Abstract
Desulfobulbus propionicus was able to grow with Fe(III), the humic acids analog anthraquinone-2,6-disulfonate (AQDS), or a graphite electrode as an electron acceptor. These results provide an explanation for the enrichment of Desulfobulbaceae species on the surface of electrodes harvesting electricity from anaerobic marine sediments and further expand the diversity of microorganisms known to have the ability to use both sulfate and Fe(III) as an electron acceptor.
Electrical energy can be harvested from marine sediments when a graphite electrode emplaced in marine sediment (anode) is connected by an electrical circuit to another electrode (cathode) in overlying aerobic water (22). Microbial activity in the sediments is required for current production (2, 8, 25, 28), and there is a specific enrichment of δ-Proteobacteria on the anode surface (2, 8, 28).
In most freshwater and marine sediment fuel cells, microorganisms in the Geobacteraceae family are the predominant microorganisms on the anodes (2, 8, 28). However, in all marine sediment fuel cells evaluated, another group of δ-proteobacterial sequences, most closely related to sulfate-reducing bacteria from the family Desulfobulbaceae, was also consistently enriched on the anode (8, 28). In fact, in one field deployment, organisms in this cluster accounted for all of the δ-proteobacterial sequences and ca. 62% of the bacterial 16S rRNA gene sequences recovered from the current-harvesting anode (8).
In order to investigate how Desulfobulbaceae might interact with anodes in sediments, studies were conducted with Desulfobulbus propionicus. Dissimilatory sulfate reduction via the incomplete oxidation of organic acids such as lactate, propionate, butyrate, and ethanol to acetate is considered the primary metabolism of D. propionicus (29, 30). However, D. propionicus can oxidize organic electron donors with nitrate (5, 30), nitrite (5), or oxygen (5, 6) and can oxidize inorganic sulfur compounds with the reduction of O2 (5, 7), nitrite (5), nitrate (5), or Mn(IV) (16). Furthermore, in the absence of an electron acceptor, D. propionicus can ferment lactate, pyruvate, or ethanol to a mixture of acetate and propionate via the succinate-propionate pathway (26, 29, 30).
Previous studies also demonstrated that cell suspensions of D. propionicus reduced Fe(III)-nitrilotriacetic acid (NTA; 10 mM) and poorly crystalline Fe(III)-oxide (100 mM) when propionate (5 to 10 mM) was provided as the electron donor (22). However, Fe(III) did not support growth in those studies. The ability to grow via reduction of Fe(III) oxides may have relevance to growth on electricity-harvesting electrodes in sediments, because both electrodes and Fe(III) oxides represent insoluble, extracellular electron acceptors.
Dissimilatory Fe(III) reduction.
The representative Desulfobulbaceae species, D. propionicus (DSMZ 2032), was obtained from DSMZ (German Collection of Microorganisms and Cell Cultures; Braunschweig, Germany) and was grown in a slightly modified version of NB Basal medium (14), which contained the following (per liter): 0.42 g of KH2PO4, 0.22 g of K2HPO4, 0.2 g of NH4Cl, 0.38 g of KCl, 0.36 g of NaCl, 0.75 g of CaCl2 · 2 H2O, 0.10 g of MgCl2, 1.8 g of NaHCO3, and 0.5 g of Na2CO3, as well as 1 μM Na2SeO4 and trace minerals and vitamins. This medium differed from that typically used to culture D. propionicus (DSMZ medium 194) in that it contained selenium and fivefold more calcium. Sulfate other than that present in the trace metal solution (300 μM) was omitted to prevent extensive sulfide production with subsequent abiotic reduction of Fe(III). Strict anaerobic techniques were used throughout, and cultures were incubated at 30°C in the dark. Organic acids (21), cell numbers (19), Fe(II), and total iron (19, 20) were monitored as previously described. Growth with various electron donors and acceptors was considered positive only after six consecutive transfers.
When D. propionicus was grown on pyruvate alone, 6.67 ± 0.33 mM (n = 3) pyruvate was fermented to 4.22 ± 0.51 mM acetate and 2.23 ± 0.35 mM propionate according to the following reaction: 3CH3COCOO− + 3H2O→2CH3COO− + CH3CH2COO− + 2HCO3− + 2H+.
These results are similar to those of previous studies that have shown that acetate and propionate are formed in a 2:1 ratio when Desulfobulbus propionicus is grown on pyruvate in the absence of an electron acceptor (26).
