Abstract
The enzyme indoleamine 2,3-dioxygenase (IDO) converts tryptophan into kynurenine metabolites that suppress effector T-cell function. In this study, we investigated IDO and its metabolite, 3-hydroxyanthranilic acid (3HAA), in regulating lung allograft rejection, using a murine orthotopic lung transplant model with a major mismatch (BALB/c donor and C57BL6 recipient). IDO was overexpressed in murine donor lungs, using an established nonviral (polyethylenimine carrier)–based gene transfer approach, whereas 3HAA was delivered daily via intraperitoneal injection. Increased IDO expression or its metabolite, 3HAA, resulted in a remarkable therapeutic effect with near normal lung function and little acute rejection, approximately A1, compared with A3 in untreated allografts (grading based on International Society for Heart and Lung Transplantation guidelines). We found that a high IDO environment for 7 days in lung allografts resulted in impaired T-cell activation, the production of multiple effector cytokines (IL-2, IL-4, IL-5, IL-6, IFN-γ, TNF-α, IL-12, and IL-13), and the generation of effector memory T cells (CD62LloCD44hi phenotype). In isolated murine splenocytes, we observed that IDO/3HAA impaired T-cell receptor (TCR)–mediated T-cell activation, and more importantly, a decrease of intracellular calcium, phospholipase C-γ1 phosphorylation, and mitochondrial mass was evident. This work further illustrates the potential role of a high IDO environment in lung transplantation, and that the high IDO environment directly impairs TCR activation via the disruption of calcium signaling.
Keywords: 3-hydroxyanthranilic acid, lung allograft rejection, nonviral gene transfer
Clinical Relevance
Lung transplant recipients experience a high rate of rejection and limited long-term outcomes. This study evaluates a novel therapy using a murine lung transplant model, with potential benefits for lung transplantation.
Lung transplantation is a well-accepted therapy for patients with endstage lung disease that is not amenable to other medical or surgical therapies. Unfortunately, acute cellular rejection (ACR) is a common complication in lung transplant recipients, despite current immunosuppressive protocols (1). ACR is problematic because it is the major risk factor for the development of bronchiolitis obliterans, believed to be the manifestation of chronic rejection, and ACR necessitates the augmentation of immune suppression, leading to increased mortality and morbidity (2). The difficulties with bronchiolitis obliterans and frequent rejections result in relatively poor outcomes for lung transplantation, compared with the transplants of other solid organs (1).
Indoleamine 2,3-dioxygenase (IDO) is an inducible enzyme that promotes tryptophan (Trp) catabolism, and has been shown to involve immune modulating activities (3). By increasing IDO activity, an environment is generated with reduced Trp and an increase in its metabolic products, resulting in the modification of dendritic cell function and the inhibition of T-cell activation and proliferation. Consistent with this concept, our laboratory previously showed that an increase in IDO within rat lung allografts resulted in less rejection, transplant-mediated injury, and impaired CD8 cell function (4–6).
In the present study, we expanded upon the potential role of IDO in preventing lung allograft rejection, using a murine lung transplant model. We first showed that augmented IDO activity is protective to the murine lung allograft, based on reduced ACR and the preservation of lung function, along with impaired T-cell cytokine release and activation. For a better understanding of the role of IDO in lung transplantation and to find a potential nongene therapeutic approach, we examined whether the metabolites of IDO activity were effective at reducing lung allograft injury. We focused on 3-hydroxyanthranilic acid (3HAA) because it is less neurally active than some of the other metabolites (7). We found that treating recipients with 3HAA similarly results in the protection of murine lung allografts. In addition, we further examined the effects of a high IDO environment, either through the overexpression of IDO in a coculture system or through the addition of 3HAA, and we found that such an environment impaired T-cell receptor (TCR) activation through the interruption of intracellular calcium (Ca2+) and of the TCR/Ca2+ signaling pathway.
Materials and Methods
Reagents
Reagents were obtained from Sigma Chemical Company (St. Louis, MO), unless otherwise specified. Antibodies for TCR activation and flow cytometry included anti-CD3 (145-2C11), anti-CD28 (37.51), anti-CD16/CD32 (2.4G2), anti-CD8a, anti-CD69, anti-CD44, anti-CD62L (BD Biosciences, San Diego, CA), anti-CD45.2, anti-CD4, anti-TCRβ (eBioscience, San Diego, CA), and anti-CD25 (BioLegend, San Diego, CA). Tissue culture reagents were obtained from GIBCO (Grand Island, NY), and Transwell Permeable inserts were obtained from Corning (Lowell, MA).
Murine Orthotopic Lung Transplants
BALB/c, C57BL/6 (B6), and GCN2−/− (B6 background) mice (Jackson Laboratory, Bar Harbor, ME) were housed in accordance with institutional and National Institute of Health guidelines, and our protocols were approved by the Animal Care Committee of Children’s Hospital Boston. Orthotopic left lung transplants were performed based on our previous rat lung transplant model (4, 8), with modifications. Briefly, donors were anesthetized with ketamine/xylazine, intubated, and ventilated with isoflurane/oxygen. Lungs were flushed, left lungs were harvested, and cuffs were placed in the pulmonary artery and vein and bronchus. Recipient mice were anesthetized and ventilated, and their left lungs were transplanted via a thoracotomy and implantation of the cuffed structures. Transplanted lungs were harvested 7 days after transplantation.
Experimental Groups, Lung Function, and Histopathology
Experimental groups included 4–8 transplants/group, including (1) normal untransplanted B6 mice, (2) B6 donor to B6 recipient (isografts), (3) BALB/c to B6 (allografts), (4) pCMV-null (vehicle)–treated allografts, (5) pCMV-human IDO (IDO)–treated allografts), (6) IDO-treated allografts plus IDO inhibitor 1-methyl-d-tryptophan (1-mT), (7) allografts receiving 3HAA (250–350 mg/kg), and BALB/c into GCN2−/− recipients with (8) and without (9) IDO or 3HAA.
