Abstract
Objective
The purpose of this study was to investigate the patterns and the risk factors of newly developed vertebral compression fractures (VCFs) after percutaneous vertebroplasty (PVP).
Methods
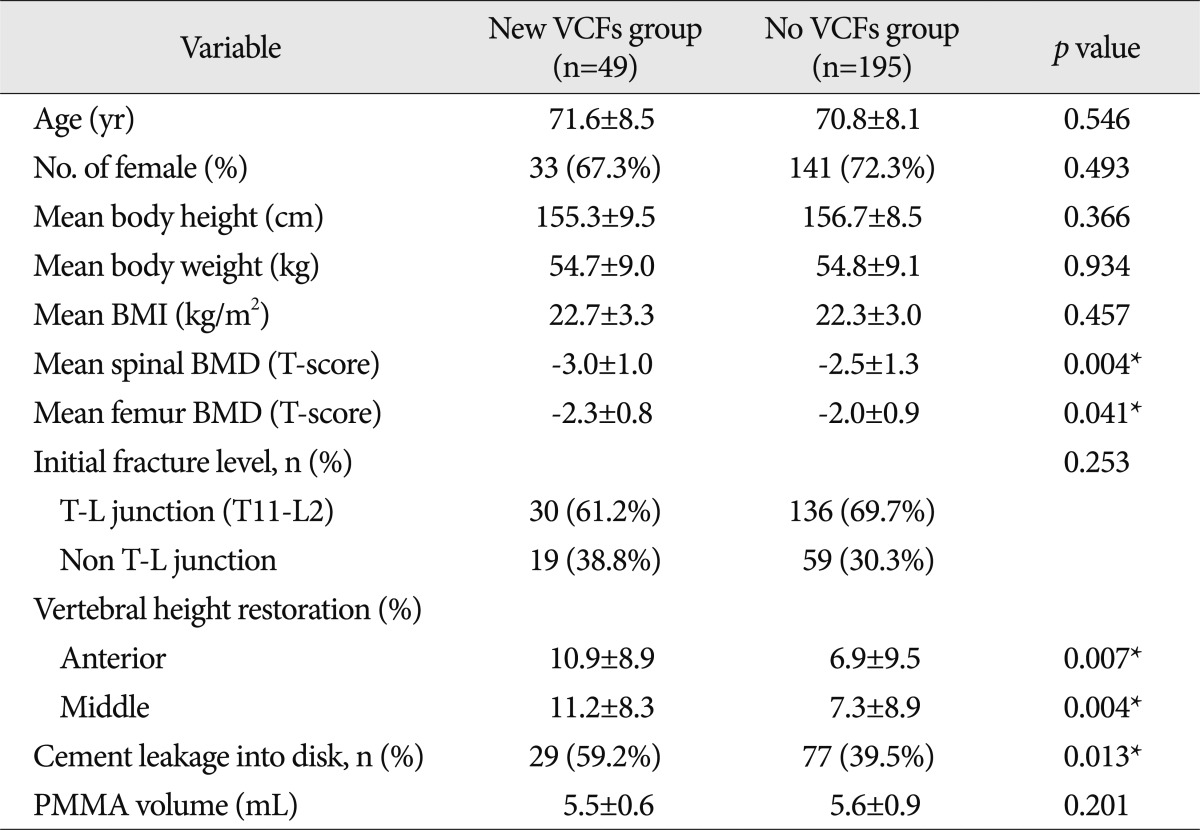
We performed a retrospective review of the 244 patients treated with PVP from September 2006 to February 2011. Among these patients, we selected 49 patients with newly developed VCFs following PVP as the new VCFs group, and the remaining 195 patients as the no VCFs group. The new VCFs group was further divided into 2 groups : an adjacent fractures group and a nonadjacent fractures group. The following data were collected from the groups : age, gender, body weight/height, body mass index (BMI), bone mineral density (BMD) score of the spine and femur, level of initial fracture, restoration rate of anterior/middle vertebral height, and intradiscal cement leakage, volume of polymethylmethacrylate (PMMA).
Results
Age, gender, mean body height/weight, mean BMI and volume of PMMA of each of the group are not statistically significantly associated with fractures. In comparison between the new VCFs group and the no VCFs group, lower BMD, intradiscal cement leakage and anterior vertebral height restoration were the significant predictive factors of the fracture. In addition, new VCFs occurrence at the adjacent spines was statistically significant, when the initial fracture levels were confined to the thoracolumbar junction, among the subgroups of new VCFs.
Conclusion
Lower spinal BMD, the greater anterior vertebral height restoration rate and intradiscal cement leakage were confirmed as risk factors for newly formed VCFs after PVP.
Keywords: Compression fracture, Vertebroplasty, Risk factor, Osteoporosis, Pattern
INTRODUCTION
Vertebroplasty using bone cement is a widely accepted, minimally invasive, treatment for a painful osteoporotic compression fracture. It was first introduced by Deramond et al.7) and Galibert et al.9) in 1987. Since then, interest in such techniques and augmentation materials has been increasing consistently, internationally. It was reported that immediate significant pain relief was achieved in 60% to 90%11,12), and pain reduction and return to normal function were observed in 70% to 90% of patients who underwent vertebroplasty2,10,29).
However, some of those patients suffered unexpected subsequent fractures, requiring further treatment. The causes of new-onset fractures after vertebroplasty are still debated : progression of the underlying disease3,20,21), augmentation of implanted cements, increased physical activity11,16,18,27) are possible contributors. The pertinent question regarding new-onset fractures after vertebroplasty is whether the next fracture level could be predicted. Almost all new-onset fractures after vertebroplasty developed in adjacent vertebra, but fractures in non-adjacent vertebra were also reported. The risk factors for these non-adjacent vertebra fractures are less understood.
