Abstract
Antibiotics-induced release of the pore-forming virulence factor pneumolysin (PLY) in patients with pneumococcal pneumonia results in its presence days after lungs are sterile and is a major factor responsible for the induction of permeability edema. Here we sought to identify major mechanisms mediating PLY-induced endothelial dysfunction. We evaluated PLY-induced endothelial hyperpermeability in human lung microvascular endothelial cells (HL-MVECs) and human lung pulmonary artery endothelial cells in vitro and in mice instilled intratracheally with PLY. PLY increases permeability in endothelial monolayers by reducing stable and dynamic microtubule content and modulating VE-cadherin expression. These events, dependent upon an increased calcium influx, are preceded by protein kinase C (PKC)-α activation, perturbation of the RhoA/Rac1 balance, and an increase in myosin light chain phosphorylation. At later time points, PLY treatment increases the expression and activity of arginase in HL-MVECs. Arginase inhibition abrogates and suppresses PLY-induced endothelial barrier dysfunction by restoring NO generation. Consequently, a specific PKC-α inhibitor and the TNF-derived tonoplast intrinsic protein peptide, which blunts PLY-induced PKC-α activation, are able to prevent activation of arginase in HL-MVECs and to reduce PLY-induced endothelial hyperpermeability in mice. Arginase I (AI)+/−/arginase II (AII)−/− C57BL/6 mice, displaying a significantly reduced arginase I expression in the lungs, are significantly less sensitive to PLY-induced capillary leak than their wild-type or AI+/+/AII−/− counterparts, indicating an important role for arginase I in PLY-induced endothelial hyperpermeability. These results identify PKC-α and arginase I as potential upstream and downstream therapeutic targets in PLY-induced pulmonary endothelial dysfunction.
Keywords: PKC, arginase, pneumococcus, pneumolysin, TNF
Clinical Relevance
This research identifies protein kinase C-α and arginase 1 as crucial targets in pneumolysin-induced pulmonary capillary leak. Because the latter represents a major complication of severe pneumonia, mainly in patients treated with antibiotics, these factors should be taken into account when developing novel therapies.
Although severe pneumonia remains the leading cause of mortality worldwide in children aged less than 5 years (1), community-acquired pneumonia represents a major cause of morbidity and mortality mainly in elderly patients (2). Despite the use of potent antibiotics and aggressive intensive care support, the fatality rate associated with Streptococcus pneumoniae, accounting for 45% of all cases of community-acquired pneumonia, is approximately 20% (1, 2). Pulmonary permeability edema, a major complication of severe pneumonia characterized by endothelial hyperpermeability, can occur days after initiation of antibiotics therapy when tissues are sterile and the pneumonia is clearing and correlates with the presence of the bacterial virulence factor pneumolysin (PLY) (3, 4). This cytoplasmic hemolytic protein is released during bacterial lysis, as occurs after treatment with β-lactam antibiotics (5). PLY-induced lung injury was suggested to result from direct pneumotoxic effects on the alveolar–capillary barrier rather than from resident or recruited phagocytic cells (6).
Upon binding of PLY to cholesterol in cell membranes, oligomerization and pore formation occur, causing increased intracellular Ca2+ levels (7), the initial pivotal signal preceding pathways leading to endothelial cell (EC) contraction (8), including myosin light chain (MLC)-dependent mechanisms and microtubule rearrangement. Depolymerization of microtubules can cause disassembly of adherens junction proteins with which they associate, such as VE-cadherin, thus increasing permeability (9). The Ca2+–dependent protein kinase C (PKC) α isoform was suggested to play a critical role in initiating endothelial cell contraction and disassembly of VE-cadherin–mediated cell–cell contacts (8, 10). Recent data suggest that lung structure and function is partly maintained by a balance between the competing l-arginine–metabolizing enzymes arginase, existing in two isoforms (AI and AII), and nitric oxide synthase (11). Arginase I and II have been found in different endothelial cell populations (12–15). Although in the lungs arginase expression and activity is increased during asthma (16), pulmonary hypertension (17) and cystic fibrosis (18), its role in models of bacterial toxin–induced pulmonary endothelial barrier loss remains largely unknown. Because complete knock-out of the arginase I (AI) gene in mice leads to the development of lethal hyperammonemia within 2 weeks of birth, we have used AI+/−/AII−/− transgenic mice (19).
Because no standard therapy is available to treat pulmonary permeability edema, the need for novel substances that can improve pulmonary capillary barrier integrity in patients with pneumonia is important (20). The main aim of this study was to identify crucial mediators of PLY-induced pulmonary endothelial hyperpermeability and to identify therapeutic targets. Our results identify PKC-α as an important upstream and arginase I as an important downstream target in PLY-induced endothelial dysfunction. We demonstrate that PKC-α inhibitors are able to blunt PLY-mediated arginase activation in vitro and can prevent PLY-induced pulmonary endothelial hyperpermeability in mice in vivo. We also observed a potent protective effect of the TNF-derived tonoplast intrinsic protein (TIP) peptide (21–24), which was shown to activate amiloride-sensitive sodium transport in alveolar epithelial and microvascular endothelial cells (24, 25), the latter of which express the α subunit of the epithelial sodium channel ENaC (26, 27). The TIP peptide was previously shown to protect from hyperpermeability induced by the cholesterol-binding pore-forming toxin listeriolysin in human lung microvascular endothelial cell (HL-MVEC) monolayers in an amiloride-sensitive and PKC-α–dependent manner (28).
Materials and Methods
Cells
HL-MVECs and human lung pulmonary artery endothelial cells (HPAECs) (Lonza, Walkersville, MD) were used up to passage 6 and were grown in EBM-2 complete medium (Lonza). Experiments were performed in serum-free medium.
Mice
Male C57BL/6 mice of (6–8 wk of age; 19–21 g) were from Harlan (Indianapolis, IN). Male AI+/−/AII−/− and AI+/+/AII−/− transgenic mice (6–8 wk of age) on a C57BL/6 background were generated as described (19) and were bred at the animal facilities at Georgia Health Sciences University.
Pneumolysin Purification
PLY was purified from a recombinant Listeria innocua 6a strain expressing PLY. The batch of PLY used in this study had a specific activity of 1.25 × 107 hemolytic units/mg.
Quantitative Analysis of Microtubule Network
Quantitative analysis of microtubules near the cell margin was done as described previously (29) using ImageJ software linked to an Excel spreadsheet (Microsoft Corp., Redmond, WA). Analysis included measurement of peripheral microtubule density in an area circumjacent to the cell periphery that was 5 μm from the cell margin by assessing their fluorescence intensity in digital images collected with a digital CCD camera. Original images were processed using ImageJ software (Gaussian filtration and background subtraction). Statistical analysis was performed using Sigma Plot 7.1 (SPSS Science, Point Richmond, CA) and Excel. Sigma Plot 7.1 software was used for graphical data presentation.
