Abstract
Objective
Detection of focal non-hemorrhagic lesion (NHL) has become more efficient in diffuse axonal injury (DAI) patients using an MRI. The aims of this study are to find out the radiological distribution, progress of NHL and its clinical significance.
Methods
Between September 2005 and October 2011, 32 individuals with NHLs on brain MRI were enrolled. NHLs were classified by brain location into 4 major districts and 13 detailed locations including cortical and subcortical, corpus callosum, deep nuclei and adjacent area, and brainstem. The severity of NHL was scored from grades 1 to 4, according to the number of districts involved. Fourteen patients with NHL were available for MRI follow-up and an investigation of the changes was conducted.
Results
Thirty-two patients had 59 NHLs. The most common district of NHL was cortical and subcortical area; 15 patients had 20 NHSs. However the most common specific location was the splenium of the corpus callosum; 14 patients had 14 lesions. The more lesions patients had, the lower the GCS, however, this was not a statistically meaningful difference. On follow-up MRI in 14 patients, out of 24 lesions, 13 NHLs resolved, 5 showed cystic change, and 6 showed atrophic changes.
Conclusion
NHLs were located most commonly in the splenium and occur frequently in the thalamus and the mesial temporal lobe. Because most NHS occur concomitantly with hemorrhagic lesions, it was difficult to determine their effects on prognosis. Since most NHLs resolve completely, they are probably less significant to prognosis than hemorrhagic lesions.
Keywords: Traumatic brain injury, Diffuse axonal injury, Magnetic resonance imaging, Corpus callosum, Non-hemorrhagic
INTRODUCTION
Diffuse axonal injury (DAI) is commonly induced by sudden acceleration-deceleration or rotational forces and the subsequent tissue injury is characterized by axonal stretching, disruption and eventual separation of nerve fibers2,3,6,21,25). The anatomical sites commonly affected are corticomedullary junctions, located in frontal and temporal regions as well as the corpus callosum, upper brainstem, and deep gray matter1,3). Three grades of DAI have been described by Adams et al.3) based on their neuropathological studies : Grade 1 DAI denotes widespread, microscopic axonal damage in any location; in Grade 2 DAI there are additional focal abnormalities in the corpus callosum; and in Grade 3 DAI, focal lesions in the rostral brainstem are also found.
As a result of recent developments in radiological techniques and widespread use of magnetic resonance imaging (MRI) in traumatic brain injury (TBI) patients, detection of focal non-hemorrhagic lesions (NHL) has become more efficient, as compared to previous X-ray or computer tomography (CT). NHLs after head trauma have been noticed as a form of DAI8,13). But, the lesions were not clearly defined compared to the hemorrhagic lesions. Until now, there were several fragmentary mentions about NHL. Gentry et al.8) defines NHL as a lesion which cannot be found by CT but can found by MRI, and Topal et al.28) defines NHL as a lesion which cannot be found by gradient echo (GRE) sequence MRI but can found by diffusion-weight (DW) sequence MRI. NHL has sometimes been described as traumatic white matter damage, shear injury DAI with its most pathological findings as retraction ball, delayed axotomy and cytoskeletal disruption4,20,21,26). However, the distribution, evolution and developmental mechanism of NHL are not well known and there have been no detailed studies.
The aims of this study are to find out the radiological distribution, progress of NHL and its clinical significance.
MATERIALS AND METHODS
Between September 2005 and October 2011, 32 patients aged 6 to 75 years with moderate to severe TBI with NHL on brain MRI were enrolled. Brain MRI was carried out 1 to 78 days (average 12 days) after trauma, and included T1, T2, T2 flair axial images, susceptibility weighted imaging (SWI), T1 sagittal and T2 coronal images. MRI was performed using a 3.0T Achieva Philips imaging system. The protocols of axial spin echo T1-weighted imaging are 25 slices, slice thickness 5 mm, TR 450.0 msec, TE 10.0 msec, FOV 220×220 mm, reconstructed voxel size 0.430 mm, axial turbo spin echo T2-weighted imaging 25 slices, slice thickness 5 mm, TR 3000.0 msec, TE 80.0 msec, FOV 220×220 mm, reconstructed voxel size 0.449 mm, maximum/minimum intensity projection SWI 25 slices, slice thickness 5 mm, TR 25.5 msec, TE 36.2 msec, FOV 220×220 mm, reconstructed voxel size 0.371 mm, coronal turbo spin echo T2-weighted imaging 25 slices, slice thickness 5 mm, TR 3000.0 msec, TE 80.0 msec, FOV 220×220 mm, reconstructed voxel size 0.449 mm, and sagittal T1-weighted imaging 23 slices, slice thickness 5 mm, TR 430.0 msec, TE 10.0 msec, FOV 220×220 mm, reconstructed voxel size 0.430 mm.
Imaging analysis - the definition, classification, and grading of NHL
Inclusion criteria
NHL is defined as demonstration of low signal at T1, and hyperintense signal change at T2 and T2 flair image but without hypointense signal change at SWI, which is most sensitive for hemorrhages10,27).
Exclusion criteria
Peri-lesional edema due to hemorrhagic lesion was excluded, as well as old lacunae infarction or degenerative white matter lesions thought to be present before the trauma.