Expression vectors were complexed with in vivo jetPEI (Qbiogene Molecular Biology, Qbiogene, Illkirch Cedex S.A., France), and were injected into donor tail veins 24 hours before transplantation. Lung function was assessed via peak airway pressures, as previously described (4, 8), and ACR was based on histologic analyses of hematoxylin and eosin staining in a blinded fashion based on International Society for Heart and Lung Transplantation (ISHLT) guidelines (9).
In Vitro Stimulation
Isolated T cells were exposed to a high-IDO environment generated by ±IDO endothelial cell cocultures or increasing concentrations of 3HAA. After 24 to 48 hours, cells were stimulated with anti-CD3 and anti-CD28 antibodies.
Outcome Measures
Cytokines were measured using the Mouse Th1/Th2/Th17 Cytokines Multi-Analyte ELISArray Kit (SABiosciences, Frederick, MD) or MILLIPLEX MAP Mouse Cytokine/Chemokine-22 Plex (Millipore, MA). Flow cytometry (Hitachi F-4500 Fluorescence Spectrophotometer or LSRII Flow Cytometer; Becton Dickinson, Franklin Lakes, NJ) was used to phenotype T cells with the antibodies already mentioned, and Ca2+ flux analysis was assessed using Indo-1/AM (Invitrogen, Grand Island, NY). The calibration procedure consisted of obtaining maximum fluorescence by lysing cells with Triton X-100, and minimum fluorescence by adding EGTA. Mitochondria were stained using MitoTracker Green (MTG) (Invitrogen). Flow cytometry was analyzed using FlowJo software version 8 (Tree Star, Ashland, OR). Western blot analysis was used to assess phospho-PLC-γ1 (Tyr783) and SLP-76 (Cell Signaling Technology, Danvers, MA), phospho-LAT (Tyr132) (Abcam, Cambridge, MA), and murine β-actin (clone C-11; Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical Analysis
Data are expressed as mean ± SEM, and statistical analyses were performed using one-way ANOVA with the Newman-Keuls test or nonparametric Kruskal-Wallis test (GraphPad, San Diego, CA). P values less than 0.05 were considered significant.
Results
IDO Treatment Impairs T-Cell Activation and Function in Murine Lung Allografts
To examine the role of IDO in lung transplantation, we established a murine lung transplant model in our laboratory. Lung transplants were performed using mice across a full major histocompatibility complex mismatch, namely, BALB/c (H-2d) to B6 (H-2b). IDO expression was augmented in murine lung allografts, using a well established gene delivery system with polyethylenimine (PEI) as the carrier of the pCMV–human IDO vector (4, 6). IDO transgene expression and activity within lung allografts are illustrated in the online supplement. In concordance with our previous observations of rat lung transplants (4, 5, 8), the IDO treatment of murine lung allografts proved effective at reducing ACR and allograft injury (Figure 1).
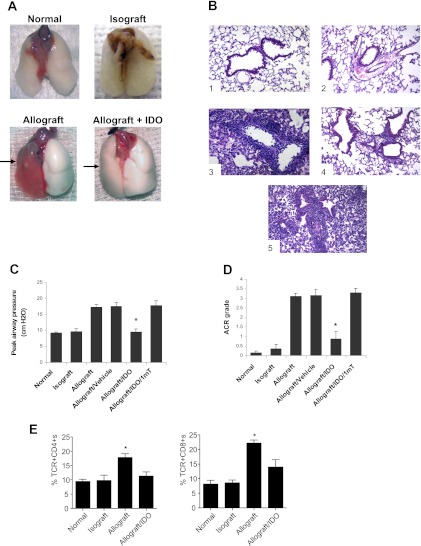
Figure 1.
Murine model of lung transplantation and increased indoleamine 2,3-dioxygenase (IDO). (A) Samples of lung blocks from normal left lung, lung isograft, untreated allograft, and IDO-treated allograft. Arrows indicate lung allografts. (B) Representative micrographs (original magnification, ×200) show hematoxylin-and-eosin staining for (1) normal left lung, (2) isograft, (3) untreated allograft, (4) IDO-treated allograft, and (5) IDO plus 1-mT (IDO inhibitor)–treated allograft, taken 7 days after transplantation. (C) Functional property of lung grafts was assessed via level of peak airway pressure. (D) Acute cellular rejection (ACR) grade was based on International Society for Heart and Lung Transplantation criteria for different lung grafts. (E) Relative percentages of CD4+ and CD8+ cells from lung grafts. Graphs or data represent assessment in a minimum of six animals. Results are presented as means ± SEM. *P < 0.05 versus allografts, allografts plus vehicle, or allografts treated with IDO plus 1-mT (B and C).
The appearance and lung function of left lung isografts (B6–B6) were similar to those in normal untransplanted lungs, whereas lung allografts presented evidence of rejection/injury (Figure 1) based on histologic analysis, and impaired lung function as evidenced by an elevated peak airway pressure with the delivery of a fixed tidal volume (Figure 1). Figures 1A and 1B illustrate representative samples of lungs and micrographs for untransplanted (normal), isografts, vehicle-treated allografts, IDO-treated allografts, and IDO plus the inhibitor 1-mT. IDO-treated lung allografts demonstrated a remarkable decrease in cellular infiltrates and lung injury, and a significant reduction in ACR grade based on ISHLT scale, with an average of approximately A3 in untreated or vehicle-treated allografts, compared with approximately A1 in IDO-treated allografts. Inhibiting IDO with 1-mT resulted in a loss of the protective effect (Figure 1). As a correlate to the reduced rejection/injury, IDO preserved lung function (Figure 1C). Moreover, a reduction was evident in the number of inflammatory TCR+CD4+ and TCR+CD8+ cells isolated from IDO-treated lung allografts (Figure 1E).