Measurement of NO Release
HL-MVEC were seeded at 105 cells per well in 24-well plates. All stimulations were performed with confluent quiescent cell monolayers in serum-free medium. Cells were treated with PLY (30 ng/ml) for 2 hours. Reactions were terminated by removal of the supernatant. Fresh serum-free medium was replaced, and cells were incubated for an additional 30 minutes. The supernatant was then removed, centrifuged, and stored at −80°C for NO analysis. The PKC-α inhibitor Ro32–40–32 (10 nM) or the arginase inhibitor (S)-(2-boronoethyl)-l-cysteine (BEC) (100 μM) were applied 30 minutes before PLY. Cell supernatants containing nitrite (NO2−), the stable breakdown product of NO in aqueous medium, were refluxed in glacial acetic acid containing sodium iodide. NO2− is quantitatively reduced to NO under these conditions, which can be quantified by a chemiluminescence detector in a NO analyzer (Sievers, Boulder, CO), as described (12).
Intratracheal Instillation and Vascular Leakage Assessment In Vivo
PLY (3.125 μg/kg in saline) was instilled intratracheally in anesthetized mice with or without TIP peptide (2.5 mg/kg) or Ro-32–4032 (49 μg/kg) Evans Blue Dye albumin (EBD) (30 mg/kg in saline) (0.5% EBD conjugated to 4% BSA, Fraction V; Sigma), was injected into the tail vein 2 hours before killing the animals. Mice were killed after 6 hours to assess vascular leak. Extravasated EBD concentration in the lungs was calculated using a standard curve (μg of EBD per g wet lung tissue). All animal studies conformed to NIH guidelines. The experimental procedure was approved by the GHSU Institutional Animal Care and Use Committee.
Other Methods
Biochemicals, immunofluorescence, Rac1, RhoA, PKC-α, and arginase expression/activation assays, transendothelial resistance, and Ca2+ influx measurements are described in the online supplement.
Statistical Analysis
All experimental data are presented as means ± SD. Control and treated samples were compared by unpaired Student's t test. For multiple group comparisons, one-way ANOVA was used. P < 0.05 was considered statistically significant.
Results
Pneumolysin Induces Hyperpermeability in HL-MVEC Monolayers in a Ca2+–Dependent Manner In Vitro
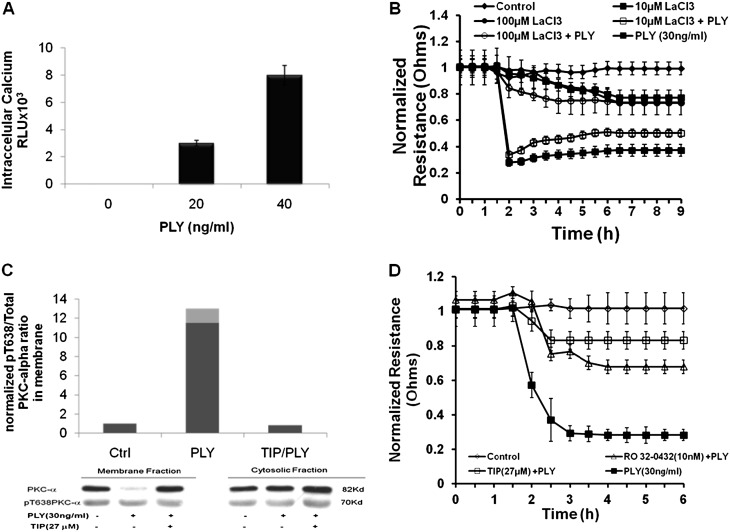
We have previously demonstrated a dose-dependent effect of PLY on permeability in HL-MVEC monolayers (30). Within 1 minute, PLY induces a dose-dependent influx of Ca2+ in HL-MVECs (Figure 1A). The permeability-increasing activity of PLY in HL-MVEC monolayers depends on Ca2+ influx because pretreatment of the cells with the nonspecific Ca2+ channel antagonist lanthanum chloride (LaCl3) (31) dose-dependently inhibits its barrier-disruptive effect in HL-MVEC monolayers (Figure 1B). Because activation of the Ca2+–dependent PKC-α isoform, characterized by an increased ratio of Thr638 phosphorylated over total PKC-α in the membrane fraction of the cells, was shown to increase pulmonary endothelial permeability (32), we have investigated the involvement of this enzyme in the effects of PLY in HL-MVEC monolayers. PLY treatment of HL-MVECs leads to a rapid activation of PKC-α within 30 minutes, as demonstrated by the increased ratio of phospho-Thr638 PKC-α over total PKC-α in the membrane fraction (Figure 1C). This PLY effect can be blunted by the TNF-derived TIP peptide, previously shown to also inhibit listeriolysin-induced PKC-α activation in HL-MVECs (28) (Figure 1C). Moreover, the PKC-α–specific inhibitor Ro32–4032 (10 nM) significantly inhibits the permeability increase induced by 30 ng/ml PLY in HL-MVEC monolayers (as well as in HPAEC monolayers; data not shown), assessed by measuring transendothelial resistance using electrical cell-substrate impedance sensing technology as described previously (28) (Figure 1D).
Figure 1.
(A) Within 1 minute, pneumolysin (PLY) treatment of human lung microvascular endothelial cells (HL-MVEC) induces a dose-dependent increase in intracellular Ca2+ concentrations (n = 8). (B) A 30-minute pretreatment with the aspecific Ca2+ channel inhibitor LaCl3 dose-dependently inhibits the barrier-disruptive effect of PLY (30 ng/ml) in HL-MVECs (n = 4). (C) PLY (30 ng/ml) activates protein kinase C (PKC)-α within 30 minutes in HL-MVECs (n = 4), which can be completely blunted upon 30-minute pretreatment of the cells with the tonoplast intrinsic protein (TIP) peptide (27 μM). (D) PLY (30 ng/ml) significantly decreases normalized transendothelial resistance (measured in ECIS; Applied Biophysics, Troy, NY) in monolayers of HL-MVECs. Pretreatment of the cells for 1 hour with PKC-α inhibitor Ro32–4032 (10 nM) or with TIP peptide (27 μM) significantly reduces this effect (n = 4).