These NHLs in our patient sample were classified by location into 4 major districts and 13 detail locations including cortical and subcortical, corpus callosum, deep nuclei and adjacent area and brainstem. Cortical and subcortical were further divided into cortical, mesial temporal (hippocampus or parahippocampal gyrus), and subcortical. Lesions in the corpus callosum were divided into splenium, splenium and body. There were no lesions of the rostrum or genu of the corpus callosum. The deep nuclei and adjacent area included internal capsule, thalamus, basal ganglia and cerebellum, and the brainstem were divided into ventral, dorsal, dorso-ventral, and cerebellar peduncle.
The severity of NHL was graded from 1 to 4, according to the number of districts involved. If limited to one district, regardless of the number of NHL, it was assigned as Grade 1. The same system was applied for Grades 2 and 3. Grade 4 indicates that NHLs were present in all 4 districts. We investigated the relativity of the grading of NHL to the Glasgow coma scale score (GCS) of the patient upon admission, and the prognosis as calculated by the Glasgow outcome scale score (GOS) system.
We also investigated hemorrhagic lesions accompanying the NHL. The presence of traumatic subarachnoid hemorrhage (T-SAH), intraventricular hemorrhage (IVH) and the location of hemorrhagic contusion (H-contusion) were taken into consideration while subdural hematoma and epidural hematoma were excluded because they are extra-axial lesions.
Fourteen patients with NHL were available for MRI follow-up and investigations of the changes in the original NHLs were conducted. The initial evaluation of patients included sex, age, type of trauma, and GCS score upon admission. The prognosis was recorded by GOS taken during the last visit to the hospital, and GOS analyses of patients who were not available for in-hospital follow-up were conducted by telephone.
RESULTS
Patient characteristics
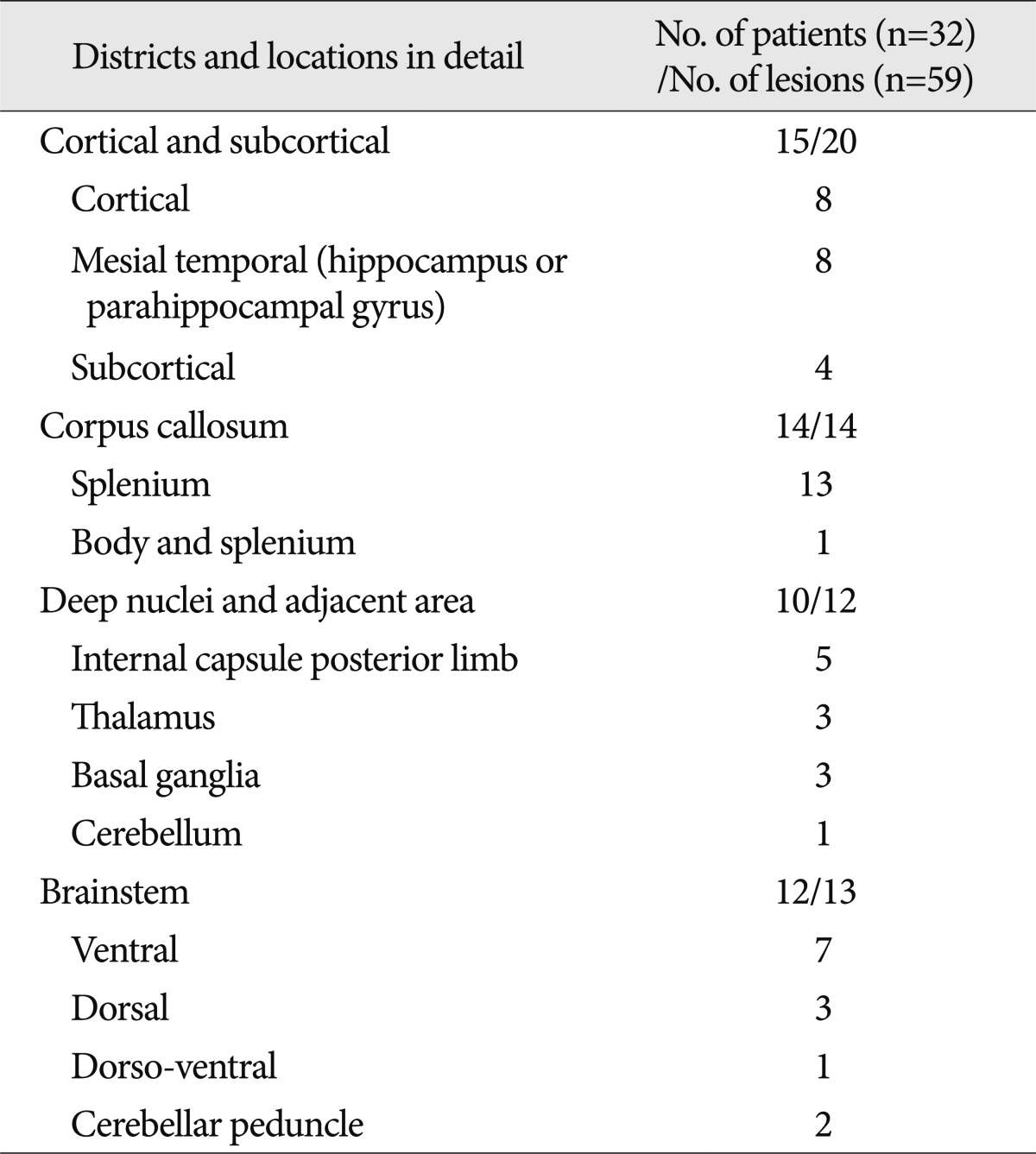
From September 2005 to October 2011, 59 NHLs were found in 32 patients who underwent post-injury (average 12 days, range 1-78 days) brain MRIs. The average age of the patients was 34.2 (range 6-75 years); 26 patients were male and 6 patients were female. The cause of trauma for 19 patients was car, motorbike or bike accidents and the rest were falls and fighting injuries. Fifteen patients had initial GCS scores of 7 or lower due to severe head injury, and 17 had scores greater than 8. Fifteen patients showed "good recovery", 4 patients "moderate disability", 8 patients "severe disability", 5 patients vegetative states, and there were no deaths (Table 1).