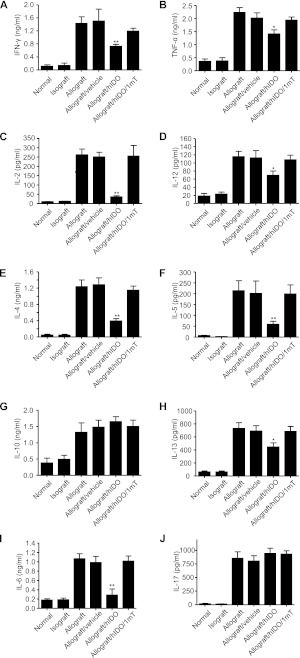
We previously observed impaired CD8 effector function/cytotoxicity with a decrease in Th1 cytokines TNF-α and IL-2, but not IFN-γ, from IDO-treated rat lung allografts (5). In this study, we expanded upon these findings and examined lung cytokine profiles, involving a population of Th1, Th2, and Th17 cytokines (Figure 2). As expected, we observed an increase in the number of cytokines in murine lung allografts, with specific increases in Th1 cytokines (IFN-γ, TNF-α, IL-2, and IL-12), Th2 cytokines (IL-4, IL-5, IL-10, and IL-13), IL-6, and IL-17. Increased IDO in lung allografts resulted in a reduction in the majority of these cytokines, which was reversed with the inhibitor 1-mT. IDO resulted in an approximately 50% reduction in IFN-γ, TNF-α, and IL-12, with a nearly complete inhibition of IL-2 concentrations. Moreover, a decrease was evident in IL-4, IL-5, and IL-13, whereas no effect on the potentially protective cytokine, IL-10, was observed. Notably, IDO treatment also resulted in a dramatic reduction in IL-6, without changes in IL-17 concentrations from lung allografts.
Figure 2.
Enhanced IDO reduces Th1 and Th2 cytokine concentrations in lung allografts. Enhanced IDO significantly reduced the alloantigen-specific production of Th1-related and Th2-related cytokines, including IFN-γ (A), TNF-α (B), IL-2 (C), IL-12 (D), IL-4 (E), IL-5 (F), IL-13 (H), IL-6 (I), and IL-17 (J) in lung grafts. Concentrations of IL-10 (G) and IL-17 (J) were not affected by IDO. Results are presented as means ± SEM (n ≥ 4 in each group). *P < 0.05 and **P < 0.01, versus all other allograft groups.
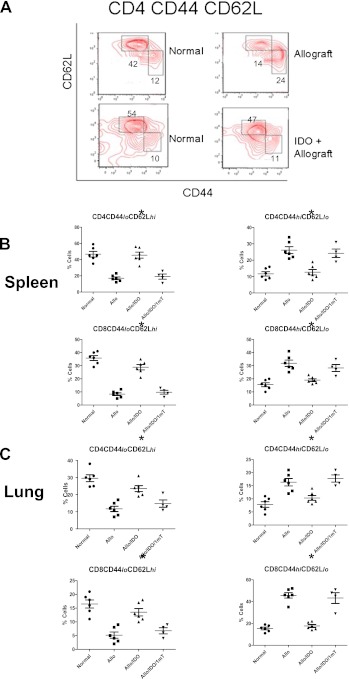
Because we observed similar findings in murine lung allografts with a reduction of cytokines and cellular profile, we further evaluated the potential role of IDO in preventing naive T cells (Tn) from developing effector function by examining the conversion of naive T cells to effector memory T cells (Tem). CD4 and CD8 T cells from the spleen and lung were evaluated 7 days after transplantation for CD62L and CD44 expression by flow cytometry. We found an increase in the Tem profile (CD44hiCD62Llo), whereas the Tn profile (CD44loCD62Lhi) was decreased (Figure 3) in mice receiving a lung transplant compared with untransplanted control mice. Mice that received an IDO-treated donor lung exhibited a Tn/Tem profile similar to that of normal mice. Blocking IDO activity with 1-mT resulted in a T-cell profile similar to that in untreated lung recipients, confirming that the response involved increased IDO expression.
Figure 3.
Enhanced IDO activity in lung allografts inhibits effector CD4 and CD8 T-cell generation. (A) Freshly harvested spleen cells were prepared and stained for CD44 expression and CD62L expression in CD4 or CD8 T cells from normal (untransplanted), untreated allograft, and IDO-treated allografts. Whereas untreated recipients showed a higher effector cell profile (CD44hiCD62Llo) than untransplanted control mice, the samples from IDO-treated recipients were similar to control samples, with more of a naive T-cell profile (CD44loCD62Lhi). One representative analysis of at least four independent experiments of each group is shown. (B) Graphs illustrate the percentages of CD44hi/lo and CD62Lhi/lo expression of both CD4-positive and CD8-positive T cells from mice that received vehicle-treated allografts, IDO-treated allografts, or IDO plus 1-mT–treated allografts, compared with normal mice. Enhanced IDO significantly inhibited the memory T-cell profile, which was reversed by treatment with the IDO inhibitor 1-mT. Results are presented as means ± SEM (n ≥ 4/group), with *P < 0.05 for IDO-treated allografts compared with the other allograft groups. (C) Graphs illustrate the percentages of CD44hi/lo and CD62Lhi/lo expression of CD4 and CD8 cells from normal lungs, lung allografts, and IDO ± 1-mT-treated lung allografts. The expression of lymphocytes from IDO-treated lung allografts is similar to normal lungs and significantly different from allografts or 1-mT–treated allografts (*P < 0.05, mean ± SEM, n ≥ 4/group).
