescence analysis of nuclear RelB, 24 hours after oropharyngeal instillation of LPS. Deparaffinized lung sections were stained for RelB (red), and nuclei were counterstained with Sytox Green. Images were captured by laser scanning confocal microscopy, using identical instrument settings. Images are representative results of three independent experiments. IgG Control: As a negative control, primary antibody was omitted. RelB, red; DNA, green; nuclear RelB, yellow.
LPS was previously shown to activate classical NF-κB signaling in lung tissue (7). To confirm whether increases in nuclear RelB occurred in lung tissue in response to LPS, C57Bl/6 mice received 5 μg LPS oropharyngeally, and lung tissue was harvested 24 hours later. Compared with PBS control samples after exposure to LPS, increases in nuclear RelB were apparent (Figure 2C). The assessment of RelB in lung tissue via confocal laser scanning microscopy demonstrated that in control mice, RelB (Figure 2C, red) was predominantly localized in the cytoplasm of bronchiolar epithelial cells. Twenty-four hours after LPS administration, RelB immunoreactivity (Figure 2C, red) was markedly enhanced in the bronchiolar epithelium, and strong colocalization with the DNA stain Sytox Green was observed (Figure 2C, yellow), indicative of the nuclear localization of RelB in the bronchiolar epithelium. In mice exposed to LPS, select cells in the parenchyma that require further identification also started to express detectable RelB.
Impact of Classical and Alternative NF-κB Signaling on Cytokine Production in Response to LPS
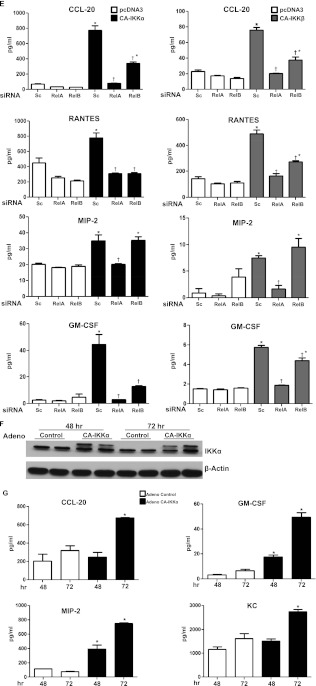
To determine the relative contributions of RelA and RelB in the regulation of LPS-induced proinflammatory responses, we used the siRNA-mediated knockdown of RelA or RelB, and assessed the content of NF-κB–dependent proinflammatory mediators in supernatants. RelA siRNA decreased the RelA content in C10 lung epithelial cells, albeit not completely (Figure 3A). As expected, LPS-induced increases in CCL-20, RANTES, KC, and IL-6 in the medium were significantly attenuated in cells transfected with RelA siRNA, compared with scrambled control cells (Figure 3B). The siRNA-mediated knockdown of RelB (Figure 4A) also resulted in marked decreases in the LPS-induced production of the proinflammatory mediators CCL-20, RANTES, and KC, compared with scrambled siRNA control cells (Figure 4B). Surprisingly, the knockdown of RelB led to a significant increase in LPS-induced IL-6 content in comparison to scrambled siRNA control cells at all time points. To determine whether IKKα, which activates alternative NF-κB signaling, exerted a similar impact on LPS-induced proinflammatory mediators such as RelB, we ablated IKKα with siRNA (Figure 5A). The results in Figure 5B demonstrate decreases in LPS-induced CCL-20 and RANTES content after the ablation of IKKα, and no effect on KC. Conversely, the siRNA-mediated knockdown of IKKα led to increases in the basal and LPS-stimulated production of IL-6, similar to the effects of RelB siRNA. In aggregate, these findings demonstrate that both the classical and alternative NF-κB pathways contribute to the LPS-induced production of diverse proinflammatory cytokines that include CCL-20 and RANTES, whereas IKKα and RelB repress the production of IL-6.
Figure 3.