The purpose of this study was to identify the patterns and risk factors for new vertebral compression fractures (VCFs) after percutaneous vertebroplasty (PVP).
MATERIALS AND METHODS
Study Design and Patient Selection
We examined our computerized database to identify all patients who had undergone PVP at our hospital from September 2006 to February 2011. A total of 365 patients with symptomatic VCFs were treated with PVP in our hospital. Patients with underlying disease, such as metastatic pathologic fractures or multiple myeloma, and patients with multiple compression fractures were excluded from the study.
Consequently, 244 patients (174 female, 70 male) were retrospectively reviewed for the study. The mean age of the patients was 70.9±8.2 years (48 to 92 years) at the time of surgery. Patients were followed up after PVP for a mean of 16.3±16.8 months (0.2-61.7 months). The initial fracture levels of the VCF patients who were treated with PVP were as follows : thoracic lesion; 42 patients (17.2%), thoracolumbar (T-L) junction (T11 through L2 spine) lesion; 167 patients (68.4%), lumbar lesion; 35 patients (14.3%). From the database, we selected cases in which the patients had undergone additional PVP to treat painful VCFs after the initial PVP. Among these patients, we selected the patients suffering from newly developed VCFs after PVP, designated as the new VCFs group, and the rest of the patients assigned to the no VCFs group. The fracture group was divided into 2 groups, those with the adjacent-level fractures and those with the non-adjacent-level fractures.
The pre-PVP radiological evaluation of the patients included conventional radiography and spine magnetic resonance imaging (MRI) for all patients. The MR imaging features were indicative of an acute/subacute fracture activity, part of the inclusion criteria; low signal intensity on T1-weighted MR images, high signal intensity on T2-weighted MR images and high signal intensity on T2-weighted with fat suppression MR images within the vertebral body indicating active edema and inflammation. In addition, the inclusion criteria for newly developed compression fractures were as follows : 1) relapse of pain after initial vertebroplasty, after an obvious pain-free interval; 2) evidence of new VCFs on both MR imaging and radiography, occurring either above or below the previously treated level; 3) additional vertebroplasty required to relieve painful symptoms due to a subsequent fracture.
We carefully reviewed the clinical and imaging records to rule out any evidence of vertebral fracture occurring after PVP, during the follow-up period.
Procedure
The PVP procedure was performed according to an established technique14). The patients were placed in the prone position on the examination table and given either general or local anesthesia. The fracture level was observed fluoroscopically, and a fluoroscopically guided uni- or bilateral transpedicular approach was performed, with an 11-gauge bone biopsy needle. The needle was pushed through the cortex, traversed the center of the pedicle, and was then directed into the bone marrow of the vertebral body. The anterior third of the body was an ideal location for the needle placement. We used the bone cement : Antibiotic simplex, Stryker Howmedica or Spine_Fix. The mixed bone cement was injected by using 1-mL syringes under the fluoroscopic control, and the procedure immediately terminated if any of the following were observed : 1) cement reaching the posterior fourth of the vertebral body; 2) cement migrating to drainage veins; 3) significant leakage.
After the procedure, plain radiographs of treated vertebral levels were assessed to characterize the deposition of cement.
Data collection
Patient demographics, including age, gender, body weight, body height, body mass index (BMI) and bone mineral density (BMD), along with the time from the date of initial intervention with PVP to the diagnosis of subsequent fractures, were obtained from clinical records. The mean BMI is calculated as the weight in kilograms divided by the square of the height in meters (kg/m2); the lumbar spine bone density and the femur bone density were measured by dual-energy X-ray absorptiometry.
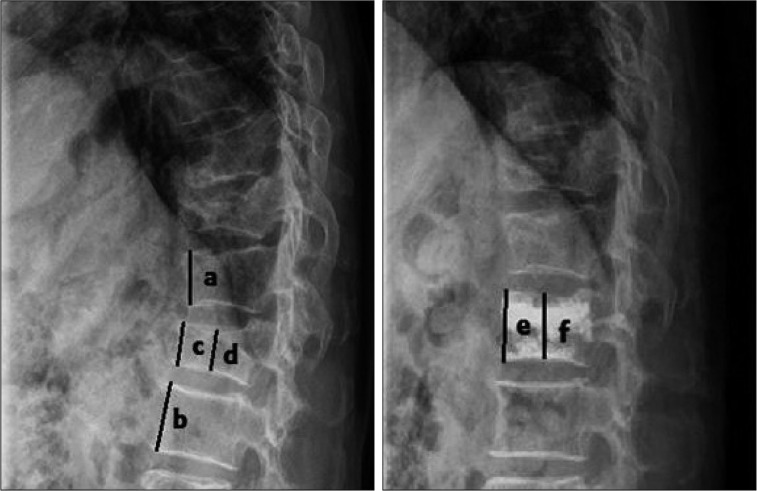
In addition, the anterior/middle vertebral height restoration rate was calculated and the intradiscal cement leakage was confirmed by checking the vertebral body height from the radiologic findings (Fig. 1). At the time of the review, the duration of follow-up was calculated. Vertebrae were categorized into two groups : those at the T-L junction and those outside the T-L junction.
Fig. 1.