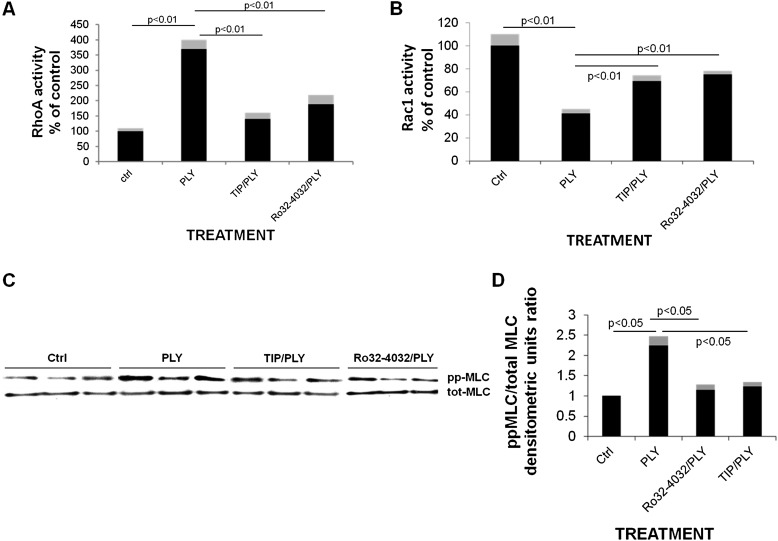
Treatment of HL-MVEC monolayers with PLY (30 ng/ml) causes a dramatic change in the RhoA/Rac1 balance within 1 hour (Figures 2A and 2B), increasing the activity of barrier-disruptive RhoA (Figure 1A) and inactivating the barrier-protective small GTP-ase Rac1 (Figure 1B). Moreover, myosin light chain (MLC) phosphorylation, a hallmark of increased endothelial dysfunction, is significantly elevated within 1 hour upon PLY treatment of HL-MVECs (Figures 1C and 1D). Both the PKC-α inhibitor Ro32–4032 (10 nM) and TNF-derived TIP peptide (27 μM) are able to significantly reduce PLY-induced RhoA/Rac-1 balance impairment and MLC phosphorylation when applied 30 minutes before PLY. We have moreover recently shown that PLY also increases the ratio of phospho-Tyr658 over total VE-cadherin and as such decreases the expression of this adherens junction protein (33). The concentration of PLY used in these experiments (30 ng/ml) does not affect viability of the cells, as assessed by the Alamar Blue incorporation viability assay (data not shown).
Figure 2.
(A) PLY (30 ng/ml) increases RhoA GTPase activity (assessed with a commercially available G-LISA kit; Cytoskeleton, Denver, CO) within 2 hours in HL-MVECs (n = 8). TIP peptide (27 μM), when applied 30 minutes before PLY, significantly blunts this effect of PLY. (B) Two-hour PLY (30 ng/ml) treatment of HL-MVECs significantly reduces Rac1 activity (n = 8; assessed with a commercially available G-LISA kit), an effect blocked upon a 30-minute pretreatment with TIP peptide (27 μM). (C) PLY (30 ng/ml) increases myosin light chain (MLC) phosphorylation within 2 hours in HL-MVECs as assessed by Western blotting (n = 3) and quantified as (D) the densitometric units ratio of ppMLC over total MLC normalized to controls (n = 6).
Pneumolysin Impairs Microtubule Network Organization and VE-Cadherin Expression in Lung Endothelial Cells
The observed PLY-induced MLC hyperphosphorylation in HL-MVECs indicates that PLY treatment may result in actin cytoskeleton reorganization, leading to stress fiber formation. On the other hand, PLY treatment may affect the microtubular network dynamics. PLY-induced increases in intracellular Ca2+ can induce disassembly of microtubules. The microtubule population in endothelial cells is heterogeneous and partly consists of posttranslationally modified (acetylated) microtubules (34). In the present study, the subpopulation of dynamic microtubules was identified by immunofluorescence staining using antibodies against β-tubulin. By contrast, stable (acetylated) microtubules were identified by antibodies against acetylated tubulin.
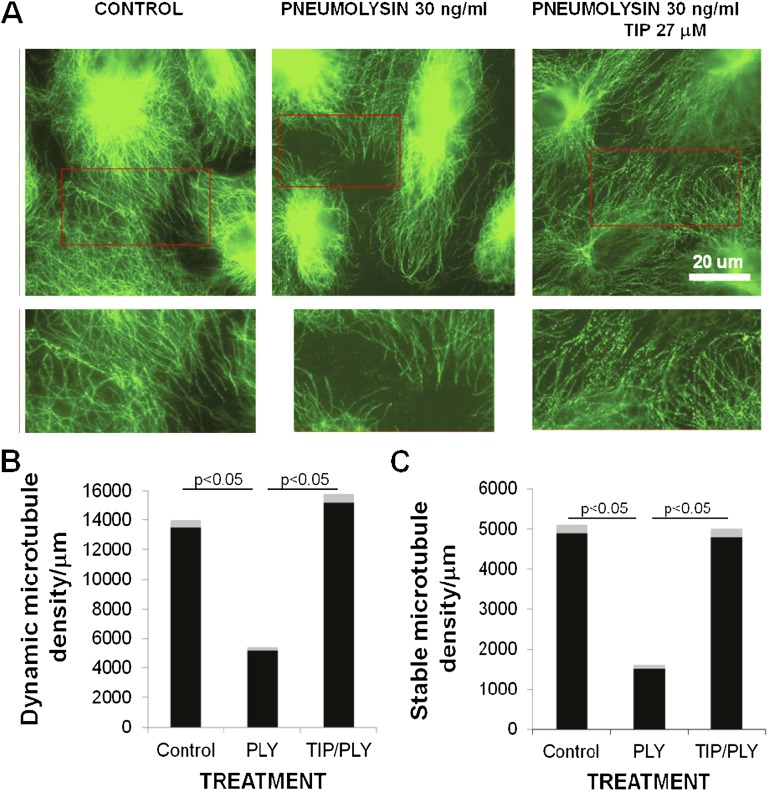
A 2-hour treatment with PLY (30 ng/ml) disrupts microtubule organization in HPAECs (Figure 3A). The dynamic microtubule network in these cells has a well defined convergent center near the nucleus, and its density is the highest in the internal cytoplasm, but it diminishes gradually in the direction of the cell margin. Single microtubules are visualized at the cell periphery. Stable microtubules are predominantly localized in the cell center and are devoid of a well defined convergence center. Quantitative analysis of microtubule density near the cell margin demonstrates that PLY induces partial disruption (microtubule depolymerization) of the dynamic and stable microtubule network at the cell periphery (Figures 3B and 3C). According to our data (Figures 3B and 3C), the density of dynamic microtubules in the 5-μm zone from the cell margin slightly increases after treatment with the TIP peptide but decreases more than twice upon PLY action. The TIP peptide completely rescues dynamic microtubules from PLY action near the cell edge. Moreover, the peptide does not influence the density of stable acetylated microtubules by itself but at least partially removes the PLY effect, which decreases their presence (Figures 3B and 3C).