LPS-induced production of proinflammatory mediators is dependent on RelA. (A) Verification of RelA knockdown in C10 cells. Whole-cell lysates were prepared from cells transfected with either scrambled (Sc) small interfering (si) RNA or RelA siRNA. Twenty micrograms of protein were separated by SDS-PAGE, to evaluate the content of RelA protein by Western blotting. β-actin served as a loading control. (B) ELISA was performed on cell supernatants from C10 cells transfected with either scrambled siRNA or RelA siRNA. Cells were treated with LPS for the indicated times, and evaluated for content of IL-6, CCL-20, regulated-on-activation normal T cells expressed and secreted (RANTES), and keratinocyte-derived chemokine (KC). *P < 0.05 (ANOVA), compared with untreated control samples. †P < 0.05 (ANOVA), compared with scrambled siRNA groups at the same time point. Results are representative of at least three independent experiments.
Figure 4.
Impact of RelB on LPS-induced production of proinflammatory mediators. (A) Verification of RelB knockdown in C10 cells. Whole-cell lysates were prepared from cells transfected with either scrambled siRNA or RelB siRNA. Twenty micrograms of protein were separated by SDS-PAGE, for evaluation of content of RelB protein by Western blotting. β-actin served as a loading control. (B) ELISA was performed on cell supernatants from C10 cells transfected with either scrambled siRNA or RelB siRNA, treated with LPS for the indicated times, and evaluated for content of IL-6, CCL-20, RANTES, and KC. *P < 0.05 (ANOVA), compared with untreated control samples. †P < 0.05 (ANOVA), compared with scrambled siRNA groups at the same time point. Results are representative of at least three independent experiments.
Figure 5.
Impact of IκB kinase complex–α (IKKα) on the LPS-induced production of proinflammatory mediators. (A) Verification of IKKα knockdown in C10 cells. Whole-cell lysates were prepared from cells transfected with either scrambled siRNA or IKKα siRNA. Twenty micrograms of protein were separated by SDS-PAGE, for the evaluation of content of IKKα protein by Western blotting. β-actin served as a loading control. (B) ELISA was performed on cell supernatants from C10 cells transfected with either scrambled siRNA or IKKα siRNA, treated with LPS for the indicated times, and evaluated for content of IL-6, CCL-20, RANTES, and KC. *P < 0.05 (ANOVA), compared with untreated control samples. †P < 0.05 (ANOVA), compared with scrambled siRNA groups at the same time point. Results are representative of at least three independent experiments.
Impact of Constitutively Active IKKα and IKKβ on the Expression of Proinflammatory Mediators in Lung Epithelial Cells
To evaluate the role of the activation of IKKα and IKKβ directly, C10 lung epithelial cells were transfected with 1 μg constitutively active (CA) variants of IKKα or IKKβ. The results in Figure 6A demonstrate that the expression of IKKα or IKKβ individually led to increases in the nuclear content of RelA, p50, RelB, and p52. The expression of CA-IKKα or CA-IKKβ individually led to increases in mRNA expression and the production of CCL-20, MIP-2, RANTES, and GM-CSF in C10 lung epithelial cells (Figure 6B). In contrast, no clear increases in the content of IL-6 and KC were evident under these conditions (data not shown). The cotransfection of C10 cells with 0.5 μg IKKα and IKKβ together led to increases in nuclear RelA and RelB, which were not observed in response to the transfection of individual constructs at these lower concentrations (Figure 6C). The dual expression of 0.25 or 0.5 μg CA-IKKα and CA-IKKβ each resulted in striking increases in the production of CCL-20, MIP-2, GM-CSF, and RANTES, which were not observed in cells transfected with individual constructs (Figure 6D) at these lower concentrations, in contrast to earlier experiments with 1 μg of plasmid (Figure 6B). These findings demonstrate clear cooperation between both kinases in eliciting proinflammatory responses in lung epithelial cells. To determine the role of RelA and RelB in CA-IKKα–dependent or CA-IKKβ–dependent proinflammatory responses, we knocked down either RelA or RelB with siRNA before the transfection of epithelial cells with CA-IKKα or CA-IKKβ. The results in Figure 6E demonstrate that the ability of CA-IKKα or CA-IKKβ to induce the expression of proinflammatory mediators depended partly on both RelA and RelB, dependent upon the individual proinflammatory mediator. RelA and RelB each contributed to CA-IKKα–mediated or CA-IKKβ–mediated increases in CCL-20, RANTES, and GM-CSF. In contrast, RelA, but not RelB, contributed to the CA-IKKα–induced or CA-IKKβ–induced expression of MIP-2, because the content of this cytokine was unchanged after RelB siRNA (Figure 6E). These findings demonstrate that both RelA and RelB are functionally important in driving the expression of CA-IKKα–induced or CA-IKKβ–induced proinflammatory genes, and that the exact contribution of these NF-κB subunits depends on the target gene.