Table 1.
Demographic summary of patients with non-hemorrhagic brain lesions after head trauma
GCS : glasgow coma scale score, GOS : glasgow outcome scale score, TBI : traumatic brain injury
Distribution and locations of NHLs
When looking at the distribution of NHL, the cortical and subcortical areas had 20 lesions in 15 patients, the corpus callosum area 14 lesions in 14 patients (Fig. 1), the deep nuclei and adjacent area 12 lesions in 10 patients (Fig. 2, 3), and the brain stem area 13 lesions in 12 patients (Fig. 4). The most common location for single lesions was the splenium of the corpus callosum, with 13 lesions, and cortical and hippocampal or parahippocampal gyral lesions followed with 8 each (Table 2). All of the internal capsular lesions were located in the posterior limb. Brainstem lesions were more common in the ventral area (n=7) than the dorsal area (n=3). One lesion occupied almost the entire lateral half (dorso-ventral) of the given axial view of the pons. Two lesions were found in the middle cerebellar peduncle.
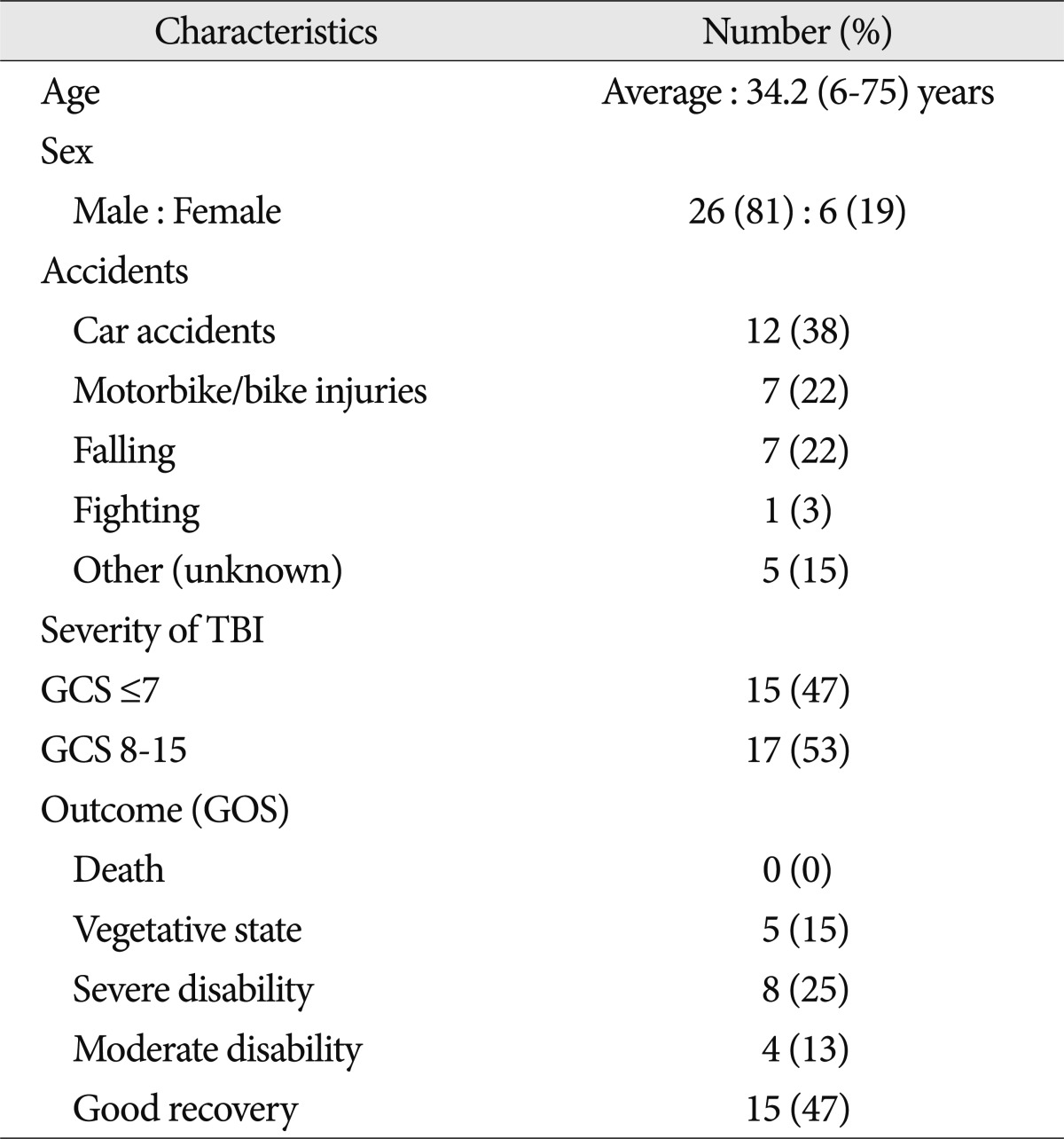
Fig. 1.