Method used for determining vertebral body height restoration rate. Vertebral body height before compression fracture (Y) : Y=(a+b)/2, anterior height restoration (A) : A=[(e-c)/Y]×100 (%), middle height restoration (M) : M=[(f-d)/Y]×100 (%).
Statistical analysis
Data are presented as the mean standard deviations for continuous variables and as percentages for categorical variables. Differences between patients with and patients without new VCFs were assessed by using Student's t-test for continuous variables and the chi-square test for categorical variables. Estimates of the risk of new VCFs were based on the Kaplan-Meier method, with censoring on the date of death or the latest follow-up assessment. Survival differences were analyzed by log-rank test. Predictors of new VCFs were ascertained from the Cox proportional hazards model. All analyses were performed using Predictive Analytics SoftWare Statistics 18.0 (SPSS Inc., Chicago, IL, USA). A 2-tailed probability value of <0.05 was considered to be statistically significant.
RESULTS
Out of 244 VCF patients, 49 of them (33 women, 16 men; mean age, 71.6±8.5 years) experienced new VCFs after undergoing PVP. Demographics of patients stratified according to the presence or the absence of new VCFs are summarized in Table 1. The age, gender, mean body height/weight, mean BMI, and the initial fracture level showed no significant difference between the new VCFs group and the no VCFs group. Additionally, the mean volume of polymethylmethacrylate (PMMA) injected per vertebral body, one of the procedure-related factors, was smaller in the new VCFs group (5.5 mL) than no VCFs group (5.6 mL) although significantly so. The comparison between adjacent fractures group (5.7 mL) and nonadjacent fractures group (5.4 mL), subgroups of the no VCFs group and new VCFs group did not differ.
Table 1.
The comparison between the new VCFs group and no VCFs group by constitutional factors