Figure 6.
Expression of constitutively active (CA) IKKα and IKKβ in lung epithelial cells increases the production of proinflammatory mediators in a cooperative manner. (A) Confirmation of expression of CA-IKKα and CA-IKKβ in C10 lung epithelial cells. C10 cells were transfected with 1 μg pcDNA3, CA-IKKα, or CA-IKKβ. Twenty-four hours later, cytosolic extracts were prepared, and 20 μg of protein were evaluated via Western blot analysis for content of IKKα and IKKβ. β-actin served as loading control. Below: Assessment of nuclear content of RelA, RelB, p50, and p52 after the expression of CA-IKKα or CA-IKKβ. Histone H3 served as loading control. (B) Assessment of mRNA expression (left) and overall content (right) of CCL-20, MIP-2, RANTES, and granulocyte monocyte–colony stimulating factor (GM-CSF) in C10 cells 24 hours after transfection with pcDNA3, CA-IKKα, or CA-IKKβ, as described in A. mRNA expression was determined via real-time PCR analysis, whereas chemokine content was evaluated by ELISA performed on cell supernatants. *P < 0.05 (ANOVA), compared with pcDNA3-transfected controls. (C) Western blotting of C10 cells transfected with indicated amounts of CA-IKKα and/or CA-IKKβ, or with pcDNA3 as a control. All transfections were equalized to 1 μg DNA/dish with pcDNA3. Twenty-four hours later, cytosolic extracts were prepared for analysis of IKKα and IKKβ to confirm enhanced expression. β-actin served as loading control. Below: Evaluation of content of RelA, RelB, p50, and p52 in nuclear extracts. Histone H3 served as loading control. (D) ELISA analysis to assess content of CCL-20, MIP-2, RANTES, and GM-CSF in cell supernatants from transfection conditions indicated in C. *P < 0.05 (ANOVA), compared with pcDNA3-transfected control samples. (E) Assessment of overall content of CCL-20, MIP-2, RANTES, and GM-CSF in C10 cells transfected with CA-IKKα or CA-IKKβ after the ablation of RelA or RelB with siRNA. Chemokine content was assessed by ELISA. *P < 0.05 (ANOVA), compared with respective pcDNA3-transfected control samples. †P < 0.05 (ANOVA), compared with CA-IKKα or CA-IKKβ and scrambled siRNA-transfected conditions. (F) Primary murine tracheal epithelial cells were infected with either adeno-null (control) vector or adenovirus expressing CA-IKKα. After 48 or 72 hours, whole-cell lysates were prepared to confirm increased expression of hemagglutinin-tagged constitutively active IKKα. (G) Assessment of content of KC, GM-CSF, macrophage inflammatory protein (MIP)-2, and CCL-20 in supernatants from cells transduced with adenovirus expressing CA-IKKα or control virus, as described in F. All data are representative of at least three independent experiments. *P < 0.05 (ANOVA), compared with control vector.
To confirm that CA-IKKα elicits proinflammatory responses in primary lung epithelial cells, we infected MTECs with adenovirus expressing CA-IKKα for 48 and 72 hours, in comparison to control virus (Figure 6F). The transduction of CA-IKKα led to increases in the production of diverse proinflammatory mediators, including KC, MIP-2, CCL-20, and GM-CSF, compared with control virus (Figure 6G). Altogether, these findings demonstrate that both IKKα and IKKβ are capable of eliciting proinflammatory responses in lung epithelial cells, and that cooperation exists between both kinases.
