Abstract
Many transcription factors that regulate lung morphogenesis during development are reactivated to mediate repairs of the injured adult lung. We hypothesized that CCAAT/enhancer binding protein–α (C/EBPα), a transcription factor critical for perinatal lung maturation, regulates genes required for the normal repair of the bronchiolar epithelium after injury. Transgenic CebpαΔ/Δ mice, in which Cebpa was conditionally deleted from Clara cells and Type II cells after birth, were used in this study. Airway injury was induced in mice by the intraperitoneal administration of naphthalene to ablate bronchiolar epithelial cells. Although the deletion of C/EBPα did not influence lung structure and function under unstressed conditions, C/EBPα was required for the normal repair of terminal bronchiolar epithelium after naphthalene injury. To identify cellular processes that are influenced by C/EBPα during repair, mRNA microarray was performed on terminal bronchiolar epithelial cells isolated by laser-capture microdissection. Normal repair of the terminal bronchiolar epithelium was highly associated with the mRNAs regulating antiprotease activities, and their induction required C/EBPα. The defective deposition of fibronectin in CebpαΔ/Δ mice was associated with increased protease activity and delayed differentiation of FoxJ1-expressing ciliated cells. The fibronectin and ciliated cells were restored by the intratracheal treatment of CebpαΔ/Δ mice with the serine protease inhibitor. In conclusion, C/EBPα regulates the expression of serine protease inhibitors that are required for the normal increase of fibronectin and the restoration of ciliated cells after injury. Treatment with serine protease inhibitor may aid in the recovery of injured bronchiolar epithelial cells, and prevent common chronic lung diseases.
Keywords: C/EBPα, antiprotease, Spink5, naphthalene, Scgb1a1
Clinial Relevance
CCAAT/enhancer binding protein–α regulates the expression of serine protease inhibitors that are required for normal increases of fibronectin and the restoration of ciliated cells after bronchiolar epithelial cell injury. Treatment with serine protease inhibitors may aid in the recovery of injured bronchiolar epithelial cells, and prevent common chronic lung diseases.
Airway epithelial cell injury is associated with increased protease activity and oxidative stress, which contribute to the pathogenesis of many common lung diseases, including chronic obstructive pulmonary disease, cystic fibrosis, asthma, chronic bronchiolitis, and bronchopulmonary dysplasia (1–6). The terminal bronchioles in the mouse are primarily composed of cuboidal nonciliated cells (Clara cells and Scgb1a1-positive [Scgb1a1+] cells) and ciliated cells (FoxJ1-positive [FoxJ1+] cells). Nonciliated cells are crucial for pulmonary homeostasis, serving as progenitor cells that proliferate and differentiate into nonciliated and ciliated cells during regeneration after epithelial cell injury (7–10). Although the normal lifespan of bronchiolar epithelial cells is relatively long (7, 11), cell proliferation occurs rapidly, peaking 24–48 hours after injury (12). The underlying molecular mechanisms for bronchiolar epithelial cell recovery after injury are still not completely understood.
The transcription factors that regulate lung morphogenesis during development are reactivated during the repair of an injured adult lung (13). CCAAT/enhancer binding protein–α (C/EBPα) is one of the important transcription factors required for normal differentiation of the fetal lung. The lung-specific deletion of Cebpa in the fetus inhibited lung maturation, resulting in death from respiratory failure at birth (14). In contrast, Cebpa gene expression was not required for pulmonary homeostasis in adult mice under unstressed conditions, and transgenic mice with a postnatal lung-specific deletion of Cebpa had a normal lifespan, with no obvious abnormalities in the lung (15). Our previous study demonstrated that C/EBPα played an important role in the protection of the alveolar epithelium after hyperoxic injury in the adult mouse. Microarray analyses of isolated alveolar Type II (ATII) cells demonstrated that hyperoxic challenge reduced the expression of many genes involved in cell injury and recovery in CebpαΔ/Δ mice compared with control mice (15). Moreover, C/EBPα is generally recognized as a transcription factor important for cell differentiation in the skin, adipocytes, and hematopoietic cells (16, 17). We hypothesized that C/EBPα regulates the genes required for normal repair of the terminal bronchiolar epithelium after injury in the adult lung.
In the present study, we demonstrated that C/EBPα regulates the protease/antiprotease balance in terminal bronchiolar epithelial cells after naphthalene injury. Intratracheal treatment with a serine protease inhibitor (bovine pancreatic trypsin inhibitor; BPTI) restored the ciliated cell differentiation required for normal regeneration of the bronchiolar epithelium.
Materials and Methods
All study protocols were approved by the Institutional Animal Care and Use Committee at Cincinnati Children’s Hospital Research Foundation.
Transgenic Mice
All transgenic mice were maintained in a mixed C57/BL6 and FVB/N genetic background. Scgb1a1-rtTA−/tg–line-2 mice were bred to (tetO)7CMV-Cretg/tg/Cebpαflox/flox mice to generate Scgb1a1-rtTA−/tg/(tetO)7CMV-Cre−/tg/Cebpα+/flox mice. These mice were bred to (tetO)7CMV-Cretg/tg/Cebpαflox/flox mice to generate triple-transgenic Scgb1a1-rtTA-/tg/(tetO)7CMV-Cretg/tg/Cebpαflox/flox mice (here termed CebpαΔ/Δ mice) (15). Littermate (tetO)7CMV-Cretg/tg/Cebpαflox/flox mice were used as controls. Doxycycline (625 mg/kg; Harlan Teklad, Madison, WI) was given in the chow to the dams starting on Embryonic Day 0 to Postnatal Day 14. Doxycycline was provided to the fetuses and newborn animals via placental transfer and milk, respectively (18).
Rosa26Rtg/tg mice were bred to CebpαΔ/Δ mice to generate Scgb1a1-rtTA−/tg/(tetO)7CMV-Cre−/tg/Rosa26R−/tg/Cebpαflox/flox mice (Rosa–CebpαΔ/Δ mice) and littermate Scgb1a1-rtTA−/tg/(tetO)7CMV-Cre−/tg/Rosa26R−/tg/Cebpα+/+ mice (Rosa–control mice). Chow containing doxycycline was given to the 5-week-old Rosa–CebpαΔ/Δ and Rosa–control mice for 3 weeks.
Laser-Capture Microdissection and RNA Microarray Analysis
The terminal bronchiolar epithelial cells, in an approximately 200-μm section of airway starting from the bronchoalveolar duct junctions, were isolated by laser-capture microdissection (LCM) from mice (19) at 0, 3, and 72 hours after naphthalene injection. The samples were pooled for each mouse (n = 5 mice/group), and the total RNA was isolated. The mRNA microarray was performed in the Cincinnati Children’s Hospital Medical Center Microarray Core on the three samples with the highest-quality RNA (n = 3 mice/group). Genotype- and time-dependent mRNA expression was assessed by ANOVA, using as thresholds of expression P < 0.01 and fold change greater than or equal to 1.5 (15) (detailed methods are presented in the online supplement). The total RNA was also used for RT-PCR (see Table E1 in the online supplement).
Protease Activity
Caseinolytic protease activity was measured in the supernatants of lung homogenates (detailed methods are provided in the online supplement).