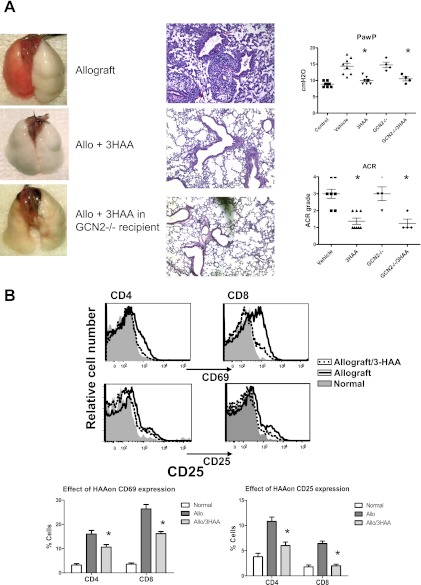
3HAA Promotes the Survival of Lung Allografts and Inhibits TCR Signaling
The generation of Trp metabolites through IDO activity is believed to comprise one method for the immune modulating activities of IDO. This study examined whether the systemic delivery (via intraperitoneal injections) of 3HAA for 7 days would inhibit transplant-mediated rejection/injury. The 3HAA treatment of murine lung allograft recipients resulted in a similar protective effect of lung allografts, with improved lung function, the inhibition of ACR, and impaired T-cell activation, as evidenced by the down-regulation of CD25 and CD69 in both CD4 and CD8 T cells (Figure 4). In addition, a reduction in a number of cytokines and chemokines (Table 1) occurred, with a profile similar to that in IDO gene transfer studies. This indicates that the protective effects of IDO are at least partly mediated through the generation of Trp metabolites, and exemplifies the potential of these metabolites as a therapeutic option in transplantation. General control nonrepressed 2 (GCN2) is a serine/threonine-protein kinase that senses amino-acid deficiency and is believed to mediate IDO-dependent effects upon T cells (10). We found that lung allografts using GCN2−/− mice as either donors or recipients resulted in rejection/injury similar to that in wild-type mice, with a similar response of GCN2−/− recipient mice to a high IDO environment (Figure 4A). The lack of a difference between GCN2−/− mice and wild-type mice suggests that IDO metabolites play a more critical role in its protective actions on murine lung allografts rather than Trp depletion, with a subsequent activation of GCN2 kinase.
Figure 4.
3-hydroxyanthranilic acid (3HAA) treatment ameliorates the clinical severity of murine lung allografts. (A) Representative lung blocks and micrographs (hematoxylin-and-eosin stain, ×200) show an allograft from an untreated recipient, a 3HAA-treated recipient, and an allograft in which the recipient is general control nonrepressed (GCN)2−/− and treated with 3HAA. Graphs illustrate lung function based on the peak airway pressure (PawP) of a constant tidal volume, and ACR-graded for normal nontransplanted lungs (Control) and from the four treatment groups, vehicle-treated allografts, 3HAA-treated allografts, and ±3HAA with GCN2−/− mice. Results show the means ± SEM for ≥ 4 transplants/group, with P < 0.05 for untreated allografts compared with the other two treatment groups. (B) Flow cytometry analysis of CD69 and CD25 expression of CD4+ and CD8+ T cells from 3HAA-treated and untreated lung allografts and normal lungs. Results are shown for representative FACS, based on CD45 TCR+CD4+ or CD8+ gates and graphs of the percentages of CD69 and CD25 cells in CD45 TCR+CD4+ or CD8+ gates (means ± SEM; n = 4). TCR, T-cell receptor.
TABLE 1.
CYTOKINE AND CHEMOKINE (PG/ML) CONCENTRATIONS 7 DAYS AFTER TRANSPLANTATION IN VEHICLE-TREATED LUNG ALLOGRAFTS AND 3HAA-TREATED ALLOGRAFTS
| Allo | Allo + 3HAA | |
| G-CSF* | 2,348 ± 307 | 856 ± 144 |
| GM-CSF | 326 ± 18 | 330 ± 24 |
| IFN-γ | 11,143 ± 188 | 699 ± 110 |
| IL-2* | 204 ± 22 | 39 ± 8 |
| IL-1β* | 183 ± 19 | 96 ± 11 |
| TNF-α* | 463 ± 55 | 288 ± 35 |
| RANTES* | 1,626 ± 208 | 836 ± 93 |
| IL-4* | 322 ± 33 | 156 ± 21 |
| IL-5* | 181 ± 25 | 101 ± 32 |
| IL-6* | 352 ± 42 | 199 ± 35 |
| IL-10 | 296 ± 35 | 238 ± 40 |
| IL-13 | 152 ± 37 | 88 ± 29 |
| IP-10 | 2,694 ± 351 | 2,006 ± 451 |
| KC | 1,978 ± 293 | 1,551 ± 187 |
| MCP-1* | 2,431 ± 166 | 1,231 ± 176 |
Definition of abbreviations: Allo, allografts; 3HAA, 3-hydroxyanthranilic acid; G-CSF, granulocyte colony-stimulating factor; GM-CSF. granulocyte/macrophage colony-stimulating factor; RANTES, regulated on activation normal T cell expressed and secreted (CCL5); IP-10, interferon-inducible protein 10 (CXCL10); KC, neutrophil-activating protein (CXCL1); MCP-1, monocyte chemotactic protein-1 (CCL2).