Requirement of IKKγ for the Activation of Classical and Alternative NF-κB Pathways in Lung Epithelial Cells
We next sought to explore the mechanism that underlies the activation of classical and alternative NF-κB pathways in epithelial cells exposed to LPS. The mechanism of activation of IKKα in response to diverse ligands is not fully known. Although IKKα was shown to be part of the classical NF-κB signalsome (consisting of IKKα, IKKβ, and IKKγ) (25), during the activation of the alternative pathway, IKKα is activated via NIK in an IKKγ-independent manner (11). To determine whether increases in the nuclear content of RelB/p52 in response to LPS occur in an IKKγ (NEMO)–dependent manner, we incubated epithelial cells with NBD peptide, which disrupts the classical NF-κB signalsome (26), and determined the impact on the LPS-induced nuclear accumulation of RelA/p50 and RelB/p52. The results in Figure 7A demonstrate that the disruption of the IKK signalsome with NBD peptide not only attenuates the LPS-induced nuclear accumulation of RelA/p50, but also blocks increases in nuclear RelB/p52, demonstrating that the activation of the alternative pathway by LPS in lung epithelial cells relies, at least in part, on the classical IKK signalsome containing IKKγ (Figure 7B).
Figure 7.
LPS-mediated activation of classical and alternative NF-κB pathways is mediated via IKKγ. (A) C10 cells were exposed to NEMO-binding domain peptide (NBD) or mutant peptide as a control. Two hours later, cells were exposed to LPS for 30 minutes or 4 hours. Nuclear extracts were prepared for the evaluation of IκBα, nuclear RelA, RelB, p52, and p50. Histone H3 served as loading control. (B) Schematic summarizes the coordinated requirement of classical and alternative NF-κB pathways controlled by IKKβ/RelA and IKKα/RelB, respectively, in regulating proinflammatory signaling in lung epithelial cells.
Discussion
The transcription factor NF-κB has been shown to play a crucial role in the orchestration of proinflammatory responses in the lung. Previous work performed in our laboratory demonstrated the nuclear presence of RelA in acute models of LPS-induced inflammation (7) and in the ovalbumin model of allergic airway disease (27). Furthermore, transgenic mice expressing a dominant negative version of IκBα in bronchial epithelial cells (to inhibit the classical NF-κB pathway) exhibited marked protection from the inflammation associated with those agents (6, 7). Conversely, in multiple, independently generated transgenic lines, the inducible activation of the IKKβ transgene in bronchiolar epithelial cells resulted in marked neutrophilic inflammation (5, 28), enhanced sensitization to and inflammatory responses to ovalbumin (5, 9, 29), and carcinogenesis (30), demonstrating the functional significance of the activation of the classical NF-κB pathway in lung epithelial cells. Despite this knowledge, little information is available about the functional requirements for the activation of the alternative NF-κB pathway in regulating the transcription of proinflammatory genes in lung epithelial cells.
Results from the present study demonstrate that classical and alternative NF-κB activation pathways are coordinately activated in lung epithelial cells in response to diverse stimuli, as evidenced by increases in the nuclear content of RelA, p50, RelB, and p52. Notably, increases in RelA tended to occur rapidly, whereas RelB increased more gradually, but in a protracted manner. Although we did not detect p100 protein in lung epithelial cells under basal conditions, in response to LPS, p100 content increased in primary tracheal epithelial cells, with corresponding increases in nuclear p52. Because p100 is an NF-κB–dependent gene (31), the activation of classical NF-κB may plausibly lead to the induction of p100, which is subsequently processed by IKKα, leading to alternative NF-κB signaling. Validation of this possible scenario will require additional studies. The functional significance of the differential timing of the activation of classical and alternative NF-κB signaling in lung epithelial cells remains unknown, and requires additional assessments of the presence of NF-κB components bound to the regulatory regions of target genes, and correlations with transcriptional activation.
In C10 epithelial cells stimulated with LPS, RelA, RelB, and IKKα, all were demonstrated to play a role in controlling the strength and nature of proinflammatory cytokine production. In addition, we demonstrated here that cooperation exists between both pathways, as indicated by the synergistic increases in cytokine production upon the coexpression of IKKα and IKKβ, illuminating an important role for both NF-κB signaling pathways in dictating proinflammatory responses in lung epithelial cells. Recent work has demonstrated the activation of NIK and IKKα in A549 lung epithelial cells in response to respiratory syncytial virus, with a causal role for this pathway in the activation of RANTES (32).