*Statistically significant. VCFs : vertebral compression fractures, BMI : body mass index, BMD : bone mineral density, T-L junction : thoracolumbar junction, PMMA : polymethylmethacrylate
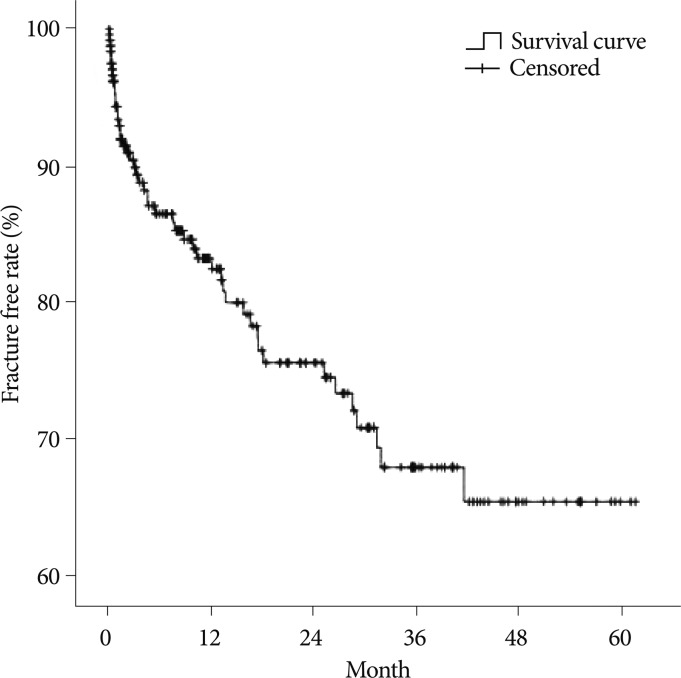
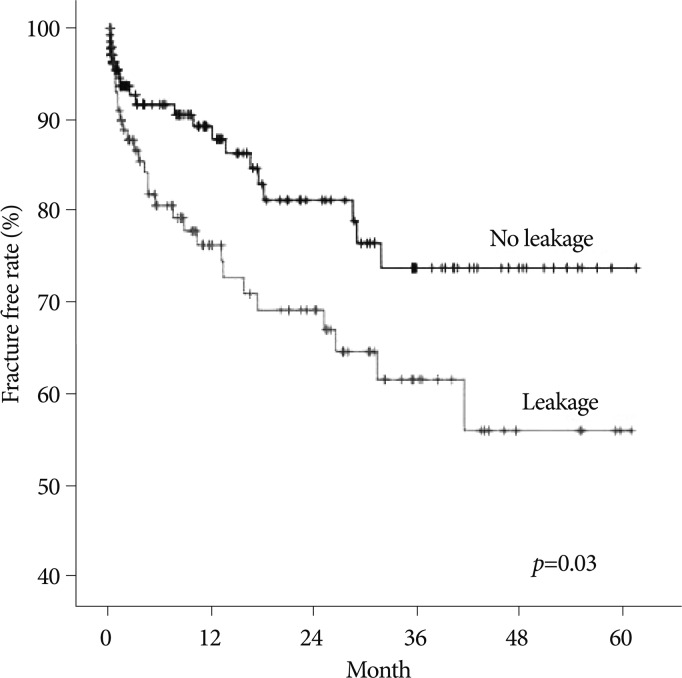
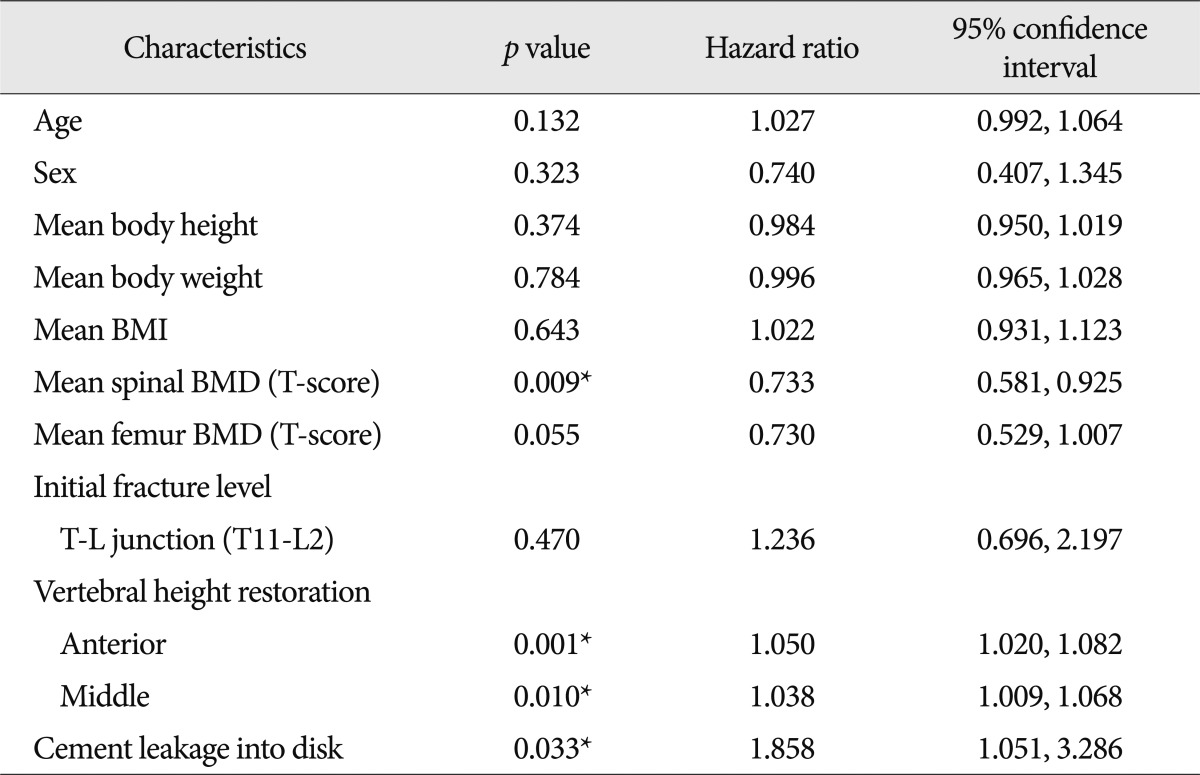
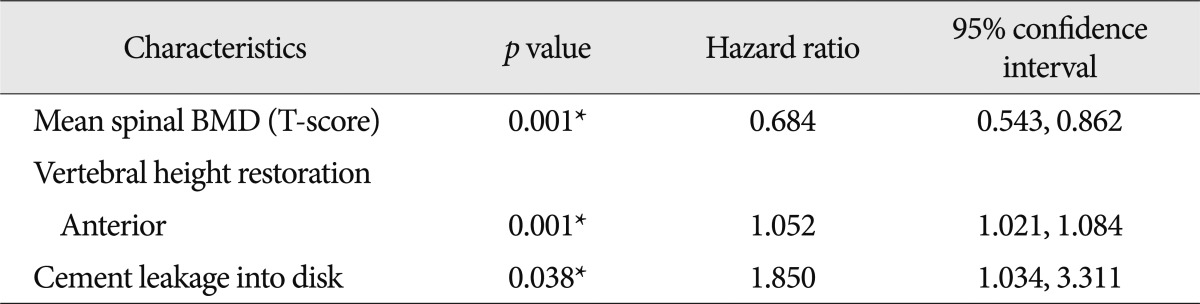
The spinal BMD and the femur BMD was lower in the new VCFs group than that of the no VCFs group. In detail, the T-score of the spinal BMD and femur BMD in the new VCFs group were -3.0±1.0 and -2.28±0.8, while the numbers were -2.5±1.3 and -2.0±0.9 in the no VCFs group. In addition, the anterior/middle vertebral height restoration rate (%) was significantly higher in the new VCFs group with the rate of 10.9±8.9/11.2±8.3, while the rate was 6.9±9.5/7.3±8.9 in the no VCFs group (p=0.007, 0.004), respectively. The presence of intradiscal cement leakage also was statistically higher in the new VCFs group (59.2%) then the no VCFs group (39.5%), (p=0.013). The mean interval between the onset of new VCFs and the initial PVP was 9.13±10.6 months (0.2-41.6 months). The Kaplan-Meier estimate of the 1-year fracture free rate after PVP was 82.5% (Fig. 2). The intradiscal cement leakage had a higher risk of new VCFs group (p=0.03 by the log-rank test) (Fig. 3). Using the Cox proportional hazards model, the significant independent variables for new VCFs after PVP were spinal BMD, anterior/middle vertebral height restoration, and intradiscal cement leakage (Table 2). Further investigation with Cox proportional hazards model revealed that spinal BMD [hazard ratio (HR) 0.684; p=0.001], anterior vertebral height restoration (HR 1.052; p=0.001), and intradiscal cement leakage (HR 1.850; p=0.038) were independent predictors of new VCFs after PVP (Table 3).
Fig. 2.
The Kaplan-Meier survival curve shows the estimated fracture-free rate of vertebrae after vertebroplasty.
Fig. 3.
The Kaplan-Meier survival curve shows the estimated fracture-free rate of vertebrae after vertebroplasty by intradiscal cement leakage.
