Abstract
Previous studies have shown that injection of extracellular products (ECP) of Pseudoalteromononas atlantica isolated from shell disease-infected edible crabs (Cancer pagurus) into healthy crabs causes rapid death. In this study we examined the nature of the active lethal factor(s) in ECP. Injection of ECP into crabs caused a rapid decline in the total number of circulating hemocytes (blood cells), and the crabs died within 60 to 90 min. The individuals that died showed eyestalk retraction, limb paralysis, and lack of antennal sensitivity, suggesting that the active factor(s) targeted the nervous system. Histopathological investigations showed that affected crabs had large aggregates of hemocytes in the gills, and there was destruction of the tubules in the hepatopancreas. The active factor in ECP was not sensitive to heat treatment (100°C for 30 min) and proteinase K digestion. As lipopolysaccharide (LPS) was a potential candidate for the lethal factor, it was purified from whole P. atlantica bacteria or ECP and subsequently injected into crabs. These crabs had all of the external symptoms observed previously with ECP, such as limb paralysis and eyestalk retraction, and they died within 90 min after challenge, although no significant decline in the number of circulating hemocytes was observed. Similarly, in vitro incubation of hemocytes with purified LPS (1 to 20 μg) from P. atlantica did not result in the clumping reaction observed with ECP but did result in a degranulation reaction and eventual cell lysis. Injection of crabs with Escherichia coli or Pseudomonas aeruginosa LPS (1 μg g of body weight−1) did not cause any of the characteristic symptoms observed following exposure to P. atlantica LPS. No mortality of crabs followed the injection of E. coli LPS, but P. aeruginosa LPS caused ca. 80% mortality at 2 h after injection. Overall, these results show that the main virulence factor of P. atlantica for edible crabs is LPS either alone or in combination with other heat-stable factors.
Shell disease syndrome is an economically important disease of crustaceans that is characterized by the degradation of the exoskeleton by chitinolytic (chitinoclastic) bacteria. After initial damage to the outer epicuticle, the underlying chitin-containing layers of the cuticle are exposed to the enzymatic activities of various chitinolytic bacteria and fungi. The members of this microbial community are likely to be highly diverse, but bacteria belonging to the genera Aeromonas, Alteromonas, Flavobacterium, Moraxella, Pasteurella, Photobacterium, and Vibrio have been isolated from areas of degraded cuticle (3) and may therefore play synergistic roles in lesion progression. Such microbial activities result in a decrease in the economic value of crustaceans, such as lobsters and crabs, because of the unsightly effects on the cuticle of the animals. Furthermore, following extensive damage to the cuticle, mortality of crustaceans may occur as a result of unsuccessful molting (13). Until recently, this disease was thought to affect only the outer cuticle of the animals, but recent studies have shown that individuals with extensive cuticular lesions also have septicemia which may lead to mortality (17). In these studies, Vogan et al. (17) isolated several different species of chitinolytic bacteria from the lesions and blood (hemolymph) of edible crabs (Cancer pagurus) with this disease. Initial studies revealed that several of these isolates produced extracellular products (ECP) that caused rapid mortality when they were injected into C. pagurus (17). One isolate that produced lethal ECP was identified as a strain of Pseudoalteromonas atlantica (unpublished observations).
Pseudoalteromonads are naturally occurring, gram-negative bacteria which are very common in marine and estuarine environments. The genus Pseudoalteromonas was created to accommodate 12 “Alteromonas” species (2). Pseudoalteromonas atlantica is well known for its extracellular agarases, which have been fully characterized (9, 15), and other extracellular enzymes, such as metalloproteases (5) and a serine protease that possesses algicidal activity (8).
In the present study we examined the nature of the lethal factor(s) in the ECP of P. atlantica and the effect of ECP on crab tissues, including the blood cells (hemocytes).
MATERIALS AND METHODS
Animals and injections.
The edible crabs (C. pagurus) used in this study were obtained from commercial pots kept off the Gower Peninsula or Guernsey, United Kingdom The animals were maintained in aerated tanks in a circulating seawater aquarium and were fed regularly with cockles and chopped whitefish. All crabs were intermolt individuals with a carapace width between 130 and 170 mm. The volume of each injection administered to a crab was calculated to be ca. 1% of the total hemolymph (blood) volume. The blood volume of crabs was determined as described previously (12). Injection and bleeding sites were surface sterilized with 70% ethanol before needles were inserted. Injections were made with disposable 23-gauge needles through the soft membrane of the coxa at the base of the fifth pereiopod.
Bacteria.
An isolate from shell disease-infected edible crabs, identified as P. atlantica by 16S ribosomal DNA sequencing by NCIMB Ltd. (Aberdeen, United Kingdom), was routinely maintained on marine agar 2216 (Becton-Dickinson, Oxford, United Kingdom).
ECP preparation.