Our findings, demonstrating that LPS can activate both classical and alternative NF-κB pathways both in primary lung epithelial cells and in lung tissue, are supported by previous work demonstrating the activation of both classical and alternative NF-κB pathways by LPS in B lymphocytes (33). Similarly, TNF-α, an agonist of the classical NF-κB pathway, has been shown to activate the alternative NF-κB pathway in murine embryonic fibroblasts (34, 35). These findings demonstrate that multiple ligands previously considered as unique activators of either classical or alternative NF-κB can indeed activate both pathways. The results of the present study show the simultaneous activation of both NF-κB pathways in lung epithelial cells in response to diverse stimuli, and demonstrate the functional relevance of both pathways in regulating proinflammatory signals activated by LPS.
IKKα has been demonstrated to play diverse roles both in regulating the activation of NF-κB in the cytosol and in the remodeling of chromatin, and previous studies demonstrated both positive and negative roles for IKKα in the classical NF-κB pathway. For example, the requirement of IKKα for the activation of NF-κB–responsive genes such as IL-8 and IκBα was demonstrated in response to TNF-α (15) and cigarette smoke (36), in association with the increased phosphorylation of histone H3. Conversely, IKKα was also shown to shut down the classical NF-κB pathway via the phosphorylation of human T-cell leukemia virus type I binding protein 1 (Tax1bp1) (13) or the protein inhibitor of activated signal transducer and activator of transcription 1 (STAT-1) (37). The loss of IKKα has been shown to prolong the activity of IKKβ and to promote inflammation (14). Moreover, the accelerated removal of RelA/p50 NF-κB subunits from proinflammatory gene promoters by IKKα has been demonstrated to limit the activation of NF-κB (15). Furthermore, IKKα can inhibit the alternative pathway NF-κB via the phosphorylation and subsequent degradation of NIK (38). Lastly, IKKα can mediate the transcriptional activation of genes independently of NF-κB (11). In aggregate, these findings demonstrate the multifaceted roles of IKKα in regulating the transcriptional output and duration of NF-κB activation in diverse cell types exposed to a variety of agonists. Our present results, demonstrating that the siRNA-mediated ablation of IKKα paradoxically leads to increases in IL-6 content but decreases in RANTES and CCL-20, indicate that the functional contribution of IKKα not only depends on stimulus and cell type, but also on the actual target genes. Additional studies will be required to assess the impact of IKKα on the transcriptional activation potential of individual NF-κB–regulated genes in lung epithelial cells.
Our present results demonstrate that the ablation of RelB with siRNA affected the production of proinflammatory mediators in response to LPS in a similar manner as the siRNA-mediated ablation of IKKα, suggesting that IKKα may exert its effects in lung epithelial cells via the regulation of RelB. Numerous studies have demonstrated anti-inflammatory roles for RelB. For example, RelB knockout animals develop spontaneous inflammation in multiple organs, including the lung (39), and the inflammation was further exacerbated by the concomitant loss of p50 (40). Aryl hydrocarbon receptor–deficient fibroblasts exposed to cigarette smoke showed increased expression of cyclooxygenase-2 and prostaglandins, in association with a loss of RelB (41). Indeed, an anti-inflammatory role for RelB in cigarette smoke–induced inflammation was recently demonstrated, as evidenced by decreased neutrophilic infiltration and the diminished content of proinflammatory mediators after the adenovirus-mediated transduction of RelB (42). In response to LPS, RelB−/− fibroblasts showed prolonged increases in proinflammatory cytokines compared with wild-type controls, suggesting a role for RelB in the resolution of inflammatory responses (43). Finally, RelB was also shown to promote tolerance to LPS, and to diminish proinflammatory responses in macrophages (44, 45). These previous findings contrast with the results of the present study, demonstrating that the siRNA-mediated knockdown of RelB diminishes the LPS-induced production of KC, CCL-20, and RANTES. However, RelB knockdown resulted in a significant increase in IL-6 production in response to LPS, illustrating a dynamic and highly specific role for RelB in controlling gene expression in epithelial cells exposed to LPS.