Pyruvate consumption by D. propionicus differed significantly from fermentation when a soluble form of iron was provided as an electron acceptor. For example, when pyruvate (mean ± standard deviation, 19.20 ± 0.68 mM; n = 3) was provided as the electron donor with Fe(III)-citrate (50 mM) as the electron acceptor, Fe(III) was reduced and 18.65 ± 0.36 mM acetate was formed, accompanied by cell growth (Fig. 1a). The stoichiometry of pyruvate consumption and Fe(III) reduction was consistent with the following reaction: CH3COCOO− + 2Fe3+ + 2H2O→CH3COO− + HCO3− + 2Fe2+ + 3H+.
FIG. 1.
(A) Growth of D. propionicus with pyruvate (19.2 mM) as the electron donor and Fe(III)-citrate (50 mM) as the electron acceptor. (B) Growth with Fe(III)-oxide (100 mmol/liter) as the electron acceptor and hydrogen (101 kPa) as electron donor. (C) Growth with Fe(III)-oxide (100 mmol/liter) as electron acceptor and pyruvate (7.15 mM) as the electron donor. The results are the means of triplicate incubations. •, cell number; ○, cell number, control (no donor); ▪, Fe(II) concentration; □, Fe(II) concentration, control (no donor); ▴, pyruvate concentration; ▵, pyruvate concentration, control (no cells).
D. propionicus was able to grow with several other soluble electron acceptors, including Fe(III)-NTA (5 mM), Fe(III)-pyrophosphate (10 mM), and anthraquinone-2,6-disulfonate (AQDS; 5 mM), with pyruvate as the electron donor. Propionate, lactate, and hydrogen also served as electron donors for growth on all forms of soluble Fe(III) evaluated as well as AQDS. The mechanism(s) for Fe(III) reduction appeared to be independent of the mechanism(s) for sulfate reduction, because D. propionicus continued to reduce Fe(III)-citrate in the presence of 1 to 10 mM molybdate, an inhibitor of sulfate reduction.
There was a mixture of pyruvate fermentation and Fe(III) reduction when poorly crystalline Fe(III)-oxide (100 mM) (17) was provided as the electron acceptor with pyruvate (7.15 mM) as the electron donor. In the presence of Fe(III)-oxide approximately twice as much acetate was formed from pyruvate oxidation than would be expected from pyruvate fermentation alone; 6.87 ± 0.75 mM (n = 3) of Fe(III)-oxide was reduced, and 5.13 ± 0.36 mM acetate and 1.84 ± 0.25 mM propionate accumulated (Fig. 1c). In addition, Fe(III)-oxide reduction during pyruvate metabolism appeared to yield energy to support cell growth, as the final cell numbers in cultures grown in the presence of Fe(III)-oxide, 1.0 × 108 ± 1.53 × 107 cells/ml (starting cell number was 1.20 × 106 ± 2.32 × 105; n = 3), were significantly higher than those in cultures grown with pyruvate alone (3.72 × 107 ± 2.5 × 106 cells/ml of culture; starting cell number was 2.43 × 106 ± 6.35 × 105; n = 3). The stoichiometry of this mixed reaction was consistent with the following reaction: 3.4CH3COCOO− + 6.8Fe3+ + 6.8H2O→3.4CH3COO− + 3.4HCO3− + 6.8Fe2+ and 3.75CH3COCOO− + 3.75H2O→2.5CH3COO− + 1.25CH3CH2COO− + 2.5HCO3− + 2.5H+ for a combined reaction of 7.15CH3COCOO− + 6.8Fe3+ + 10.55H2O→5.9CH3COO− + 5.9HCO3− + 6.8Fe2+ +1.25CH3CH2COO− + 12.7H+.
D. propionicus could also be continually cultured with hydrogen as the electron donor and poorly crystalline Fe(III)-oxide as the electron acceptor when acetate (0.1 mM) was provided as a carbon source (Fig. 1b).
Electron transfer to a graphite electrode.