ECP of P. atlantica were produced by the cellophane overlay method of Zhang and Austin (19). Briefly, sterilized cellophane sheets were placed on tryptic soy agar plates (Becton-Dickinson) with 2% NaCl and inoculated with 200-μl portions of overnight cultures grown in tryptic soy broth (Becton-Dickinson) supplemented with 2% NaCl. After incubation for 72 h at 28°C, the bacteria grown on the cellophane together with associated ECP were scraped into 1.5 ml of ice-cold, isotonic, sterile, calcium-free marine saline (MS) (0.4 M NaCl, 9 mM KCl, 30 mM NaSO4, 3 mM NaHCO3; pH 7.4). Following centrifugation at 12 000 × g for 30 min at 4°C, the supernatant (ECP) was sterilized by filtration (0.45-μm-pore-size filters followed by 0.22-μm-pore-size filters). Aliquots of ECP were stored at −80°C until they were used. For heat treatment experiments, ECP was heated at 100°C for 30 min and centrifuged at 12,000 × g for 10 min before it was used to remove any precipitate. The protein concentration of the ECP was determined by a commercial bicinchoninic acid (BCA) assay (Pierce Warriner, Chester, United Kingdom) by using bovine serum albumin as a standard according to the manufacturer's instructions. The protein concentration of most batches of ECP was adjusted to ca. 2.0 mg ml−1 prior to use.
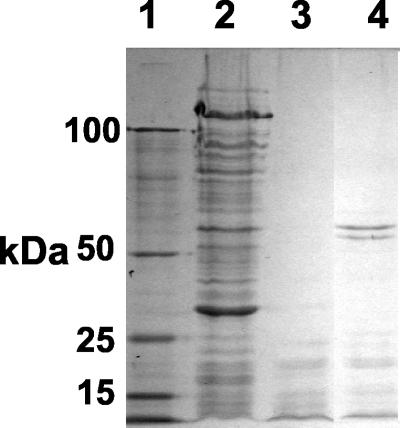
For proteinase K treatment of P. atlantica ECP, ECP was mixed at an ECP/proteinase K ratio of either 6:1 or 5:1 with a solution containing 2.5 mg of proteinase K (Sigma Chemical Co., Poole, United Kingdom) per ml and incubated at 60°C for 1 h. As a control, ECP was replaced in the reaction mixture by distilled water. To avoid any potential proteolytic digestion of crab tissues by the proteinase K, at the end of the reaction period ca. 250 μl of Complete protease inhibitor (Roche Diagnostics GmbH, Mannheim, Germany) was added to both types of samples. Samples of ECP treated with proteinase K for different times and with different concentrations were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (12% acrylamide) and stained with Coomassie blue.
Blood cell (hemocyte) counts.
Total hemocyte counts (THC) were determined at different times following intrahemocoelic injection of P. atlantica ECP, purified P. atlantica lipopolysaccharide (LPS), Escherichia coli 0111:B4 LPS (Sigma), or Pseudomonas aeruginosa 10 LPS (Sigma) (all at a concentration of 1 μg g of body weight−1) or following intrahemocoelic injection of sterile MS. A 0.2-ml sample of hemolymph was collected in an equal volume of ice-cold Baker's formalin fixative (3 M formaldehyde, 60 mM calcium acetate, 5 mM NaCl), and the THC were determined by using an improved Neubauer hemocytometer.
LPS isolation and purification from P. atlantica.
P. atlantica was grown overnight at 25°C in tryptic soy broth with 2% NaCl, washed by centrifugation several times, and resuspended in a sterile 3.2% NaCl solution. The final pellet was resuspended in distilled water and lyophilized. LPS was purified from the lyophilized material by the phenol-water method (18), with modifications described by Gu et al. (4). Approximately 0.2 g of lyophilized bacteria was suspended in 14 ml of ultrapure water at 68°C, and an equal volume of 90% phenol, preheated to 68°C, was then added with vigorous stirring. The mixture was incubated at 68°C for 15 min and then cooled to 10°C. The resulting emulsion was centrifuged at 1,500 × g and 4°C for 45 min. The upper aqueous phase was aspirated off, and the remainder was reextracted with ultrapure water as described above. To reduce phospholipid contamination, sodium acetate (5 mg ml−1) was added to the combined aqueous phases, and LPS was precipitated with 2 volumes of cold acetone. The pellet was then washed twice with 70% ethanol to reduce trace concentrations of phenol in the sample and suspended in ultrapure water. RNase and DNase (final concentration, 80 μg ml−1; Sigma) were added, and the digestion mixture was incubated at 37°C for 4 h. Proteinase K (final concentration, 25 μg ml−1; Sigma) was added, and the preparation was incubated at 60°C overnight. The digested mixture was centrifuged at 150,000 × g for 5 h, and the gel-like LPS was lyophilized and stored at −20°C. The LPS yield was ca. 1% of the dry weight of bacteria.