Although previous reports demonstrated cross-regulation between classical and alternative NF-κB pathway activation (11), it remains unknown whether the inducible activation of both pathways can cooperatively enhance the production of NF-κB–dependent cytokines. Results in the present study demonstrated that the coexpression of the constitutively active kinases IKKα and IKKβ, in the absence of any other ligands, led to a cooperative increase in the expression of proinflammatory cytokines, which corresponded to increases in nuclear RelA and RelB. Furthermore, the present study showed that the nuclear translocation of RelA, RelB, p50, and p52 all are, in part, regulated by the IKKγ-containing signalsome complex, indicating at least one common regulatory mechanism whereby IKKα and IKKβ facilitate NF-κB–dependent gene activation.
Interactions between RelA and RelB have been described previously, and have been shown to regulate transcription. In response to the priming of cells with TNF-α and lymphotoxin β, both RelA and RelB were recruited to the GM-CSF promoter, in association with increased transcription (46). However, RelB has also been shown to replace RelA at the IL-12p35 promoter, shutting down transcription (47). In response to the stimulation of dectin-1, the v-raf-1 murine leukemia viral oncogene homolog (Raf-1)–induced phosphorylation of RelA was shown to lead to inactive RelA–RelB dimers that inhibit transcription (48), and similar inhibitory effects of RelA-mediated on RelB-mediated transcription after the phosphorylation of RelA were reported in response to TNF-α (49). Future studies are needed to determine whether the enhanced association between RelA and RelB contributes to the gene-specific stimulatory effects (CCL-20, RANTES, and KC) or inhibitory effects (IL-6) observed in the present study.
Convincing studies demonstrate the contribution of classical NF-κB to lung inflammation. However, the role of the alternative NF-κB activation pathway in the regulation of pulmonary inflammation is only emerging. Adenovirus expressing phosphomimetic, and hence constitutively active, IKKα introduced into airways, was sufficient to induce neutrophilic infiltration and increased mRNA expression of a variety of proinflammatory genes, similar to adenovirus expressing CA-IKKβ (50). In support of these findings, the present study demonstrates similar proinflammatory effects of CA-IKKα in primary tracheal epithelial cells. In support of the contributions of both IKKα and IKKβ to pulmonary inflammation, a recent study showed the increased activity of IKKβ and IKKα in both healthy smokers and patients with chronic obstructive pulmonary disease (51). The siRNA-mediated knockdown of IKKα in peripheral blood mononuclear cells isolated from these patients resulted in decreases in IL-8 production, illustrating a proinflammatory role for IKKα. Based on those findings, the results of the present study, and recent reports that illuminated the cardinal role of epithelial cells in the orchestration of inflammation (3), additional studies are well warranted to unravel further the mechanisms whereby IKKα and RelB facilitate or repress inflammatory signaling in lung epithelial cells.
Supplementary Material
Acknowledgments
The authors thank Drs. Charles Irvin, Nicholas Heintz, Matthew Poynter, and Albert van der Vliet (University of Vermont, Burlington, VT) for their review of this work.
Footnotes
This work was supported by National Institutes of Health grants T32 HL076122 and R01 HL060014 (Y.M.W.J.-H.).