Table 2.
Results of univariate Cox proportional hazards regression analysis
*Statistically significant. BMI : body mass index, BMD : bone mineral density, T-L junction : thoracolumbar junction
Table 3.
Results of multivariate Cox proportional hazards regression analysis
*Statistically significant. BMD : bone mineral density
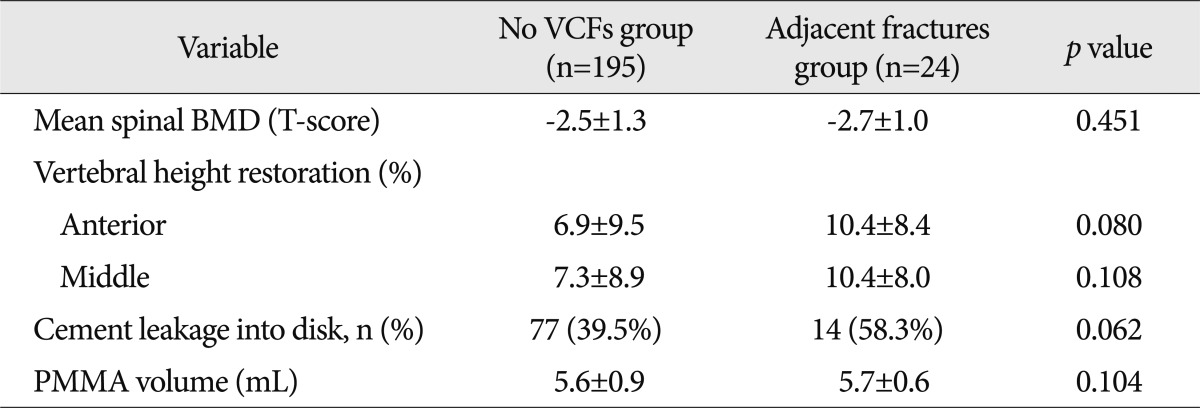
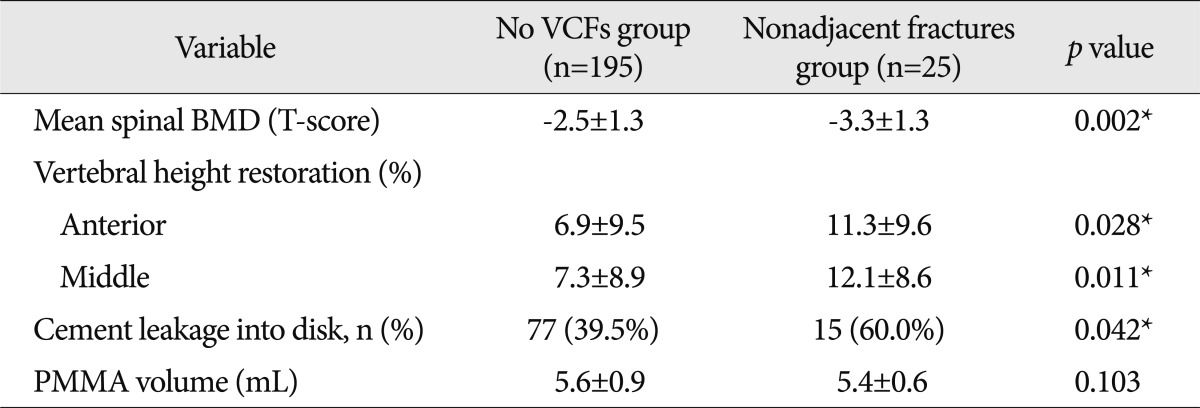
The spinal BMD, vertebral height restoration, intradiscal cement leakage, which are known risk factors, were compared and analyzed among no VCFs group, adjacent fractures group, and non-adjacent fractures group. Although there was no significant difference between no VCFs group and adjacent fractures group (Table 4), there was a significant difference between no VCFs group and nonadjacent fractures group in all those factors (Table 5).
Table 4.
The comparison between the no VCFs group and adjacent fractures group by risk factors
BMD : bone mineral density, VCFs : vertebral compression fractures, PMMA : polymethylmethacrylate
Table 5.