N= 5/group.
P < 0.05.
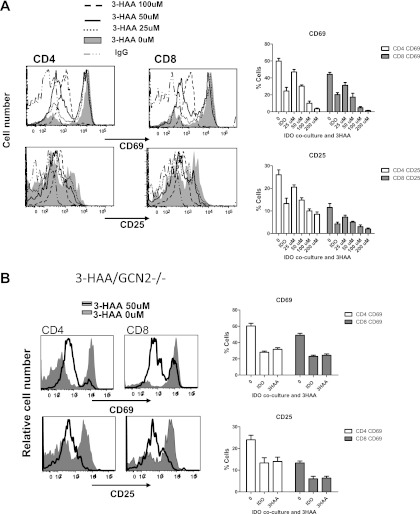
For a better understanding of the effects of IDO and 3HAA upon T-cell activation and TCR signaling, we used an in vitro model of TCR activation, applying anti-CD3 and anti-CD28 antibodies to activate isolated murine T cells directly. A high IDO environment was generated through a coculture system of IDO-overexpressing cells with isolated murine splenocytes, or by exposing splenocytes to increasing concentrations of 3HAA. We first verified that both increasing IDO activity and treatment with 3HAA resulted in impaired activation. Similar to the findings in previous reports (10, 11), a high IDO environment with either the IDO transgene or 3HAA resulted in the decreased activation (CD25 and CD69 expression) in both CD4+ and CD8+ T cells (Figure 5). Because a similar response to increased IDO activity and exposure to its metabolite 3HAA was evident, we focused mostly on the effects of 3HAA. Figure 5A illustrates representative flow cytometry and the results of TCR activation in response to increasing concentrations of 3HAA, indicating the impaired activation of T cells by 3HAA. Both IDO overexpression and 3HAA also inhibited the TCR activation of T cells isolated from GCN2-deficient mice, triggered by CD3/CD28 costimulation (Figure 5B). These data indicate that the generation of metabolites through IDO activity is also critical for the inhibitory actions of IDO upon the TCR activation of T cells.
Figure 5.
Impaired T-cell activation in response to 3HAA. Spleen cells from naive C57BL/6 mice (A) or GCN2−/− mice (B) were stimulated for 2 days with anti-CD3 (1 μg/ml) and anti-CD28 (5 μg/ml) in the presence of increasing concentrations of 3HAA, namely, 0–100 μM (A) or up to 50 μM in isolated GCN2−/− cells (B). CD69 and CD25 expression was analyzed by flow cytometry. Representative samples of the experiments are shown. The graphs show the mean ± SEM for percentages of CD69 and CD25 expression in response to increasing doses of 3HAA (0–200 μM; n = 3 for complete dose response and IDO coculture, n = 5 for 50 μM and 100 μM 3HAA, n = 2 for IDO coculture with GCN2−/− cells, and n = 3 for 50 μM 3HAA with GCN2−/− cells).
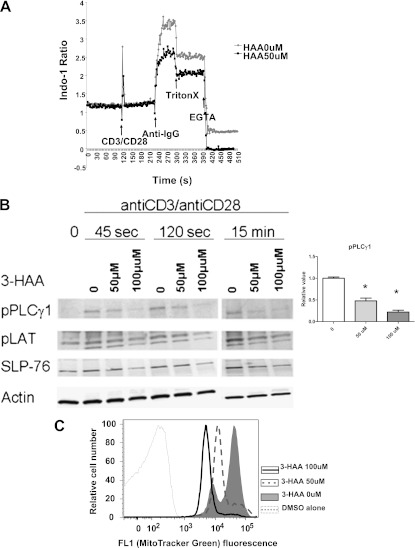
Calcium signaling is critical in the TCR activation of Tn and the generation of effector T cells. To investigate the defect underlying the failure of T cells to proliferate in the presence of IDO or its metabolites, we analyzed the effects of IDO or its metabolites on TCR-mediated signaling. A high IDO environment reduced Ca2+ influx in T cells upon TCR activation (anti-CD3/anti-CD28) (Figure 6A), whereas no effect upon Ca2+ gradients in response to the Ca2+ ionophore, ionomycin, was evident (data not shown). The increase in Ca2+ upon TCR activation is controlled through the PLC-γ1–mediated production of IP3 (12). Therefore, we evaluated PLC-γ1 phosphorylation and its upstream T-cell effector signals LAT (linker for the activation of T cells) and SLP-76 (SH-2 domain–containing leukocyte protein of 76 kD). 3HAA treatment resulted in a significant inhibition of pPLC-γ1 expression early in the activation process, with less of an effect upon phosphorylated LAT and SLP-76, suggesting that 3HAA inhibits Ca2+ signaling upon TCR engagement by reducing PLC-γ1 phosphorylation (Figure 6B).
Figure 6.
IDO and 3HAA inhibit TCR-mediated Ca2+ mobilization, signaling molecules, and mitochondrial content. (A) A representative example (n = 5) of decreased Ca2+ influx in T cells after TCR (anti-CD3/anti-CD28) activation in the presence of 3HAA (50 μM), as measured by indo-1 staining. Arrows indicate the timing of cell activation with respect to Ca2+ measurements. (B) Immunoblots of lysates from naive C57BL/6 cells stimulated with anti-CD3/anti-CD28 activation after 45 seconds, 120 seconds, and 15 minutes, with and without 3HAA exposure. A representative analysis (n = 3 independent experiments) is illustrated for phosphorylated (p) PLCγ1, pLAT, and SLP-76, with β-actin as internal loading control. The graph illustrates the relative pPLCγ1 concentration at 120 seconds for untreated cells and 3HAA treatment. (C) Decreased mitochondrial content of CD4+ and CD8+ T cells was measured by MitoTracker Green staining. Splenocytes cultured with the indicated concentrations of 3HAA were stained with anti-CD4 and anti-CD8, as well as 75 nM MitoTracker Green, for 20 minutes at 37°C. Splenocytes were then washed and their fluorescence was determined by flow cytometry. The fluorescence histogram is representative of three independent experiments.