A non-hemorrhagic lesion of the corpus callosum. A 21-year-old male was admitted due to a bicycle accident. Upon admission his Glasgow coma scale score was 6 points. MRI shows a non-hemorrhagic lesion (arrows) at the splenium of the corpus callosum. Non-hemorrhagic lesions show high signal intensity at T2W1 (A) and FLAIR (B), but dark signals cannot be seen on SWI (C).
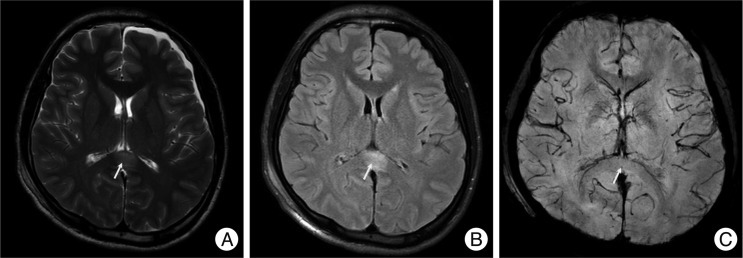
Fig. 2.
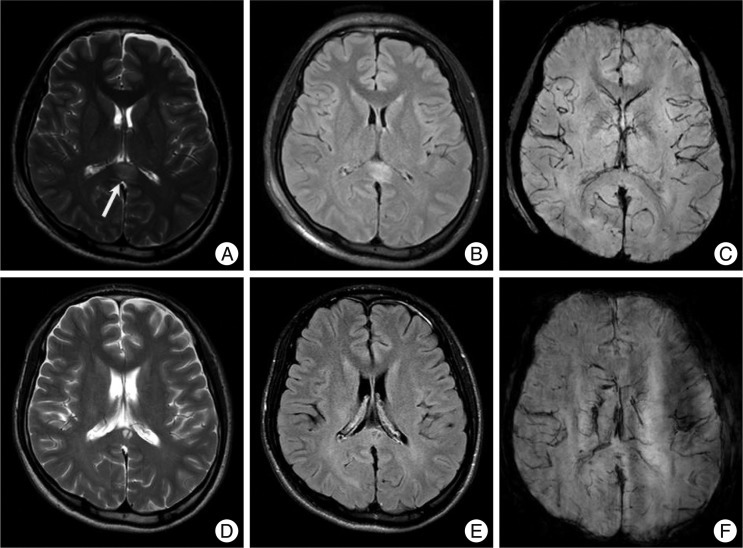
A non-hemorrhagic lesion of the thalamus area. A 57-year-old male was admitted due to a pedestrian car accident, with a Glasgow coma scale of 7 on admission. MRI shows a non-hemorrhagic lesion at the right thalamus (arrow). Non-hemorrhagic lesions show high signal intensity at T2W1 (A) and FLAIR (B), but a dark signal cannot be seen on SWI (C). However, at one month follow-up, the previous high signal at T2W1 (D), FLAIR (E) is not seen and SWI (F) still showed no dark signals.
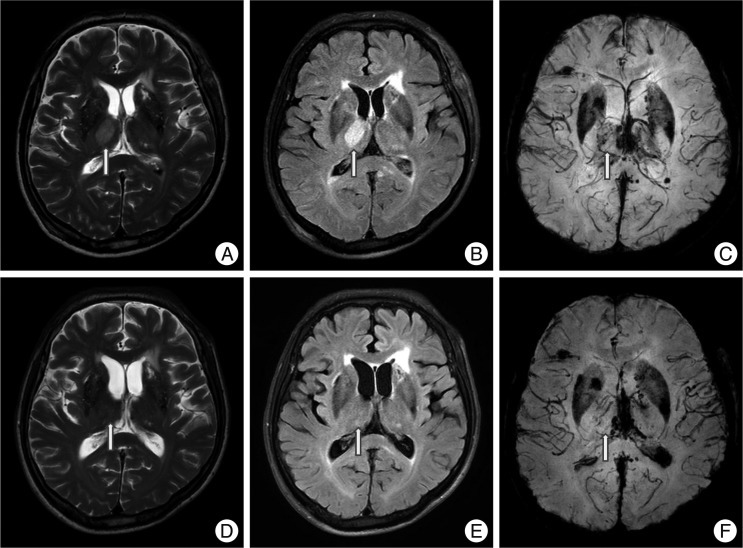
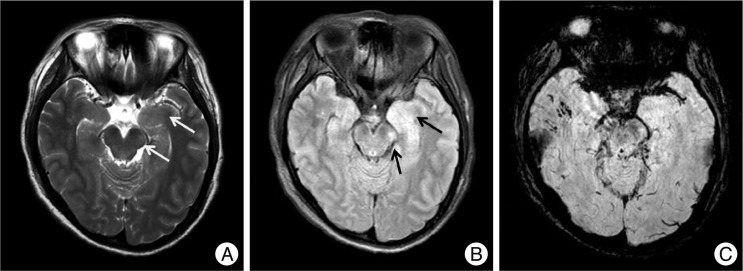
Fig. 3.