The comparison between the no VCFs group and nonadjacent fractures group by risk factors
*Statistically significant. BMD : bone mineral density, VCFs : vertebral compression fractures, PMMA : polymethylmethacrylate
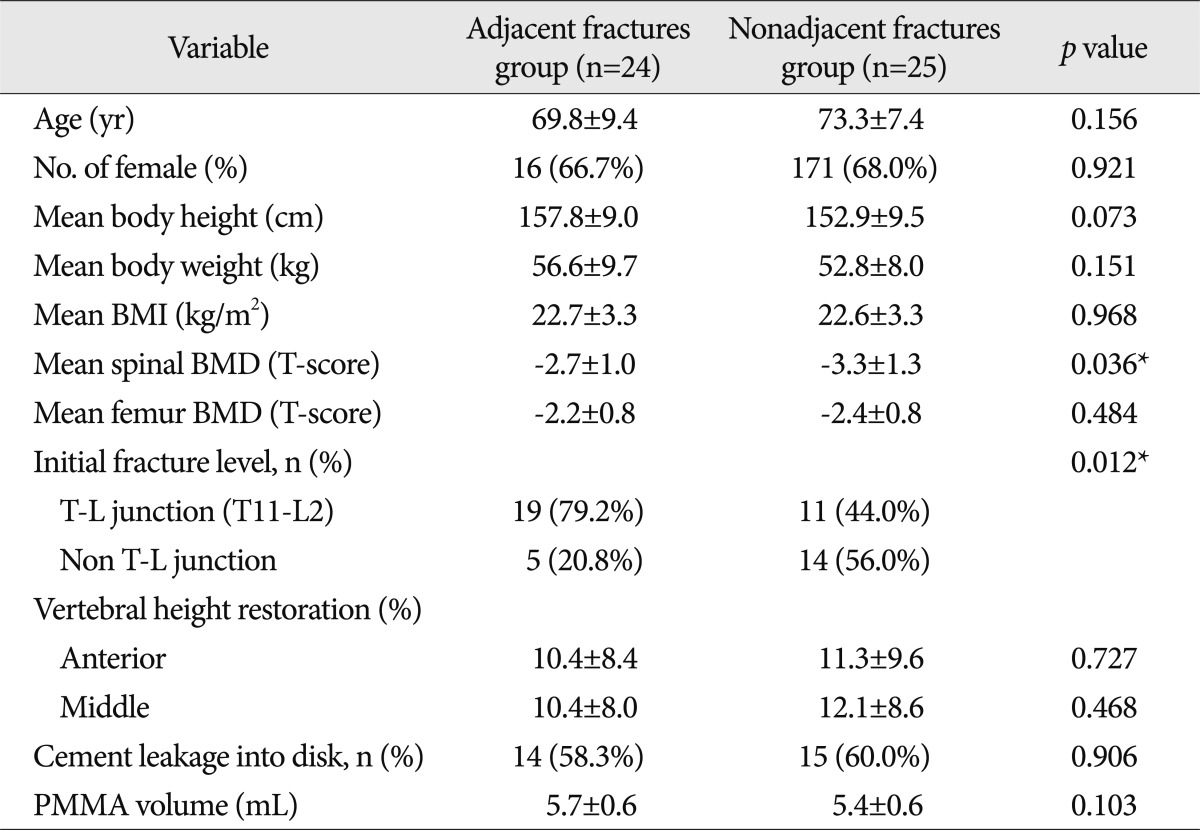
Demographics of patients stratified according to the adjacent or the nonadjacent fracture group of new VCFs are summarized in Table 6. The comparison of the spinal BMD and the initial fracture level between the subgroups of the new VCFs group, adjacent fractures group and the non-adjacent fractures group, showed statistically different results. The spinal BMD of the non-adjacent fractures group was -3.3±1.3, which was lower than the BMD of the adjacent fractures group (-2.7±1.0, p=0.036). Also, the proportion of T-L junctional fracture in the initial fracture level differed significantly in the adjacent fractures group (19, 79.2%) from the nonadjacent fractures group (11, 44.0%, p=0.012).
Table 6.
The comparison between the adjacent fractures group and nonadjacent fractures group by constitutional factors
*Statistically significant. BMI : body mass index, BMD : bone mineral density, T-L junction : thoracolumbar junction, PMMA : polymethylmethacrylate
DISCUSSION
The PVP is an effective treatment for vertebral compression fractures. However, unexpected vertebral fractures commonly occur after PVP, and these require additional PVP as a part of the treatment process. A number of studies have reported the incidence of serial vertebral fractures after PVP from 12% to 52%11,18,25,27,28). The cause and development of newly formed vertebral fractures were reviewed in several articles, but it is uncertain whether the PVP itself works as a cause of the fracture or not3,20,21). Therefore, we aimed to estimate the relationship of vertebral fracture with risk factors, and ways to reduce the fractures through the analysis of patterns of newly formed vertebral fractures.
In general, it is known that the degree of osteoporosis is risk factor for subsequent fracture24,25). However, there have been few reports about the effect of constitutional factors on the development of subsequent spinal fracture after vertebroplasty. As a result of univariate analysis and multivariate analysis in our study, lower BMD is an important constitutional factor of subsequent fractures after PVP. Spinal BMD in the new VCFs group was -3 in average, while it was -2.5 in the no VCFs group (p<0.05). The comparisons of lower BMD between nonadjacent fractures group and no VCFs group were significantly different. This result suggests spinal degenerative change as an important risk factor, in addition to the increased risk of fractures after procedures like PVP. Uppin et al.28) noted that when osteoporosis worsens, patients easily develop new fractures in adjacent vertebrae. Belkoff et al.5) reported that cement augmentation increases the strength and stiffness of the individual fractured vertebral bodies, which may place greater stress on the adjacent vertebrae. However, this study did not show significant differences in BMD between adjacent fractures group and no VCFs group. On the other hand, the initial fracture level of T-L junction was greater in adjacent fractures group than the nonadjacent fractures group, which suggested higher BMD, adjacent fractures after PVP can be explained since T-L junction has higher dynamic motility. In T-L junction level vertebral fractures, the increase in adjacent nucleus pulposus and endplate pressure change of untreated adjacent vertebra is caused by dynamic motility of this level although BMD is higher, leading to the development of fractures.