Originally Published in Press as DOI: 10.1165/rcmb.2012-0014OC on May 31, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev 2004;18:2195–2224 [DOI] [PubMed] [Google Scholar]
- 2.Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene 2006;25:6685–6705 [DOI] [PubMed] [Google Scholar]
- 3.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the “epimmunome.” Nat Immunol 2010;11:656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahy JV, Locksley RM. The airway epithelium as a regulator of Th2 responses in asthma. Am J Respir Crit Care Med 2011;184:390–392 [DOI] [PubMed] [Google Scholar]
- 5.Pantano C, Ather JL, Alcorn JF, Poynter ME, Brown AL, Guala AS, Beuschel SL, Allen GB, Whittaker LA, Bevelander M, et al. Nuclear factor–kappaB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med 2008;177:959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol 2004;173:7003–7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappaB activation in lipopolysaccharide-induced airway inflammation. J Immunol 2003;170:6257–6265 [DOI] [PubMed] [Google Scholar]
- 8.Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, McElwain S, Karin M. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta–dependent genes in airway epithelium. Proc Natl Acad Sci USA 2005;102:17723–17728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ather JL, Hodgkins SR, Janssen-Heininger YM, Poynter ME. Airway epithelial NF-kappaB activation promotes allergic sensitization to an innocuous inhaled antigen. Am J Respir Cell Mol Biol 2011;44:631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, et al. Activation by IKKalpha of a second, evolutionarily conserved, NF-kappaB signaling pathway. Science 2001;293:1495–1499 [DOI] [PubMed] [Google Scholar]
- 11.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol 2011;12:695–708 [DOI] [PubMed] [Google Scholar]
- 12.Xiao G, Rabson AB, Young W, Qing G, Qu Z. Alternative pathways of NF-kappaB activation: a double-edged sword in health and disease. Cytokine Growth Factor Rev 2006;17:281–293 [DOI] [PubMed] [Google Scholar]
- 13.Shembade N, Pujari R, Harhaj NS, Abbott DW, Harhaj EW. The kinase IKKalpha inhibits activation of the transcription factor NF-kappaB by phosphorylating the regulatory molecule TAX1BP1. Nat Immunol 2011;12:834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 2005;434:1138–1143 [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature 2003;423:655–659 [DOI] [PubMed] [Google Scholar]
- 16.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase–alpha in NF-kappaB–dependent gene expression. Nature 2003;423:659–663 [DOI] [PubMed] [Google Scholar]
- 17.Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YM. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J Cell Sci 2008;121:1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu R, Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro 1982;18:800–812 [DOI] [PubMed] [Google Scholar]
- 19.Malkinson AM, Dwyer-Nield LD, Rice PL, Dinsdale D. Mouse lung epithelial cell lines: tools for the study of differentiation and the neoplastic phenotype. Toxicology 1997;123:53–100 [DOI] [PubMed] [Google Scholar]
- 20.Chung S, Sundar IK, Yao H, Ho YS, Rahman I. Glutaredoxin 1 regulates cigarette smoke–mediated lung inflammation through differential modulation of I{kappa}B kinases in mice: impact on histone acetylation. Am J Physiol Lung Cell Mol Physiol 2010;299:L192–L203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Velden JL, Schols AM, Willems J, Kelders MC, Langen RC. Glycogen synthase kinase 3 suppresses myogenic differentiation through negative regulation of NFATc3. J Biol Chem 2008;283:358–366 [DOI] [PubMed] [Google Scholar]
- 22.Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-kappaB activation pathways by NF-kappaB–inducing kinase. Immunity 2004;21:477–489 [DOI] [PubMed] [Google Scholar]
- 23.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med 2009;180:720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 2009;15:410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive NF-kappaB kinase that activates the transcription factor NF-kappaB. Nature 1997;388:548–554 [DOI] [PubMed] [Google Scholar]
- 26.May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science 2000;289:1550–1554 [DOI] [PubMed] [Google Scholar]
- 27.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor–kappaB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol 2002;160:1325–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-kappaB pathway. J Immunol 2007;178:6504–6513 [DOI] [PubMed] [Google Scholar]