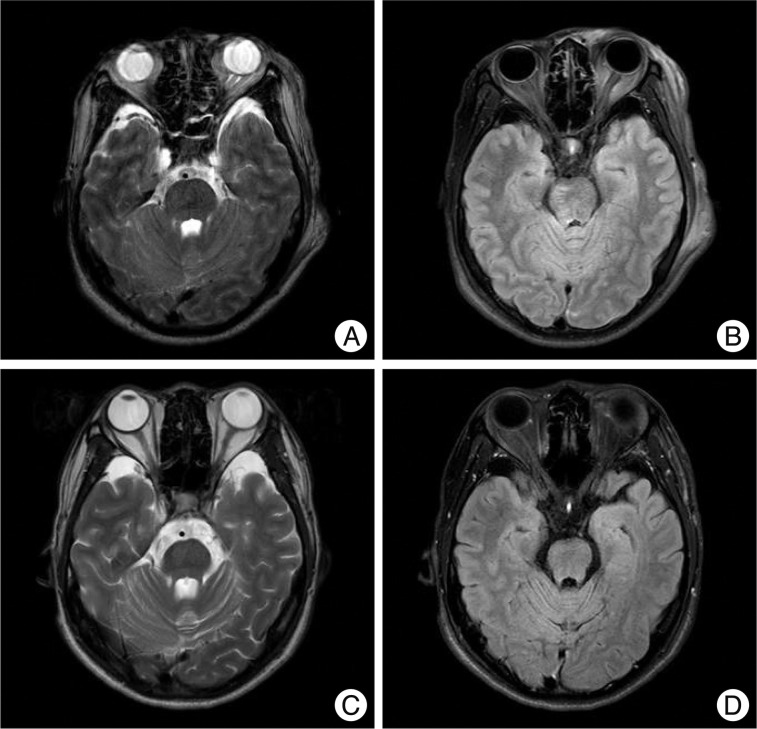
A non-hemorrhagic lesion of the internal capsule. A 45-year-old male fell and was admitted to the emergency room. His initial Glasgow coma scale was 4 points and he was in a semi-coma. MRI shows a non-hemorrhagic lesion in the left internal capsule posterior limb at T2W1 (arrow, A) and FLAIR (B) with no dark signal at SWI (C). Hemorrhagic lesions are also noted in the corpus callosum, thalamus, brainstem and cerebellum.
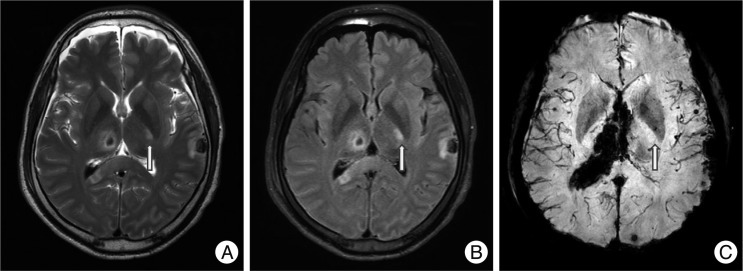
Fig. 4.
Non-hemorrhagic lesions of the brainstem and hippocampus. A 35-year-old woman was admitted in a semicomatose state after a fall. T2WI (A) and FLAIR (B) shows a bright dot on the left crus cerebri of the midbrain (arrows) and SWI (C) does not show low signal intensity. Another non-hemorrhagic lesion is suspected on the left hippocampus. FLAIR (B) shows a bright signal intensity lesion from the left pes hippocampus to the body of the hippocampus, which is not prominent on T2WI (A) except slightly swollen pes hippocampus. Several petechial hemorrhagic lesions are also noted in the right temporal subcortical area and left mesial temporal area.
Table 2.
Locations of non-hemorrhagic brain lesions
Severity of NHL
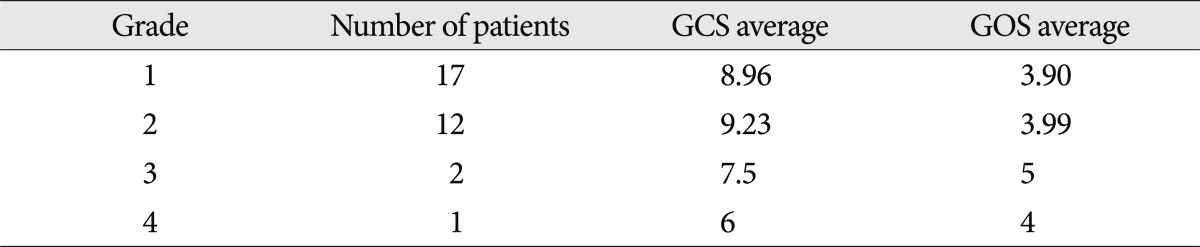
Grades 1, 2, 3 and 4 included 17, 12, 2 and 1 patients, respectively. The higher the grade of NHL, the lower the GCS, however, this was not a statistically meaningful difference. GOS was worst in the NHL grade 3 group but NHL grade was not correlated with GOS (Table 3).
Table 3.