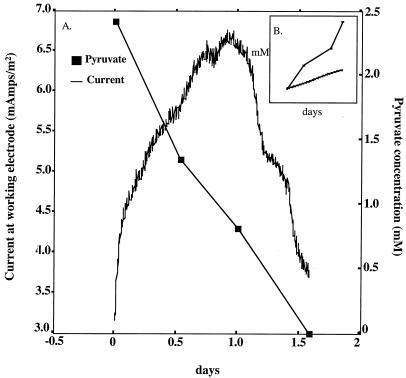
In order to evaluate the ability of D. propionicus to transfer electrons to an electrode, the inoculum was grown fermentatively on lactate (10 mM). The cells were pelleted via centrifugation, washed, and then resuspended in 20 ml of fresh anoxic medium lacking electron donor or acceptor. Ten milliliters of this cell suspension was inoculated into the anaerobic anodic chamber (250 ml of medium) of a two-chambered electrode system, constructed as previously described (2, 3). The electrodes were (in centimeters) 2.34 by 7.02 by 1.17 sticks of unpolished graphite (grade G10; Graphite Engineering and Sales, Greenville, Mich.). The anode was poised with a potentiostat (AMEL instruments, Milan, Italy) at a constant potential of +0.52 V in reference to a standard H2 electrode, which preliminary studies demonstrated was the optimal potential to support growth. D. propionicus was able to use the electrode surface as an electron acceptor when pyruvate (2.42 mM) (Fig. 2), lactate (1 mM) (Fig. 3), propionate (2 mM) (data not shown), or hydrogen (data not shown) was provided as electron donor. Almost identical current profiles were observed when lactate (Fig. 3) or propionate (data not shown) was provided as electron donor; current rose rapidly and then declined as lactate or propionate was depleted. Maximum current production with lactate and propionate reached 28.35 ± 4.72 mA/m2 (mean ± standard deviations; n = 3) and 26.77 ± 7.87 mA/m2, respectively, and between 21 to 27% of the electrons available from the incomplete oxidation of lactate and propionate were transferred to the electrode. Current produced with pyruvate and hydrogen was significantly lower: only 8.27 ± 1.97 mA/m2 from pyruvate (Fig. 2) and 5.91 ± 1.18 mA/m2 of current from hydrogen (data not shown). Similar to Fe(III) reduction, the addition of molybdate (5 mM) to cultures grown in the anodic chamber with lactate (2 mM) did not have a significant effect on current production (data not shown).
FIG. 2.
(A) Current production by D. propionicus when pyruvate (2.42 mM) was provided as the electron donor, and a poised electrode (+0.52 V in reference to a standard H2 electrode) served as the electron acceptor. (B) Number of electrons available from the incomplete oxidation of pyruvate that were transferred to the electrode surface. The results are the means of triplicate incubations.
FIG. 3.
(A) Continued current production when lactate-depleted medium was replaced with fresh medium and lactate (1 mM) at times designated by the arrows. (B) Number of electrons available from the incomplete oxidation of lactate that were transferred to the electrode surface. The results are the means of triplicate incubations.
Evaluation of end products and electron recovery from pyruvate oxidation suggests that electron transfer to an insoluble graphite electrode can be attributed to the same mixed metabolism as observed with insoluble Fe(III)-oxide. For example, when D. propionicus was grown in the electrode chamber with 2.42 ± 0.23 mM pyruvate provided as the electron donor with a poised electrode as the sole electron acceptor, 1.79 ± 0.33 mM acetate and 0.64 ± 0.29 mM propionate was formed. Thus, 26.4% of the pyruvate metabolized was associated with oxidation of pyruvate to acetate coupled to electron transfer to the electrode.
When spent medium from a lactate-grown culture was replaced with fresh medium containing more lactate (1 mM), current production immediately resumed (Fig. 3). This suggested that, as was previously seen with Geobacter sulfurreducens (3) and Rhodoferax ferrireducens (4), cells of D. propionicus attached to the electrode, rather than planktonic cells, were primarily responsible for the current production.
When S0 (20 g/liter) was added as a potential electron donor for electron transfer to the electrode, sulfate was produced in the presence of D. propionicus but not in the absence of cells (Fig. 4). Cells did not produce sulfate in the absence of the electrode.
FIG. 4.
Sulfate production by D. propionicus when elemental S0 was provided as the electron donor, and an electrode poised at +0.522 V (in reference to a standard H2 electrode) was the electron acceptor. Sulfate was measured with ion chromatography as previously described (16). The results are the means of triplicate incubations.
Implications.
The ability of D. propionicus to transfer electrons to Fe(III), AQDS, and electrodes has implications for anaerobic respiration in sedimentary environments. Only one other organism, the gram-positive bacterium Desulfotomaculum reducens, has been reported to grow with both sulfate and Fe(III) as terminal electron acceptors (27). D. propionicus is the first sulfate-reducing organism found to conserve energy to support growth from the reduction of insoluble Fe(III)-oxide, the most abundant form of microbially reducible Fe(III) in most sedimentary environments (12). Microorganisms that can use both sulfate and Fe(III) as electron acceptors may be most competitive at the interface between the zones of Fe(III) reduction and sulfate reduction in aquatic sediments where Fe(III) is still available but is not in sufficient concentrations to inhibit sulfate reduction (15, 18).