In addition to BMD, the cement leakage to the disk space can also be a risk factor of newly formed VCFs. We verified this result; the risk of new VCF in patients with cement leakage is 4.6 times higher than in patients without the cement leakage17). This study revealed intradiscal cement leakage as important risk factors, but there was no significant difference between no fractures group and adjacent fractures group. Many studies report that intradiscal cement leakage increases risk of new VCFs at the adjacent regions of PVP after new VCFs18,25). They reported that the intradiscal cement leakage increased the newly formed VCFs in an already weak disc or discs weakened by intradiscal leakage. These biomechanical changes caused by intradiscal cement leakage may affect new VCFs formation. Unlike such hypothesis, this study demonstrated intradiscal cement leakage to be a significant risk factor in nonadjacent fractures group. This may support the study of Ahn et al.1), which demonstrated the mechanism of nonadjacent-segment fracture after initial PVP. If the adjacent segment is already rigid, the pillar effect is not prominent through the adjacent segment. In that case, the augmentation strength may affect a mobile remote segment. The mobility gradient between a rigid adjacent segment and a relatively mobile remote segment may cause a subsequent fracture. By this mechanism, intradiscal cement leakage can works as factor that increase augmentation strength that affecting mobile remote segment. In addition, increased anterior vertebral height restoration is one of the well-known risk factor of VCFs. In particular, it increases the risk of the newly formed VCFs because of the restoration of vertebral height, and greater loading of adjacent vertebra16). Although this study did not showe significant differences between the no fractures group and adjacent fractures group, the fracture can increase due to the dynamic hammer effect in the same mechanism with cement intradiscal leakage, since the anterior vertebral restoration affect the development of remote segmental fractures in the comparison with nonadjacent fractures group. In conclusion, remote vertebra with lower BMD increases intradiscal cement leakage and an anterior vertebral body height, which enhances the stiffness and the strength gradient and caused environmental basement of the new VCFs formation in remote vertebral segment.
Elderly patients with a severely collapsed or deformed fracture at the T-L junction were at high risk of developing a non-union of the fracture22). In our study, new VCFs pattern showed that adjacent fracture occurred more frequently, if the initial fracture level is at the T-L junction, in comparison between the subgroups of new VCFs group, the adjacent fracture group, and the nonadjacent fracture group. In general, the T-L junction is the most dynamic in the flexion and extension of the spine23). Thus new VCFs occurrence was higher in an adjacent location, due to high dynamic motility in T-L junction. Also, new VCFs had lower BMD in a nonadjacent location (Table 6). The fracture that appears at a nonadjacent level can be explained by the vertebral body's disease activity, which develops through the degenerative change of the spine itself, due to lower BMD than an adjacent level.
The cement volume is the one of the major concerns regarding vertebroplasty. A large volume of cement is injected increasing stress on adjacent vertebra, raising risk of subsequent fracture. Many researchers have studied such problem, and found higher instances of fractures related to large volume of cement injection4,6,25). However, others insisted that there is no specific association between cement volume and consequent fractures13,15). In this review, PMMA volume and subsequent vertebral fractures not related.
Low BMI and low body weight are known to increase the risk of recurrent fracture on the spine or the hip8,26). In one study, lower BMI caused direct pillar effect on the adjacent vertebra and the weakened vertebra1). In another study, authors reported that patients with BMI less than 22 kg/m2 had a significantly greater chance of developing new VCFs after vertebroplasty19). The results of this study showed that comparison of weight, height and mean BMI of the adjacent and nonadjacent fracture groups did not result in statistically significant differences. Further, the age and gender was not significantly different between these groups.
In summary, the significant predictive factors for the post-PVP development of new VCFs were lower BMD, intradiscal cement leakage, anterior body restoration. Also, the comparison between no fractures group with nonadjacent fractures group and subgroups of new fractures group, may support the dynamic hammer effect explained by Ahn et al.1) in the case of nonadjacent segmental fracture.
Differences between our study and others could be because our study is a retrospective review, performed in only one institution and included only patients who underwent repeated intervention, not those patients with subsequent fracture treated with conservative management. In addition, the efficacy of the PVP, the degree of pain relief, medical treatment, physical activity, acquisition of follow up images of the patients after PVP were not reviewed. This review included patients who underwent repeated vertebroplasty, not conservative treatment. This limited our estimates of prevalence of subsequent vertebral fracture. However, our study also has considerable validity. The patients of each study group were compared under strict inclusion criteria, and the cases were selected from a relatively large patient population (365 consecutive patients). Further multi-institutional, prospective randomized controlled studies to determine the real risks with greater accuracy for future studies are required.
CONCLUSION
We analyzed patterns and risk factors of newly formed VCFs after PVP. This tended to occur more often with lower spinal BMD, the higher restoration rate of anterior vertebral height, and more leakage of cement to the disc space. In particular, the nonadjacent level fracture was found to be influenced by risk factors mentioned above. The assessment of risk factor on nonadjacent fracture group revealed that the fracture of the remote segment level occur more frequently by dynamic hammer effect. Also, the adjacent level fracture occurred more frequently when the initial fracture level was at the T-L junction. These results suggest the importance of medications to stabilize bone density, pain medication after PVP, wearing a back brace to reduce motion, meticulous technique when PVP is performed, and requirement of careful physical activity during daily life.