Kallikrein activities in frozen lung sections were studied by in situ zymography as described previously (20). Briefly, frozen lung sections were incubated with buffer, EDTA (10 mΜ), or BPTI (10 mg/ml) at 37°C for 1 hour, followed by incubation with D-Val-Leu-Arg-7- amino-4-methylcoumarin (1 mM in 200 mM Tris, pH 8.2; Sigma Chemical Co., St. Louis, MO) at 37°C for 1 hour. The slides were washed with 1% Tween-20 and mounted with water. The fluorescence of amino-4-methylcoumarin signals was detected by a Zeiss Imaging Microscope (Carl Zeiss Microimaging, Thornwood, NY), using the same exposure time for all samples. The fluorescence of terminal bronchiolar epithelial cells was evaluated using Image J software (National Institutes of Health, Bethesda, MD). The signal intensity was converted to a serial rainbow color image, ranging from background (black) to high kallikrein activity (red).
Methods for the RT-PCR, immunohistochemistry, X-gal staining (21), mitotic index, Scgb1a1+ cell isolation (22), and administration of naphthalene (23, 24), BPTI (25), and EDTA to mice are provided in the online supplement.
Statistical Analysis
Data are expressed as the means ± SEM. Statistical analysis was performed using StatView, version 5 (SAS Institute, Cary, NC). Comparisons between two groups were performed according to the Mann-Whitney U test. For multiple comparisons, ANOVA was used, followed by the Bonferroni post hoc test for significance.
Results
Conditional Deletion of Cebpα in the Respiratory Epithelium
To generate CebpαΔ/Δ mice, the rtTA system was applied to control Cebpa expression in mice (26), using the Scgb1a1 gene promoter. In this transgenic murine line, the Scgb1a1 promoter directs the expression of rtTA in Clara cells on Embryonic Days 16–17 and in a subset of ATII cells after birth (27). Thus, in the CebpαΔ/Δ mice, the deletion of Cebpa occurred in the Clara cells from Embryonic Days 16–17 of gestation, and in subsets of ATII cells after birth. In contrast to the SP-C-rtTA CebpαΔ/Δ mice that died at birth from pulmonary immaturity and respiratory failure (14), Cebpa was not deleted from the fetal ATII cells in the Scgb1a1-rtTA CebpαΔ/Δ mice, and lung development was normal.
In a previous study, analyses of littermate controls lacking either the rtTA or Cre genes identified no genotype-related differences (15). In the present study, Cebpαflox/flox littermates lacking rtTA served as the control mice. The control lungs were normal, and the lung structure and expression of Cebpα mRNA in the lungs were similar to those of wild-type C57/BL6 mice (control mice, 1.00 ± 0.02; C57/BL6 mice, 1.01 ± 0.08; n = 5/group). In the control lungs (Figure 1A), immunohistochemical and immunofluorescence staining for the C/EBPα protein was detected in Scgb1a1+ cells (Figure 1C), pro–SP-C–positive ATII cells (Figure 1E), and alveolar macrophages (Figure 1A, arrow). Similar to a previous study (28), the C/EBPα protein was not detected in the FoxJ1+ ciliated cells (Figure 1D). A substantial loss of C/EBPα staining was observed in the Scgb1a1+ cells and the ATII cells of CebpαΔ/Δ mice (Figure 1B). As expected because of the specificity of the Scgb1a1 gene promoter, C/EBPα staining was not altered in alveolar macrophages (Figures 1A and 1B, arrow). In the terminal bronchiolar epithelial cells isolated by LCM, the level of Cebpa mRNA was lower than in whole lungs (Figure 1F) in the CebpαΔ/Δ mice, which is likely related to the expression of C/EBPα in alveolar macrophages. Using Western blot analysis, C/EBPα was shown to be decreased in the Scgb1a1+ cells isolated from CebpαΔ/Δ mice (Figure 1G) compared with control mice.
Figure 1.
Conditional deletion of CCAAT/enhancer binding protein–α (C/EBPα) from the respiratory epithelium. Immunohistochemical staining for C/EBPα in lung tissues from 9-week-old control mice (A) and CebpαΔ/Δ mice (B) is shown. C/EBPα was deleted from the airway epithelial cells in CebpαΔ/Δ mice. Alveolar macrophages (arrows) are stained for C/EBPα in both the control and CebpαΔ/Δ mice. (C) In control mice, C/EBPα (red) was expressed in the Scbg1a1-positive (green) cells and ATII cells (E) (pro–SP-C–positive, green) but not in the FoxJ1-positive (green) ciliated cells (D). (C, D, and E) DAPI (4′,6-diamidino-2-phenylindole) staining of the reagent bound to DNA (blue). (F) Quantitative RT-PCR analyses of Cebpa mRNA in the whole lung and terminal bronchiolar epithelial cells isolated by laser-capture microdissection (LCM). The Cebpα mRNA was significantly decreased in CebpαΔ/Δ terminal bronchiolar epithelial cells of the CebpαΔ/Δ mice compared with the whole lung. This likely represents the residual expression of C/EBPα in alveolar macrophages. (G) Western blot analysis was used to assess the expression of C/EBPα protein and β-actin in isolated Scgb1a1+ cells. C/EBPα was decreased in Scgb1a1+ cells from CebpαΔ/Δ mice. Immunostaining for Scgb1a1 (H and I) and C/EBPα (J and K) in 18-month-old control and CebpαΔ/Δ mice is shown. Scgb1a1 expression and lung structure were not influenced by the long-term deletion of Cebpα. Scale bars, 100 μm; insets, 10 μm (n = 4/group). *P < 0.05 versus control mice, according to ANOVA.
In a previous study, we demonstrated that CebpαΔ/Δ mice were normal under unstressed conditions, with no apparent abnormalities detected by either light or electron microscopy of lung tissue, including ATII cells (15). Likewise, the surfactant lipid metabolism and lung mechanics were normal, and the concentrations of proinflammatory cytokines (IL-1β, IL-6, and macrophage inflammatory protein-2) in CebpαΔ/Δ lungs were low (15). Consistent with these previous findings, the composition and number of terminal bronchiolar epithelial cells (Figure 2B, Day 0) and the expression of protease genes (Figure 4, 0 h) were similar in the control and CebpαΔ/Δ mice under unstressed conditions. The CebpαΔ/Δ mice were morphologically indistinguishable from the control mice at 18 months of age (Figures 1H–1K). The long-term deletion of Cebpa (18 months; Figures 1J and 1K) did not influence Scgb1a1 staining in the lungs of CebpαΔ/Δ mice (Figure 1I). In summary, under unstressed conditions, C/EBPα is not required for the maintenance of lung structure, lung function, or Scgb1a1 expression, and the CebpαΔ/Δ mice had a normal lifespan.
Figure 2.
C/EBPα is required for the regeneration of the terminal bronchiolar epithelia and the reestablishment of column-ciliated cells after injury. (A) Scgb1a1 and FoxJ1 staining of the terminal bronchiolar epithelium is shown before (Day 0) and after (Days 2, 5, and 14) naphthalene injury. Bronchoalveolar duct junctions are included at the right end in the microphotographs. Scgb1a1+ cells were depleted after naphthalene injury, and recovered in both the control and CebpαΔ/Δ mice. FoxJ1+ cells were decreased in the CebpαΔ/Δ mice after naphthalene injury. Scale bars, 50 μm; insets, 20 μm. (B) The numbers of Scgb1a1-stained and FoxJ1-stained cells lining 200 μm of the terminal bronchiolar epithelium from the bronchoalveolar duct junction were counted in the control and CebpαΔ/Δ mice from 0–185 days after naphthalene injury. The Scgb1a1+-stained cells were increased by the deletion of Cebpa. The deletion of C/EBPα significantly decreased the number of FoxJ1+ cells after naphthalene injury, and the cells did not recover. *P < 0.05 versus control mice, according to the Mann-Whitney U test (n = 4/group).