Figure 3.
(A) Two-hour PLY-treatment (30 ng/ml) of human lung pulmonary artery endothelial cells causes a profound reorganization of microtubules (top panels were immunostained with beta-tubulin antibodies for microtubule detection; bottom panels are magnified images [insets] that show microtubule depolymerization at the cell periphery), characterized by a reduced (B) dynamic and (C) stable, acetylated microtubule density (n = 6). The TIP peptide (27 μM) inhibits the PLY-mediated effects on microtubule reorganization (n = 6; microtubules stained in green).
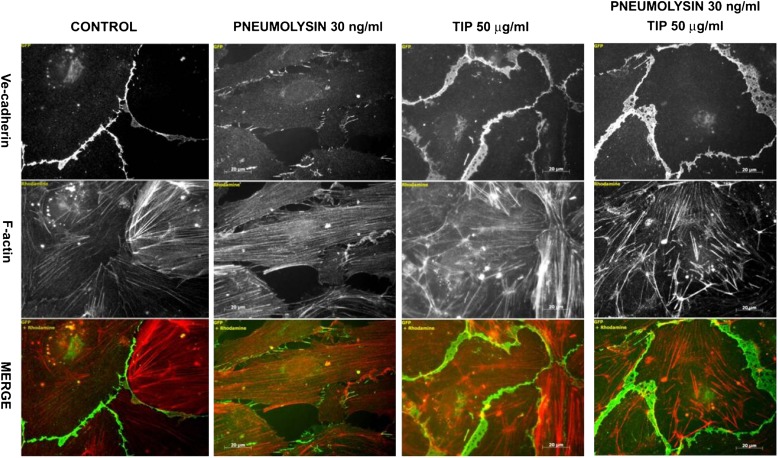
Microtubule organization is implicated in the exocytosis and endocytosis of adherens junction proteins, such as VE-cadherin. PLY induces a significant decrease in VE-cadherin presentation and an increased gap formation after 2 hours of treatment of HPAECs (Figure 4). The TIP peptide restores microtubule organization, VE-cadherin expression, and monolayer integrity (Figures 3A–3C, 4).
Figure 4.
Pneumolysin (30 ng/ml) significantly decreases VE-cadherin expression in human lung pulmonary artery endothelial cells within 2 hours of treatment. The TIP peptide (27 μM) blunts this effect (n = 6) (V-cadherin expression shown in green; actin filaments stained in red).
Pneumolysin Causes Increased Arginase Expression and Activity in HL-MVECs
Our results demonstrate that PLY activates the small GTPase RhoA in HL-MVECs (Figure 2A). Because RhoA was proposed to be implicated in the induction of arginase activity, which has been shown to be involved in endothelial dysfunction (12, 13), we investigated whether PLY can increase arginase expression and activity.
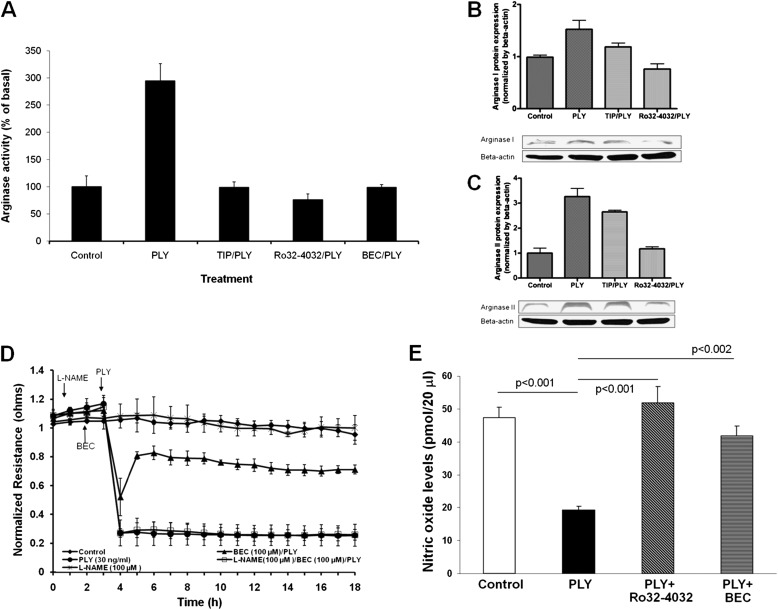
PLY increases arginase activity in HL-MVECs within 2 hours (Figure 5A), an effect prevented by the PKC-α inhibitor Ro32–0432 and by the TIP peptide, strongly suggesting that PKC-α acts upstream of arginase activation. PLY increases the expression of arginase I and II in HL-MVECs, an effect abolished by PKC-α inhibition and partially inhibited by the TIP peptide (Figures 5B and 5C). To understand whether arginase is implicated in PLY-mediated endothelial hyperpermeability, we pretreated HL-MVEC monolayers for 30 minutes with the arginase inhibitor BEC (100 μM). BEC pretreatment significantly blunts PLY-induced the EC monolayer integrity loss (Figure 5D). This effect is at least in part due to the restoration of nitric oxide (NO) production in the PLY-treated EC monolayers because a 1-hour pretreatment of the cells with the nitric oxide synthase (NOS) inhibitor l-NAME (100 μM) before the application of BEC prevents the protective effect of the arginase inhibitor. Moreover, PLY treatment of HL-MVECs for 2 hours with PLY (30 ng/ml) leads to a significantly reduced basal NO generation in these cells (Figure 5E). A 30-minute pretreatment of the cells with arginase inhibitor BEC (100 μM) or with PKC-α inhibitor Ro32–0432 (10 nM) restores NO generation to control levels (Figure 5E). Taken together, these results indicate that PLY-mediated arginase activation reduces endothelial NOS-mediated NO generation, diminishing the barrier-protective effect of NO in this in vitro model.
Figure 5.
(A) PLY (30 ng/ml) induces a significant increase in arginase activity within 2 hours in HL-MVECs (n = 6). This effect is blunted upon pretreating the cells for 30 minutes with PKC-α inhibitor Ro32–4032 (10 nM), TIP peptide (27 μM), or the arginase inhibitor (S)-(2-boronoethyl)-l-cysteine (BEC) (100 μM). PLY (30 ng/ml) significantly increases arginase I expression (B) and arginase II expression (C) in HL-MVEsC as assessed by Western blotting. This effect is partially or completely blunted upon pretreating cells with TIP peptide (27 μM) or the PKC-α inhibitor Ro32–4032 (10 nM), respectively (n = 4). (D) The arginase inhibitor BEC (100 μM), upon 1 hour pretreatment, partially blunts PLY (30 ng/ml)-mediated hyperpermeability as assessed by transendothelial resistance in HL-MVECs. Treatment of the cells with the NOS inhibitor l-NAME (100 μM) 1 hour before the BEC treatment abolishes the protective effect of the latter. (E) A 2-hour PLY treatment (30 ng/ml) of HL-MVECs significantly reduces basal NO production in these cells. This PLY effect is prevented upon pretreating the cells 30 minutes before PLY with the PKC-α inhibitor Ro32–4032 (10 nM) or with the TNF-derived TIP peptide (27 μM).