The ability of D. propionicus to use a graphite electrode as an electron acceptor provides an explanation for the consistent enrichment of closely related organisms in the family Desulfobulbaceae on electrodes harvesting electricity from marine sediments. As previously observed with such dissimilatory Fe(III)-reducing microorganisms as species within the family Geobacteraceae (2, 3), Shewanella putrefaciens (9, 10), Clostridium butyricum (23), Rhodoferax ferrireducens (4), Aeromonas hydrophila (24), and Geothrix fermentans (D. R. Bond and D. R. Lovley, unpublished data). D. propionicus did not require the addition of exogenous electron-shuttling compounds for electricity production.
In addition, the finding that D. propionicus is able to oxidize elemental S0 with an electrode serving as the electron acceptor provides further insight into the biological mechanisms involved in current production by the marine sediment fuel cell. It has previously been shown that when Mn(IV) oxides are added to anoxic marine sediments containing sulfides, there is a production of sulfate that requires biological activity (1, 11). Mn(IV) is able to chemically oxidize sulfides to S0, which is then oxidized by microorganisms to sulfate with the reduction of Mn(IV). When a current-harvesting electrode is placed in anoxic sediments, similar processes are observed. Elemental S0 is known to precipitate on the anodes of marine sediment fuel cells as the result of abiotic sulfide oxidation at the anode surface (28), and elevated sulfate levels have been measured in sediments closest to the current-harvesting anode (28). These geochemical results coupled with the fact that S0 oxidation with an electrode serving as the electron acceptor was observed in pure-culture studies of D. propionicus suggests that the oxidation of elemental sulfur on the anode surface may be an important biological process in the marine sediment fuel cell.
However, the results also suggest that Desulfobulbaceae species are unlikely to play an important role in coupling the oxidation of organic matter to the reduction of the electrode in marine sediment fuel cells. Desulfobulbaceae are not known to oxidize acetate, which is likely to be the primary electron donor for electricity production. Rather, D. propionicus can only metabolize such organic acids as propionate, lactate, and pyruvate, which are less likely to be important extracellular intermediates in sediments (13). Furthermore, electron transfer to the electrode from the mixed metabolism of organic acids by D. propionicus is relatively inefficient; only ca. 25% of the electrons available from the incomplete oxidation of pyruvate, lactate, and propionate were transferred to the electrode surface. In contrast, several Geobacteraceae species can oxidize acetate and are able to quantitatively transfer all of the electrons available from the complete oxidation of organic acids to CO2 to an electrode (2, 3). Therefore, the consistent enrichment of Desulfobulbaceae 16S rRNA gene sequences on current-harvesting marine anodes (2, 8, 28) is probably due to their ability to oxidize S0 on the electrode surface.
In summary, this study provides the first example of a sulfate-reducing organism that can also conserve energy to support growth via electron transfer to insoluble electron acceptors, such as Fe(III) oxide and electrodes. Further studies are warranted to determine whether the mechanisms involved in electron transfer by D. propionicus to these extracellular electron acceptors are similar to those in more well-studied Fe(III)-reducing microorganisms.