Figure 4.
C/EBPα influences the expression of antiproteases during repair. “Peptidase inhibitor activity” was one of the significantly enriched functionalities of the mRNAs induced at 72 hours in the control mice, but not in the CebpαΔ/Δ mice, as assessed by mRNA microarray. Quantitative RT-PCR was performed on the mRNAs from the terminal bronchiolar epithelial cells collected by LCM. (A) The serine protease inhibitor (Spink5, Serpina3n, and Slpi) and cysteine protease inhibitor (Cstb) mRNAs were significantly increased in control mice compared with CebpαΔ/Δ mice 72 hours after injury. Spink5 mRNA was most significantly influenced by the deletion of Cebpα. Note that the scale of the vertical axis for Spink5 is different from that of the others. (B) Protease, Dpep2, and Lgmn of Cebpα at 72 hours after injury. Mean value of control mice at 0 hour = 1 (n = 3/group). *P < 0.05 versus control mice, according to the Mann-Whitney U test.
Cell Recovery after Naphthalene-Induced Injury
To investigate the role of C/EBPα in the regeneration of terminal bronchiolar epithelial cells after injury, 9-week-old mice received an intraperitoneal injection of naphthalene (23, 24). No gender-related differences were evident regarding sensitivity to naphthalene in the CebpαΔ/Δ mice. Therefore, both male and female mice were used in this study. The expression of Cebpa in terminal bronchiolar epithelial cells isolated by LCM was evaluated using an mRNA microarray (see Figure E1A in the online supplement) and RT-PCR (Figure E1B). The amount of Cebpa mRNA was more than threefold higher in control mice compared with CebpαΔ/Δ mice, both before and after naphthalene injection. The Cebpa mRNA in CebpαΔ/Δ mice showed no further alterations attributable to naphthalene injury. Destruction of the terminal bronchiolar epithelial cells peaked at 48 hours after the naphthalene injection, and the terminal bronchiolar epithelial cells could not be isolated by LCM for mRNA analyses. The acute recovery of C/EBPα-positive cells in the terminal bronchioles of control mice after naphthalene injury was performed using immunohistochemistry (Figure E1C).
The nonciliated (Scgb1a1+) and ciliated (FoxJ1+) cells in the 200-μm terminal bronchioles were evaluated by immunohistochemistry after naphthalene injury (Figure 2A). A bronchoalveolar duct junction is included at the right end in all of the microphotographs in this study. The numbers of Scgb1a1+ and FoxJ1+ cells were counted using MetaMorph imaging software (Universal Imaging, West Chester, PA) (23) (Figure 2B). The Scgb1a1+ cells were similarly depleted from both the control and CebpαΔ/Δ mice on Day 2 after naphthalene injury. During the recovery period (3–185 d), the number of Scgb1a1+ cells increased in CebpαΔ/Δ mice faster than in control mice. Likewise, cell proliferation, as evaluated according to the mitotic index at the peak of proliferation (3 days after naphthalene treatment), was higher in the CebpαΔ/Δ mice than in control mice (control mice, 20.4% ± 0.4%; CebpαΔ/Δ mice, 32.5% ± 1.4%; P < 0.01; n = 3/group). Scgb1a1+ cells are critical airway progenitor cells and proliferate after injury, whereas FoxJ1+ cells do not proliferate (29). The terminal bronchiolar Scgb1a1+ cell proliferation was increased by the deletion of Cebpa, a finding consistent with previous studies demonstrating that C/EBPα inhibited cell proliferation in adipocytes (30). The FoxJ1+ cells were decreased on Day 2, reflecting squamous metaplasia in both the control and CebpαΔ/Δ mice (Figure 2B). In control mice, FoxJ1+ cells were detectable from Day 3, reflecting the restoration of the cuboidal epithelium. The deletion of C/EBPα impaired FoxJ1+ cell recovery after injury, and the number of FoxJ1+ cells in CebpαΔ/Δ mice was reduced by 50% compared with control mice throughout the recovery period.
Ciliated cells become squamous within hours after naphthalene injury, and spread beneath injured Clara cells to maintain the integrity of the bronchiolar epithelium (12). To better assess the transformation of FoxJ1+ cells after naphthalene injury, mice expressing a FoxJ1–enhanced green fluorescent protein (eGFP) tag (FoxJ1–eGFP mice; Jackson Laboratory, Bar Harbor, ME) were injected with naphthalene. The ciliated cells were identified by FoxJ1 immunohistochemistry before injury (Figure E1D). Two days after injury, the ciliated cells were difficult to detect by immunohistochemistry, but were readily visualized by eGFP, suggesting a lower sensitivity of the immunohistochemistry. The decreased staining for FoxJ1+ cells in CebpαΔ/Δ mice after injury likely reflects a delayed recovery from squamous to cuboidal cells, and an altered differentiation from Scgb1a1+ cells to FoxJ1+ cells.
Rosa–CebpαΔ/Δ Mice
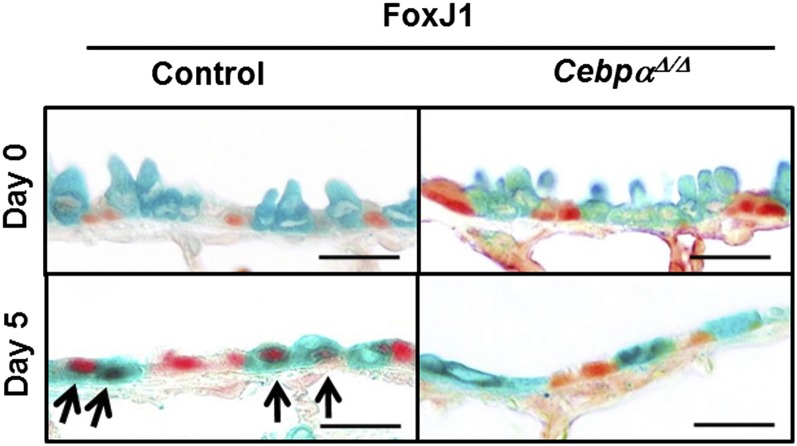
To assess cell lineage during repair, Clara cells were labeled using the conditional expression of Cre-recombinase under control of the Scgb1a1 promoter to recombine the Rosa26 floxed locus, and were then treated with naphthalene (Figure 3). The mice (at 5 weeks of age) were treated with doxycycline for 3 weeks. FoxJ1/LacZ double-positive cells were not detected before injury (Figure 3, Day 0), but were detected in the Rosa–control mice on Day 5 after naphthalene treatment, which is consistent with the differentiation of naphthalene-resistant (labeled) progenitor cells into ciliated cells after injury. In contrast, the FoxJ1/LacZ double-positive cells were markedly decreased in the Rosa–CebpαΔ/Δ mice, indicating a decreased differentiation to ciliated cells (Figure 3).
Figure 3.
Lineage tracing by the colocalization of FoxJ1 (red) and LacZ (green) in the Rosa–CebpαΔ/Δ mice on Day 5 after naphthalene injury. In the Rosa–control mice terminal bronchioles, FoxJ1/LacZ double-positive cells were detected 5 days after naphthalene injury (arrows). Double-positive cells were rarely seen in the Rosa–CebpαΔ/Δ mice 5 days after naphthalene injury, demonstrating decreased cell differentiation of the progenitor cells to ciliated cells. Scale bars, 20 μm (n = 3/group).