Inhibition of PKC-α or Arginase Activity Blunts PLY-Mediated Endothelial Hyperpermeability In Vivo
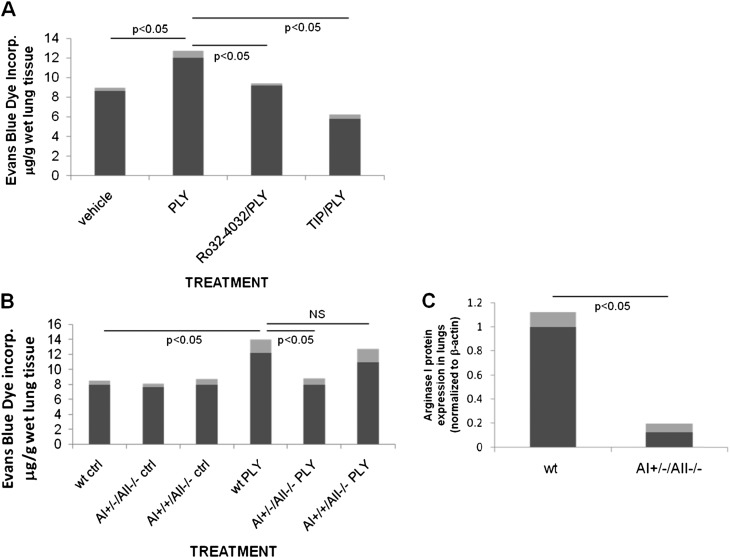
Intratracheal instillation of 3.125 μg PLY/kg in male 6- to 8-wk-old C57BL/6 mice induces a significantly increased pulmonary endothelial permeability within 6 hours of treatment as assessed by Evans Blue Dye (EBD) incorporation injected intravenously 2 hours before the end of the experiment (Figure 6A). Cotreatment of the mice with the PKC-α inhibitor Ro32–0432 (49 μg/kg, intratracheally) or with the TIP peptide (2.5 mg/kg, intratracheally) significantly inhibits PLY-induced capillary leak. Moreover, we detected a significant increase in EBD levels in the bronchoalveolar lavage fluid from PLY-instilled wild-type (wt) mice, as compared with TIP/PLY-treated or Ro32–4032/PLY-treated wt mice, respectively, indicating an increased permeability of the alveolar epithelial barrier upon PLY instillation (data not shown). We assessed the role of arginase in PLY-induced pulmonary endothelial barrier loss in vivo. Because arginase I and II have been proposed to play a role in lung diseases (14–18) and because PLY increases the expression of both isoforms (Figures 5B and 5C), we have compared the sensitivity of AI+/−/AII−/− and AI+/+/AII−/− mice (19) with their wt counterparts. As shown in Figure 6B, AI+/−/AII−/− mice, which express a 10-fold lower amount of AI in the lungs as compared with wt mice (Figure 6C), have a significantly reduced sensitivity to PLY-induced pulmonary endothelial hyperpermeability, assessed upon EBD incorporation 6 hours after intratracheal injection of PLY, as compared with wt or AI+/+/AII−/− mice. These results indicate an important role for arginase I, rather than arginase II, in PLY-mediated endothelial dysfunction in vivo.
Figure 6.
(A) PLY (3.125 μg/kg, intratracheally) induces pulmonary endothelial hyperpermeability within 6 hours in male C57BL6 mice. Upon cotreatment, the specific PKC-α inhibitor Ro32–4032 (49.5 μg/kg) and the TIP peptide (2.5 mg/kg) significantly blunt this activity (n = 6). (B) A+/−/−/− C57BL/6 mice, which have a 10-fold lower expression of arginase I in the lungs, as compared with wt mice (C), but not A+/+/−/− mice, are significantly protected from PLY (3.125 μg/kg)-induced hyperpermeability (n = 6).
Discussion
PLY, a 53-kD protein produced by virtually all clinical isolates of S. pneumoniae (35), represents the key virulence factor in pneumococcal pneumonia. Although not secreted by live bacteria, PLY can be released after aggressive antibiotics therapy of pneumococcal pneumonia (5). As such, in view of the powerful direct pore-forming effects of PLY in all cells expressing cholesterol in their membranes, a sudden release of large concentrations of the toxin in the alveolar and interstitial space of the lungs can cause a barrier dysfunction in alveolar epithelial and capillary endothelial compartments. In this study, we investigated the effects of PLY on endothelial monolayer resistance and on lung barrier integrity in C57BL/6 mice to identify the crucial mediators of PLY-induced endothelial barrier dysfunction.
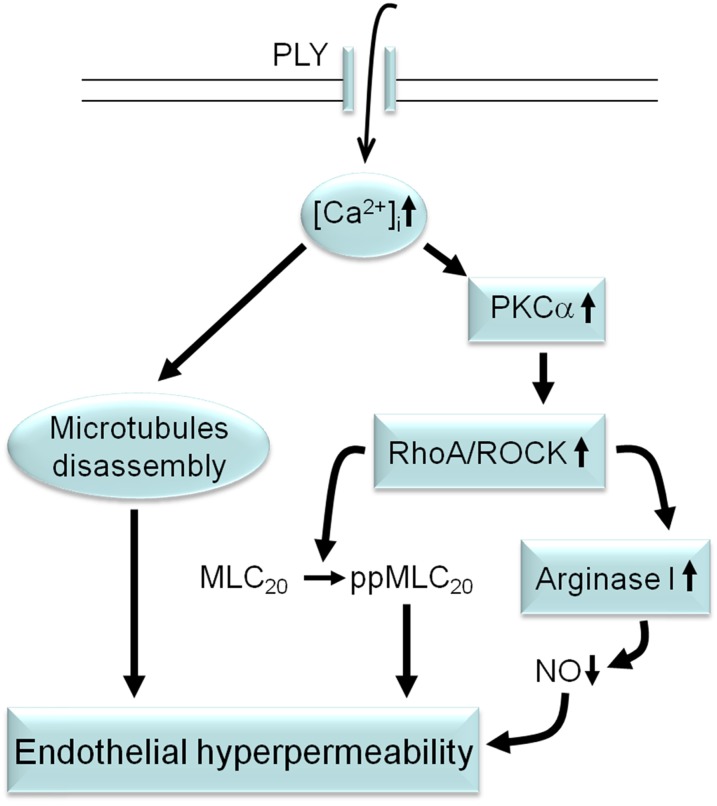
Our results indicate that the conventional PKC-α, which is stimulated by PLY-induced and pore-mediated Ca2+ influx, represents one of the early targets activated by PLY in HL-MVECs (Figure 7). PKC-α activation was demonstrated to stimulate RhoA/ROCK in ECs (36, 37) (Figure 7). Activation of RhoA compromises the endothelial barrier function, increasing MLC phosphorylation, actomyosin contractility, and intercellular gap formation. By contrast, Rac1, which may act upstream of RhoA, is required for the assembly, maintenance, and recovery of endothelial intercellular junctions (38). We found that PLY treatment activates RhoA and reduces Rac1 activity in HL-MVECs, thereby increasing the ratio of RhoA/Rac1 activity and favoring barrier-compromising processes and resulting in a dramatic loss of VE-cadherin expression (Figure 4). In contrast to our findings in HL-MVECs, sublytic concentrations of PLY were shown to preferentially activate RhoA and Rac1 in neuroblastoma cells (39, 40). Although Rac1 enforces the endothelial junctions, it can also become part of a barrier-disturbing mechanism as an activator of ROS-generating NADPH oxidase (41).