Severity of non-hemorrhagic lesions and initial and final neurologic state
GCS : glasgow coma scale score, GOS : glasgow outcome scale score
The distribution of hemorrhagic lesions accompanying NHLs
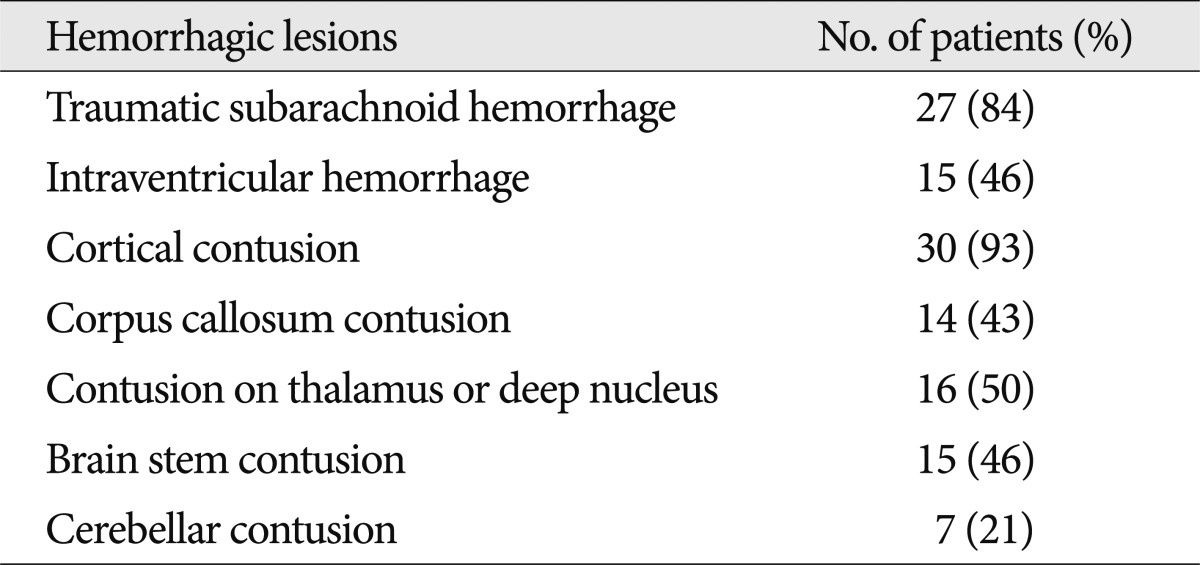
Out of 32 patients with NHL, all but one had hemorrhagic lesions. There were a total of 124 hemorrhagic lesions, which is much greater than the number of NHLs. Twenty-seven patients had T-SAH. Fifteen patients had IVH. H-contusions in subcortical area were noted in 30 patients, in the thalamus or deep nuclei in 16 patients, in the brainstem in 15 patients and in the cerebellum in 7 patients (Table 4).
Table 4.
Combined hemorrhagic lesions
Evolution of NHL
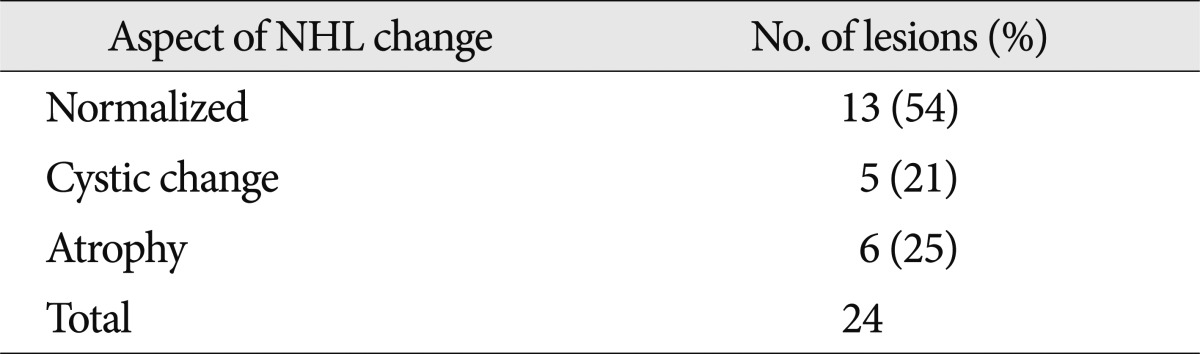
Twenty-four NHLs in 14 patients were available for MRI follow-up. Thirteen of the 24 NHLs disappeared with no traces (Fig. 2), 5 showed cystic changes (Fig. 5), and 6 showed atrophy (Fig. 6) (Table 5).
Fig. 5.
Cystic changes of non-hemorrhagic lesions on the corpus callosum. A 22-year-old man with a splenial non-hemorrhagic lesion (arrow, A : T2WI, B : FLAIR, C : SWI). Follow-up images in three months show a small cyst on the same area of splenium (D : T2WI, E : FLAIR, F : SWI).
Fig. 6.
Atrophy of non-hemorrhagic lesion on brainstem. A 34-year-old man with a lesion on the right dorso-ventral midbrain. Follow-up images at two years show no abnormal signal in the midbrain, however, the volume of the midbrain seems to be smaller than the initial images especially on the right side (A : initial T2WI, B : initial FLAIR, C : 2-year follow-up T2WI, D : 2-year follow-up FLAIR).
Table 5.
Evolution of non-hemorrhagic lesions
NHL : non-hemorrhagic lesion
DISCUSSION
DAI was referred to by several names such as shearing injury, diffuse damage to white matter of immediate impact type, diffuse white matter shearing injury or inner cerebral trauma4,9,20,26,30). Adams et al.3) questioned whether focal damage could occur in the brain stem as a result of a head injury as an isolated event and have suggested by themselves DAI was a clinical syndrome of primary brain injury and classified it as three grades based on involved locations. Their study was a histopathological result of 434 cases of non-missle head injuries and as they mentioned this study has limitations in that DAI might have been obscured by other type of contusions and hematomas or brain damage secondary to increased intracranial pressure, hypoxia or infarctions and in that their study was performed in forensic work, the identification and definition of less severe forms of DAI was not fully performed. There needs a brief review of their reports to clarify the difference with our study. According to their reports, the focal lesions in the corpus callosum were typically hemorrhagic and tended to lie to one side of the midline, occurred most frequently in the inferior part of the corpus callosum and occasionally restricted to the splenium. The brain stem lesions were in the dorsolateral quadrants of the rostral brain stem. They also noticed that there were some lesions with no hemorrhage and those lesions were histological evidence of vacuolation and rarefaction of the affected tissue3).