LPS was also partially purified from P. atlantica ECP by using the same approach but without the DNase-RNase and proteinase K steps. The resultant LPS yield was ca. 0.1% (wt/vol) of the original ECP.
The concentrations of LPS in ECP and in purified LPS preparations were estimated by using the 1,9-dimethylmethylene blue (DMB) assay (6) and a commercial Limulus amebocyte kinetic chromogenic assay (Cambrex Bio Science, Verviers, Belgium). To test for the presence of any contaminating protein in these purified LPS preparations, samples were reacted with a BCA kit for total protein. Purified LPS from P. atlantica was also electrophoresed on SDS-PAGE gels (14% acrylamide with 4 M urea) and stained for LPS by using the silver stain method of Tsai and Frasch (14).
Histological examination of crab tissues following injection of P. atlantica ECP or LPS.
To determine the effect of undiluted ECP, proteinase K-treated ECP, or purified P. atlantica LPS (1 μg g of body weight−1) on the hemocytes and tissues of C. pagurus, crabs (five or six animals for each treatment) were injected with these preparations (the volume injected was ≤1% of the total hemolymph volume), and immediately following death ca. 30 ml of Bouin's seawater fixative was injected into each crab. The gills and hepatopancreas were dissected into an excess volume of fixative and stored in the dark until further processing. Following dehydration, clearing, and wax impregnation, sections (thickness, ca. 10 μm) were cut and stained with Cole's hematoxylin and eosin. At least four blocks from each tissue per animal were cut. Photographs were taken with an Olympus digital camera by using an Olympus BX50 microscope (Olympus Optical, London, United Kingdom).
Hemocyte incubation with P. atlantica LPS and ECP.
Monolayers of edible crab blood cells were prepared to investigate the effects of P. atlantica ECP or purified LPS (1 to 20 μg ml−1) on these cells in vitro. Hemolymph (0.5 ml) was collected in an equal volume of ice-cold marine anticoagulant (0.4 M NaCl, 0.1 M glucose, 30 mM trisodium citrate, 25 mM citric acid, 9 mM EDTA), centrifuged, and replaced with calcium-free MS, and the number of cells was adjusted to 1 × 106 cells ml−1. Fifty microliters of this cell suspension was placed on each glass microscope slide, and the cells were left to attach for 20 min at room temperature. The blood cell monolayers were then incubated with 100 μl of either undiluted ECP (in MS), purified LPS from P. atlantica (1 to 20 μg ml−1) in MS, or MS (control) for different periods of time (>90 min) prior to examination and photography.
Statistical analyses.
Statistical analyses were performed with Instat2 (GraphPad Software, San Diego, Calif.) by using Student's t test or analysis of variance (ANOVA) with a Bonferroni posttest as appropriate.
RESULTS
Effect of injection of ECP from P. atlantica on crabs.
In crabs injected with undiluted ECP (protein concentration, ca. 2.0 mg ml−1) there was a rapid onset of behavioral changes like those observed previously following injection of live P. atlantica bacteria or broth in which these bacteria had been grown (17). In particular, there were early changes in the ventilatory activity of crabs, with an initial increase followed by a later decrease. At a later time following injection (ca. 30 min), the eyestalks retracted and the antennae became fixed. At this stage most crabs failed to react to either visual or mechanical stimuli of the antennae. Finally, by 60 min in most crabs there was a lack of limb turgor; raising the animals resulted in all of the walking legs sagging with no response to mechanical stimulation. The time scale of such changes varied, but usually crabs showed no reaction to all stimuli (antennae, eyestalks, and limbs) after 50 to 120 min (mean, 74 min; n = 5). Such crabs were considered to be dead. Injection of crabs with a fivefold dilution of ECP had similar behavioral effects, but the time taken for symptoms to become apparent increased and death occurred at 70 to 100 min (mean, 85 min; n = 5). Only 40% of the crabs injected with the fivefold dilution of ECP died. The other 60% had none of the symptoms, such as eyestalk retraction and loss of limb activity, and these crabs survived. Injection of a 10-fold dilution of ECP resulted in no mortality.
Effect of P. atlantica ECP on the number of circulating hemocytes.