Figure 7.
Proposed sequence of events leading to PLY-induced endothelial hyperpermeability in the lungs. PLY induces pore formation in HL-MVECs, causing a rapid influx of Ca2+. Increased intracellular Ca2+ levels induce microtubule disassembly and cause the activation of the conventional PKC-α isoform. PKC-α activation, upon activating RhoA/ROCK, leads to increased MLC phosphorylation and increased arginase 1 activity, the latter of which leads to reduced NO generation. Endothelial permeability is increased upon microtubule disassembly, MLC phosphorylation, and reduced NO generation.
Although PLY has been demonstrated to excessively stabilize microtubules upon increased acetylation in neuroblastoma cells, astrocytes, and fibroblasts (40), our observations that PLY, upon increasing intracellular Ca2+ levels, induces microtubule disassembly and decreases Rac1 activity in HL-MVEC point toward important cell- and organ type-specific differences in PLY-induced signaling. This could imply that local, rather than systemic, treatments should be developed to tackle PLY-induced barrier dysfunction in the lungs during pneumococcal pneumonia. The endothelial microtubule network is heterogeneous, which is consistent with the dynamic characteristics of its constituent microtubules. The microtubule population can be divided into two subpopulations: (1) stable, modified (acetylated) and (2) dynamic microtubules. Acetylated microtubules are more stable than dynamic microtubules and thus are more resistant to the effects of external factors. It is possible that under conditions compromising vascular endothelial integrity, the stable microtubules may confer stability to the endothelial microtubule network (29). However, in our experiments, dynamic and stable microtubules were depolymerized significantly upon PLY action. Thus, the effect of the PLY treatment was equally strong on dynamic as on stable microtubules, such that microtubular stabilization did not rescue them from the toxin’s effects. The TNF-derived TIP peptide was very effective in rescuing dynamic and stable microtubules from PLY’s actions.
Reduction of NO generation in vivo induces microvessel leakage in the pulmonary circulation of eNOS-null mice (42). These data thus strongly suggest a barrier-protective effect of NO in the pulmonary microvasculature, but the mechanisms remain unclear. Arginase expressed in the endothelium serves as an endogenous competitor of NOS, reducing the availability of the substrate l-arginine to NOS and thus plays a counteracting role in NO-mediated vasodilatory function (12–18, 43). It also has been suggested that limitations in arginine availability or specific phosphorylation of the enzyme can switch eNOS from an NO generating to an O2– generating enzyme (44, 45). Little is known about the role of arginase in microvascular permeability. Our results show for the first time an involvement of arginase in PLY-mediated endothelial hyperpermeability. Indeed, PLY increases the expression of arginase I and II and increases arginase activity in HL-MVEC in vitro in a PKC-α–dependent manner. Because inhibition of the RhoA pathway was shown to blunt and its activation by PKC-α was demonstrated to activate arginase (12, 13), we propose that the subsequent activation of PKC-α and RhoA is involved in PLY-mediated arginase activation and the subsequent reduction in NO generation (Figure 7). Although arginase I and II were suggested to be associated with eNOS dysfunction and although the expression of both isoforms is increased by PLY in the lungs, in our study we only found an important role for arginase I in PLY-induced barrier dysfunction in vivo. We can only speculate why, apart from the crucial arginase I, arginase II expression is also up-regulated. Recent work by others (46) has suggested that arginase II overexpression can restore iNOS-mediated growth inhibition in LPS/TNF-treated pulmonary endothelial cells by reducing the pool of l-arginine. As such, up-regulation of arginase II can function as a negative feedback mechanism to a stress response in these cells.
The arginase inhibitor BEC is able to blunt PLY-mediated barrier disturbance in HL-MVECs, most likely by means of restoring NO generation because the NOS inhibitor l-NAME prevents its protective effect. Even though BEC does not completely inhibit PLY-mediated permeability, it completely restores NO generation in PLY-treated cells, suggesting that, apart from eNOS dysfunction, other mechanisms are involved in PLY-induced endothelial dysfunction. Apart from inhibiting arginase, BEC was also proposed to directly activate soluble guanylate cyclase in a NOS-independent manner (47).
We could not obtain a complete inhibitory activity on PLY-induced hyperpermeability in HL-MVECs with BEC, TIP peptide, or Ro32–4032, which indicates that alternative mechanisms from the ones identified here are involved in PLY-induced endothelial dysfunction, such as the induction of reactive oxygen species. However, reducing arginase 1 and PKC-α activity in vivo provides a more potent inhibition than that observed in our in vitro experiments. This could be due to the combined effects of these enzymes on other cell types (i.e., apart from the endothelium), such as the alveolar type II cells. Indeed, we could recently show that epithelial sodium channel-dependent sodium uptake, which is crucial for alveolar liquid clearance, is impaired by PLY in H441 cells (33). PKC-α activation has been shown to impair ENaC function and expression (48).