Microarray Analyses
Terminal bronchiolar epithelial cells isolated by LCM were used for the mRNA microarray. The complete dataset has been submitted to the Gene Expression Omnibus (GEO Accession Number GSE29285). Using the 0-hour control samples as a common reference, mRNAs that were influenced by the deletion of Cebpa in terminal bronchiolar epithelial cells were identified. The heat map represents the average intensity of the differentially expressed genes (n = 3 mice/group) after 0, 3, and 72 hours of naphthalene treatment relative to the mean of the 0-hour control mice (P ≤ 0.01, and fold change ≥ 1.5; Figure E2A). The heat map of the differentially expressed genes at 72 hours after naphthalene injury in each CebpαΔ/Δ mouse relative to the mean of the 72-hour control mice is shown in Figure E2B, demonstrating the consistency of the microarray analyses among the three mice in each group. The total number of mRNAs that were changed in comparison with the control group at 0 hours is presented in Figure E2C. A large number of mRNAs were induced 72 hours after naphthalene treatment in the control mice, although the same genes were unchanged in the CebpαΔ/Δ mice. Gene ontology analysis of the mRNAs demonstrated that the most abundantly induced genes in the control mice at 72 hours were related to cell death (23%), cellular growth and proliferation (20%), and the cell cycle (13%). The most enriched biological processes at 72 hours in the control mice were “cell cycle, M phase” (P = 1.34E-16), “microtubule-based process” (P = 3.02E-06), and “angiogenesis” (P = 9.66E-04). The most enriched molecular functions were “microtubule motor activity” (P = 4.07E-04) and “peptidase inhibitor activity” (P = 1.17E-03) (Figure E2D). Neither “angiogenesis” nor “peptidase inhibitor activity” was induced in CebpαΔ/Δ mice at 72 hours.
Antiprotease Genes
The mRNAs in the “peptidase inhibitor activity” cluster were enriched with antiproteases, including Spink5, Serpina3n, Serpina7, Timp1, and Slpi, and the majority of the corresponding genes contain C/EBPα binding sites (Table 1). The dynamic expression patterns of mRNAs encoding antiproteases and proteases were confirmed by quantitative RT-PCR (Figure 4) of the terminal bronchiolar epithelial cells collected by LCM. Among these, Spink5 (serine peptidase inhibitor) was the most significantly altered gene in the control mice. Spink5 was induced (9.5-fold increase in the microarray, and 27-fold increase in RT-PCR) at 72 hours after naphthalene injury in the control mice. In contrast, Spink5 expression was unchanged across all time points in CebpαΔ/Δ mice (Figure 4A). The Spink5 gene encodes the lymphoepithelial Kazal-type–related protease inhibitor (LEKTI) that inhibits kallikrein, trypsin, plasmin, subtilism A, cathepsin G, and elastase in vitro (20). Spink5 expression was significantly decreased by the deletion of Cebpa (Figure 4A, Spink5 at 0 hours). However, in the absence of stress, the Spink5 deficiency was not associated with any pathological changes. Conserved C/EBPα binding sites are present in the Spink5 gene promoter (∼ 400 base pairs upstream of the transcription start site) in the human, mouse, rat, monkey, and chicken genomes, suggesting that Spink5 may be a direct transcriptional target of Cebpα.
TABLE 1.
ANTIPROTEASE GENES WERE UP-REGULATED AT 72 HOURS AFTER NAPHTHALENE INJURY IN CONTROL MICE BUT NOT IN CEBPαΔ/Δ MICE
| Control Mice at 72 h/Control at 0 h |
CebpαΔ/Δ Mice at 72 h/Control Mice at 0 h |
|||||
| AffyID | Gene | Ratio | P Value | Ratio | P Value | Cebpα Binding Site* |
| 10587383 | Cd109 | 2.3 | 1.50E-02 | ns | 3 | |
| 10356520 | Col6a3 | 1.9 | 5.72E-04 | ns | 2 | |
| 10476915 | Cst8 | 1.6 | 1.69E-02 | −1.3 | 0.04 | 1 |
| 10533085 | Pebp1 | 1.7 | 2.44E-02 | ns | ||
| 10379727 | Gm11428 | 3.9 | 1.99E-03 | ns | ||
| 10489463 | Slpi | 2.0 | 8.19E-03 | ns | 3 | |
| 10606928 | Serpina7 | 1.9 | 2.65E-02 | ns | 2 | |
| 10398075 | Serpina3n | 3.6 | 3.21E-03 | ns | 2 | |
| 10408600 | Serpinb6a | 1.6 | 7.17E-03 | ns | 4 | |
| 10455395 | Spink5 | 9.5 | 1.82E-04 | ns | 3 | |
| 10598976 | Timp1 | 3.4 | 4.94E-03 | ns | 1 | |
| 10455647 | Tnfaip8 | 1.5 | 1.41E-02 | 1.2 | 0.02 | 2 |
Definition of abbreviations: C/EBPα, CCAAT/enhancer binding protein–α; ns, not significant.
Cebpa binding sites were searched in a 600–base-pair promoter region of the genes induced at 72 hours after naphthalene injury in the control mice but not in the CebpαΔ/Δ mice, as determined using Matlnspector (Gienomatrix Software GmbH, Munich, Germany).
Protease Activity
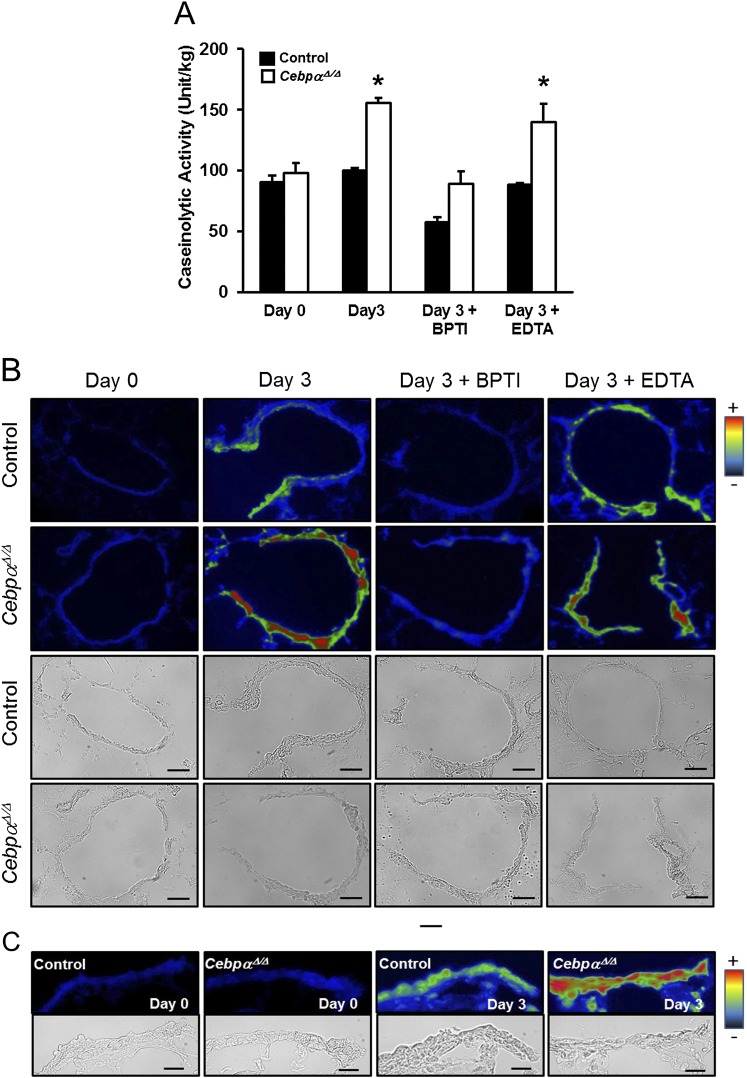
Increased protease activity after injury has been shown to play a role in the pathogenic processes leading to airway tissue remodeling (31). The caseinolytic protease activity was comparably low in both the control and CebpαΔ/Δ murine lungs before the naphthalene injection (Figure 5A). Although caseinolytic activity remained unchanged in control mice, it increased by 60% in CebpαΔ/Δ murine lungs 3 days after naphthalene injury. Kallikrein activity was assessed in the terminal bronchiolar epithelial region by in situ zymography (Figures 5B and 5C). Three days after the naphthalene-induced injury, kallikrein activity was increased in the CebpαΔ/Δ mice (Figures 5B and 5C, green to red), but changes in the control mice were minimal (Figures 5B and 5C, green). The cellular sites of increased kallikrein activity in the terminal bronchiolar epithelial cells were identified using higher-magnification microphotographs (Figure 5C).