We previously observed an impairment of mitochondrial membrane potential in rat T cells in a high IDO environment (5). Mitochondria are believed to help regulate Ca2+ release upon TCR activation, with an increase in mitochondrial mass (13) inversely correlated with the Ca2+ response (14). We evaluated mitochondrial mass using the mitochondria-specific dye MTG, a lipophilic thiol-reactive dye that selectively labels the mitochondrial inner membrane and matrix (15). The anti-CD3/anti-CD28 activation of T cells increased mitochondrial content, based on MTG staining. A decrease in the amount of mitochondrial membrane content upon exposure to 3HAA suggests a role of mitochondrial homeostasis in the inhibitory actions of a high IDO environment upon T cells.
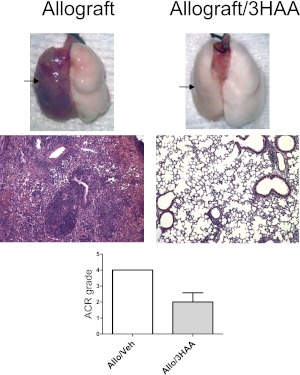
To address better whether 3HAA is a potential therapeutic agent, we evaluated the response of murine lung allografts after 4 weeks (Figure 7). Our findings in murine lung allografts in which a high IDO environment was generated by the systemic delivery of its metabolite were similar to our previous findings in a rat lung transplant model where a high IDO environment was generated using the Sleeping Beauty transposon/transposase system (8). Delivery of the metabolite resulted in less lung rejection compared with nontreated lung allografts, with mild to moderate rejection at approximately A2, compared with severe rejection at A4 with four transplants in each group.
Figure 7.
3HAA reduced murine lung allograft injury/rejection 3 weeks after transplantation. Lung allografts from recipient mice treated with 250 μg/kg of 3HAA for 4 weeks after transplantation demonstrated less rejection/injury, compared with vehicle-treated mice. Illustrated are representative examples of both untreated and treated explants (arrows show transplanted left lung) and hematoxylin-and-eosin–stained micrographs (×100). The graph illustrates the ACR grade for vehicle and 3HAA-treated lung allografts, presented as means ± SEM (n = 4 in each group) with P < 0.05.
Discussion
In this study, we used the murine lung transplant model to assess the role of IDO and its metabolites in lung transplantation, and to examine some of the mechanisms involved in limiting T-cell activation. We first verified that, similar to the rat lung transplant model (4, 5), a high IDO lung allograft environment generated using the PEI/IDO gene delivery system resulted in reducing acute lung allograft rejection and injury, while improving lung function. Blocking IDO using the chemical inhibitor 1-mT resulted in a complete reversal of the protective effects of IDO and evidence of severe acute rejection and impaired lung function. We also evaluated the IDO−/−-deficient mouse as both donor and recipient, and found no significant effect on early acute rejection in compareison with wild-type mice (data not shown). Interestingly, a lack of IDO exerts little impact, whereas the overexpression of IDO results in a significant reduction of the rejection process. This lack of a significant effect in IDO−/− mice was observed previously, and may reflect compensatory or redundant immunosuppressive mechanisms (16). Alternatively, differing tissues and disease processes may be contributory, because in another immune-mediated disease, namely collagen-induced arthritis, the lack of IDO resulted in earlier and more severe disease (17).
The array of secreted cytokines within the local environment plays a critical role in the activation of Tn to effector and memory cells. Although we observed a remarkable reduction in the acute rejection grade and a reduction in lung inflammatory cells, including T cells, in IDO-treated allografts, the T cells present still showed some evidence of activation, similar to cells present in untreated allografts. IL-2 is necessary for the second phase of the immune response for the differentiation into cytokine-producing effector cells, with a subsequent progression to memory cells (18, 19). We observed a dramatic decrease in IL-2 concentrations, along with a reduction of both Th1 and Th2 cytokines including IL-4, IL-5, and IL-6, and to a lesser degree IFN-γ, TNF-α, IL-12, and IL-13. Surprisingly, no decrease of IL-17 cells was evident in our model. Previous studies using cardiac transplantation, mycobacterium, fungus, and collagen-induced arthritis models indicated that IDO altered the expression of IL-17 (17, 20–23). This may be reflected by the lack of IL-2 induction, so that the negative regulatory effect of IL-2 upon Th17 development is missing. However, we also observed a significant decrease in IL-6 concentrations, which are important in the development of Th17 (24).
IL-2, IL-4, IL-12, and IL-6 are involved in the development of effector memory cells (19, 25–28). Therefore, we further examined the potential role of IDO in suppressing the ability of the immune system to generate effector memory cells. Memory T cells generated by exposure to an alloantigen are believed to be a barrier to maintaining allograft survival (29). Compared with Tn, Tem responded more rapidly to lower concentrations of antigen, and were less dependent upon costimulation. Not only are memory cells more resistant to apoptosis, with a relatively long lifespan, but they are also more resistant to suppression by alloreactive regulatory T cells and resistant to immunosuppressive agents (30, 31). Therefore, therapies that reduce the generation of Tem may be beneficial in maintaining lung allograft survival. Tn cells upon alloactivation undergo changes in cell-surface adhesion molecules that alter their trafficking characteristics. The conversion from the CD44loCD62Lhi (Tn) to a CD44hiCD62Llo (Tem) phenotype confers the ability to enter nonlymphoid tissue (32). We found that at least in the early phase, an augmented IDO environment results in an immune profile for both CD4 and CD8 cells that favors the maintenance of Tn rather than the generation of Tem. This suggests that IDO not only inhibits early T-cell responses such as cytotoxic T-lymphocyte activity, as previously described (5), but may also reduce effector memory cells, thereby moderating the ability to develop ACR, with the potential to prolong allograft survival. At present, it is unclear whether the decrease in the CD44hiCD62Llo population in IDO-treated mice represents the differential recruitment or activation and survival of T-cell subsets.