REFERENCES
- 1.Aller, R. C., and P. D. Rude. 1988. Complete oxidation of solid phase sulfides by manganese and bacteria in anoxic marine sediments. Geochim. Cosmochim. Acta 52:751-765. [Google Scholar]
- 2.Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483-485. [DOI] [PubMed] [Google Scholar]
- 3.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhuri, S. K., and D. R. Lovley. 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21:1229-1232. [DOI] [PubMed]
- 5.Dannenberg, S., M. Kroder, W. Dilling, and H. Cypionka. 1992. Oxidation of H2, organic compounds and inorganic sulfur compounds coupled to reduction of O2 or nitrate by sulfate-reducing bacteria. Arch. Microbiol. 158:93-99. [Google Scholar]
- 6.Dilling, W., and H. Cypionka. 1990. Aerobic respiration in sulfate-reducing bacteria. FEMS Microbiol. Lett. 71:123-128. [Google Scholar]
- 7.Fuseler, K., and H. Cypionka. 1995. Elemental sulfur as an intermediate of sulfide oxidation with oxygen by Desulfobulbus propionicus. Arch. Microbiol. 164:104-109. [Google Scholar]
- 8.Holmes, D. E., D. R. Bond, R. A. O'Neil, C. E. Reimers, and D. R. Lovley. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol., in press. [DOI] [PubMed]
- 9.Kim, B. H., H. J. Kim, M. S. Hyun, and D. S. Park. 1999. Direct electrode reaction of Fe(III) reducing bacterium, Shewanella putrefaciens. J. Microb. Biotechnol. 9:127-131. [Google Scholar]
- 10.Kim, H. J., H. S. Park, M. S. Hyun, I. S. Chang, M. Kim, and B. H. Kim. 2002. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb. Technol. 30:145-152. [Google Scholar]
- 11.King, G. M. 1990. Effects of added manganic and ferric oxides on sulfate reduction and sulfide oxidation in intertidal sediments. FEMS Microbiol. Ecol. 73:131-138. [Google Scholar]
- 12.Lovley, D. R. 2000. Fe(III) and Mn(IV)-reducing prokaryotes. In M. Dworkin, S. Falkow, E. Rosenberg, K. Schleifer, and E. Stackebrandt (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed. [Online.] Springer-Verlag, New York, N.Y. http://link.springer-ny.com/link/service/books/10125/.
- 13.Lovley, D. R., and F. H. Chapelle. 1995. Deep subsurface microbial processes. Rev. Geophys. 33:365-381. [Google Scholar]
- 14.Lovley, D. R., J. L. Fraga, J. D. Coates, and E. L. Blunt-Harris. 1999. Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1:89-98. [DOI] [PubMed] [Google Scholar]
- 15.Lovley, D. R., and S. Goodwin. 1988. Hydrogen concentrations as an indicator of the predominant terminal electron accepting reactions in aquatic sediments. Geochim. Cosmochim. Acta 52:2993-3003. [Google Scholar]
- 16.Lovley, D. R., and E. J. P. Phillips. 1994. Novel processes for anoxic sulfate production from elemental sulfur by sulfate-reducing bacteria. Appl. Environ. Microbiol. 60:2394-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovley, D. R., and E. J. Phillips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovley, D. R., and E. J. P. Phillips. 1987. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl. Environ. Microbiol. 53:2636-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovley, D. R., and E. J. P. Phillips. 1987. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 53:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovley, D. R., and E. J. P. Phillips. 1989. Requirement for a microbial consortium to completely oxidize glucose in Fe(III)-reducing sediments. Appl. Environ. Microbiol. 55:3234-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovley, D. R., E. E. Roden, E. J. P. Phillips, and J. C. Woodward. 1993. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Marine Geol. 113:41-53. [Google Scholar]
- 23.Park, H. S., B. H. Kim, H. S. Kim, H. J. Kim, G. T. Kim, M. Kim, I. S. Chang, Y. K. Park, and H. I. Chang. 2001. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7:297-306. [Google Scholar]
- 24.Pham, C. A., S. J. Jung, N. T. Phung, J. Lee, I. S. Chang, B. H. Kim, H. Yi, and J. Chun. 2003. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Ecol. 223:129-134. [DOI] [PubMed] [Google Scholar]
- 25.Reimers, C. E., L. M. Tender, S. Fertig, and W. Wang. 2001. Harvesting energy from the marine sediment-water interface. Environ. Sci. Technol. 35:192-195. [DOI] [PubMed] [Google Scholar]
- 26.Tasaki, M., Y. Kamagata, K. Nakamura, K. Okamura, and K. Minami. 1993. Acetogenesis from pyruvate by Desulfotomaculum thermobenzoicum and differences in pyruvate metabolism among three sulfate-reducing bacteria in the absence of sulfate. FEMS Microbiol. Lett. 106:259-264. [Google Scholar]
- 27.Tebo, B. M., and A. Y. Obraztsova. 1998. Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiol. Lett. 162:193-198. [Google Scholar]
- 28.Tender, L. M., C. E. Reimers, H. A. Stecher, D. E. Holmes, D. R. Bond, D. A. Lowy, K. Pilobello, S. J. Fertig, and D. R. Lovley. 2002. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 20:821-825. [DOI] [PubMed] [Google Scholar]
- 29.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In H. G. T. A. Balows, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes. Springer, Berlin, Germany.
- 30.Widdel, F., and N. Pfennig. 1982. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. II. Incomplete oxidation of propionate by Desulfobulbus propionicus gen. nov., sp. nov. Arch. Microbiol. 131:360-365. [DOI] [PubMed] [Google Scholar]