Figure 5.
Increased protease activity in CebpαΔ/Δ mice after injury. (A) Caseinolytic protease activity was assessed on the supernatants of the lung homogenates harvested 3 days after naphthalene injury. Protease activity was significantly increased in the CebpαΔ/Δ mice 3 days after naphthalene injury. The addition of bovine pancreatic trypsin inhibitor (BPTI) decreased the protease activity in the lungs of CebpαΔ/Δ mice to low levels. EDTA, an inhibitor of matrix metalloproteinases (MMPs), did not influence the increased protease activity in CebpαΔ/Δ mice. *P < 0.01, versus Day 0 CebpαΔ/Δ murine lungs. In situ zymography for kallikrein activity is shown in the terminal bronchioles of the control and CebpαΔ/Δ mice. (B and C) The lower columns represent bright-field images. The color gradient from black to red represents kallikrein activity. On Day 0, kallikrein activity was comparably low (blue) in the terminal bronchiolar epithelial region of the control and CebpαΔ/Δ mice. In contrast to control mice, kallikrein activity was increased in CebpαΔ/Δ mice on Day 3 after injury (green to red) in the terminal bronchiolar epithelium, and the addition of BPTI (Day 3 + BPTI) decreased the kallikrein activity. The addition of EDTA did not influence kallikrein activity (Day 3 + EDTA). Scale bars, 50 μm. (C) Microphotographs of terminal bronchioles at higher magnifications demonstrate the sites of protease activity in the terminal bronchiolar epithelial cells at 0 and 3 days after naphthalene injury. For all microphotographs, the bronchoalveolar duct junctions are at the right end. Scale bars, 20 μm.
Degradation of Fibronectin by Protease
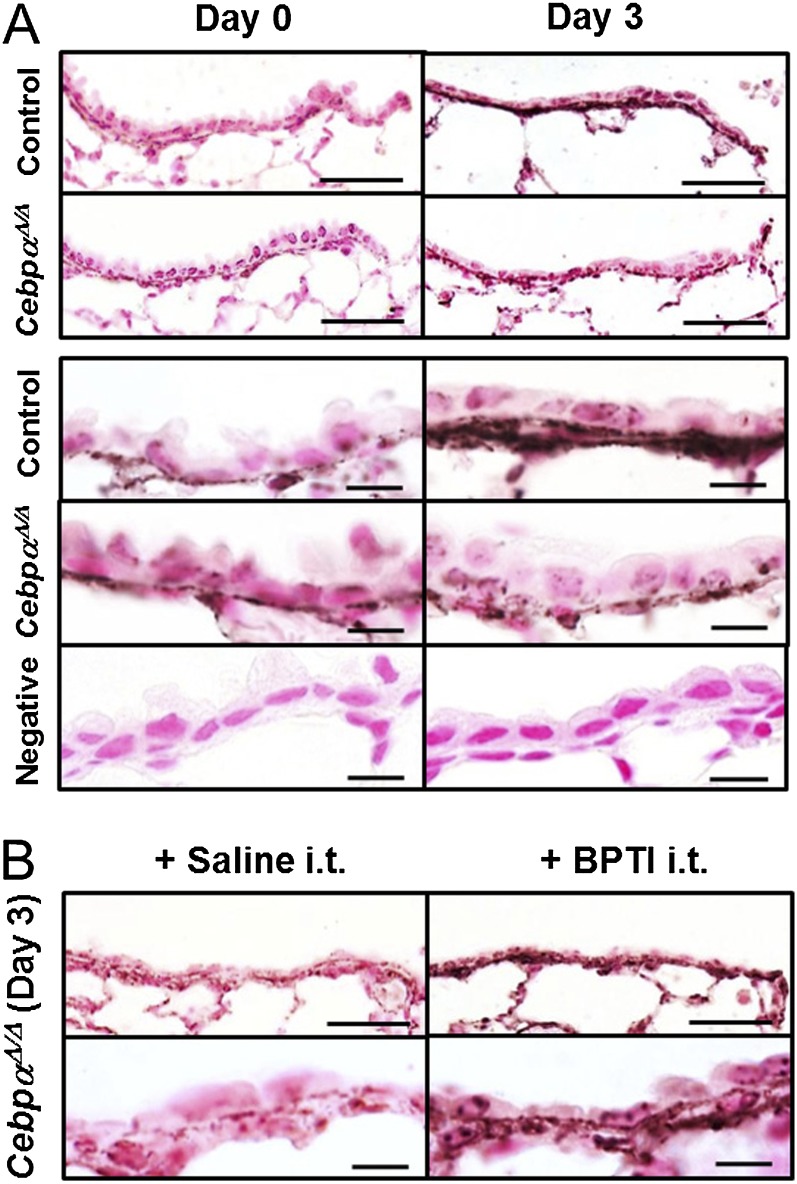
Degradation of the extracellular matrix (ECM), including fibronectin, as caused by increased protease activity, is a significant component of chronic pulmonary disease (31). Fibronectin is known to increase after injury, including naphthalene injury, and is associated with the release of cellular growth factors and wound healing (32, 33). Increased concentrations of fibronectin were detected by immunohistochemistry 3 days after naphthalene injury in control mice (Figure 6A). In contrast, no increase in fibronectin was evident in the CebpαΔ/Δ mice after injury. These data support the concept that the increased protease activity in CebpαΔ/Δ mice enhanced the degradation of fibronectin during the repair process.
Figure 6.
C/EBPα is required for fibronectin deposition during terminal bronchiolar repair. (A) On Day 3 after naphthalene injury, fibronectin deposition was detected in the terminal bronchioles of the control mice but not the CebpαΔ/Δ mice. Scale bars, 50 μm; for the higher magnifications in the lower columns, 10 μm. The negative control was a murine lung with an isotype-specific control antibody. (B) Intratracheal (i.t.) treatment with BPTI 2 days after naphthalene injury prevented the degradation of fibronectin deposition in CebpαΔ/Δ mice. Bronchoalveolar duct junctions are at the right end (n = 3/group).