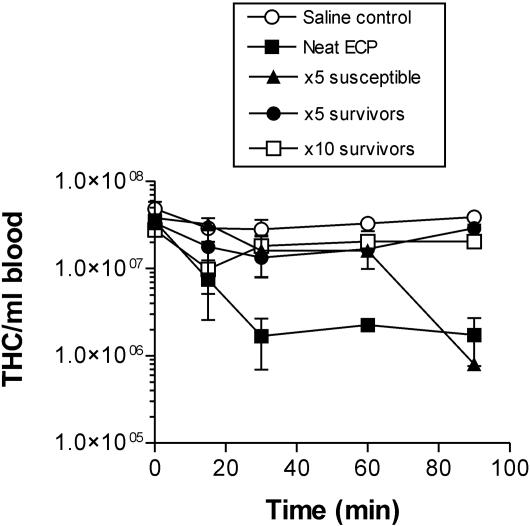
The effect of injection of ECP on the THC is shown in Fig. 1. Injection of undiluted P. atlantica ECP caused a rapid reduction in the THC, and only ca. 4.8% of the cells remained in circulation at 90 min after injection. In the case of the fivefold dilution of ECP, in crabs that eventually died following injection there was also a rapid reduction in the THC, while in the crabs that survived there was no significant reduction in the THC compared to the THC of the saline-injected crabs (Fig. 1). In crabs injected with the 10-fold dilution of ECP, all of which survived, there were also no significant changes in the THC (Fig. 1).
FIG. 1.
Effect of ECP injection on the THC in the edible crab C. pagurus. Crabs (ca. 150 g) were injected with undiluted ECP (Neat ECP) (2 mg of total protein ml−1) or a fivefold (×5) or 10-fold (×10) dilution of ECP in MS. For the crabs that were injected with the fivefold dilution of ECP, blood cell counts were determined for susceptible animals (crabs that died) and animals that were not susceptible. The values are means ± standard errors of the means (n = 5).
Histopathology after P. atlantica ECP injection.
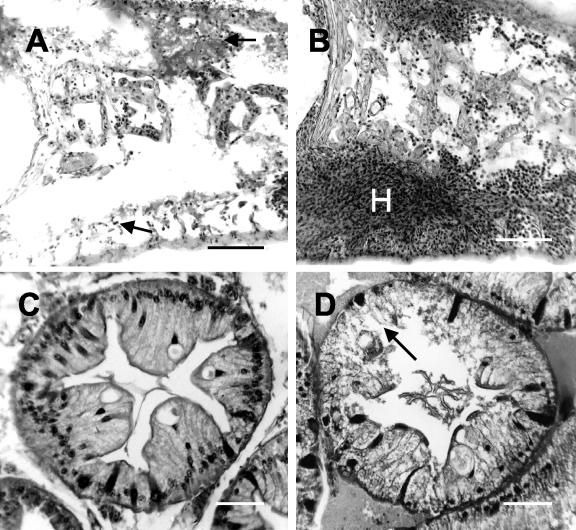
To determine the fate of the hemocytes following injection of undiluted ECP, crabs were sacrificed and tissues were processed for histology. The gills of ECP-injected crabs had large accumulations of hemocytes in both the central branchial septa and the lamellae that branch from these structures. In the branchial septa such accumulations were found at the margins of the hemal (blood) channels (Fig. 2B). Such accumulations were not seen in control (saline-injected) crabs, in which hemocytes occurred mainly as individual cells embedded in a flocculated (precipitated) hemolymph (Fig. 2A). Diffuse clumps of hemocytes were often observed in the lamellae of ECP-injected crabs, particularly at the terminal ends of these structures (data not shown). Such clumps were absent from the gills of saline-injected individuals (data not shown).
FIG. 2.
Effect of injection of undiluted ECP (final concentration, 1% of calculated hemolymph volume) on the gills (A and B) and hepatopancreas (C and D) of edible crabs. (A and B) Histological sections through the branchial septum in the gills of crabs injected with either saline (A) or ECP (B). Note the presence of extensive hemocyte clumps (H) in the gills of ECP-injected crabs and the lack of similar clumps in the control (arrows). Scale bars = 100 μm. (C and D) Sections through crab hepatopancreatic tubules after injection of saline (C) or ECP (D). Note the apparent tubule necrosis (arrow) following injection of ECP. Scale bars = 50 μm. The sections were stained with hematoxylin and eosin.
As described previously, during septicemic infections of crabs, the hepatopancreatic tubules show a variable amount of damage depending on the level of intrahemocoelic infection (16). Following exposure to undiluted ECP, similar tubule damage was observed in the hepatopancreas, and there were clear signs of necrosis, including flocculation of the cytoplasm and sloughing of cell contents into the central lumen of the tubules (Fig. 2D). In contrast, sections of hepatopancreas from saline-injected animals were mainly intact, and there was no sign of cell debris in the central lumen (Fig. 2C).
Effect of P. atlantica ECP on hemocytes in vitro.