References
- 1.Ahn Y, Lee JH, Lee HY, Lee SH, Keem SH. Predictive factors for subsequent vertebral fracture after percutaneous vertebroplasty. J Neurosurg Spine. 2008;9:129–136. doi: 10.3171/SPI/2008/9/8/129. [DOI] [PubMed] [Google Scholar]
- 2.Amar AP, Larsen DW, Esnaashari N, Albuquerque FC, Lavine SD, Teitelbaum GP. Percutaneous transpedicular polymethylmethacrylate vertebroplasty for the treatment of spinal compression fractures. Neurosurgery. 2001;49:1105–1114. discussion 1114-1115. [PubMed] [Google Scholar]
- 3.Ananthakrishnan D, Berven S, Deviren V, Cheng K, Lotz JC, Xu Z, et al. The effect on anterior column loading due to different vertebral augmentation techniques. Clin Biomech (Bristol, Avon) 2005;20:25–31. doi: 10.1016/j.clinbiomech.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Baroud G, Heini P, Nemes J, Bohner M, Ferguson S, Steffen T. Biomechanical explanation of adjacent fractures following vertebroplasty. Radiology. 2003;229:606–607. doi: 10.1148/radiol.2292030378. author reply 607-608. [DOI] [PubMed] [Google Scholar]
- 5.Belkoff SM, Mathis JM, Jasper LE, Deramond H. The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine (Phila Pa 1976) 2001;26:1537–1541. doi: 10.1097/00007632-200107150-00007. [DOI] [PubMed] [Google Scholar]
- 6.Berlemann U, Ferguson SJ, Nolte LP, Heini PF. Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J Bone Joint Surg Br. 2002;84:748–752. doi: 10.1302/0301-620x.84b5.11841. [DOI] [PubMed] [Google Scholar]
- 7.Deramond H, Depriester C, Toussaint P, Galibert P. Percutaneous vertebroplasty. Seminars in Musculoskeletal Radiology. 1997;Vol 1:285–296. doi: 10.1055/s-2008-1080150. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara S, Kasagi F, Yamada M, Kodama K. Risk factors for hip fracture in a Japanese cohort. J Bone Miner Res. 1997;12:998–1004. doi: 10.1359/jbmr.1997.12.7.998. [DOI] [PubMed] [Google Scholar]
- 9.Galibert P, Deramond H, Rosat P, Le Gars D. [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty] Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- 10.Garfin SR, Yuan HA, Reiley MA. New technologies in spine : kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine (Phila Pa 1976) 2001;26:1511–1515. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 2000;39:1410–1414. doi: 10.1093/rheumatology/39.12.1410. [DOI] [PubMed] [Google Scholar]
- 12.Heini PF, Wälchli B, Berlemann U. Percutaneous transpedicular vertebroplasty with PMMA : operative technique and early results. A prospective study for the treatment of osteoporotic compression fractures. Eur Spine J. 2000;9:445–450. doi: 10.1007/s005860000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodler J, Peck D, Gilula LA. Midterm outcome after vertebroplasty : predictive value of technical and patient-related factors. Radiology. 2003;227:662–668. doi: 10.1148/radiol.2273011930. [DOI] [PubMed] [Google Scholar]
- 14.Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures : technical aspects. AJNR Am J Neuroradiol. 1997;18:1897–1904. [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufmann TJ, Trout AT, Kallmes DF. The effects of cement volume on clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2006;27:1933–1937. [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SH, Kang HS, Choi JA, Ahn JM. Risk factors of new compression fractures in adjacent vertebrae after percutaneous vertebroplasty. Acta Radiol. 2004;45:440–445. doi: 10.1080/02841850410005615. [DOI] [PubMed] [Google Scholar]
- 17.Komemushi A, Tanigawa N, Kariya S, Kojima H, Shomura Y, Komemushi S, et al. Percutaneous vertebroplasty for osteoporotic compression fracture : multivariate study of predictors of new vertebral body fracture. Cardiovasc Intervent Radiol. 2006;29:580–585. doi: 10.1007/s00270-005-0138-5. [DOI] [PubMed] [Google Scholar]
- 18.Lin EP, Ekholm S, Hiwatashi A, Westesson PL. Vertebroplasty : cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR Am J Neuroradiol. 2004;25:175–180. [PMC free article] [PubMed] [Google Scholar]
- 19.Lin WC, Cheng TT, Lee YC, Wang TN, Cheng YF, Lui CC, et al. New vertebral osteoporotic compression fractures after percutaneous vertebroplasty : retrospective analysis of risk factors. J Vasc Interv Radiol. 2008;19:225–231. doi: 10.1016/j.jvir.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Lindsay R, Burge RT, Strauss DM. One year outcomes and costs following a vertebral fracture. Osteoporos Int. 2005;16:78–85. doi: 10.1007/s00198-004-1646-x. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 22.Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26:1634–1640. [PMC free article] [PubMed] [Google Scholar]
- 23.Pope MH. Biomechanics of the lumbar spine. Ann Med. 1989;21:347–351. doi: 10.3109/07853898909149219. [DOI] [PubMed] [Google Scholar]
- 24.Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919–923. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 25.Syed MI, Patel NA, Jan S, Harron MS, Morar K, Shaikh A. Intradiskal extravasation with low-volume cement filling in percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2005;26:2397–2401. [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas T, Barou O, Vico L, Alexandre C, Lafage-Proust MH. Recurrence of vertebral fracture with cyclical etidronate therapy in osteoporosis : histomorphometry and X-Ray microanalysis evaluation. J Bone Miner Res. 1999;14:198–205. doi: 10.1359/jbmr.1999.14.2.198. [DOI] [PubMed] [Google Scholar]
- 27.Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty : adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol. 2006;27:217–223. [PMC free article] [PubMed] [Google Scholar]
- 28.Uppin AA, Hirsch JA, Centenera LV, Pfiefer BA, Pazianos AG, Choi IS. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 29.Winking M, Stahl JP, Oertel M, Schnettler R, Böker DK. Treatment of pain from osteoporotic vertebral collapse by percutaneous PMMA vertebroplasty. Acta Neurochir (Wien) 2004;146:469–476. doi: 10.1007/s00701-004-0259-7. [DOI] [PubMed] [Google Scholar]