Ex Vivo and In Vivo Treatment of BPTI
BPTI is a serine protease inhibitor with activity similar to that of LEKTI (34). The associations between increased protease activity in CebpαΔ/Δ mice and the delayed recovery of terminal bronchiole epithelial cells after injury was studied ex vivo and in vivo using BPTI. The addition of BPTI to lung homogenates from CebpαΔ/Δ mice decreased caseinolytic activity on Day 3 after naphthalene treatment (Figure 5A, Day 3 + BPTI). In contrast, the inhibition of matrix metalloproteinases (MMPs) by the addition of EDTA did not alter protease activity (Figure 5A, Day 3 + EDTA). These results suggest that the increased protease activity in the injured bronchioles of CebpαΔ/Δ mice was primarily related to serine proteases rather than MMPs. Kallikrein activity was evaluated on cryosections of lung tissue prepared 3 days after naphthalene treatment and incubated in the presence or absence of BPTI for 1 hour. The BPTI markedly decreased the kallikrein activity (blue) in the terminal bronchioles of CebpαΔ/Δ mice (Figure 5B, Day 3 + BPTI).
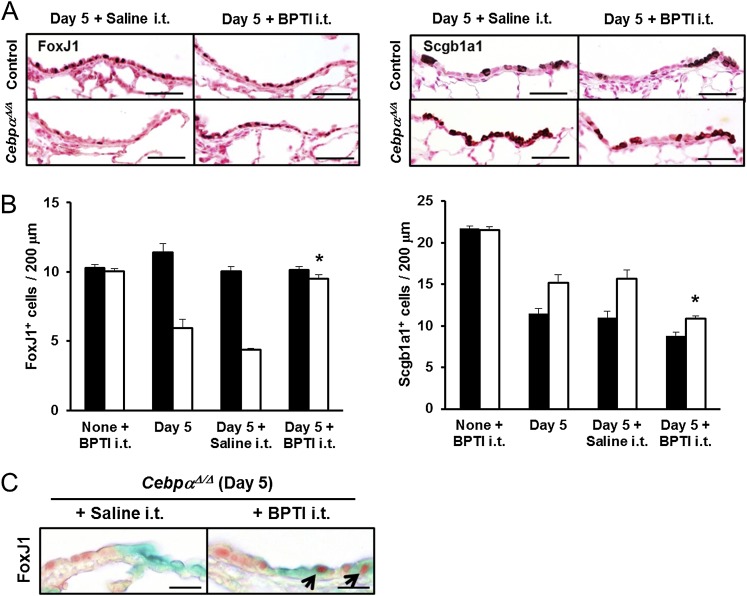
To demonstrate the efficacy of antiserine protease treatment during the repair of terminal bronchiolar epithelial cells, BPTI was intratracheally administered to the CebpαΔ/Δ and control mice daily, beginning 2 days after naphthalene injection. Fibronectin deposition in the CebpαΔ/Δ mice was restored by an intratracheal instillation of BPTI, resulting in increased fibronectin staining by Day 3 after naphthalene injection (Figure 6B). BPTI also increased the number of ciliated cells (FoxJ1+) in the terminal bronchiolar epithelium on Day 5 after naphthalene injury (Figures 7A and 7B). In the CebpαΔ/Δ mice, the total number of terminal bronchiolar epithelial cells was similar both before and after BPTI treatment (data not shown). However, the number of Scgb1a1+ cells was decreased by BPTI treatment (P < 0.05; Figures 7A and 7B), whereas the number of FoxJ1+ cells increased.
Figure 7.
Intratracheal treatment with BPTI restored FoxJ1+ cells in vivo. (A) Immunohistochemistry of FoxJ1 and Scgb1a1 in the terminal bronchioles is shown on Day 5 after naphthalene injury, with or without daily BPTI treatment. FoxJ1+ cells were increased after BPTI treatment in the CebpαΔ/Δ mice to a level similar to that in control mice. Scale bars, 50 μm. (B) The numbers of stained FoxJ1 and Scgb1a1 cells lining 200 μm of the terminal bronchiolar epithelium from the bronchoalveolar duct junctions were quantitated. The number of FoxJ1+ cells in the terminal bronchioles of the CebpαΔ/Δ mice was restored by BPTI treatment. The intratracheal injection of BPTI into noninjured mice (None + BPTI i.t.) did not influence the number of FoxJ1+ and Scgb1a1+ cells. In CebpαΔ/Δ mice, the proportion of Scgb1a1-stained cells decreased after an intratracheal injection of BPTI, consistent with the increase in FoxJ1+-stained cells. *P < 0.05 versus Day 5 + Saline, according to ANOVA (n = 4/group). (C) Intratracheal treatment with BPTI increased the double-positive (FoxJ1, red; Lac Z, green) cells in the terminal bronchioles of Rosa–CebpαΔ/Δ mice, supporting the concept that BPTI restored the regeneration of ciliated cells in Rosa–CebpαΔ/Δ mice. Scale bars, 20 μm (n = 3/group).
Intratracheal BPTI injection also restored FoxJ1/LacZ reactive cells in the Rosa–CebpαΔ/Δ mice on Day 5 after naphthalene injury (Figure 7C). Of the total FoxJ1+ cells, the percentage of FoxJ1/LacZ double-positive cells was 55.2% ± 5.7% in the Rosa–control mice, 12.0% ± 1.7% in the Rosa–CebpαΔ/Δ mice, and 40.9% ± 9.6% in the Rosa–CebpαΔ/Δ mice treated with BPTI. Overall, the deletion of Cebpa in the respiratory epithelium resulted in increased serine protease activity after injury, which in turn inhibited the normal increase of fibronectin and the regeneration of normal ciliated cuboidal epithelium.
Discussion
Many of the concepts regarding lung-cell differentiation and proliferation after injury are derived from developmental studies. The present study demonstrated that C/EBPα, a transcription factor critical for perinatal lung maturation, regulates a large number of genes required for the repair of bronchiolar epithelium after injury. We included mRNAs related to cell death, cellular proliferation, and the cell cycle. Among all of the mRNAs identified, the expression of the serine peptidase inhibitor Spink5 was altered to the greatest extent in the CebpαΔ/Δ mice compared with control mice. The present study demonstrated that Cebpa regulates the protease/antiprotease balance after airway injury, thereby enhancing the recovery of ciliated cells lining the terminal bronchiole (summarized in Figure E3).
The lung-specific postnatal deletion of Cebpa did not alter lung morphology and function under unstressed conditions. In the present study, we used a well-established model of naphthalene injection to induce airway epithelial cell injury. Naphthalene is converted to a toxic epoxide in Scgb1a1+ cells, destroying the naphthalene-sensitive Scgb1a+ cells and leaving progenitor cells (naphthalene-resistant Scgb1a1+ cells) (29, 35) and ciliated cells that undergo squamous metaplasia. The deletion of C/EBPα in murine lungs impaired the recovery of the ciliated cells after naphthalene injury. Microarray and RT-PCR analyses of the terminal bronchiolar epithelial cell RNA demonstrated that protease genes were up-regulated after naphthalene injury in both the control and CebpαΔ/Δ mice. In contrast, the postinjury induction of a number of antiprotease genes, particularly serine protease inhibitors, was significantly increased only in control mice. The induction of serine protease inhibitors was blocked and protease activity was increased in the bronchioles of CebpαΔ/Δ mice, resulting in decreased fibronectin deposition and impaired recovery of ciliated cells. The intratracheal injection of the serine protease inhibitor BPTI increased fibronectin and induced ciliated cell regeneration in the naphthalene-injured CebpαΔ/Δ lung.