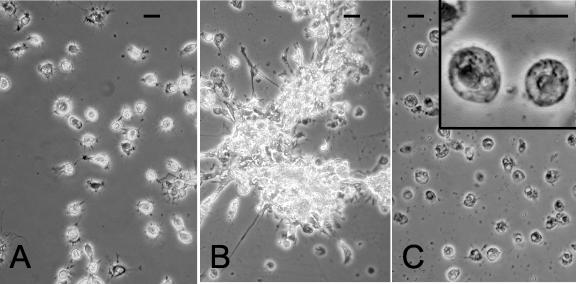
Exposure of crab hemocyte monolayers to ECP resulted in a rapid clumping reaction without any apparent loss of hemocyte viability in the time scale of the experiment (>90 min), while hemocytes incubated in saline showed no such clumping reaction (compare Fig. 3A and B).
FIG. 3.
Phase-contrast micrographs showing the appearance of crab (C. pagurus) hemocyte monolayers after 30 min of incubation with either 100 μl of saline (A), 100 μl of undiluted P. atlantica ECP (B), or 100 μl of a solution containing 5 μg of purified LPS from P. atlantica bacteria ml−1 (C). Note the vacuolated appearance of hemocytes exposed to LPS (panel C and inset) and the extensive clumping reaction of ECP-treated hemocytes. In contrast, cells incubated in saline alone remained viable and unclumped. Scale bars = 10 μm.
Nature of active factors in P. atlantica ECP.
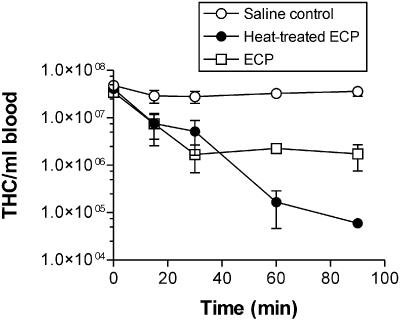
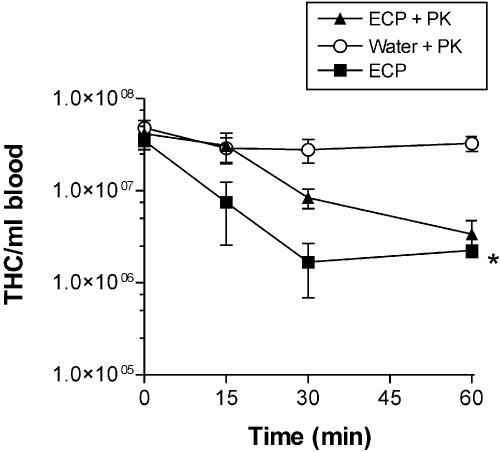
To determine the heat stability of the factors involved in the changes in THC and mortality, crabs were injected with either undiluted ECP or heat-treated (100°C for 30 min) undiluted ECP. Crabs in both groups exhibited progressive behavioral changes like those described above that resulted in death after 30 to 60 min. Similarly, injection of both native and heat-treated ECP into crabs resulted in rapid decreases in the THC that exhibited similar dynamics for the first 30 min postinjection (Fig. 4). A statistical comparison of the decreases in the THC showed that there were no significant differences between the two treatments (P > 0.05, as determined by ANOVA with a Bonferroni posttest). Proteinase K treatment of ECP was investigated to determine if degradation of protein in ECP affected either the crab mortality or the hemocyte response to injection. Incubation of ECP with proteinase K (final concentration, 25 μg ml−1) (ratio, 5:1) for 60 min was found to remove all of the major proteins observed in Coomassie blue-stained SDS-PAGE gels (Fig. 5). Injection of proteinase K-treated ECP from P. atlantica resulted in rapid mortality of crabs like that observed with ECP alone, and although the initial rate of decline in the THC observed following injection of proteinase K-treated ECP was apparently less than that seen with native ECP, the difference was not statistically different (Fig. 6). Statistical comparison showed that there was a significant difference at 60 min between the numbers of hemocytes in the control and both proteinase K-treated ECP and untreated ECP (P < 0.05, as determined by ANOVA with a Bonferroni posttest). However, there were no significant differences between the THC from crabs after injection of either ECP or proteinase K-treated ECP at any single time. All crabs injected with proteinase K alone (following treatment with protease inhibitor) survived with no significant changes in the THC (Fig. 6).
FIG. 4.
Effect of heat treatment (100°C for 30 min) of ECP on the THC in C. pagurus. The values are means ± standard errors of the means (n = 3.)
FIG. 5.
Coomassie blue-stained SDS-PAGE gels of molecular weight markers (lane 1), undiluted ECP (lane 2), ECP treated with proteinase K (ratio, 5:1) for 60 min (lane 3), and ECP treated with proteinase K (ratio, 6:1) for 60 min (lane 4).
FIG. 6.
Effect of treatment of ECP from P. atlantica with proteinase K (PK) (ratio, 5:1) for 60 min on the number of circulating hemocytes per milliliter of blood (THC). *, P < 0.05 for a comparison of proteinase K-treated ECP or ECP with the control containing water and proteinase K alone. The values are means ± standard errors of the means (n = 5).