- 29.Sheller JR, Polosukhin VV, Mitchell D, Cheng DS, Peebles RS, Blackwell TS. Nuclear factor kappa B induction in airway epithelium increases lung inflammation in allergen-challenged mice. Exp Lung Res 2009;35:883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaynagetdinov R, Stathopoulos GT, Sherrill TP, Cheng DS, McLoed AG, Ausborn JA, Polosukhin VV, Connelly L, Zhou W, Fingleton B, et al. Epithelial nuclear factor–kappaB signaling promotes lung carcinogenesis via recruitment of regulatory T lymphocytes. Oncogene 2011;31:3164–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liptay S, Schmid RM, Nabel EG, Nabel GJ. Transcriptional regulation of NF-kappaB2: evidence for NF-kappaB–mediated positive and negative autoregulation. Mol Cell Biol 1994;14:7695–7703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choudhary S, Boldogh S, Garofalo R, Jamaluddin M, Brasier AR. Respiratory syncytial virus influences NF-kappaB–dependent gene expression through a novel pathway involving MAP3K14/NIK expression and nuclear complex formation with NF-kappaB2. J Virol 2005;79:8948–8959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Souvannavong V, Saidji N, Chaby R. Lipopolysaccharide from Salmonella enterica activates NF-kappaB through both classical and alternative pathways in primary B lymphocytes. Infect Immun 2007;75:4998–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JY, Morgan M, Kim DG, Lee JY, Bai L, Lin Y, Liu ZG, Kim YS. TNFalpha induced noncanonical NF-kappaB activation is attenuated by RIP1 through stabilization of TRAF2. J Cell Sci 2011;124:647–656 [DOI] [PubMed] [Google Scholar]
- 35.Adli M, Merkhofer E, Cogswell P, Baldwin AS. IKKalpha and IKKbeta each function to regulate NF-kappaB activation in the TNF-induced/canonical pathway. PLoS ONE 2010;5:e9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edirisinghe I, Caito S, Adenuga D, Henry R, Fromm G, et al. IKK alpha causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol 2008;38:689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, Yang R, Mink S, Shultz D, Bellone CJ, et al. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell 2007;129:903–914 [DOI] [PubMed] [Google Scholar]
- 38.Razani B, Zarnegar B, Ytterberg AJ, Shiba T, Dempsey PW, Ware CF, Loo JA, Cheng G. Negative feedback in noncanonical NF-kappaB signaling modulates NIK stability through IKKalpha-mediated phosphorylation. Sci Signal 2010;3:ra41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappaB/Rel family. Cell 1995;80:331–340 [DOI] [PubMed] [Google Scholar]
- 40.Weih F, Durham SK, Barton DS, Sha WC, Baltimore D, Bravo R. P50–NF-kappaB complexes partially compensate for the absence of RelB: severely increased pathology in p50(−/−)RelB(−/−) double-knockout mice. J Exp Med 1997;185:1359–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, Sime PJ. The aryl hydrocarbon receptor attenuates tobacco smoke–induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. J Biol Chem 2008;283:28944–28957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMillan DH, Baglole CJ, Thatcher TH, Maggirwar S, Sime PJ, Phipps RP. Lung-targeted overexpression of the NF-kappaB member RelB inhibits cigarette smoke–induced inflammation. Am J Pathol 2011;179:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia Y, Pauza ME, Feng L, Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol 1997;151:375–387 [PMC free article] [PubMed] [Google Scholar]
- 44.Yoza BK, Hu JY, Cousart SL, Forrest LM, McCall CE. Induction of RelB participates in endotoxin tolerance. J Immunol 2006;177:4080–4085 [DOI] [PubMed] [Google Scholar]
- 45.El Gazzar M, Liu T, Yoza BK, McCall CE. Dynamic and selective nucleosome repositioning during endotoxin tolerance. J Biol Chem 2010;285:1259–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki CY, Ghosh P, Longo DL. Recruitment of RelB to the CSF2 promoter enhances RelA-mediated transcription of granulocyte–macrophage colony–stimulating factor. J Biol Chem 2011;286:1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saccani S, Pantano S, Natoli G. Modulation of NF-kappaB activity by exchange of dimers. Mol Cell 2003;11:1563–1574 [DOI] [PubMed] [Google Scholar]
- 48.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, Geijtenbeek TB. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol 2009;10:203–213 [DOI] [PubMed] [Google Scholar]
- 49.Jacque E, Tchenio T, Piton G, Romeo PH, Baud V. RelA repression of RelB activity induces selective gene activation downstream of TNF receptors. Proc Natl Acad Sci USA 2005;102:14635–14640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadikot RT, Han W, Everhart MB, Zoia O, Peebles RS, Jansen ED, Yull FE, Christman JW, Blackwell TS. Selective I kappa B kinase expression in airway epithelium generates neutrophilic lung inflammation. J Immunol 2003;170:1091–1098 [DOI] [PubMed] [Google Scholar]
- 51.Gagliardo R, Chanez P, Profita M, Bonanno A, Albano GD, Montalbano AM, Pompeo F, Gagliardo C, Merendino AM, Gjomarkaj M. IkappaB kinase–driven nuclear factor–kappaB activation in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 2011;128:635–645 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.