The ECM is composed of basement membrane collagens, interstitial collagens, various proteoglycans, and fibronectin. In addition to providing support and anchorage for cells, segregating tissues from one another, and regulating intercellular communications, the ECM also acts as a local depot for a wide range of cellular growth factors (36). The ECM, particularly fibronectin (32), is markedly increased after tissue injury, and cellular growth factors are released from the ECM. This allows the rapid and local growth factor–mediated activation of cell regeneration without de novo synthesis (37). The serine protease kallikrein is known to cleave fibronectin-binding sites on cell surfaces, inhibiting cell adhesion in vitro (38, 39). Thus, the formation of fibronectin is essential for wound healing (35). The critical role of the ECM in the repair response to naphthalene injury was shown previously (33). The present study demonstrated that C/EBPα maintains a protease/antiprotease balance in the injured lung, thereby promoting fibronectin deposition and the re-epithelization of airways.
Under normal conditions, the turnover of cells in the lung is quite slow and requires several months (7, 11, 40). Because of their permanent contact with the external environment, airway epithelial cells are at risk of injury and are able to respond through rapid cell proliferation and differentiation during repair (10, 12). Although the present study was limited to terminal bronchiolar cells and the study period was limited to 185 days after injury, the delayed and incomplete recovery of these cells after injury may contribute to the pathogenesis of chronic pulmonary diseases, including chronic obstructive pulmonary disease, asthma, chronic bronchiolitis, and bronchopulmonary dysplasia. The protease/antiprotease imbalance is central to the pathogenic process in these diseases. Most of the studies investigating the association between the protease/antiprotease imbalance and chronic lung disease have focused on alveolar injury, in which alveolar macrophages and neutrophils comprise the main sources of proteases, including MMP-12, MMP-8, and cathepsins S and L (31, 41). The ability of MMP inhibitors to restore lung architecture after alveolar injury has been experimentally demonstrated (42). In contrast, injury to the bronchiolar epithelium, caused by naphthalene or sulfur mustard, was amenable to treatment with serine protease inhibitors. Treatment with an MMP inhibitor or antioxidant was not effective in the repair of bronchiolar injury caused by sulfur mustard (43), or in the repair of naphthalene-injured lungs in CebpαΔ/Δ mice. MMPs are induced by alveolar injury, whereas bronchiolar injury is associated with increased serine proteases.
The Notch signaling pathway plays a critical role in the differentiation of bronchiolar epithelial cells in the developing lung (44), and regulates cell regeneration after injury in the adult lung (45, 46). However, the deletion of Cebpα did not alter the Notch signaling pathway. The expressions of the genes in the Notch signaling pathway, including Jag1, Jag2, Dll1, Notch1, Notch2, Notch3, Notch4, Hes1, Hes2, Hes5, Hey1, and Hey2, were similar in the control and CebpαΔ/Δ mice at 0, 3, and 72 hours after injury (Figure E4). Thus, the altered ciliated cell regeneration in CebpαΔ/Δ mice was unlikely attributable to impaired Notch signaling.
Of the eight Clara-cell maturation markers evaluated, four mRNAs (Cldn10, Aox3, Fmo3, and Pon1) (47) were down-regulated (≥ 1.5-fold) in the terminal bronchiolar epithelial cells of CebpαΔ/Δ mice compared with control mice at 0 hours, suggesting that the deletion of Cebpa may influence Clara-cell maturation (Table 2). However, as shown in Figure 2B, the recovery of Clara cells after naphthalene injury was not delayed in CebpαΔ/Δ mice.
TABLE 2.
CLARA-CELL MATURATION MARKERS WITH ≥ 1.5-FOLD CHANGE OF mRNA EXPRESSION
| Gene | CebpαΔ/Δ Mice at 0 h vs. Control Mice at 0 h |
| Cyp2f2 | |
| Cldn10 | −1.6 |
| Ces1 | |
| Gabrp | |
| Aox3 | −2.0 |
| Fmo3 | −1.9 |
| Scgb3a2 | |
| Pon1 | −3.8 |
Previous studies on the role of C/EBPα in the regulation of Scgb1a1+ expression were controversial. In vitro studies demonstrated a requirement for C/EBPα binding to the Scgb1a1 promoter for gene expression in cell lines and isolated Scgb1a1+ cells (28, 48). In contrast, a recent in vivo study (49) demonstrated that interference of the promoter-binding ability of C/EBP did not alter Scgb1a1 expression in the lung. In the present study, the expression of Scgb1a1 in the lung was not influenced by the long-term (18-mo) deletion of C/EBPα, consistent with the hypothesis that C/EBPα does not regulate Scgb1a1 expression in vivo.
C/EBPα activity may decrease with aging (30) and in diseases of the lung, including asthma (50). The present study demonstrated that C/EBPα plays a role in increasing the expression of serine protease inhibitors and maintaining the protease/antiprotease balance after terminal bronchiolar epithelial cell injury. Treatment with serine protease inhibitors provides a potential therapeutic strategy to accelerate the recovery from bronchiolar epithelium injury and prevent airway diseases.
Supplementary Material
Acknowledgments
The authors thank Shawn Grant, Angelica Schehr, and Michael Minnick for excellent technical support, and Dr. Timothy Weaver for editorial support.