NHLs have been identified and noticed in several literatures but not clearly defined. Topal et al.28) reported 5 cases of DAIs who had had mild head injury of GCS score between 13 and 15. They indicated one of the 5 cases had a lesion showing hyperintense on FLAIR and DW image but no signal change on GRE image and defined the lesion as 'non-hemorrhagic'. Gentry et al.8) described that acute callosal lesions were commonly nonhemorrhagic in nature on MRI features because 21 of 31 cases (67.7%) showed nonhemorrhagic nature. However, these cases seemed to include the edema surrounding the hemorrhagic lesion in that they mentioned as 'Usually, large nonhemorrhagic zones of injury surrounded the foci of blood'. The same authors reported brain stem injuries as well but just mentioned as NHLs are more detectable on T2-weighted MRI than T1-weighted MRI or CT7).
Location and injury mechanism
The greatest number of NHLs in our study was found in the most exterior part of brain, the cortical and subcortical areas. When looking at a subdivided category, however, the most common area was the splenium of the corpus callosum, in which 13 of 59 lesions were found. This distribution may be caused by traumatic laceration by the free edge of the falx20). However, this explanation is not feasible except for a small number of cases11,12,15,16). Lindenberg et al.15-17) stated that shear-strain forces and direct blows to the vertex of the head, specifically the superior level of the corpus callosum, result in traumatic lacerations. One explanation gaining prominence is that shear-strain deformation is an essential element of such injuries. Gennarelli et al.6) performed an important series of primate experiments and demonstrated that nonimpact rotational acceleration of the head in the lateral, or oblique-lateral directions uniformly produces traumatic corpus callosum injury. Although the falx does not play a direct role in corpus callosum injuries, it may play an indirect role8). Shear-strain forces do not develop between tissues of the same density if they are free to move as a unit. When some portions of the accelerating and rotating brain lag behind the faster adjacent moving areas, shear strains develop between the tissues. This can easily occur in the callosal region because the bulky and independently mobile cerebral hemispheres are connected by the less mobile corpus callosum. The rigid falx prevents the cerebral hemispheres from moving across the midline. Shear strains, therefore, develop across the connecting point (corpus callosum) of the two hemispheres8).
Five NHLs were found in the posterior limb of the internal capsule. The corticospinal, corticobulbar, and corticorubral tracts pass through the posterior limb of the internal capsule, pathways that may be prone to deformation by shear strain force because their vertical paths are long.
There are also numerous lesions in the thalamus, basal ganglia, cerebellum, and the medial temporal lobe including the hippocampus and parahippocampal gyrus. These portions are thought to be underestimated with hemorrhagic lesions. The injury mechanism is also to be solved issue.
The brainstem lesions are more common in the ventral portion rather than the dorsolateral area. The especially high prevalence of lesions at the crus cerebri of the midbrain is thought to be due to shear injury to the long tract that descends from the posterior limb of the internal capsule. This result differs from Adams diffuse axonal injury classification in terms of location. The mechanism of the injury to the dorsolateral aspect was explained that the area may contact the free edge of the tentorium at the tentorial incisura7,13). However, the tentorial incisura is actually in relation to the midbrain. Anteriorly the incisura extends above the optic chiasm to the lamina terminalis, the middle incisural space is located between the midbrain and tentorial edge and posterior incisural space is located the posterior midbrain and encompasses the quadrigeminal cistern24). Our study includes two cases of cerebellar peduncular lesions where cerebellar afferent and efferent fibers either entered or exited. With these results and the anatomical characteristics of cerebral and cerebellar peduncles which are main transfer zone of long tracts, NHLs in brainstem may represent a kind of shear injury
Clinical significance
When examining GCS upon admission and GOS, Grades 1 and 2 were similar in terms of GCS but Grades 3 and 4 had lower scores. However, this was not a statistically meaningful difference. GOS did not show much of a difference. As observed during the follow-up of 14 patients, NHLs have a 50% probability of disappearing completely on MRI, suggesting that NHLs are less detrimental to neurologic state than hemorrhagic lesions. Furthermore, as the effect of hemorrhagic lesions on neurologic state is severe, it seems inappropriate to examine only the severity of NHL lesions. When examining changes that occurred in the 24 NHL lesions in 14 patients with MRI follow-up, more than half of the patients showed complete resolution, which indicates that NHL predicts good prognosis or has less of an effect on prognosis. In the other patients, 5 showed cystic change, and 6 showed atrophic changes. It is likely that the NHLs in these patients did not resolve and that delayed axotomy progressed and resulted in deformation18,19,22,23).
Potential limitations of our study include small sample size and lack of histologic confirmation of the findings. Furthermore, initial and last follow-up MRI period after trauma is not uniform. It can cause errors in analyzing images because lesions change over time. For detecting NHLs, DW images can be helpful5,13,14,29).
CONCLUSION
NHLs that are clearly visualized on brain MRI are less well known than hemorrhagic lesions. We found that NHLs occur mostly in the splenium of the corpus callosum and also occur frequently in the thalamus and the mesial temporal lobe. However, most occur concomitantly with hemorrhagic lesions, making it difficult to determine their effects on prognosis. Since most NHLs resolve completely, they are probably less significant to prognosis than hemorrhagic lesions. Location and character of NHLs are similar to the DAI lesions. A prospective study with using DW images in more patients might be helpful for detecting the NHLs and clarifying its characteristics.