The generation of metabolites through IDO activity upon Trp is believed to be a pathway for the immune modulating activities of IDO. Therefore, we examined whether the metabolite 3HAA would produce similar lung allograft-protective actions. If so, this would constitute a potential therapy, because it would not be dependent upon the difficulties of using a gene transfer approach. The delivery of 3HAA proved to constitute an effective therapy in reducing murine lung allograft injury/rejection, with a reduction in T-cell activation and a remarkable reduction in the number of proinflammatory cytokines and chemokines. We observed similar findings using the metabolite kynurenine, although 3HAA appears to exert a more potent effect (data not shown). Previous studies indicated that Trp depletion via IDO resulted in the activation of an amino acid–sensitive pathway, GCN2, which mediates the inhibitory actions of IDO upon T cells (10). T cells lacking GCN2 were not sensitive to the inhibitory effects of IDO. Therefore, we expected to find that a high IDO environment would be less effective at reducing lung allograft injury/rejection in GCN2−/− mice. Interestingly, we found no difference when using either IDO transgene or 3HAA in preventing allograft rejection/injury in GCN2−/− mice compared with wild-type mice, suggesting a critical role for the generation of metabolites in the protective effect of IDO in lung transplantation. Jasperson and colleagues (33) reported similar findings in a graft-versus-host disease murine model in which donor T cells did not require GCN2 kinase to respond to IDO.
At this point, it remains unclear to how a high IDO environment impairs T-cell activation. We previously found that a high IDO environment inhibited the activity of complex 1 of the electron transport chain, with impairment in the energy supply of CD8 cells (5). In T cells, mitochondrial function is coupled to calcium signaling, which is one of the key triggering signals of TCR-mediated activation. In this study, we found that IDO/3HAA impairs TCR signaling with a reduction in the entry of extracellular calcium and the phosphorylation of PLC-γ1, a regulator of the calcium signaling pathway (34). No effect upon ionomycin stimulation was evident, and thus the cells are still capable of generating Ca2+ gradients. The inhibitory results of a high IDO environment upon TCR activation were the same in wild-type and GCN2-deficient mice, illustrating a direct role of the metabolites in suppressing T-cell activation.
Calcium release and gradients upon TCR engagement are related to mitochondrial activity (35), and we previously found that a high IDO environment impaired ATP release and mitochondrial membrane potential (5). Because an inverse relationship to mitochondrial mass and intracellular Ca2+ is evident in lymphocytes (14), we examined mitochondrial content in response to 3HAA. Mitochondrial mass was evaluated using MTG, because its staining of mitochondria is independent of mitochondrial membrane potential (36). Our results here, together with our previous data (5), show that mitochondrial content is also reduced upon exposure to a high IDO environment, with a greater response to 100 μM than to 50 μM. In general, we found a greater inhibition of TCR responses to higher doses of 3HAA, which may be related to the increased apoptosis of T cells with higher doses (data not shown). However, we still observed a significant inhibition of TCR-dependent events, including mitochondrial mass, even at 50 μM, whereas no significant increase in apoptosis was evident. Early apoptosis exerts little effect upon mitochondrial content/mass with no change of MTG expression, whereas membrane potential is reduced (36–39).
In conclusion, a high IDO environment, whether based on an increase in IDO activity or an increase in its metabolites, reduces lung allograft injury. A high IDO environment is able to impair both CD4 and CD8 T-cell responses, with a loss of TCR activation. A focal point appears to occur in mitochondria, with altered Ca2+ gradients and signaling. This study further suggests a role for IDO or its metabolites as a potential therapy in prolonging lung allografts.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grant RO1 HL088191 (G.A.V.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0438OC on April 19, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Knoop C, Estenne M. Acute and chronic rejection after lung transplantation. Semin Respir Crit Care Med 2006;27:521–533 [DOI] [PubMed] [Google Scholar]
- 2.Burton CM, Iversen M, Carlsen J, Mortensen J, Andersen CB, Steinbruchel D, Scheike T. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J Heart Lung Transplant 2009;28:888–893 [DOI] [PubMed] [Google Scholar]
- 3.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 2007;117:1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Liu L, Fletcher BS, Visner GA. Novel action of indoleamine 2,3-dioxygenase attenuating acute lung allograft injury. Am J Respir Crit Care Med 2006;173:566–572 [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Liu L, Liu K, Bizargity P, Hancock WW, Visner GA. Reduced cytotoxic function of effector CD8+ T cells is responsible for indoleamine 2,3-dioxygenase–dependent immune suppression. J Immunol 2009;183:1022–1031 [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Liu L, Visner GA. Nonviral gene delivery with indoleamine 2,3-dioxygenase targeting pulmonary endothelium protects against ischemia–reperfusion injury. Am J Transplant 2007;7:2291–2300 [DOI] [PubMed] [Google Scholar]