As the initial experiments suggested that the active lethal factor in ECP was not a heat-sensitive protein, we decided to investigate whether ECP contained LPS, another potentially toxic molecule. By using the Limulus amebocyte lysate chromogenic assay, the concentration of LPS in undiluted ECP (ca. 2 mg of protein ml−1) was found to be >500 EU ml−1. The DMB assay could not be used to estimate the LPS concentration in these samples due to the presence of other interfering contaminants.
Effect of purified LPS from P. atlantica on crabs.
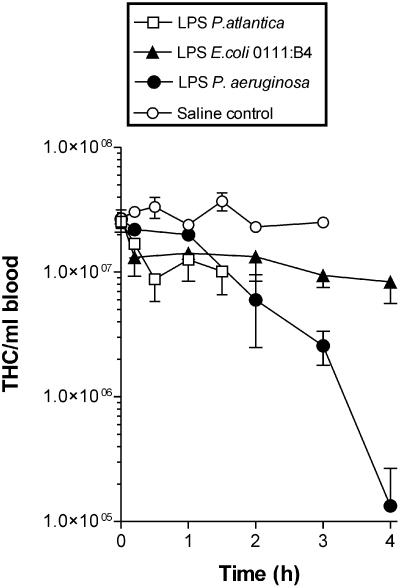
When purified LPS from the P. atlantica isolate was injected into crabs at a concentration of 1 μg g of body weight−1, all individuals displayed the same pattern of behavioral changes and mortality that was originally observed with the P. atlantica ECP or viable bacteria. By 120 min, all individuals were considered to be moribund. In terms of changes in the THC, the reduction was significantly less than that observed with ECP (compare Fig. 1 with Fig. 7). Indeed, statistical analysis showed that there was no significant difference between LPS injections and control (saline) injections in terms of the THC (P > 0.05, as determined by ANOVA with a Bonferroni posttest). Histological examination of the gills from LPS-injected crabs showed that there were small clumps of hemocytes in the branchial septal region which were not seen in the saline-injected controls, while in the hepatopancreas there was some indication of early tubular damage (data not shown).
FIG. 7.
Effect of purified LPS (1 μg g of body weight−1) from either P. atlantica, E. coli, or P. aeruginosa on the total number of circulating hemocytes (THC) per milliliter after injection. The values are means ± standard errors of the means (n = 3).
Effect of purified LPS from P. atlantica on hemocytes in vitro.
Incubation of purified LPS (1 to 20 μg) from P. atlantica with hemocytes resulted in degranulation and vacuolization of the cells. By 30 min, the hemocytes were swollen, the pseudopodia were withdrawn, and the nuclei became pyknotic (compare Fig. 3A and C). Unlike what was observed with ECP, no clumps of hemocytes were formed following exposure to purified P. atlantica LPS.
Effects of E. coli LPS and P. aeruginosa LPS on crabs.
For a comparison with the experiments performed with LPS purified from P. atlantica, crabs were injected with either E. coli LPS or P. aeruginosa LPS, and the resulting changes in the THC and mortality were examined. P. aeruginosa LPS (1 μg g of body weight−1) caused a rapid, time-dependent reduction in THC that was significantly different from the response to injection of either saline or P. atlantica LPS (Fig. 7). At 120 min, 80% of the crabs injected were considered dead, while by 240 min all of the test animals were dead. The symptoms observed following injection of P. aeruginosa LPS were different from those observed after injection of P. atlantica LPS because the crabs simply became quiescent and at the time of death their legs sagged and failed to respond to mechanical stimuli. Injection of E. coli LPS (1 μg g of body weight−1) had no significant effect on the THC compared to the effect of the saline controls (Fig. 7). Furthermore, no crabs died in the test period (>48 h postinjection).
DISCUSSION
The present study showed that ECP and LPS from P. atlantica have a potent effect on crabs that leads to rapid paralysis and mortality. As discussed previously (17), the observed effects on the crabs are consistent with a neurotoxic activity of the ECP that causes paralysis and loss of sensory ability of the injected crabs. Similar neurotoxic activity has been reported for both Vibrio alginolyticus and Vibrio anguillarum in bony fish (1). The other LPS preparations tested in the present study with edible crabs (E. coli and P. aeruginosa) failed to produce any of the characteristic neurotoxic symptoms produced by P. atlantica.