Acknowledgements
This research was supported by the Chung-Ang University Research Grants in 2008.
References
- 1.Adams JH. Diffuse axonal injury in non-missile head injury. Injury. 1982;13:444–445. doi: 10.1016/0020-1383(82)90105-x. [DOI] [PubMed] [Google Scholar]
- 2.Adams JH. The autopsy in fatal non-missile head injuries. Curr Top Pathol. 1988;76:1–22. doi: 10.1007/978-3-642-71353-8_1. [DOI] [PubMed] [Google Scholar]
- 3.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury : definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 4.Adams H, Mitchell DE, Graham DI, Doyle D. Diffuse brain damage of immediate impact type. Its relationship to 'primary brain-stem damage' in head injury. Brain. 1977;100:489–502. doi: 10.1093/brain/100.3.489. [DOI] [PubMed] [Google Scholar]
- 5.Ezaki Y, Tsutsumi K, Morikawa M, Nagata I. Role of diffusion-weighted magnetic resonance imaging in diffuse axonal injury. Acta Radiol. 2006;47:733–740. doi: 10.1080/02841850600771486. [DOI] [PubMed] [Google Scholar]
- 6.Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564–574. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- 7.Gentry LR, Godersky JC, Thompson BH. Traumatic brain stem injury : MR imaging. Radiology. 1989;171:177–187. doi: 10.1148/radiology.171.1.2928523. [DOI] [PubMed] [Google Scholar]
- 8.Gentry LR, Thompson B, Godersky JC. Trauma to the corpus callosum : MR features. AJNR Am J Neuroradiol. 1988;9:1129–1138. [PMC free article] [PubMed] [Google Scholar]
- 9.Grcević N. The concept of inner cerebral trauma. Scand J Rehabil Med Suppl. 1988;17:25–31. [PubMed] [Google Scholar]
- 10.Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 11.Hardman JM. The pathology of traumatic brain injuries. Adv Neurol. 1979;22:15–50. [PubMed] [Google Scholar]
- 12.Komatsu S, Sato T, Kagawa S, Mori T, Namiki T. Traumatic lesions of the corpus callosum. Neurosurgery. 1979;5:32–35. doi: 10.1227/00006123-197907010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kubal WS. Updated imaging of traumatic brain injury. Radiol Clin North Am. 2012;50:15–41. doi: 10.1016/j.rcl.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Le TH, Gean AD. Radiology of traumatic brain injury. In: Winn HR, editor. Youman's Neurological Surgery. Vol 4. Philadeophia: Elsevier; 2011. pp. 3342–3361. [Google Scholar]
- 15.Lindenberg R. Significance of the tentorium in head injuries from blunt forces. Clin Neurosurg. 1964;12:129–142. doi: 10.1093/neurosurgery/12.cn_suppl_1.129. [DOI] [PubMed] [Google Scholar]
- 16.Lindenberg R, Fisher RS, Durlacher SH, Lovitt WV, Jr, Freytag E. Lesions of the corpus callosum following blunt mechanical trauma to the head. Am J Pathol. 1955;31:297–317. [PMC free article] [PubMed] [Google Scholar]
- 17.Lindenberg R, Freytag E. The mechanism of cerebral contusions. A pathologic-anatomic study. Arch Pathol. 1960;69:440–469. [PubMed] [Google Scholar]
- 18.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury : a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- 19.Meaney DF, Margulies SS, Smith DH. Diffuse axonal injury. J Neurosurg. 2001;95:1108–1110. doi: 10.3171/jns.2001.95.6.1108. [DOI] [PubMed] [Google Scholar]
- 20.Peerless SJ, Rewcastle NB. Shear injuries of the brain. Can Med Assoc J. 1967;96:577–582. [PMC free article] [PubMed] [Google Scholar]
- 21.Povlishock JT. Pathophysiology of neural injury : therapeutic opportunities and challenges. Clin Neurosurg. 2000;46:113–126. [PubMed] [Google Scholar]
- 22.Povlishock JT. Traumatically induced axonal injury : pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- 23.Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans : a review of current thoughts. J Neurotrauma. 1995;12:555–564. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- 24.Rhoton AL., Jr Tentorial incisura. Neurosurgery. 2000;47:S131–S153. doi: 10.1097/00006123-200009001-00015. [DOI] [PubMed] [Google Scholar]
- 25.Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatry. 1956;19:163–185. doi: 10.1136/jnnp.19.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strich SJ. Lesions in the cerebral hemispheres after blunt head injury. J Clin Pathol Suppl (R Coll Pathol) 1970;4:166–171. doi: 10.1136/jcp.s3-4.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong KA, Ashwal S, Holshouser BA, Shutter LA, Herigault G, Haacke EM, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury : improved detection and initial results. Radiology. 2003;227:332–339. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- 28.Topal NB, Hakyemez B, Erdogan C, Bulut M, Koksal O, Akkose S, et al. MR imaging in the detection of diffuse axonal injury with mild traumatic brain injury. Neurol Res. 2008;30:974–978. doi: 10.1179/016164108X323799. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Rasmussen IA, Lagopoulos J, Håberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J Neurotrauma. 2007;24:753–765. doi: 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman RA, Bilaniuk LT, Genneralli T. Computed tomography of shearing injuries of the cerebral white matter. Radiology. 1978;127:393–396. doi: 10.1148/127.2.393. [DOI] [PubMed] [Google Scholar]