Footnotes
This work was supported by National Institutes of Health grant HL095464 (M.I.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0239OC on May 31, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653 [DOI] [PubMed] [Google Scholar]
- 2.Tulic MK, Hamid Q. New insights into the pathophysiology of the small airways in asthma. Clin Chest Med 2006;27:41–52 (vi.) [DOI] [PubMed] [Google Scholar]
- 3.Allen TC. Pathology of small airways disease. Arch Pathol Lab Med 2010;134:702–718 [DOI] [PubMed] [Google Scholar]
- 4.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 2001;164:S28–S38 [DOI] [PubMed] [Google Scholar]
- 5.Bartling TR, Drumm ML. Oxidative stress causes IL8 promoter hyperacetylation in cystic fibrosis airway cell models. Am J Respir Cell Mol Biol 2009;40:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MK, Pryhuber GS, Schwarz MA, Smith SM, Pavlova Z, Sunday ME. Developmental regulation of p66Shc is altered by bronchopulmonary dysplasia in baboons and humans. Am J Respir Crit Care Med 2005;171:1384–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009;4:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stripp BR, Reynolds SD, Plopper CG, Boe IM, Lund J. Pulmonary phenotype of CCSP/UG deficient mice: a consequence of CCSP deficiency or altered Clara cell function? Ann N Y Acad Sci 2000;923:202–209 [DOI] [PubMed] [Google Scholar]
- 9.Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest 1978;38:648–653 [PubMed] [Google Scholar]
- 10.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA 2009;106:9286–9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol 2008;295:L231–L234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzaki Y, Xu Y, Ikegami M, Besnard V, Park KS, Hull WM, Wert SE, Whitsett JA. STAT3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. J Immunol 2006;177:527–537 [DOI] [PubMed] [Google Scholar]
- 13.Whitsett JA, Matsuzaki Y. Transcriptional regulation of perinatal lung maturation. Pediatr Clin North Am 2006;53:873–887 [DOI] [PubMed] [Google Scholar]
- 14.Martis PC, Whitsett JA, Xu Y, Perl AK, Wan H, Ikegami M. C/EBP{alpha} is required for lung maturation at birth. Development 2006;133:1155–1164 [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Saegusa C, Schehr A, Grant S, Whitsett JA, Ikegami M. C/EBP{alpha} is required for pulmonary cytoprotection during hyperoxia. Am J Physiol Lung Cell Mol Physiol 2009;297:L286–L298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez RG, Garcia-Silva S, Moore SJ, Bereshchenko O, Martinez-Cruz AB, Ermakova O, Kurz E, Paramio JM, Nerlov C. C/EBPalpha and beta couple interfollicular keratinocyte proliferation arrest to commitment and terminal differentiation. Nat Cell Biol 2009;11:1181–1190 [DOI] [PubMed] [Google Scholar]
- 17.Timchenko NA, Wilde M, Kosai KI, Heydari A, Bilyeu TA, Finegold MJ, Mohamedali K, Richardson A, Darlington GJ. Regenerating livers of old rats contain high levels of C/EBPalpha that correlate with altered expression of cell cycle associated proteins. Nucleic Acids Res 1998;26:3293–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res 2002;11:21–29 [DOI] [PubMed] [Google Scholar]
- 19.Betsuyaku T, Senior RM. Laser capture microdissection and mRNA characterization of mouse airway epithelium: methodological considerations. Micron 2004;35:229–234 [DOI] [PubMed] [Google Scholar]
- 20.Deraison C, Bonnart C, Lopez F, Besson C, Robinson R, Jayakumar A, Wagberg F, Brattsand M, Hachem JP, Leonardsson G, et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol Biol Cell 2007;18:3607–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci 2009;123:213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkinson JJ, Adair-Kirk TL, Kelley DG, Demello D, Senior RM. Clara cell adhesion and migration to extracellular matrix. Respir Res 2008;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park K-S, Ikegami M, Muller W, Whitsett JA. GP130–STAT3 regulates epithelial cell migration required for repair of the bronchiolar epithelium. Am J Pathol 2008;172:1542–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plopper CG, Macklin J, Nishio SJ, Hyde DM, Buckpitt AR. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity: III. Morphometric comparison of changes in the epithelial populations of terminal bronchioles and lobar bronchi in mice, hamsters, and rats after parenteral administration of naphthalene. Lab Invest 1992;67:553–565 [PubMed] [Google Scholar]
- 25.Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, Li B, Donnelly EF, Holburn GE, Lewis KG, et al. Increased and prolonged pulmonary fibrosis in surfactant protein C–deficient mice following intratracheal bleomycin. Am J Pathol 2005;167:1267–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lubbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA 1996;93:10933–10938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L, Lim L, Costa RH, Whitsett JA. Thyroid transcription factor–1, hepatocyte nuclear factor–3beta, surfactant protein B, C, and Clara cell secretory protein in developing mouse lung. J Histochem Cytochem 1996;44:1183–1193 [DOI] [PubMed] [Google Scholar]
- 28.Nord M, Cassel TN, Braun H, Suske G. Regulation of the Clara cell secretory protein/uteroglobin promoter in lung. Ann N Y Acad Sci 2000;923:154–165 [DOI] [PubMed] [Google Scholar]
- 29.Perl AK, Riethmacher D, Whitsett JA. Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis. Am J Respir Crit Care Med 2011;183:511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang GL, Shi X, Salisbury E, Sun Y, Albrecht JH, Smith RG, Timchenko NA. Cyclin D3 maintains growth-inhibitory activity of C/EBPalpha by stabilizing C/EBPalpha-CDK2 and C/EBPalpha-BRM complexes. Mol Cell Biol 2006;26:2570–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc 2008;5:475–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limper AH, Roman J. Fibronectin: a versatile matrix protein with roles in thoracic development, repair and infection. Chest 1992;101:1663–1673 [DOI] [PubMed] [Google Scholar]
- 33.Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. Am J Respir Cell Mol Biol 2009;40:633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goettig P, Magdolen V, Brandstetter H. Natural and synthetic inhibitors of kallikrein-related peptidases (KLKs). Biochimie 2010;92:1546–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 2010;298:L715–L731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buczek-Thomas JA, Lucey EC, Stone PJ, Chu CL, Rich CB, Carreras I, Goldstein RH, Foster JA, Nugent MA. Elastase mediates the release of growth factors from lung in vivo. Am J Respir Cell Mol Biol 2004;31:344–350 [DOI] [PubMed] [Google Scholar]
- 37.Uebersax L, Merkle HP, Meinel L. Biopolymer-based growth factor delivery for tissue repair: from natural concepts to engineered systems. Tissue Eng Part B Rev 2009;15:263–289 [DOI] [PubMed] [Google Scholar]
- 38.Asakura S, Hurley RW, Skorstengaard K, Ohkubo I, Mosher DF. Inhibition of cell adhesion by high molecular weight kininogen. J Cell Biol 1992;116:465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramani VC, Haun RS. The extracellular matrix protein fibronectin is a substrate for kallikrein 7. Biochem Biophys Res Commun 2008;369:1169–1173 [DOI] [PubMed] [Google Scholar]
- 40.Blenkinsopp WK. Proliferation of respiratory tract epithelium in the rat. Exp Cell Res 1967;46:144–154 [DOI] [PubMed] [Google Scholar]
- 41.Abboud RT, Vimalanathan S. Pathogenesis of COPD: part I. The role of protease–antiprotease imbalance in emphysema. Int J Tuberc Lung Dis 2008;12:361–367 [PubMed] [Google Scholar]
- 42.Srivastava PK, Dastidar SG, Ray A. Chronic obstructive pulmonary disease: role of matrix metalloproteases and future challenges of drug therapy. Expert Opin Investig Drugs 2007;16:1069–1078 [DOI] [PubMed] [Google Scholar]
- 43.Anderson DR, Taylor SL, Fetterer DP, Holmes WW. Evaluation of protease inhibitors and an antioxidant for treatment of sulfur mustard–induced toxic lung injury. Toxicology 2009;263:41–46 [DOI] [PubMed] [Google Scholar]
- 44.Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 2009;136:2297–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi W, Chen F, Cardoso WV. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009;6:558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 2011;8:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zemke AC, Snyder JC, Brockway BL, Drake JA, Reynolds SD, Kaminski N, Stripp BR. Molecular staging of epithelial maturation using secretory cell–specific genes as markers. Am J Respir Cell Mol Biol 2009;40:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nord M, Lag M, Cassel TN, Randmark M, Becher R, Barnes HJ, Schwarze PE, Gustafsson JA, Lund J. Regulation of CCSP (PCB-BP/uteroglobin) expression in primary cultures of lung cells: involvement of C/EBP. DNA Cell Biol 1998;17:481–492 [DOI] [PubMed] [Google Scholar]
- 49.Kido T, Tomita T, Okamoto M, Cai Y, Matsumoto Y, Vinson C, Maru Y, Kimura S. FoxA1 plays a role in regulating secretoglobin 1a1 expression in the absence of CAATT/enhancer binding protein activities in lung in vivo. Am J Physiol Lung Cell Mol Physiol 2011;300:L441–L452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miglino N, Roth M, Tamm M, Borger P. House dust mite extract downregulates C/EBPalpha in asthmatic bronchial smooth muscle cells. Eur Respir J 2011;38:50–58 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.