The effect of the ECP on the number of hemocytes following injection is similar to the effect reported previously for challenge of crabs with the live P. atlantica isolate or culture filtrate from this bacterium (17). Following injection, there was a rapid decline in the number of circulating hemocytes, and histological examination of the gills showed that many of the cells that disappeared from circulation clumped in the gills at the edges of the hemal channels that feed the lamellae. In some cases these clumps were so extensive that blockage of the gills could have occurred, potentially leading to a reduction in the efficiency of gaseous exchange at this site, but this alone was unlikely to account for the death of the crabs. These clumps, particularly those in the lamellae, were structurally similar to the hemocytic accumulations (termed nodules) reported to form following injection of bacteria and other foreign particulates into crustaceans (12). The main difference in the structure of the accumulations following injection of P. atlantica ECP and injection of bacteria appears to be that the nodules formed in response to bacteria are more compact and consist of a central core of hemocytes and melanized debris surrounded by a sheath of flattened hyaline cells (12). In the present study these accumulations were more diffuse, and this process was not accompanied by melanization. Incubation of hemocytes with ECP in vitro also resulted in the extensive clumping reaction seen in vivo.
Injection of purified LPS from P. atlantica into crabs had no significant effect on the number of circulating hemocytes compared with the effect of the saline challenge. This is markedly different from the results observed after injection of the same dose of P. aeruginosa LPS into edible crabs, which were characterized by a rapid, time-dependent decrease in the THC. Injection of E. coli LPS had no significant effect on the number of circulating hemocytes. Lorenzon et al. (10) recently reported that injection of E. coli 0111:B4 LPS caused a rapid reduction in the number of hemocytes in a variety of species of shrimps and crabs. They also found that the crustacean species most sensitive to LPS (in terms of toxicity) exhibited the greatest drop in the THC, which did not always return to normal within the 48-h experimental period. When LPS did cause death of the crustaceans examined by Lorenzon et al. (10), the time scale of this event was much longer than that observed in the present study with either P. aeruginosa LPS or P. atlantica LPS. In the present study, incubation of crab hemocytes with purified P. atlantica LPS (1 to 20 μg ml−1) resulted in degranulation and vacuolization of the cells, but there was not the aggregatory response seen with the crude P. atlantica ECP, which contained LPS together with other contaminating factors, such as proteins. These observations suggest that the factor(s) responsible for hemocyte aggregation observed both in vivo and in vitro and therefore the reduction in THC is not LPS but the other contaminating factors (proteins or peptides?) in the ECP.
The experiments with proteinase K-treated ECP and the purified LPS from whole P. atlantica bacteria or ECP suggest that the lethal (and potentially neurotoxic) activity is in the LPS fraction. For example, treatment of ECP with proteinase K had no effect on the ability of the preparations to cause death of the crabs or the characteristic symptoms, such as eyestalk retraction, limb paralysis, and loss of antennal sensitivity. Purified LPS extracted from either whole P. atlantica bacteria or ECP injected into crabs produced the same key symptoms as crude ECP produced, and these preparations also resulted in rapid death of such animals. As the LPS preparations from P. atlantica whole bacteria were treated with proteinase K, DNase, and RNase and BCA assays failed to detect any protein in the preparations, such contaminants are unlikely to be the lethal factor. However, the possibility of a synergistic role of other low-molecular-weight, heat-stable factors that contribute to the death of crabs cannot be totally excluded.
It is premature to comment on the importance of the P. atlantica isolate in shell disease syndrome and subsequent intrahemocoelic infection. Indeed, although this isolate is chitinolytic and therefore capable of degrading the cuticle of crustaceans (17), the synergistic role of the other bacteria and fungi present during lesion development should also be considered. For the P. atlantica isolate to be pathogenic for edible crabs, it would have to grow rapidly either in the hemocoel or on the inner margins of the cuticle to produce the levels of LPS required to cause mortality. Initial experiments on the growth of P. atlantica in either whole hemolymph or lysates of the hemocytes have revealed that this bacterium is resistant to any naturally occurring antibacterial factors in the blood (unpublished observations). These results imply that P. atlantica is able to multiply in the hemolymph to a sufficient level to yield the amounts of LPS needed to cause the death of crabs.
Overall, the results of the present study strongly suggest that LPS is the main lethal factor of the P. atlantica isolate and that the reduction in the number of hemocytes observed following injection of ECP or whole bacteria (17) is not caused by LPS. Although there have been no reports of P. atlantica infections in other crustaceans, the pathogenicity of a range of Vibrio spp. for such animals is well documented. In the prawn Nephrops norvegicus, LPS isolated from ECP of a virulent strain of Vibrio harveyi is thought to be the main lethal factor (11). In other vibrio infections of crustaceans, the virulence factors have been reported to be enzymatic. For example, in V. alginolyticus infections of the Kuruma prawn, Penaeus japonicus, a purified serine protease was shown to cause death upon injection (7). Hence, the virulence factors in bacterial diseases of crustaceans are likely to be diverse in terms of structure and mode of action.
Acknowledgments
We thank Claire Vogan for her advice and assistance throughout these studies and Jim Beesley and John Dodd (Guernsey Fishermans Trading Company) for help in collecting crabs.