Although our study has not assessed the effects of PKC-α or arginase inhibition during a pneumococcal infection, it nevertheless has a clinical impact because it models late stages of severe pneumonia in patients treated with antibiotics when live bacteria are virtually absent and PLY itself is released. It has been reported that these patients can still die days after their lungs are sterile, thus stressing the clinical importance of tackling PLY-induced lung dysfunction. Because PKC-α has been proposed to be important for T-cell proliferation and IFN-γ production (49), and as such can be involved in antibacterial defense mechanisms, direct PKC-α inhibitors can have potential negative effects on, for example, adaptive immunity. Furthermore, because arginase is involved in macrophage-mediated antibacterial responses and in wound repair (50), it is possible that substances inhibiting its activation, although conferring resistance to PLY-mediated vascular leak, can interfere with macrophage-mediated innate immunity to pneumococci and wound healing. Direct arginase inhibitors block arginase activity not only in the endothelium but also in other tissues, such as the liver, where its activity is needed in the urea cycle. Therefore, alternative substances interfering with the activation of these enzymes, rather than with their activity per se, such as the TNF-derived TIP peptide, which activates amiloride-sensitive sodium uptake in alveolar epithelial and microvascular endothelial cells and which is currently being evaluated in a clinical trial, should be investigated more in detail.
In conclusion, this study has identified PKC-α and arginase I as important upstream and downstream mediators, respectively, of PLY-induced endothelial hyperpermeability. Our results suggest that antibiotic therapy toward pneumonia should be accompanied by treatments reducing the activation of these enzymes.
Supplementary Material
Acknowledgments
The authors thank Connie Snead for providing help with the endothelial cell cultures, Martina Hudel for pneumolysin purification, and Dr. Christiana Dimitropoulou for assistance in obtaining institutional approval for the animal protocols.
Footnotes
Supported by National Institutes of Health/National Heart, Lung and Blood Institute grants R01HL094609 (R.L.), R01HL070215 (R.W.C.), RO1EY11766 (R.B.C.), RO1HL51854 (M.A.M.), RO1GM086416 (J.F.P.), R01HL093460 (J.D.C.), RO1HL093460 (J.D.C.), RO1HL067307 (A.D.V.), and P01HL101902 (D.J.F., A.D.V., J.D.C.), by Russian Federation for Basic Research grants 09–04–00363 and 12–04–00488 (I.B.A.), and by a grant from the Deutsche Forschungsgemeinschaft through the Transregio Initiative TRR84 Project A4 (T.C.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0332OC on May 10, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008;86:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waterer GW, Rello J, Wunderink RG. Management of community-acquired pneumonia in adults. Am J Respir Crit Care Med 2011;183:157–164 [DOI] [PubMed] [Google Scholar]
- 3.Rubins JB, Charboneau D, Fasching C, Berry AM, Paton JC, Alexander JE, Andrew PW, Mitchell TJ, Janoff EN. Distinct roles for pneumolysin’s cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am J Respir Crit Care Med 1996;153:1339–1346 [DOI] [PubMed] [Google Scholar]
- 4.Witzenrath M, Gutbier B, Hocke AC, Schmeck B, Hippenstiel S, Berger K, Mitchell TJ, de los Toyos JR, Rosseau S, Suttorp N, et al. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med 2006;34:1947–1954 [DOI] [PubMed] [Google Scholar]
- 5.Anderson R, Steel HC, Cockeran R, von Gottberg A, de Gouveia L, Klugman KP, Mitchell TJ, Feldman C. Comparison of the effects of macrolides, amoxicillin, ceftriaxone, doxycycline, tobramycin and fluoroquinolones, on the production of pneumolysin by Streptococcus pneumoniae in vitro. J Antimicrob Chemother 2007;60:1155–1158 [DOI] [PubMed] [Google Scholar]
- 6.Maus UA, Srivastava M, Paton JC, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlöndorff D, Seeger W, Lohmeyer J. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J Immunol 2004;173:1307–1312 [DOI] [PubMed] [Google Scholar]
- 7.Stringaris AK, Geisenhainer J, Bergmann F, Balshüsemann C, Lee U, Zysk G, Mitchell TJ, Keller BU, Kuhnt U, Gerber J, et al. Neurotoxicity of pneumolysin, a major pneumococcal virulence factor, involves calcium influx and depends on activation of p38 mitogen-activated protein kinase. Neurobiol Dis 2002;11:355–368 [DOI] [PubMed] [Google Scholar]
- 8.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca(2+) signalling and PKC-α activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol 2001;533:433–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Crit Care Med 2003;28:574–581 [DOI] [PubMed] [Google Scholar]
- 10.Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and Rho activation and regulates the endothelial cell barrier function. J Biol Chem 2001;276:22614–22620 [DOI] [PubMed] [Google Scholar]
- 11.Cederbaum SD, Yu H, Grody WW, Kern RM, Yoo P, Iyer RK. Arginases I and II: do their functions overlap? Mol Genet Metab 2004;81:S38–S44 [DOI] [PubMed] [Google Scholar]
- 12.Romero M, Platt D, Tawfik H, Labazi M, ElRemessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 2008;102:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra S, Romero M, Shatanawi A, Alkilany A, Caldwell RB, Caldwell RW. Oxidative species increase arginase activity in endothelial cells through RhoA/Rho kinase pathway. Br J Pharmacol 2012;165:506–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J 2001;15:1264–1266 [DOI] [PubMed] [Google Scholar]
- 15.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L60–L68 [DOI] [PubMed] [Google Scholar]
- 16.North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol 2009;296:L911–L920 [DOI] [PubMed] [Google Scholar]
- 17.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 2004;18:1746–1748 [DOI] [PubMed] [Google Scholar]
- 18.Grasemann H, Schwiertz R, Matthiesen S, Racke K, Ratjen F. Increased arginase activity in cystic fibrosis airways. Am J Respir Crit Care Med 2005;172:1523–1528 [DOI] [PubMed] [Google Scholar]
- 19.Deignan JL, Livesay JC, Yoo PK, Goodman SI, O'Brien WE, Iyer RK, Cederbaum SD, Grody WW. Ornithine deficiency in the arginase double knockout mouse. Mol Genet Metab 2006;89:87–96 [DOI] [PubMed] [Google Scholar]
- 20.Diaz V, Brower R, Calfee CS, Matthay MA. Therapeutic strategies for severe acute lung injury. Crit Care Med 2010;38:1644–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elia N, Tapponnier M, Matthay MA, Hamacher J, Pache JC, Brundler MA, Totsch M, De Baetselier P, Fransen L, Fukuda N, et al. Functional identification of the alveolar edema reabsorption activity of murine tumor necrosis factor-alpha. Am J Respir Crit Care Med 2003;168:1043–1050 [DOI] [PubMed] [Google Scholar]