- 7.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood–brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 1991;56:2007–2017 [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Liu L, Fletcher BS, Visner GA. Sleeping Beauty–based gene therapy with indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. FASEB J 2006;20:2384–2386 [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26:1229–1242 [DOI] [PubMed] [Google Scholar]
- 10.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 2005;22:633–642 [DOI] [PubMed] [Google Scholar]
- 11.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase–expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med 2002;196:447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh-Hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol 2008;20:250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Souza AD, Parikh N, Kaech SM, Shadel GS. Convergence of multiple signaling pathways is required to coordinately up-regulate mtDNA and mitochondrial biogenesis during T cell activation. Mitochondrion 2007;7:374–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustavsson N, Abedi G, Larsson-Nyren G, Lindstrom P. Timing of Ca2+ response in pancreatic beta-cells is related to mitochondrial mass. Biochem Biophys Res Commun 2006;340:1119–1124 [DOI] [PubMed] [Google Scholar]
- 15.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol 2009;182:4046–4055 [DOI] [PubMed] [Google Scholar]
- 16.Baban B, Chandler P, McCool D, Marshall B, Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol 2004;61:67–77 [DOI] [PubMed] [Google Scholar]
- 17.Criado G, Simelyte E, Inglis JJ, Essex D, Williams RO. Indoleamine 2,3 dioxygenase–mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum 2009;60:1342–1351 [DOI] [PubMed] [Google Scholar]
- 18.Dooms H, Kahn E, Knoechel B, Abbas AK. IL-2 induces a competitive survival advantage in T lymphocytes. J Immunol 2004;172:5973–5979 [DOI] [PubMed] [Google Scholar]
- 19.Hoyer KK, Dooms H, Barron L, Abbas AK. Interleukin-2 in the development and control of inflammatory disease. Immunol Rev 2008;226:19–28 [DOI] [PubMed] [Google Scholar]
- 20.Desvignes L, Ernst JD. Interferon-gamma–responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity 2009;31:974–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 2008;451:211–215 [DOI] [PubMed] [Google Scholar]
- 22.Romani L, Zelante T, De Luca A, Fallarino F, Puccetti P. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J Immunol 2008;180:5157–5162 [DOI] [PubMed] [Google Scholar]
- 23.Yu G, Dai H, Chen J, Duan L, Gong M, Liu L, Xiong P, Wang CY, Fang M, Gong F. Gene delivery of indoleamine 2,3-dioxygenase prolongs cardiac allograft survival by shaping the types of T-cell responses. J Gene Med 2008;10:754–761 [DOI] [PubMed] [Google Scholar]
- 24.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 2007;26:371–381 [DOI] [PubMed] [Google Scholar]
- 25.Ben-Sasson SZ, Makedonski K, Hu-Li J, Paul WE. Survival and cytokine polarization of naive CD4(+) T cells in vitro is largely dependent on exogenous cytokines. Eur J Immunol 2000;30:1308–1317 [DOI] [PubMed] [Google Scholar]
- 26.Huang LR, Chen FL, Chen YT, Lin YM, Kung JT. Potent induction of long-term CD8+ T cell memory by short-term IL-4 exposure during T cell receptor stimulation. Proc Natl Acad Sci USA 2000;97:3406–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev 2006;211:81–92 [DOI] [PubMed] [Google Scholar]
- 28.Rochman I, Paul WE, Ben-Sasson SZ. IL-6 increases primed cell expansion and survival. J Immunol 2005;174:4761–4767 [DOI] [PubMed] [Google Scholar]
- 29.Brook MO, Wood KJ, Jones ND. The impact of memory T cells on rejection and the induction of tolerance. Transplantation 2006;82:1–9 [DOI] [PubMed] [Google Scholar]
- 30.Liang H, Zhao Y, San Z, Liao C, Sha C, Xie B, Chen J, Xia J, Wang Y, Qi Z. The recall alloresponse following retransplantation is more intense compared with the T cell memory-transfer model. Immunol Invest 2010;39:39–53 [DOI] [PubMed] [Google Scholar]
- 31.Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am J Transplant 2006;6:647–651 [DOI] [PubMed] [Google Scholar]
- 32.Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J Exp Med 2000;191:1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood 2009;114:5062–5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells AD, Liu QH, Hondowicz B, Zhang J, Turka LA, Freedman BD. Regulation of T cell activation and tolerance by phospholipase C gamma–1–dependent integrin avidity modulation. J Immunol 2003;170:4127–4133 [DOI] [PubMed] [Google Scholar]
- 35.Quintana A, Hoth M. Apparent cytosolic calcium gradients in T-lymphocytes due to fura-2 accumulation in mitochondria. Cell Calcium 2004;36:99–109 [DOI] [PubMed] [Google Scholar]
- 36.Poot M, Pierce RH. Detection of changes in mitochondrial function during apoptosis by simultaneous staining with multiple fluorescent dyes and correlated multiparameter flow cytometry. Cytometry 1999;35:311–317 [DOI] [PubMed] [Google Scholar]
- 37.Camilleri-Broet S, Vanderwerff H, Caldwell E, Hockenbery D. Distinct alterations in mitochondrial mass and function characterize different models of apoptosis. Exp Cell Res 1998;239:277–292 [DOI] [PubMed] [Google Scholar]
- 38.Metivier D, Dallaporta B, Zamzami N, Larochette N, Susin SA, Marzo I, Kroemer G. Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1–triggered apoptosis of Jurkat T lymphoma cells: comparison of seven mitochondrion-specific fluorochromes. Immunol Lett 1998;61:157–163 [DOI] [PubMed] [Google Scholar]
- 39.Petrovas C, Chaon B, Ambrozak DR, Price DA, Melenhorst JJ, Hill BJ, Geldmacher C, Casazza JP, Chattopadhyay PK, Roederer M, et al. Differential association of programmed death–1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J Immunol 2009;183:1120–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.