REFERENCES
- 1.Balebona, M. C., K. Krovacek, M. A. Moriñigo, L. Mansson, A. Faris, and J. J. Borrego. 1998. Neurotoxic effect on two fish species and a PC12 cell line of the supernate of Vibrio alginolyticus and Vibrio anguillarum. Vet. Microbiol. 63:61-69. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier, G., M. Gauthier, and R. Christen. 1995. Phylogenetic analysis of the genera Alteromonas, Shewanella, and Moritella using genes coding for small-subunit ribosomal RNA sequences and division of the genus Alteromonas into two genera, Alteromonas (emended) and Pseudoalteromonas gen. nov., and proposal of 12 new species combinations. Int. J. Syst. Bacteriol. 45:755-761. [DOI] [PubMed] [Google Scholar]
- 3.Getchell, G. C. 1989. Bacterial shell disease in crustaceans: a review. J. Shellfish Res. 8:1-6. [Google Scholar]
- 4.Gu, X., C. Tsai, M. A. Apicella, and D. J. Lim. 1995. Quantification and biological properties of released and cell-bound lipooligosaccharides from nontypeable Haemophilus influenzae. Infect. Immun. 63:4115-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman, M., and A. W. Decho. 2000. Proteolytic enzymes in the marine bacterium Pseudoalteromonas atlantica: post-secretional activation and effects of environmental conditions. Aquat. Microb. Ecol. 23:29-39. [Google Scholar]
- 6.Keler, T., and A. Nowotny. 1986. Metachromatic assay for the quantitative determination of bacterial endotoxins. Anal. Biochem. 156:189-193. [DOI] [PubMed] [Google Scholar]
- 7.Lee, K.-K., S.-R. Yu, and P.-C. Liu. 1997. Alkaline serine protease is an exotoxin of Vibrio alginolyticus in Kuruma prawn, Penaeus japonicus. Curr. Microbiol. 34:110-117. [DOI] [PubMed] [Google Scholar]
- 8.Lee, S. O., J. Kato, N. Takiguchi, A. Kuroda, T. Ikeda, A. Mitsutani, and H. Ohtake. 2000. Involvement of an extracellular protease in algicidal activity of the marine bacterium Pseudoalteromonas sp. strain A28. Appl. Environ. Microbiol. 66:4334-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leon, O., L. Quintana, G. Peruzzo, and J. C. Slebe. 1992. Purification and properties of an extracellular agarase from Alteromonas sp. strain C-1. Appl. Environ. Microbiol. 58:4060-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenzon, S., S. De Guarrini, V. J. Smith, and E. A. Ferrero,. 1999. Effects of LPS injection on circulating haemocytes in crustaceans in vivo. Fish Shellfish Immunol. 9:31-50. [Google Scholar]
- 11.Montero, A., and B. Austin. 1999. Characterization of extracellular products from an isolate of Vibrio harveyi recovered from diseased post-larval Penaeus vannamei (Bonne). J. Fish Dis. 22:377-386. [Google Scholar]
- 12.Smith, V. J., and N. A. Ratcliffe. 1980. Cellular defense reactions of the shore crab, Carcinus maenas: in vivo hemocytic and histopathological responses to injected bacteria. J. Invertebr. Pathol. 35:65-74. [Google Scholar]
- 13.Smolowitz, R. M., R. A. Bullis, and D. A. Abt. 1992. Pathological cuticular changes of winter impoundment shell disease preceding and during intermolt in the American lobster, Homarus americanus. Biol. Bull. (Woods Hole) 183:99-112. [DOI] [PubMed] [Google Scholar]
- 14.Tsai, C.-M., and C. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 15.Vera, J., R. Alvarez, E. Murano, J. C. Slebe, and O. Leon. 1998. Identification of a marine agarolytic Pseudoalteromonas isolate and characterization of its extracellular agarase. Appl. Environ. Microbiol. 64:4378-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogan, C. L., C. Costa-Ramos, and A. F. Rowley. 2001. A histological study of shell disease syndrome in the edible crab Cancer pagurus. Dis. Aquat. Org. 47:209-217. [DOI] [PubMed] [Google Scholar]
- 17.Vogan, C. L., C. Costa-Ramos, and A. F. Rowley. 2002. Shell disease syndrome in the edible crab Cancer pagurus—isolation, characterization and pathogenicity of chitinolytic bacteria. Microbiology 148:743-754. [DOI] [PubMed] [Google Scholar]
- 18.Westphal, O., and K. Jann. 1965. Bacterial lipopolysacharides. Extraction with phenol-water and further applications of the procedure, p. 83-91. In R. L. Whistler (ed.), Methods in carbohydrate chemistry, vol. 5. Academic Press, Inc., New York, N.Y.
- 19.Zhang, X., and B. Austin. 2000. Pathogenicity of Vibrio harveyi to salmonids. J. Fish Dis. 23:93-102. [Google Scholar]