- 22.Vadász I, Schermuly RT, Ghofrani HA, Rummel S, Wehner S, Mühldorfer I, Schäfer KP, Seeger W, Morty RE, Grimminger F, et al. The lectin-like domain of tumor necrosis factor-alpha improves alveolar fluid balance in injured isolated rabbit lungs. Crit Care Med 2008;36:1543–1550 [DOI] [PubMed] [Google Scholar]
- 23.Hamacher J, Stammberger U, Roux J, Kumar S, Yang G, Xiong C, Schmid RA, Fakin RM, Chakraborty T, Hossain HM, et al. The lectin-like domain of TNF improves lung function after rat lung transplantation: potential role for a reduction in reactive oxygen species generation. Crit Care Med 2010;38:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazemi P, Tzotzos SJ, Fischer B, Andavan GS, Fischer H, Pietschmann H, Lucas R, Lemmens-Gruber R. Essential structural features of TNF-α lectin-like domain derived peptides for activation of amiloride-sensitive sodium current in A549 cells. J Med Chem 2011;53:8021–8029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hribar M, Bloc A, van der Goot FG, Fransen L, De Baetselier P, Grau GE, Bluethmann H, Matthay MA, Dunant Y, Pugin J, et al. The lectin-like domain of tumor necrosis factor-alpha increases membrane conductance in microvascular endothelial cells and peritoneal macrophages. Eur J Immunol 1999;29:3105–3111 [DOI] [PubMed] [Google Scholar]
- 26.Kusche-Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk-Zloy V, Schwab A, Schillers H, Oberleithner H. Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch 2008;455:849–857 [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Meng F, Mohan S, Champaneri B, Gu Y. Functional ENaC channels expressed in endothelial cells: a new candidate for mediating shear force. Microcirculation 2009;16:276–287 [DOI] [PubMed] [Google Scholar]
- 28.Xiong C, Yang G, Kumar S, Aggarwal S, Leustik M, Snead C, Hamacher J, Fischer B, Umapathy NS, Hossain H, et al. The lectin-like domain of TNF protects from listeriolysin-induced hyperpermeability in human pulmonary microvascular endothelial cells: a crucial role for protein kinase C-alpha inhibition. Vascul Pharmacol 2010;52:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alieva IB, Zemskov EA, Kireev II, Gorshkov BA, Wiseman DA, Black SM, Verin AD. Microtubules growth rate alteration in human endothelial cells. J Biomed Biotechnol 2010;2010:671536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catravas JD, Snead C, Dimitropoulou C, Chang AS, Lucas R, Verin AD, Black SM. Harvesting, identification and barrier function of human lung microvascular endothelial cells. Vascul Pharmacol 2010;52:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boucek MM, Snyderman R. Calcium influx requirement for human neutrophil chemotaxis: inhibition by lanthanum chloride. Science 1976;193:905–907 [DOI] [PubMed] [Google Scholar]
- 32.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 2001;91:1487–1500 [DOI] [PubMed] [Google Scholar]
- 33.Lucas R, Sridhar S, Rick FG, Gorshkov B, Umapathy NS, Yang G, Oseghale A, Verin AD, Matthay MA, Chakraborty T, et al. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc Natl Acad Sci USA 2012;109:2084–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci 2008;1123:134–145 [DOI] [PubMed] [Google Scholar]
- 35.Rubins JB, Charboneau D, Paton JC, Mitchell TJ, Andrew PW, Janoff EN. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J Clin Invest 1995;95:142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, et al. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol 2004;201:55–70 [DOI] [PubMed] [Google Scholar]
- 37.Huang F, Subbaiah PV, Holian O, Zhang J, Johnson A, Gertzberg N, Lum H. Lysophosphatidylcholine increases endothelial permeability: role of PKCalpha and RhoA cross talk. Am J Physiol Lung Cell Mol Physiol 2005;289:L176–L185 [DOI] [PubMed] [Google Scholar]
- 38.Waschke J, Baumgartner W, Adamson RH, Zeng M, Aktories K, Barth H, Wilde C, Curry FE, Drenckhahn D. Requirement of Rac activity for maintenance of capillary endothelial barrier properties. Am J Physiol Heart Circ Physiol 2004;286:H394–H401 [DOI] [PubMed] [Google Scholar]
- 39.Iliev AI, Djannatian JR, Nau R, Mitchell TJ, Wouters FS. Cholesterol-dependent actin remodeling via RhoA and Rac1 activation by the Streptococcus pneumoniae toxin pneumolysin. Proc Natl Acad Sci USA 2007;104:2897–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iliev AI, Djannatian JR, Opazo F, Gerber J, Nau R, Mitchell TJ, Wouters FS. Rapid microtubule bundling and stabilization by the Streptococcus pneumoniae neurotoxin pneumolysin in a cholesterol-dependent, non-lytic and Src-kinase dependent manner inhibits intracellular trafficking. Mol Microbiol 2009;71:461–477 [DOI] [PubMed] [Google Scholar]
- 41.Wojciak-Stothard B, Torondel B, Zhao L, Renné T, Leiper JM. Modulation of Rac1 activity by ADMA/DDAH regulates pulmonary endothelial barrier function. Mol Biol Cell 2009;20:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol 2005;289:L371–L381 [DOI] [PubMed] [Google Scholar]
- 43.Mundy AL, Dorrington KL. Inhibition of nitric oxide synthesis augments pulmonary oedema in isolated perfused rabbit lung. Br J Anaesth 2000;85:570–576 [DOI] [PubMed] [Google Scholar]
- 44.Ogonowski AA, Kaesemeyer WH, Jin L, Ganapathy V, Leibach FH, Caldwell RW. Effects of NO donors and synthase agonists on endothelial cell uptake of L-Arg and superoxide production. Am J Physiol 2000;278:C136–C143 [DOI] [PubMed] [Google Scholar]
- 45.Michell BJ, Chen Zp, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem 2001;276:17625–17628 [DOI] [PubMed] [Google Scholar]
- 46.Chicoine LG, Stenger MR, Cui H, Calvert A, Evans RJ, English BK, Liu Y, Nelin LD. Nitric oxide suppression of cellular proliferation depends on cationic amino acid transporter activity in cytokine-stimulated pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 2011;300:L596–L604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huynh NN, Harris EE, Chin-Dusting JF, Andrews KL. The vascular effects of different arginase inhibitors in rat isolated aorta and mesenteric arteries. Br J Pharmacol 2009;156:84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen XJ, Seth S, Yue G, Kamat P, Compans RW, Guidot D, Brown LA, Eaton DC, Jain L. Influenza virus inhibits ENaC and lung fluid clearance. Am J Physiol Lung Cell Mol Physiol 2004;287:L366–L373 [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Qiao G, Ying H, Zhang J, Yin F. TCR-induced Akt serine 473 phosphorylation is regulated by protein kinase C-alpha. Biochem Biophys Res Commun 2010;400:16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3:23–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.